Published online Sep 25, 2024. doi: 10.5527/wjn.v13.i3.95262
Revised: June 15, 2024
Accepted: June 26, 2024
Published online: September 25, 2024
Processing time: 165 Days and 20 Hours
Flavonoids, the main class of polyphenols, exhibit antioxidant and antihypertensive properties.
To prospectively investigate the impact of flavonoids on arterial stiffness in patients with chronic kidney disease (CKD) stages I-IV.
In this prospective, single-arm study, CKD patients with arterial hypertension and diabetes mellitus were enrolled. Baseline demographic, clinical, and laboratory variables were recorded. Patients received daily treatment with a phenol-rich dietary supplement for 3 months. Blood pressure, arterial stiffness (carotid-femoral pulse wave velocity, central pulse pressure), and oxidative stress markers (protein carbonyls, total phenolic compound, total antioxidant capacity) were measured at baseline and at study end.
Sixteen patients (mean age: 62.5 years, 87.5% male) completed the study. Follo
Flavonoid supplementation in CKD patients shows promise in improving blood pressure, arterial stiffness, and oxidative stress markers.
Core Tip: Daily flavonoid supplementation in patients with chronic kidney disease stage I-IV demonstrated significant reductions in peripheral systolic blood pressure, carotid-femoral pulse wave velocity, and central pulse pressure, along with improvements in oxidative stress markers. These findings suggest that flavonoids hold promise as an adjunctive therapy to manage hypertension, arterial stiffness, and oxidative stress in chronic kidney disease patients, potentially mitigating cardiovascular risk in this population.
- Citation: Vagopoulou A, Theofilis P, Karasavvidou D, Haddad N, Makridis D, Tzimikas S, Kalaitzidis R. Pilot study on the effect of flavonoids on arterial stiffness and oxidative stress in chronic kidney disease. World J Nephrol 2024; 13(3): 95262
- URL: https://www.wjgnet.com/2220-6124/full/v13/i3/95262.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i3.95262
Flavonoids, encompassing various subclasses such as flavonols, flavones, catechins, anthocyanins, isoflavones, dihydroflavonols, and chalcones, are prevalent in vegetables, fruits, nuts, spices, herbs, red wine, and tea[1]. As primary polyphenols, flavonoids exert pharmaceutical, antioxidant, anti-inflammatory, and cardioprotective effects[2]. Further studies support the diuretic action of the flavonoids via natriuresis as well as anti-diabetic effects[3]. Researchers have linked the diverse effects of flavonoids on vascular health, particularly arterial stiffness, an independent cardiovascular risk factor[4]. This study aimed to investigate the impact of flavonoids on arterial stiffness in patients with chronic kidney disease (CKD) stages I-IV following the intake of polyphenol-rich dietary supplements.
This pilot prospective, single-arm intervention study took place at the Nephrology Clinic “C. Katsinas” of the General Hospital of Ptolemaida “Mpodosakeio” from November 2021 to April 2022. Patients meeting inclusion criteria [aged ≥ 18 years, cognitively competent, diagnosed with arterial hypertension (per European Society of Cardiology/European Society of Hypertension 2018 guidelines or on antihypertensive medication), type 2 diabetes mellitus, and CKD stage I-IV) participated. Exclusion criteria encompassed chronic atrial fibrillation, congestive heart failure New York Heart Association class III-IV, recent acute myocardial infarction or anginal symptoms, mental illness or dementia, active malignancy, alcohol abuse, inability/unwillingness to provide consent, or known flavonoid allergies.
Participants received a daily oral dietary supplement rich in phenols (200 mg) for 3 months. The supplement composition included fats (36.5 g/100 g), proteins (10 g/100 g), and carbohydrates (40.8 g/100 g), providing 532 kcal/100 g. Patients underwent clinical examinations, laboratory tests, and arterial stiffness assessments via carotid-femoral pulse wave velocity (cfPWV) at enrollment and after 3 months. Oxidative stress indicators (total phenols, total antioxidant capacity, protein carbonyls) were also evaluated.
All patients provided written informed consent after receiving comprehensive study information. The Ethics Committee of the General Hospital of Ptolemaida approved the study (80/18-11-2021), which adhered to the Declaration of Helsinki (1989).
Arterial stiffness was evaluated by measurement of cfPWV at enrollment and 3 months later by an experienced operator blinded to the aims of the study. PWV measures the speed at which a pressure wave travels along the aorta and great arteries, reflecting the elasticity and compliance of these vessels. cfPWV is generally accepted as the “gold standard” measurement in evaluating aortic stiffness and is an important predictor of cardiovascular events. It can be calculated by dividing the distance traveled between two recording sites by the pulse transit time (PWV = distance in meters/by transit time in seconds). In this study, cfPWV was measured using the validated SphygmoCor device (AtCor Medical, IL, United States)[5-8]. All participants were positioned in a quiet environment with stable temperatures and rested in a supine position for a minimum of 10 min.
The measurements were conducted on the right common carotid and the right femoral artery. Participants were instructed to maintain silence during the measurements. Distances were measured directly with a tape measure: From the suprasternal notch to the femoral artery, and from the carotid artery to the suprasternal notch. The difference between these two measurements was calculated[6]. PWV was calculated by determining the pulse transit time and the distance between the two measurement sites. An electrocardiogram was recorded concurrently to align the pressure waves. All measurements were performed by the same experienced operator.
The radial pressure waveforms were calibrated using sphygmomanometric systolic and diastolic blood pressures taken from the brachial artery. The augmentation index of the central (aortic) pressure waveform was then estimated to assess wave reflection. This index reflects the interplay between wave reflection magnitude and arterial stiffness, with arterial stiffness influencing the timing of wave reflections.
The concentration of total phenols was estimated according to the Folin-Ciocalteu method[9]. For the definition of the plasma total antioxidant capacity (TAC), the 2,2-diphenyl-1-picrylhydrazyl method was used. It is based on the use of the free radicals used for assessing the potential of substances to serve as hydrogen providers or free-radical scavengers. The results were shown as a percentage of antioxidant ability according to the following equation: AC% = (AT–AD) × 100/AT, where AT (means) represents the blind absorption to photometry to 520nm and AD represents sample absorption to photometry to 520 nm.
Finally, the concentration of carbonyls was detected based on their reaction with 2,4-dinitrophenylhydrazine to form a 2,4-dinitrophenylhydrazone, and it was expressed as nmol/mL[10].
The normality of the distribution of continuous variables was assessed by the Shapiro-Wilk test. Continuous variables that follow a normal distribution were presented as mean ± SD. In the case of non-normally distributed continuous variables, data were presented as median with the interquartile range. Categorical variables were displayed as percentages. For the over-time comparison of normally continuous variables, we used the parametric paired sample t-test and the non-parametric Wilcoxon signed ranked test, accordingly. For the assessment of differences in cfPWV across the different CKD stages, a repeated measures analysis of variance was conducted, using the Benferroni correction.
All statistical calculations were performed using SPSS software (version 25.0; SPSS Inc., Chicago, IL, United States). All reported P values were based on two-sided hypotheses, with a P value of 0.05 being considered statistically significant.
Seventeen patients participated in the study between November 2021 and April 2022. One patient died due to acute myocardial infarction, and 16 patients completed the study. The mean age of the participants was 62.5 years and 87.5% were male. The remaining baseline characteristics and laboratory tests of the study population are presented in Tables 1 and 2. During the study, we did not observe any significant changes in serum creatinine of the participants (P = 0.75).
| Parameter | Value |
| Age, years | 62.5 ± 8.2 |
| Male | 14 (87.5) |
| CKD stage | |
| I | 6 (37.5) |
| II-IIIa | 6 (37.5) |
| IIIb-IV | 4 (25.0) |
| eGFRCKD-EPI, mL/min/1.73m2 | 84 (47.50, 98.75) |
| Parameter | Value |
| Hct, % | 42.3 ± 5.2 |
| Hb, g/dL | 14.3 ± 1.9 |
| PLT, 103/μL | 222 ± 51 |
| Urea, mg/dL | 51 (26, 70) |
| Creatinine, mg/dL | 1.3 (0.77, 1.52) |
| Urine albumin, mg/24 h | 183 (91.2, 949.0) |
| Sodium, mmol/L | 139 ± 2 |
| Potassium, mmol/L | 4.9 (4.72, 5.00) |
| LDH, IU/L | 183 ± 43 |
| AST, IU/L | 23 (15, 27) |
| ALT, IU/L | 30 (18, 37) |
| Total serum protein, g/dL | 6.7 ± 0.7 |
| Serum albumin, g/dL | 3.9 ± 0.8 |
| Total cholesterol, mg/dL | 152 ± 41 |
| LDL-C, mg/dL | 80 ± 31 |
| Triglycerides, mg/dL | 148 ± 59 |
| Uric acid, mg/dL | 5.8 ± 1.6 |
| Calcium, mg/dL | 9.6 (9.4, 9.9) |
| PTH, pg/mL | 68 (40, 72) |
| CRP, mg/dL | 0.52 (0.06, 0.31) |
| Fe, mg/dL | 86 ± 32 |
| Ferritin, ng/mL | 201 (97, 299) |
| TIBC, mg/dL | 288 (241, 341) |
| HbA1c, % | 7.58 ± 1.63 |
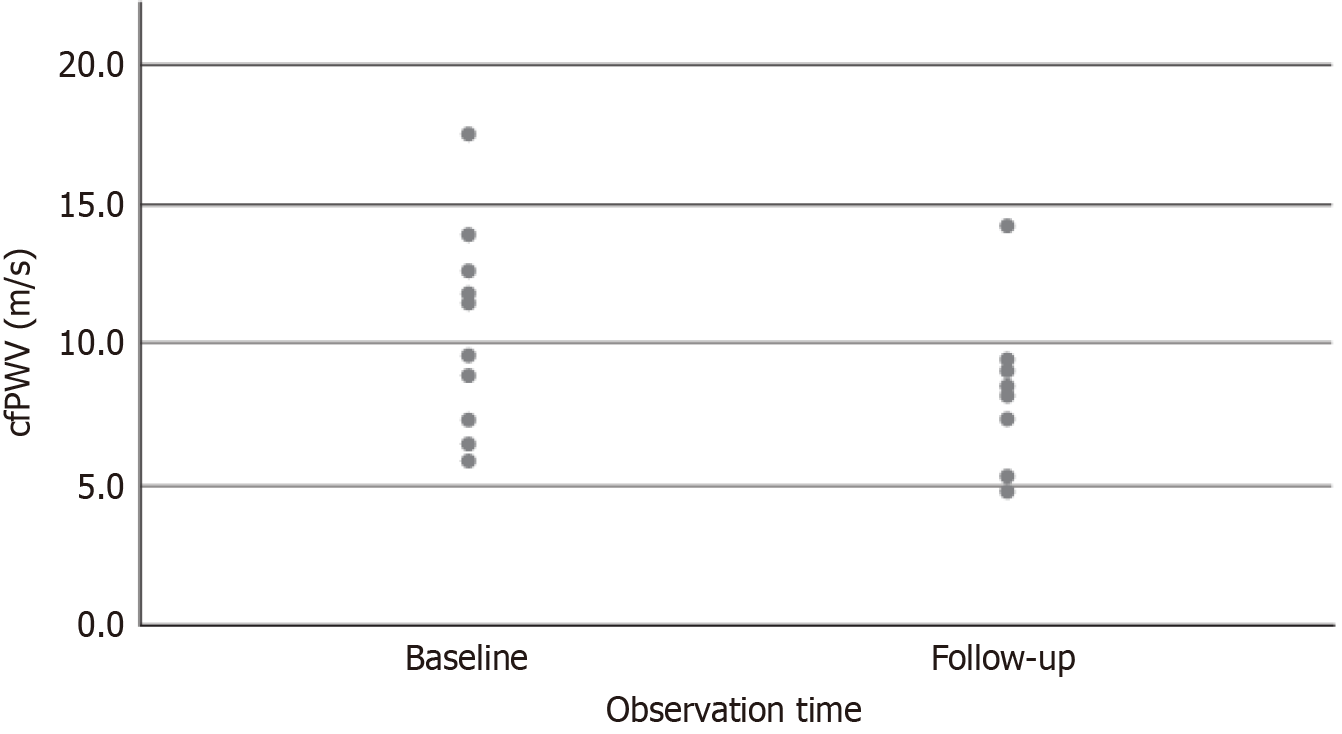
The alterations in arterial stiffness measurements following the administration of flavonoids are presented in Table 3. After the intervention, there was a statistically significant reduction in the peripheral systolic blood pressure by 14 mmHg (P < 0.001). The mean cfPWV at baseline was estimated at 8.9 m/s (6.7, 11.8) and was markedly reduced to 8.2 m/s (5.1, 9.2); a difference that was statistically significant (P < 0.001) (Figure 1). The central pulse pressure was reduced after flavonoid administration from 59 mmHg (44, 69) at baseline to 48 mmHg (37, 60) at the end of the study (P = 0.003).
| Parameter | Baseline | End of study | P value |
| PSBP, mmHg | 148 ± 16 | 134 ± 13 | < 0.001 |
| PDBP, mmHg | 81.00 ± 8.00 | 76.00 ± 6.32 | 0.051 |
| cfPWV, m/s | 8.85 (6.7, 11.8) | 8.20 (5.1, 9.2) | < 0.001 |
| CSBP, mmHg | 156 ± 23 | 137 ± 16 | 0.004 |
| CDBP, mmHg | 90 ± 14 | 80 ± 10 | 0.002 |
| CPP, mmHg | 59 (44, 69) | 48 (37, 60) | 0.003 |
| CHR, pulse/min | 69 ± 14 | 66 ± 9 | 0.370 |
| AIx, % | 26.5 ± 10.0 | 32.5 ± 13.3 | 0.510 |
| Ap | 17.44 (9.50, 21.00) | 14.25 (9.50, 18.75) | 0.780 |
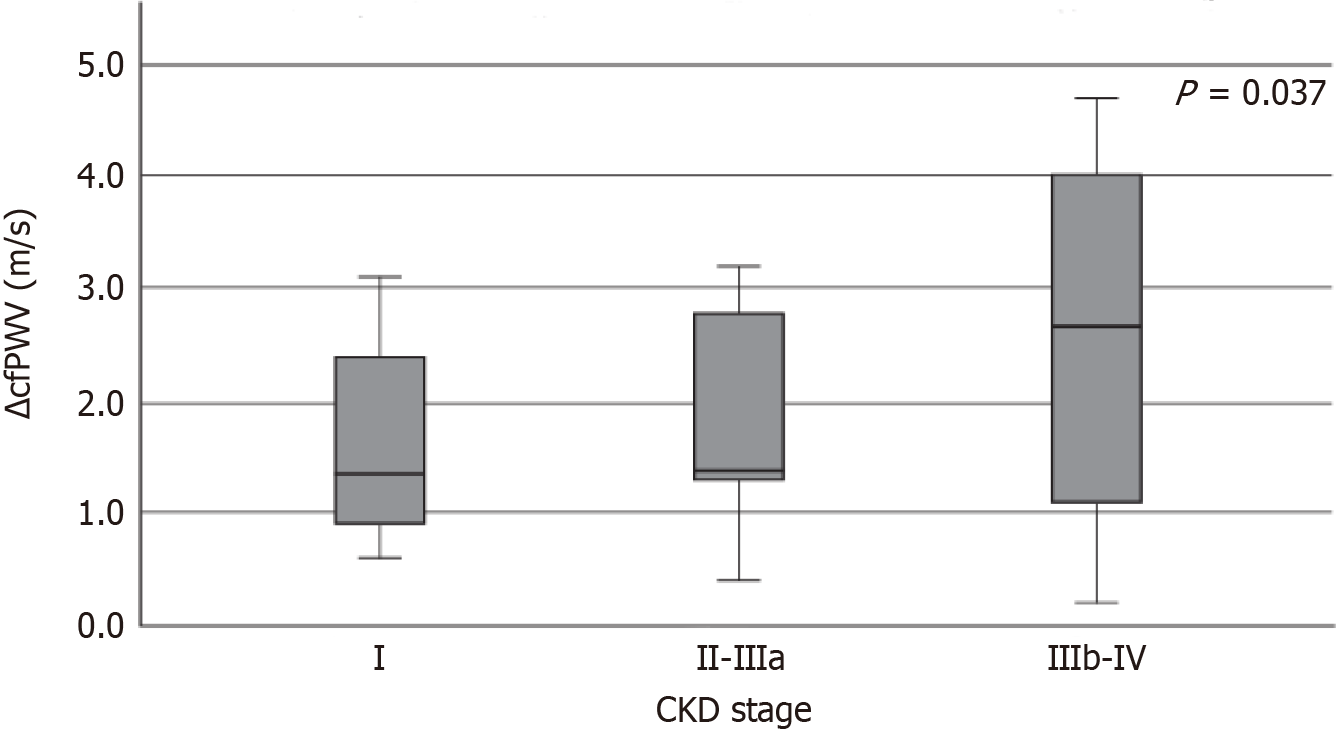
We also examined whether the alteration of cfPWV differed proportionally according to the stage of CKD. The analysis showed that the changes in cfPWV differed significantly among the CKD stages (P = 0.037) (Figure 2). After pairwise analysis among the CKD stages and the alteration of cfPWV, a statistically significant difference was noted between stages I and IIIb-IV (P = 0.042).
The changes in parameters of oxidative stress before and after flavonoid administration are displayed in Table 4. After the flavonoid administration, a reduction of plasma proteinic carbonyl was observed (73.50 ± 18.65 nmol/mL at baseline vs 52.54 ± 25.04 nmol/mL at the 3-month follow-up, P < 0.001). Moreover, the total phenolic compound concentration was significantly enhanced [25.11 mg/mL (16.95, 30.29) at baseline vs 31.91 mg/mL (30.49, 47.51) at the 3-month follow-up, P = 0.001]. The TAC also appeared augmented after the intervention [3.55% (1.15, 6.38) at baseline vs 12.51% (6.26, 17.66) at the 3-month follow-up, P = 0.013].
| Parameter | Baseline | End of study | P value |
| Proteinic carbonyls, nmol/mL | 73.50 ± 18.65 | 52.54 ± 25.04 | < 0.001 |
| TPC, mg/mL | 25.11 (16.95, 30.29) | 31.91 (30.49, 47.51) | 0.001 |
| ΤAC, % | 3.55 (1.15, 6.38) | 12.51 (6.26, 17.66) | 0.013 |
According to the recent guidelines for the non-pharmaceutical interventions of the International Society of Hypertension[11], antioxidant therapeutics are recommended as a means to reduce the production of reactive oxygen species and oxidative stress. It is well known that the abundance of reactive oxygen species and the promotion of oxidative stress play an important role in the pathogenesis of hypertension[12]. This phenomenon further leads to endothelial dysfunction and an impaired balance of vasoactive compounds such as the vasodilating nitric oxide and the vasoconstrictive angiotensin II and endothelin[12,13].
Polyphenols, which are commonly known antioxidant substances, have proven their beneficial role in hypertension[14]. The antihypertensive effect of flavonoids is mostly based on the protection of the endothelium[15] and the inhibition of the renin-angiotensin-aldosterone system[16]. In our study, we noted significant alterations in the parameters of diastolic blood pressure and central pressure after 3 months of treatment with flavonoids. Such findings are in line with previously published data[17].
Critically, we also observed a significant amelioration of arterial stiffness measures after treatment with flavonoids. Several studies have reported that flavonoids may affect arteriosclerosis and arterial stiffness. Specifically, Curtis et al[18] noted a reduction of PWV and central pressure in post-menopausal females with the administration of chocolate rich in cocoa with flavonoids after a 1-year follow-up. Nestel et al[19] made similar conclusions since they observed a reduction in PWV and blood pressure after the consumption of flavonoids by overweight males and post-menopausal females. The same reduction in PWV after the intake of flavonoids was observed in the general population. However, evidence is lacking in the CKD population. Our study is the sole prospective study to assess the importance of flavonoid administration in patients with CKD.
It is noteworthy that different types of flavonoids have been used as antioxidants across the studies. In this study, we utilized a flavonoid mixture containing cocoa, lemon balm rich in rosmarinic acid, caffeic acid, rockrose, and pome
Flavonoids act as exogenous antioxidants due to their ability to give electrons to the roots of hydrogen Peroxide, hydroxyl, and hyperoxyl, stabilizing the aforementioned roots by reducing the levels of free radicals in the human body[23]. In our study, after the administration of flavonoids, we observed a reduction of the plasma proteinic carbonyls and an increase of the total phenolic content and TAC. Our data suggests that the antioxidant effects of flavonoids may also extend to patients with CKD. Previous studies have assessed the effects of polyphenols and especially flavonoids in CKD[17]. Cao et al[17], in a recent review, analyzed the effects of flavonoids in different types of CKD such as diabetic nephropathy, glomerulonephritis, and lupus nephritis. There seems to be an improved antioxidant effect in patients with CKD, which appears to be sufficient in decelerating the progression of the disease. In our study, this hypothesis could not be adequately tested due to the small sample size and the brief period of observation (3 months).
In conclusion, this pilot study showed that the administration of flavonoids in patients with CKD appears to have a positive effect on blood pressure, arterial stiffness, and oxidative stress measures. Future studies are needed to further test their potential in this patient population.
| 1. | Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673-751. [PubMed] |
| 2. | Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5019] [Cited by in RCA: 4515] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 3. | Vargas F, Romecín P, García-Guillén AI, Wangesteen R, Vargas-Tendero P, Paredes MD, Atucha NM, García-Estañ J. Flavonoids in Kidney Health and Disease. Front Physiol. 2018;9:394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 4. | Lilamand M, Kelaiditi E, Guyonnet S, Antonelli Incalzi R, Raynaud-Simon A, Vellas B, Cesari M. Flavonoids and arterial stiffness: promising perspectives. Nutr Metab Cardiovasc Dis. 2014;24:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Kim HL, Kim SH. Pulse Wave Velocity in Atherosclerosis. Front Cardiovasc Med. 2019;6:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 6. | Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1380] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 7. | O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 761] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 8. | Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S-10S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 559] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 9. | Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology: Academic Press, 1999: 152-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8745] [Cited by in RCA: 7841] [Article Influence: 301.6] [Reference Citation Analysis (0)] |
| 10. | Patsoukis N, Zervoudakis G, Panagopoulos NT, Georgiou CD, Angelatou F, Matsokis NA. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci Lett. 2004;357:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Buelt A, Richards A, Jones AL. Hypertension: New Guidelines from the International Society of Hypertension. Am Fam Physician. 2021;103:763-765. [PubMed] |
| 12. | Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 632] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 14. | Larson AJ, Symons JD, Jalili T. Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv Nutr. 2012;3:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Lin X, Han T, Fan Y, Wu S, Wang F, Wang C. Quercetin improves vascular endothelial function through promotion of autophagy in hypertensive rats. Life Sci. 2020;258:118106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Bruno RM, Ghiadoni L. Polyphenols, Antioxidants and the Sympathetic Nervous System. Curr Pharm Des. 2018;24:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Cao YL, Lin JH, Hammes HP, Zhang C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Curtis PJ, Potter J, Kroon PA, Wilson P, Dhatariya K, Sampson M, Cassidy A. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: a double-blind randomized controlled trial. Am J Clin Nutr. 2013;97:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Nestel P, Fujii A, Zhang L. An isoflavone metabolite reduces arterial stiffness and blood pressure in overweight men and postmenopausal women. Atherosclerosis. 2007;192:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Migliori M, Cantaluppi V, Mannari C, Bertelli AA, Medica D, Quercia AD, Navarro V, Scatena A, Giovannini L, Biancone L, Panichi V. Caffeic acid, a phenol found in white wine, modulates endothelial nitric oxide production and protects from oxidative stress-associated endothelial cell injury. PLoS One. 2015;10:e0117530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Du Y, Han J, Zhang H, Xu J, Jiang L, Ge W. Kaempferol Prevents Against Ang II-induced Cardiac Remodeling Through Attenuating Ang II-induced Inflammation and Oxidative Stress. J Cardiovasc Pharmacol. 2019;74:326-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Spencer JP. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2843] [Cited by in RCA: 1977] [Article Influence: 164.8] [Reference Citation Analysis (1)] |