Published online Mar 25, 2024. doi: 10.5527/wjn.v13.i1.90402
Peer-review started: December 4, 2023
First decision: December 28, 2023
Revised: January 3, 2024
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: March 25, 2024
Processing time: 108 Days and 11.4 Hours
Polycystic kidney disease (PKD) is the most common genetic cause of kidney disease. It is a progressive and irreversible condition that can lead to end-stage renal disease and many other visceral complications. Current comprehensive data on PKD patterns in Africa is lacking.
To describe the prevalence and outcomes of PKD in the African population.
A literature search of PubMed, African journal online, and Google Scholar databases between 2000 and 2023 was performed. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses were followed to design the study. Clinical presentations and outcomes of patients were extracted from the included studies.
Out of 106 articles, we included 13 studies from 7 African countries. Ten of them were retrospective descriptive studies concerning 943 PKD patients with a mean age of 47.9 years. The accurate prevalence and incidence of PKD were not known but it represented the third causal nephropathy among dialysis patients. In majority of patients, the diagnosis of the disease was often delayed. Kidney function impairment, abdominal mass, and hypertension were the leading symptoms at presentation with a pooled prevalence of 72.1% (69.1–75.1), 65.8% (62.2–69.4), and 57.4% (54.2–60.6) respectively. Hematuria and infections were the most frequent complications. Genotyping was performed in few studies that revealed a high proportion of new mutations mainly in the PKD1 gene.
The prevalence of PKD in African populations is not clearly defined. Clinical symptoms were almost present with most patients who had kidney function impairment and abdominal mass at the diagnostic. Larger studies including genetic testing are needed to determine the burden of PKD in African populations.
Core Tip: Polycystic kidney disease is the most common genetic disorder affecting the kidney. The two main forms are autosomal dominant polycystic disease and autosomal recessive polycystic disease. It can lead to numerous complications with a natural progression leading to End stage kidney disease. Though the disease is well known and described in developed countries, its characteristics are still poorly understood in Africa. Indeed, as it appear in the present review, few studies regarding this disease were performed in the continent but reveal that advanced symptoms are already present in most of patients at the time of the diagnostic and the few studies with genetic testing revealed many new mutations.
- Citation: Ndongo M, Nehemie LM, Coundoul B, Diouara AAM, Seck SM. Prevalence and outcomes of polycystic kidney disease in African populations: A systematic review. World J Nephrol 2024; 13(1): 90402
- URL: https://www.wjgnet.com/2220-6124/full/v13/i1/90402.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i1.90402
Polycystic kidney disease (PKD) is the leading hereditary cause of chronic kidney disease. Autosomal dominant PKD (ADPKD) is its most frequent type with a reported prevalence to be between 1 in 400 and 1 in 1000 Live births in the world and is typically diagnosed later in life than autosomal recessive PKD (ARPKD)[1]. The prevalence, clinical and prognosis patterns of the disease are now well-documented in high-income countries. These advances have led to new therapeutic approaches that help slowing disease progression[2]. However, in low-resource settings such as in African countries, the lack of robust data on epidemiology, clinical presentation and prognosis of PKD are scarce. Also, a later diagnosis, fewer access to healthcare and new treatments are all factors that can explain a different epidemiology. We performed this systematic review to clarify the prevalence and outcomes of PKD in the African population.
This systematic review was conducted in October 2023 to assess the prevalence and outcomes of PKD in the African populations. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were followed to shape the study design[3].
A literature search of relevant articles published from January 2000 to September 2023 were performed on the online database PubMed, African journal online and google scholars. We also screened references of included articles to identify other potential studies. The keywords used for searching included: “Polycystic kidney disease” and “each of the 54 African countries name”. The search was realized by using different combination of these terms.
We included: (1) Observational studies with a description of the number of PKD cases; (2) Studies that offer a description of clinical manifestations at presentation; (3) Studies published in English or French; and (4) Case reports and case series with descriptions of genetic anomalies were also included.
Studies were excluded if they presented any one or more of the following criteria: case report, case series, abstracts, commenters or letter to the editor, systematic review and meta-analysis; language other than French or English, and study with age restriction of the participant.
After eliminating duplicates, the titles and abstracts of all articles were reviewed and full texts of all articles designated for inclusion was obtained to ensure that they met the criteria for inclusion in this analysis.
For each study, we extracted the following data: study design, country, number of subjects included, demographic characteristics of patients, symptoms (hypertension, flank pain, hematuria, kidney function impairment), genetic mutation, complications, and prognosis.
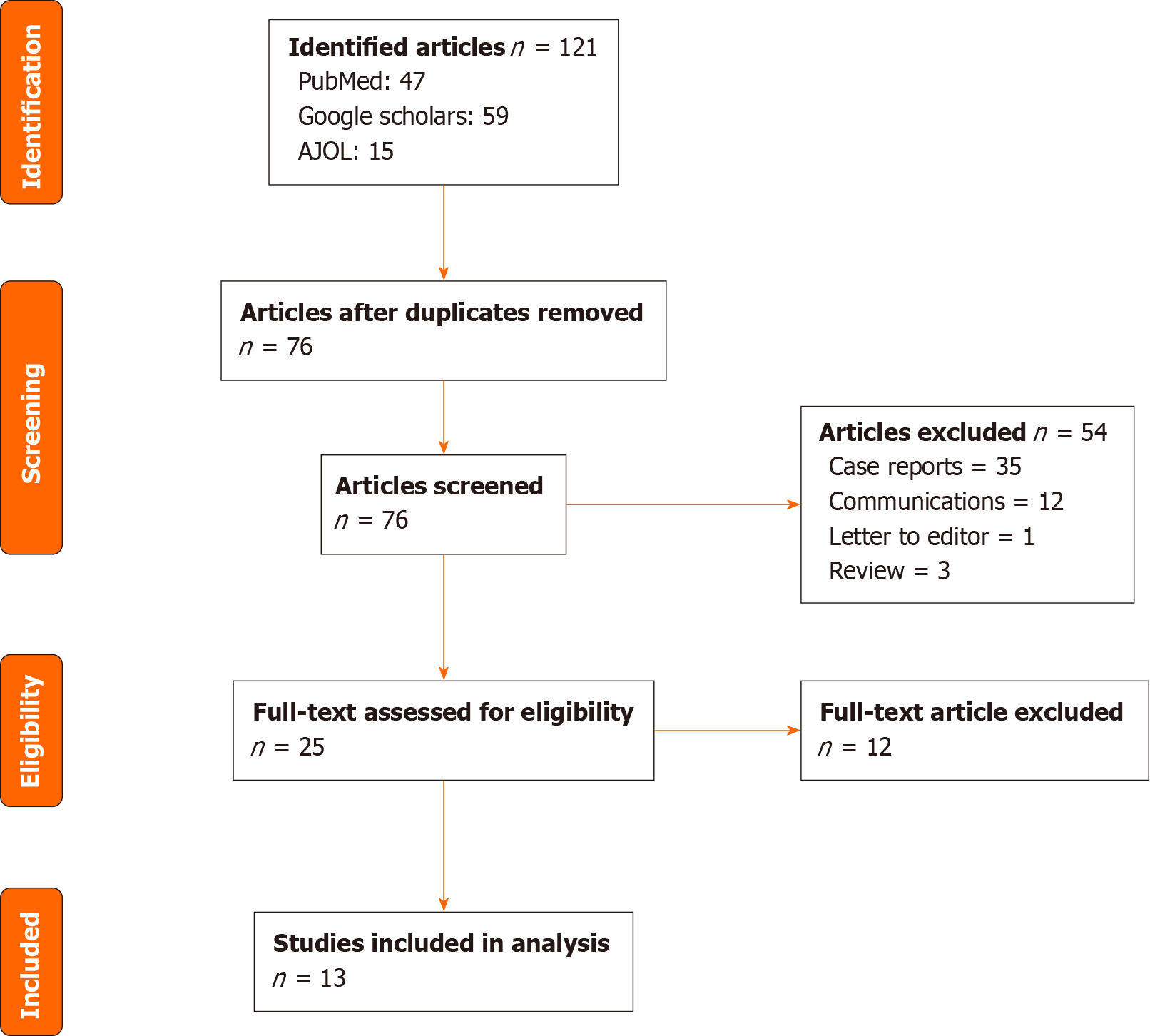
Figure 1 present the PRISMA flow diagram detailing the review shape and studies selection process. We included 13 studies from different countries as detailed in Table 1. Ten of them were retrospective observational descriptive studies[4-13] and 3 were cases reports with genetic testing performed[14-16].
| Ref. | Country | Patients | Design | Study period | Number of cases | Mean age (yr) | Gender M/F |
| Hajji et al[4], 2019 | Tunisia | ADPKD | Observational descriptive | 1969-2016 | 569 | 48.5 | 297/272 |
| Arogundade et al[5], 2018 | Nigeria | ADPKD | Observational descriptive | 1996-2010 | 41 | 48.6 4.6 | 23/18 |
| Chijioke et al[6], 2010 | Nigeria | ADPKD | Observational descriptive | 1994-2009 | 78 | 49.8 3.6 | 52/26 |
| Ogiator et al[7], 2021 | Nigeria | ADPKD | Observational descriptive | 2013-2020 | 19 | 42.8 16.9 | 10/9 |
| Mawufemo et al[8], 2018 | Togo | ADPKD | Observational descriptive | 2010-2017 | 27 | 51.6 16.4 | 10/17 |
| Fary Ka et al[9], 2010 | Senegal | ADPKD | Observational descriptive | 1995-2005 | 55 | 47.0 5.0 | 31/24 |
| Okyere et al[10], 2021 | Ghana | ADPKD | Observational descriptive | 2007-2018 | 82 | 43.8 15.7 | 43/39 |
| Laleye et al[11], 2012 | Benin | ADPKD | Observational descriptive | 2000-2010 | 32 | 47.2 | 17/15 |
| Abdelwahed et al[12], 2022 | Tunisia | ADPKD | Observational descriptive | NA | 19 | 47 18 | 10/9 |
| Abdelwahed et al[13], 2018 | Tunisia | ADPKD | Observational descriptive | NA | 18 | 45 | 8/10 |
| Seck et al[14], 2013 | Senegal | ADPKD | Case report | NA | 1 | 41 | 0/1 |
| Sahnoun et al[16], 2015 | Tunisia | ADPKD | Case report | NA | 1 | 52 | 1/0 |
| Nabhan et al[15], 2015 | Egypt | ARPKD | Case report | NA | 1 | 2.5 | 0/1 |
| Total | 943 | 47.9 | 502/441 |
A total of 943 patients with PKD were collected. The mean age were 47.9 years with a sex-ratio M/F of 1.14.
Clinical symptoms were described in all the descriptive studies. Overall, kidney function impairment, abdominal mass and hypertension were the most frequent finding at presentation, present in 72.1%, 65.8% and 57.4% of patients res
| Ref. | HTN (%) | Pain (%) | Hematuria (%) | Abdominal mass (%) | KFI (%) | ESKD (%) |
| Hajji et al[4], 2019 | 58.8 | 51.9 | 24.6 | 66.0 | 74.7 | 23.0 |
| Arogundade et al[5], 2018 | 87.8 | 68.3 | 36.6 | 82.9 | 100.0 | 19.5 |
| Chijioke et al[6], 2010 | 26.9 | 14.1 | NA | NA | 32.0 | NA |
| Ogiator et al[7], 2021 | 42.1 | 68.4 | 31.6 | NA | 63.2 | 15.8 |
| Mawufemo et al[8], 2018 | 77.8 | 63.0 | 22.2 | 63.0 | 63.0 | 25.9 |
| Fary Ka et al[9], 2010 | 65.4 | 52.7 | 25.4 | NA | NA | NA |
| Okyere et al[10], 2021 | 50.0 | 39.0 | 2.4 | NA | 81.7 | 15.9 |
| Laleye et al[11], 2012 | 59.0 | 62.0 | 46.0 | 43.0 | 72.0 | NA |
| Abdelwahed et al[12], 2022 | 57.9 | NA | 26.3 | NA | 89.5 | NA |
| Abdelwahed et al[13], 2018 | 72.2 | NA | NA | NA | NA | NA |
| Pooled prevalence [95%IC] | 57.4 [54.2–60.6] | 49.1 [45.8–52.3] | 24.0 [21.1–26.9] | 65.8 [62.2–69.4] | 72.1 [69.1–75.1] | 21.9 [18.9–24.9] |
Genetics testing were performed in 5 study with a cumulated total of 40 patients[12-16]. All these patients had genetic disorders with 13 novels mutation/single nucleotide polymorphism detected. The most frequently reported mew mutations were c.496 C>T, p.L166 among exon4; c.696 T>G, p.C232W among exon5; c.7290_7291delinsCTGCA among exon18 and c.12276 A>G, p.A4092 among exon45 in the PKD1 gene (Table 3). The mutations concerned in 92.5% of cases the PKD1 gene and in 7.5% the PKD2 gene. In sub-Saharan Africa, seven new mutations were reported from Benin and one from a Senegal[11,14].
| Gene | Exon/Intron | Nucleotide change | Aminoacid change | Number of patients | Ref. |
| PKD 1 | 4 | c.496 C>T | p.L166 | 2 | [12] |
| 5 | c.696 T>G | p.C232W | 2 | [12,13] | |
| 5 | c.688 T>G | p.C230G | 1 | [12] | |
| 5 | c.690 C>G | p.C230W | 1 | [12] | |
| 7 | c.1522 T>C | p.C508R | 1 | [12] | |
| 8 | c.1702 G>A | p.A568T | 1 | [12] | |
| 9 | c.1745_1761dup | NA | 1 | [11] | |
| 15 | c.4264 G>A | p.A1422T | 1 | [12] | |
| 15 | c.5577 T>C | p.A1859 | 1 | [12] | |
| 15 | c.4495 C>T | p.L1499 | 2 | [12] | |
| 15 | NA | p.Q1651X | 1 | [11] | |
| 15 | NA | p.W1666X | 1 | [11] | |
| 15 | NA | c.6575_6581del | 1 | [11] | |
| 18 | c.7290_7291delinsCTGCA | NA | 2 | [11,14] | |
| IVS22 | c.8161-1 G>A | Likely silent | 4 | [12] | |
| IVS42 | c.11712+28 C>T | Likely silent | 1 | [12] | |
| 23 | c.8679 C>G | p.S893 | 1 | [12] | |
| 23 | c.8715 C>T | p.V2905 | 1 | [12] | |
| 23 | c.8748 T>C | p.P2916 | 2 | [12] | |
| 23 | c.8522 A>G | p.N2841S | 1 | [12] | |
| 23 | NA | p.Q2824X | 1 | [11] | |
| 26 | c.9397+1_9397+8del | NA | 1 | [11] | |
| 28 | c.9669 G>A | p.T3223 | 2 | [12] | |
| 30 | c.3367G>A | p.G1123S | 1 | [15] | |
| 31 | c.10165 G>C | p.E3389Gln | 1 | [12] | |
| 44 | c.12133 A>G | p.I4045V | 1 | [12] | |
| 45 | c.12276 A>G | p.A4092 | 2 | [12,16] | |
| PKD 2 | 1 | c.568 G>A | p.A190T | 2 | [12] |
| 1 | c.83 G>C | p.A28P | 2 | [12] | |
| IVS1 | c.596-16 C>T | Likely silent | 1 | [12] | |
| PKHD1 | c.3367G>A | p.G1123S | 1 | [15] |
One case of ARPKD were reported in Egypt with a mutation (c.3367G>A, p.G1123S) in PKHD1[15].
PKD is a major public health problem that concerns all continents and ethnic groups. It is an incurable condition with a natural evolution leading to end-stage renal disease and can cause many other visceral complications. ADPKD and ARPKD are its two main types. ADPKD is commonly described in adults, whereas ARPKD is less frequent and usually presents during early childhood. ADPKD is the most frequent genetic cause of renal failure in adults, accounting for 6%–10% of end-stage renal disease cases. Its reported prevalence is similar around the globe. In the United States the reported diagnostic prevalence of ADPKD was 4.3 per 10000[17]. A large review study including 19 European countries revealed a prevalence of 3.96 per 10000[18]. However, as it appears in this review, the prevalence of PKD in Africa remains difficult to establish. Indeed, only one study from the Seychelles has reported a nationwide prevalence of 5.7 per 10000[19]. A broad range of mutations in PKD genes can lead to ADPKD. These disorders are widely distributed and can occur across the entire sequence of these genes, named PKD1 and PKD2. The PKD1 gene region is larger and counts 46 exons and its mutations are responsible for around 85% of cases[20]. The PKD2 region is shorter and comprise only 15 exons with mutations causing 15% of ADPKD cases[20]. More than 1500 mutations of these two genes are indexed in ADPKD mutation databases[21]. Genotyping is usually necessary in persons with suspected PKD who do not meet the echographic criteria and/or compatible familial history. In the United Kingdom, new PKD1 mutations represented 5% of ADPKD patients[22]. Results of a few genetic tests performed in African patients found that 90% of mutations were located in the PKD1 gene and 48.1% of them were new mutations not previously described in non-African populations. Such findings expose the need for broader genetic testing for a better PKD description in the continent. Despite similar clinical manifestations, mutations in the PKD1 gene are associated with an earlier onset of symptoms and ESKD compared to PKD2 mutation[23]. In the literature, the reported ages at ADPKD diagnosis were 42 and 56 years, res
Furthermore, in the majority of patients, clinical symptoms are already present at diagnosis. Hypertension, abdominal mass, flank pain, hematuria, urolithiasis, infection, and kidney function impairment were the main symptoms reported in African patients with ADPKD. The pooled proportion of ESKD was 21.9% and comparable to data from France and Canada where 22% and 25% respectively presented with ESKD at the time of diagnosis[25,26].
In the United States, ADPKD is the fourth leading cause of ESKD requiring dialysis and transplantation[27].
Less common than ADPKD, ARPKD is a childhood-onset disease with symptoms that can appear in perinatal. It is linked to the mutations PKHD1 gene with an estimated prevalence of 1 in 20000 live births in Caucasians[28]. In Africa, its prevalence is still not known, one case was reported in an Egyptian child. A mean age at diagnosis of 4 years was reported with around 60% of patients with ESKD before adulthood[29].
PKD represents the most frequent genetic disorder. ADPKD is by far more frequent than ARPKD. In Africa, little data on the prevalence, clinical presentation, and evolution of this disease are available, and genetic testing is even more lacking. Clinical symptoms were almost present with most patients who had kidney function impairment and abdominal mass at the diagnosis. As shown in this review, many new mutations were found in the PKD1 gene. More large-scale studies are needed to describe the patterns of these diseases.
Polycystic kidney disease is known as the most common genetic cause of chronic kidney disease. Its natural evolution lead to end-stage kidney disease. However, unlike developed countries, clinical and prognosis outcomes data of the disease are lacking in African population.
Mapping the data of polycystosis in African population and emphasize the gap between data from international literature and those available in our specific population and outline points for further studies.
Describe the prevalence, clinical, and genetic aspects of polycystic kidney disease in an African population.
A literature review and meta-analysis of available data were performed from January 2000 to September 2023 to identify reported data of prevalence, clinical manifestation, and genetics anomalies of patients with polycystic kidney disease in the continent.
A total of 943 patients with polycystic kidney disease were reported in the period of research but the real prevalence of the disease is not known in the continent. Most patients present with symptoms at diagnosis mainly kidney function impairment and abdominal mass. Nevertheless, the mean age at diagnosis is similar to the literature data. Genetic testing was not frequent, however, they showed a high proportion of new mutations.
Most African patients with polycystic kidney disease present with severe symptoms and complications at diagnosis. A high proportion of new mutations were reported in this population particularly in the PKD1 gene.
Further researches are needed to better assess the real prevalence of PKD and the spectrum of mutations in the continent.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Senegal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia S-Editor: Liu JH L-Editor: A P-Editor: Zhao YQ
| 1. | Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 416] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Nobakht N, Hanna RM, Al-Baghdadi M, Ameen KM, Arman F, Nobahkt E, Kamgar M, Rastogi A. Advances in Autosomal Dominant Polycystic Kidney Disease: A Clinical Review. Kidney Med. 2020;2:196-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8013] [Article Influence: 534.2] [Reference Citation Analysis (2)] |
| 4. | Hajji M, Barbouch S, Harzallah A, Hedri H, Kaaroud H, Abderrahim E, Goucha R, Hamida FB, Gorsane I, Abdallah TB. Clinical study on autosomal dominant polycystic kidney disease among North Tunisians. Saudi J Kidney Dis Transpl. 2019;30:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Arogundade FA, Akinbodewa AA, Sanusi AA, Okunola O, Hassan MO, Akinsola A. Clinical presentation and outcome of autosomal dominant polycystic kidney disease in Nigeria. Afr Health Sci. 2018;18:671-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Chijioke A, Aderibigbe A, Olanrewaju TO, Makusidi AM, Oguntoyinbo AE, Braimoh KT. The prevalence and clinical characteristics of adult polycystic kidney disease in Ilorin, Nigeria. Port J Nephrol Hypert. 2010;24:159-163. |
| 7. | Ogiator MO, Ijachi OO, Ukpabi DE, Ojobi J. Clinical Characteristics and Outcome of Patients with Autosomal Dominant Polycystic Kidney Disease at a Teaching Hospital in North Central, Nigeria: Eight Year Review. West J Med Biomed Sci. 2021;2:87-92. |
| 8. | Mawufemo TY, Yoan AE, Agbeko DK, Akomola SK, Dzidzonu NK, Kossi K, Georges TK, Badomta D, Eugene A, D'daah H, Awalou DM. Epidemiological, Clinical and Evolutive Profile of Autosomal Dominant Polycystic Kidney Disease (ADPKD) in Togo. O J Neph. 2018;8:117-123. [DOI] [Full Text] |
| 9. | Fary Ka E, Seck SM, Niang A, Cisse MM, Diouf B. Patterns of autosomal dominant polycystic kidney diseases in black Africans. Saudi J Kidney Dis Transpl. 2010;21:81-86. [PubMed] |
| 10. | Okyere P, Ephraim RKD, Okyere I, Attakorah J, Serwaa D, Essuman G, Abaka-Yawson A, Adoba P. Demographic, diagnostic and therapeutic characteristics of autosomal dominant polycystic kidney disease in Ghana. BMC Nephrol. 2021;22:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Laleye A, Awede B, Agboton B, Azonbakin S, Biaou O, Sagbo G, Adjagba M, Audrezet MP, Ferec C, Darboux R. Autosomal dominant polycystic kidney disease in University Clinic of Nephrology and Haemodialysis of Cotonou: clinical and genetical findings. Genet Couns. 2012;23:435-445. [PubMed] |
| 12. | Abdelwahed M, Hilbert P, Ahmed A, Dey M, Bouomrani S, Kamoun H, Ammar-Keskes L, Belguith N. Autosomal dominant polycystic kidney disease (ADPKD) in Tunisia: From molecular genetics to the development of prognostic tools. Gene. 2022;817:146174. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Abdelwahed M, Hilbert P, Ahmed A, Mahfoudh H, Bouomrani S, Dey M, Hachicha J, Kamoun H, Keskes-Ammar L, Belguith N. Mutational analysis in patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD): Identification of five mutations in the PKD1 gene. Gene. 2018;671:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Seck SM, Guèye S, Diouf B. A New PKD-1 Mutation Discovered in a Black African Woman With Autosomal Polycystic Kidney Disease. Nephrourol Mon. 2013;5:769-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Nabhan MM, Abdelaziz H, Xu Y, El Sayed R, Santibanez-Koref M, Soliman NA, Sayer JA. Case Report: Whole-exome analysis of a child with polycystic kidney disease and ventriculomegaly. Genet Mol Res. 2015;14:3618-3624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Sahnoun S, Barbouch S, Hadj Fredj S, Khedher A, Messaoud T. Autosomal dominant polycystic kidney disease: identification of two polymorphisms. Ann Biol Clin (Paris). 2015;73:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Willey C, Kamat S, Stellhorn R, Blais J. Analysis of Nationwide Data to Determine the Incidence and Diagnosed Prevalence of Autosomal Dominant Polycystic Kidney Disease in the USA: 2013-2015. Kidney Dis (Basel). 2019;5:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Willey CJ, Blais JD, Hall AK, Krasa HB, Makin AJ, Czerwiec FS. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. 2017;32:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 19. | Yersin C, Bovet P, Wauters JP, Schorderet DF, Pescia G, Paccaud F. Frequency and impact of autosomal dominant polycystic kidney disease in the Seychelles (Indian Ocean). Nephrol Dial Transplant. 1997;12:2069-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 404] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 21. | Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019;393:919-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 22. | Rossetti S, Strmecki L, Gamble V, Burton S, Sneddon V, Peral B, Roy S, Bakkaloglu A, Komel R, Winearls CG, Harris PC. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet. 2001;68:46-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 380] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Vikrant S, Parashar A. Autosomal dominant polycystic kidney disease: Study of clinical characteristics in an Indian population. Saudi J Kidney Dis Transpl. 2017;28:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Parfrey PS, Bear JC, Morgan J, Cramer BC, McManamon PJ, Gault MH, Churchill DN, Singh M, Hewitt R, Somlo S. The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 227] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Simon P. Prognosis of autosomal dominant polycystic kidney disease. Nephron. 1995;71:247-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Paul BM, Vanden Heuvel GB. Kidney: polycystic kidney disease. Wiley Interdiscip Rev Dev Biol. 2014;3:465-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Bergmann C. Genetics of Autosomal Recessive Polycystic Kidney Disease and Its Differential Diagnoses. Frontiers in Pediatrics [Internet]. 2018 [cite 22 Nov 2023]. Available from: https://www.frontiersin.org/articles/10.3389/fped.2017.00221. |
| 29. | Bergmann C, Senderek J, Windelen E, Küpper F, Middeldorf I, Schneider F, Dornia C, Rudnik-Schöneborn S, Konrad M, Schmitt CP, Seeman T, Neuhaus TJ, Vester U, Kirfel J, Büttner R, Zerres K; APN (Arbeitsgemeinschaft für Pädiatrische Nephrologie). Clinical consequences of PKHD1 mutations in 164 patients with autosomal-recessive polycystic kidney disease (ARPKD). Kidney Int. 2005;67:829-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |