Published online Dec 25, 2023. doi: 10.5527/wjn.v12.i5.168
Peer-review started: August 20, 2023
First decision: September 14, 2023
Revised: September 20, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: December 25, 2023
Processing time: 123 Days and 13.9 Hours
Hypertension is commonly observed in patients living with chronic kidney disease (CKD). Finding an optimal treatment regime remains challenging due to the complex bidirectional cause-and-effect relationship between hypertension and CKD. There remains variability in antihypertensive treatment practices.
To analyze data from the Salford Kidney Study database in relation to antihypertensive prescribing patterns amongst CKD patients.
The Salford Kidney Study is an ongoing prospective study that has been recruiting CKD patients since 2002. All patients are followed up annually, and their medical records including the list of medications are updated until they reach study endpoints [starting on renal replacement therapy or reaching estimated glomerular filtration rate (eGFR) expressed as mL/min/1.73 m2 ≤ 10 mL/min/1.73 m2, or the last follow-up date, or data lock on December 31, 2021, or death]. Data on antihypertensive prescription practices in correspondence to baseline eGFR, urine albumin-creatinine ratio, primary CKD aetiology, and cardiovascular disease were evaluated. Associations between patients who were prescribed three or more antihypertensive agents and their clinical outcomes were studied by Cox regression analysis. Kaplan-Meier analysis demonstrated differences in survival probabilities.
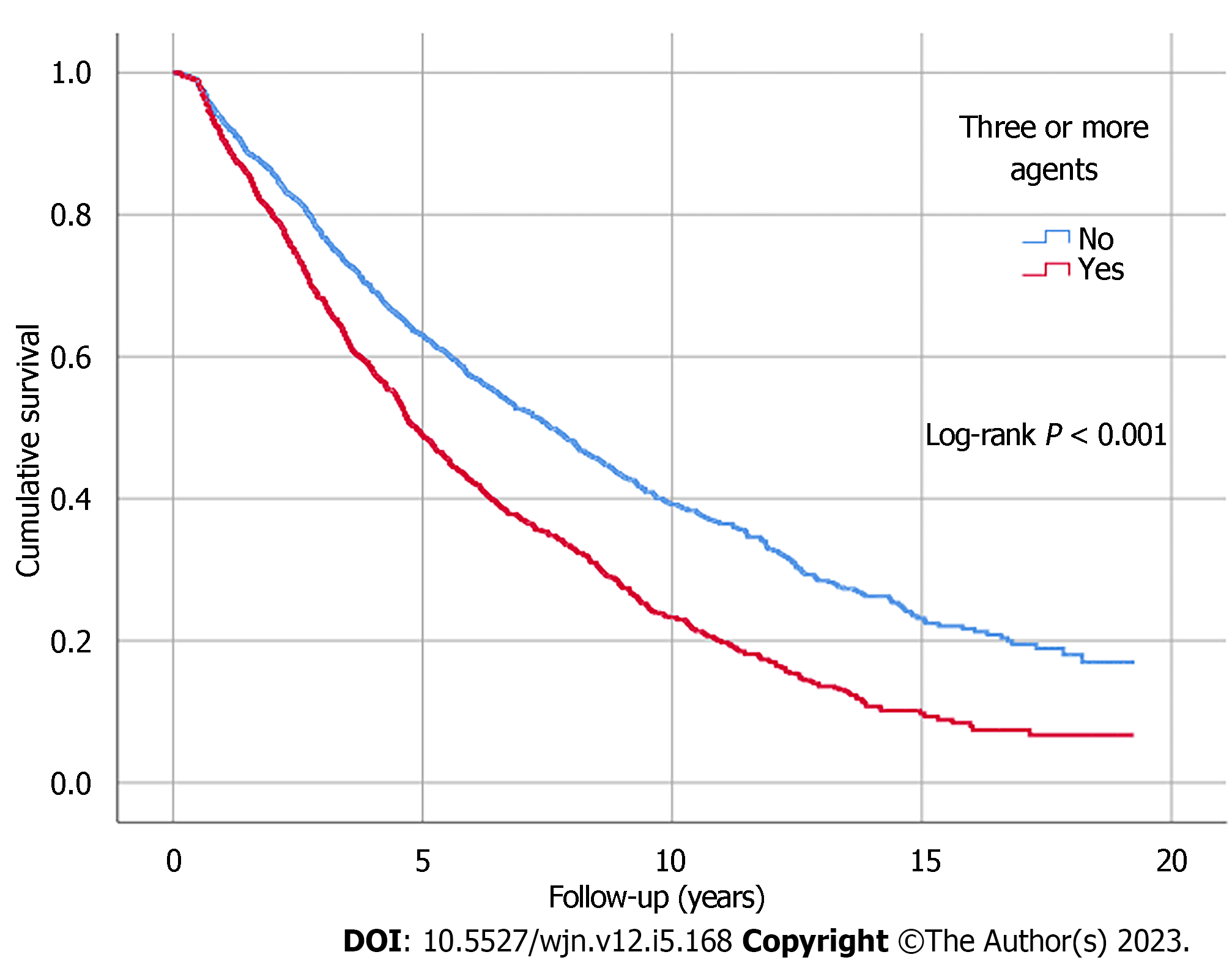
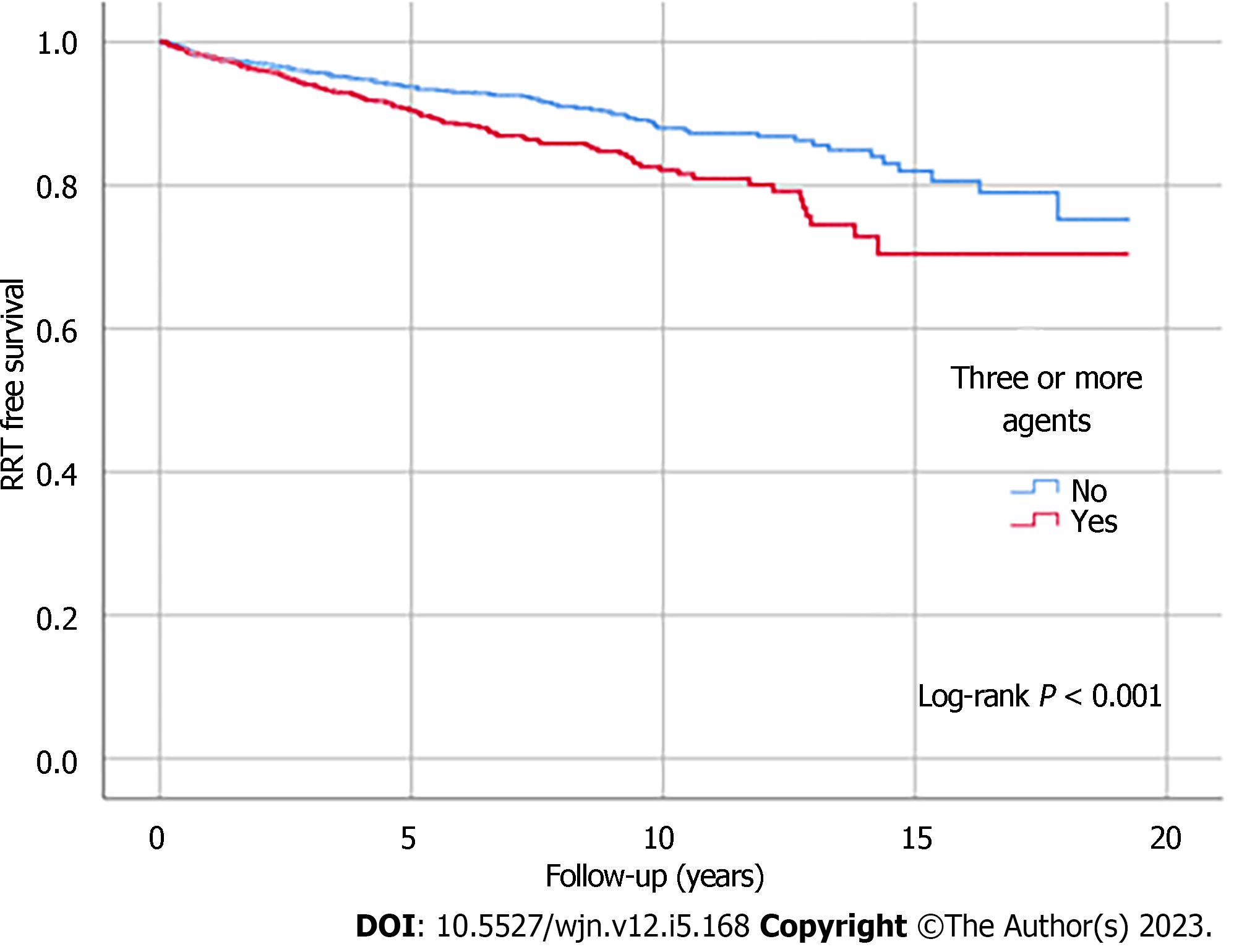
Three thousand two hundred and thirty non-dialysis-dependent CKD patients with data collected between October 2002 and December 2019 were included. The median age was 65 years. A greater proportion of patients were taking three or more antihypertensive agents with advancing CKD stages (53% of eGFR ≤ 15 mL/min/1.73 m2 vs 26% of eGFR ≥ 60 mL/min/1.73 m2, P < 0.001). An increased number of patients receiving more classes of antihypertensive agents was observed as the urine albumin-creatinine ratio category increased (category A3: 62% vs category A1: 40%, P < 0.001), with the upward trends particularly noticeable in the number of individuals prescribed renin angiotensin system blockers. The prescription of three or more antihypertensive agents was associated with all-cause mortality, independent of blood pressure control (hazard ratio: 1.15; 95% confidence interval: 1.04-1.27, P = 0.006). Kaplan-Meier analysis illustrated significant differences in survival outcomes between patients with three or more and those with less than three antihypertensive agents prescribed (log-rank, P < 0.001).
Antihypertensive prescribing patterns in the Salford Kidney Study based on CKD stage were consistent with expectations from the current United Kingdom National Institute of Health and Care Excellence guideline algorithm. Outcomes were poorer in patients with poor blood pressure control despite being on multiple antihypertensive agents. Continued research is required to bridge remaining variations in hypertension treatment practices worldwide.
Core Tip: This is an observational study that prospectively evaluated antihypertensive prescribing patterns in 3230 non-dialysis chronic kidney disease (CKD) patients over a 20-year period. Antihypertensive prescribing patterns based on CKD stage were consistent with expectations from the United Kingdom National Institute of Health and Care Excellence guideline algorithm and other international guidelines in relation to hypertension management in CKD.
- Citation: Chinnadurai R, Wu HHL, Abuomar J, Rengarajan S, New DI, Green D, Kalra PA. Antihypertensive prescribing patterns in non-dialysis dependent chronic kidney disease: Findings from the Salford Kidney Study. World J Nephrol 2023; 12(5): 168-181
- URL: https://www.wjgnet.com/2220-6124/full/v12/i5/168.htm
- DOI: https://dx.doi.org/10.5527/wjn.v12.i5.168
Chronic kidney disease (CKD) is a progressive disease defined by the presence of structural or functional abnormalities within the kidney for 3 mo or more according to the Kidney Disease Improving Global Outcomes (KDIGO)[1]. Touted as an emerging public health issue of the 21st century, the prevalence of CKD is exponentially growing and is projected to become the fifth-leading cause of mortality globally by 2040[2]. The aetiology of CKD is multidimensional and complex, of which there are various causes and consequences[3-5]. Other than diabetes mellitus, hypertension is a major contributor towards the progression of CKD and a leading consequence of CKD[6]. Depending on the stage of CKD, the prevalence of hypertension in CKD populations varies (ranging between 67% and 92%, according to previously published data), but the majority of patients with CKD are likely to have hypertension[7,8]. Given the potential health consequences of hypertension over time, namely its associated cardiovascular risks and risk of further kidney damage, adequate control of blood pressure (BP) in the CKD population is of vital importance to improve clinical outcomes[9,10].
It is not known to what extent clinicians adopt guideline-recommended or preferred antihypertensive treatment approaches for their CKD patients in the real-world setting[7,11]. Previous electronic health record and prospective longitudinal studies noted clinicians primarily prescribed angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) for CKD patients with hypertension, but varying combinations of antihypertensive prescription over time was noted when all antihypertensive agents are considered[12-16]. It remains largely unestablished which combination(s) of antihypertensive agents would generate the best results in terms of BP control and other clinical outcomes. Aiming to address these unknowns, our study evaluated trends and patterns relating to antihypertensive prescription over a 20-year period in patients identified with non-dialysis-dependent CKD included in the Salford Kidney Study (SKS).
Our investigation was conducted on patients enrolled in the non-dialysis-dependent CKD arm of the SKS (SKS-CKD). The SKS-CKD is an ongoing long-term prospective observational study that has been recruiting patients with CKD since the year 2002. Details of study recruitment in the SKS have been described in previously published literature[17,18]. In brief, any CKD patient above the age of 18 years and able to provide informed consent is recruited. At study baseline (i.e. date of recruitment), data including demographic information, comorbidities, physical parameters (weight, height, BP, heart rate, etc) and a detailed medication history is recorded. The patients are then followed up annually to update their comorbidity status and medication list until they reach a study endpoint [which may include death, starting on renal replacement therapy (RRT), reaching estimated glomerular filtration rate (eGFR) ≤ 10 mL/min/1.73 m2, the last follow-up date, or data lock of December 31, 2021 for this study]. All haematological and biochemical variables at study and routine clinic visits are recorded from electronic patient records.
In the SKS, hypertensive status was defined as being on antihypertensive agents or recorded as having a history of hypertension in general practitioner records. A smoking history was defined as current or past history of smoking, and a similar definition was followed in the collection of alcohol history. Body mass index was calculated using weight in kilograms and height in meters (kg/m2). End stage kidney disease (ESKD) was defined as starting on RRT or reaching an eGFR ≤ 10 mL/min/1.73 m2 for patients who opted for conservative care. We defined renin angiotensin system (RAS) blockers as being ACEI or ARB or renin inhibitors.
The baseline characteristics of the cohort were grouped based on CKD stages (categorized by eGFR) and proteinuria [categorized by urine albumin-creatinine ratio (uACR)]. uACR was calculated from urine protein-creatinine ratio (uPCR). This involved initial conversion from mg/mmol to mg/g by multiplication of 8.84, and further conversion from uPCR (uPCR: the standard measure in this real world population) to uACR as per the standardized formula in the kidney failure risk equation[19].
Prescription patterns of antihypertensive agents at baseline were presented corresponding to eGFR categories, uACR categories, primary kidney disease aetiology, and cardiovascular disease. Antihypertensive agent(s) prescribing trends were also examined at the 12-mo and 24-mo follow-up.
When presenting results from our statistical analyses, continuous variables were expressed with the median value (interquartile range). After checking for the normality of distribution, the P value was calculated by the Kruskal-Wallis H test. Categorical variables are expressed as frequencies in absolute number form (percentage), with P values calculated by the χ2 test. The association between being prescribed three or more antihypertensive agents and clinical outcomes (i.e. all-cause mortality and reaching ESKD) was studied by univariate and multivariate Cox regression analysis. The multivariable models were developed by including variables in a stepwise manner. Kaplan-Meier analysis was used to demonstrate the differences in survival probabilities, with the log-rank test used to calculate the P values. The annual rate of decline in eGFR (delta eGFR) was calculated using all available eGFRs between the study baseline and endpoints by linear regression analysis. Only patients with three or more eGFRs and at least 1 year of follow-up data were included in the delta eGFR analysis. All statistical analyses were performed using IBM SPSS Version 26, registered with the University of Manchester.
The SKS received ethical approval for all of the observational studies conducted in relation to its database, with individual patient consent. The research ethics number is 15/NW/0818.
A total of 3230 patients with complete datasets were included in this analysis. The median age of the cohort was 65 years with a predominance of the male sex (60%) and those of white ethnicity (96%). At baseline, the majority of study participants (66%) had an eGFR between 15 and 45 mL/min/1.73 m2. As baseline eGFR declined, the median systolic BP of the cohort was noted to have increased, and a higher proportion of those with lower eGFR also had a history of hypertension, diabetes, and cardiovascular events (P < 0.001). Biochemical variables showed decreases in haemoglobin and calcium levels and increases in phosphate levels in correspondence to worsening eGFR (P < 0.001) (Table 1).
| Demographic variables | eGFR ≥ 60, n = 2181 | eGFR 45-59, n = 4911 | eGFR 30-45, n = 9621 | eGFR 15-29, n = 11741 | eGFR < 15, n = 3851 | Total, n = 32301 | P value1 |
| Age, yr | 53 (44-63) | 62 (50-70) | 68 (56-75) | 70 (60-78) | 71 (60-78) | 67 (56-76) | < 0.001 |
| Sex, male | 126 (57.8) | 308 (62.7) | 573 (59.6) | 691 (58.9) | 251 (65.2) | 1949 (60.3) | 0.143 |
| Ethnicity, white | 198 (90.8) | 467 (95.1) | 924 (99.6) | 1127 (96.0) | 375 (97.4) | 3091 (95.7) | 0.003 |
| BMI, kg/m2 | 28.3 (24.6-33.2) | 28.2 (25.0-32.4) | 28.0 (25.0-32.4) | 28.0 (25.0-32.6) | 27.4 (24.0-33.0) | 28.0 (24.7-32.6) | 0.490 |
| Systolic BP, mmHg | 132 (120-148) | 135 (122-150) | 139 (125-153) | 140 (126-155) | 143 (130-160) | 139 (125-154) | < 0.001 |
| Diastolic BP, mmHg | 76 (70-82) | 76 (68-82) | 75 (67-81) | 72 (65-80) | 75 (66-82) | 75 (66-81) | 0.001 |
| Smoking history | 124 (56.9) | 300 (61.1) | 630 (65.5) | 781 (65.5) | 255 (66.2) | 2090 (64.7) | 0.027 |
| Alcohol history | 121 (55.5) | 252 (51.3) | 465 (48.3) | 504 (42.9) | 149 (38.7) | 1491 (46.2) | < 0.001 |
| Hypertension | 166 (76.1) | 414 (84.3) | 862 (89.6) | 1091 (92.9) | 362 (94.0) | 2895 (89.6) | < 0.001 |
| Diabetes mellitus | 37 (17.0) | 109 (22.2) | 291 (30.2) | 456 (38.8) | 148 (38.4) | 1041 (32.2) | < 0.001 |
| IHD | 21 (9.6) | 75 (15.3) | 221 (23.0) | 275 (23.4) | 75 (19.5) | 667 (20.7) | < 0.001 |
| MI | 14 (6.4) | 61 (12.4) | 149 (15.5) | 199 (17.0) | 59 (15.3) | 482 (14.9) | 0.001 |
| CCF | 18 (8.3) | 52 (10.6) | 169 (17.6) | 233 (19.8) | 84 (21.8) | 556 (17.2) | < 0.001 |
| CVA | 8 (3.7) | 25 (5.1) | 75 (7.8) | 100 (8.5) | 41 (10.6) | 249 (7.7) | 0.004 |
| PVD | 19 (8.7) | 47 (9.6) | 122 (12.7) | 169 (14.4) | 58 (15.1) | 415 (12.8) | 0.016 |
| COPD | 32 (14.7) | 74 (15.1) | 179 (18.6) | 219 (18.7) | 64 (16.6) | 568 (17.6) | 0.260 |
| CLD | 8 (3.7) | 19 (3.9) | 30 (3.1) | 33 (2.8) | 9 (2.3) | 99 (3.1) | 0.683 |
| Malignancy | 19 (8.7) | 39 (7.9) | 109 (11.3) | 136 (11.6) | 50 (13.0) | 353 (10.9) | 0.094 |
| Laboratory variables | |||||||
| Haemoglobin, g/L | 134 (121-145) | 131 (120-141) | 126 (115-137) | 120 (110-130) | 113 (104-122) | 123 (112-135) | < 0.001 |
| Albumin, g/L | 44 (41-46) | 43 (41-45) | 43 (40-45) | 42 (40-44) | 42 (39-44) | 43 (40-45) | < 0.001 |
| Corrected calcium, mmol/L | 2.32 (2.22-2.40) | 2.33 (2.24-2.41) | 2.31 (2.23-2.39) | 2.30 (2.20-2.39) | 2.28 (2.17-2.37) | 2.31 (2.22-2.39) | < 0.001 |
| Phosphate, mmol/L | 1.05 (0.91-1.10) | 1.03 (0.91-1.16) | 1.07 (0.95-1.21) | 1.16 (1.02-1.31) | 1.39 (1.21-1.59) | 1.12 (0.98-1.29) | < 0.001 |
| ALP, U/L | 71 (57-85) | 76 (59-97) | 82 (66-102) | 86 (69-112) | 89 (69-111) | 83 (66-105) | < 0.001 |
| uACR, mg/g2 | 15.9 (8.3-57.2) | 21.0 (10.6-60.5) | 24.0 (11.7 -77.0) | 43.0 (16.4-132.7) | 106.5 (44.4-232.6) | 32.7 (13.4-111.1) | < 0.001 |
Amongst patient groups with a lower eGFR, there were greater proportions that were receiving three or more antihypertensive agents (53% of eGFR ≤ 15 mL/min/1.73 m2 vs 26% of eGFR ≥ 60 mL/min/1.73 m2, P < 0.001). The most prescribed antihypertensive agents were RAS blockers (61%), followed by diuretics (47%), dihydropyridine calcium channel blockers (CCB) (39%), and beta blockers (34%). Alpha-blockers were also a popularly prescribed antihypertensive and prescribed more frequently in lower eGFR ranges. The proportion of patients receiving RAS blockers decreased with a lower eGFR, whereas the proportion on diuretics, dihydropyridine CCBs, and beta blockers increased (P < 0.001) (Table 2).
| Antihypertensive class | eGFR>60, n = 2181 | eGFR 45-59, n = 4911 | eGFR 30-45, n = 9621 | eGFR 15-29, n = 11741 | eGFR < 15, n = 3851 | Total, n = 32301 | P value1 |
| None | 54 (24.8) | 73 (14.9) | 97 (10.1) | 78 (6.6) | 26 (6.8) | 328 (10.2) | < 0.001 |
| Three or more agents | 57 (26.1) | 158 (32.2) | 397 (41.3) | 565 (48.1) | 206 (53.5) | 1383 (42.8) | < 0.001 |
| Diuretic (thiazide and loop) | 58 (26.6) | 172 (35.0) | 441 (45.8) | 641 (54.6) | 213 (55.3) | 1525 (47.2) | < 0.001 |
| CCB (dihydropyridine) | 58 (26.6) | 149 (30.3) | 364 (37.8) | 480 (40.9) | 205 (53.2) | 1256 (38.9) | < 0.001 |
| CCB (non- dihydropyridine) | 6 (2.8) | 11 (2.2) | 44 (4.6) | 50 (4.3) | 23 (6.0) | 134 (4.1) | 0.055 |
| Beta-blocker | 51 (23.4) | 133 (27.1) | 324 (33.7) | 443 (37.7) | 156 (40.5) | 1107 (34.3) | < 0.001 |
| Alpha-blocker | 28 (12.8) | 72 (14.7) | 186 (19.3) | 335 (28.5) | 158 (41.0) | 779 (24.1) | < 0.001 |
| Central agents | 9 (4.1) | 15 (3.1) | 34 (3.5) | 59 (5.0) | 33 (8.6) | 150 (4.6) | 0.001 |
| Vasodilators | 2 (0.9) | 2 (0.4) | 6 (0.6) | 13 (1.1) | 7 (1.8) | 30 (0.9) | 0.189 |
| RAS blocker | 126 (57.8) | 316 (64.4) | 616 (64.0) | 733 (62.4) | 192 (50.0) | 1983 (61.4) | < 0.001 |
| Dual RAS blockers | 22 (10.1) | 34 (7.0) | 50 (5.2) | 67 (5.7) | 14 (31.6) | 187 (5.8) | 0.014 |
| Spironolactone/eplerenone | 7 (3.2) | 17 (3.5) | 36 (3.7) | 44 (3.7) | 8 (2.1) | 112 (3.5) | 0.599 |
Furthermore, the distribution of antihypertensive agent prescriptions for patients across the spectrum of primary kidney disease aetiologies illustrated that RAS blockers were the predominant agents for most diagnoses, followed by diuretics and dihydropyridine CCBs (Table 3).
| Primary aetiology of CKD | Three or more1 | RAS blocker1 | Diure | Beta blocker1 | Alpha blocker1 | CCB (dihydro-pyridine)1 | CCB (non-dihydro-pyridine)1 | Central agents1 |
| Diabetes, n = 636 | 386 (61) | 463 (73) | 416 (65) | 239 (38) | 220 (35) | 281 (44) | 32 (5) | 53 (8) |
| Hypertension, n = 471 | 246 (52) | 294 (62) | 245 (52) | 210 (45) | 154 (33) | 222 (47) | 33 (7) | 21 (5) |
| Renovascular disease, n = 256 | 163 (64) | 142 (56) | 178 (70) | 121 (47) | 92 (36) | 122 (48) | 20 (8) | 27 (11) |
| Pyelonephritis, n = 200 | 40 (20) | 102 (51) | 54 (27) | 50 (25) | 23 (12) | 61 (31) | 4 (2) | 3 (2) |
| ADPKD, n = 197 | 66 (34) | 149 (76) | 69 (35) | 55 (28) | 40 (20) | 78 (40) | 2 (1) | 6 (3) |
| Tubulointerstitial nephritis, n = 116 | 9 (8) | 40 (35) | 18 (16) | 27 (23) | 8 (7) | 35 (30) | 3 (3) | 2 (20) |
| Glomerulonephritis, n = 375 | 171 (46) | 305 (81) | 174 (46) | 95 (25) | 73 (20) | 147 (39) | 9 (2) | 21 (6) |
| Vasculitis, n = 118 | 31 (26) | 61 (52) | 34 (29) | 35 (30) | 19 (16) | 40 (34) | 2 (2) | 1 (1) |
| Haematological disease, n = 31 | 8 (26) | 14 (45) | 9 (29) | 8 (26) | 4 (13) | 7 (23) | 0 | 1 (3) |
| Other/unknown aetiology, n = 830 | 263 (32) | 413 (50) | 328 (40) | 267 (32) | 146 (18) | 263 (32) | 29 (4) | 15 (2) |
A greater proportion of patients were receiving three or more antihypertensive agents with each higher uACR category (category A1: 40% vs category A2: 43% vs category A3: 62%, P < 0.001). There was a trend of increased numbers of individuals prescribed ACEI or ARB (Table 4).
| Antihypertensive class | uACR < 30, n = 13551,2 | uACR 30-300, n = 12361,2 | uACR > 300, n = 2481,2 | Total, n = 28391,3 | P value1 |
| None | 148 (10.9) | 112 (9.1) | 12 (4.8) | 272 (9.6) | < 0.001 |
| Three or more agents | 547 (40.4) | 530 (42.9) | 155 (62.5) | 1232 (43.4) | < 0.001 |
| Diuretic (thiazide and loop) | 656 (48.4) | 542 (43.9) | 148 (59.7) | 1346 (47.4) | < 0.001 |
| CCB (dihydropyridine) | 477 (35.2) | 516 (41.7) | 122 (49.2) | 1115 (39.3) | < 0.001 |
| CCB (non- dihydropyridine) | 61 (4.5) | 54 (4.4) | 11 (4.4) | 126 (4.4) | 0.987 |
| Beta-blocker | 458 (33.8) | 418 (33.8) | 93 (37.5) | 969 (34.1) | 0.504 |
| Alpha blocker | 265 (19.6) | 335 (27.1) | 87 (35.1) | 687 (24.2) | < 0.001 |
| Central agent | 45 (3.3) | 69 (5.6) | 23 (9.3) | 137 (4.8) | < 0.001 |
| Vasodilator | 11 (0.8) | 16 (1.3) | 1 (0.4) | 28 (1.0) | 0.288 |
| RAS blocker | 840 (62.0) | 753 (61.0) | 174 (70.2) | 1767 (62.2) | 0.023 |
| Dual RAS blockers | 49 (3.6) | 80 (6.5) | 41 (16.5) | 170 (6.0) | < 0.001 |
| Spironolactone/eplerenone | 52 (3.8) | 29 (2.3) | 11 (4.4) | 92 (3.2) | 0.054 |
When comparing between patients with and without a history of congestive cardiac failure (CCF), diuretics (68% vs 43%, P < 0.001), potassium-sparing diuretics (spironolactone or eplerenone) (10% vs 2%, P < 0.001), and beta blockers (45% vs 32%, P < 0.001) were prescribed more frequently amongst those diagnosed with CCF. Similar prescription patterns were noted for any other form of cardiovascular disease. Interestingly, there were no significant differences in the pattern of prescription of RAS blockers alone, based on cardiovascular disease status (Table 5).
| Antihypertensive class | CCF, n = 5561 | No CCF, n = 26741 | P value1 | CVE, n = 18291 | No CVE, n = 14011 | P value1 |
| None | 22 (4.0) | 306 (11.4) | < 0.001 | 72 (5.1) | 256 (14.0) | < 0.001 |
| Diuretic (thiazide and loop) | 381 (68.4) | 1144 (42.8) | < 0.001 | 818 (58.4) | 707 (38.7) | < 0.001 |
| CCB (dihydropyridine) | 192 (34.5) | 1064 (39.8) | 0.021 | 561 (40.0) | 695 (38.0) | 0.238 |
| CCB (non-dihydropyridine) | 32 (5.8) | 102 (3.8) | 0.037 | 92 (6.6) | 42 (2.3) | < 0.001 |
| Beta blocker | 248 (44.6) | 859 (32.1) | < 0.001 | 621 (44.3) | 486 (26.6) | < 0.001 |
| Alpha blocker | 140 (25.2) | 639 (23.9) | 0.520 | 385 (27.5) | 394 (21.7) | < 0.001 |
| Central agent | 29 (5.2) | 121 (4.5) | 0.481 | 73 (5.2) | 77 (4.2) | 0.180 |
| Vasodilator | 4 (0.7) | 26 (1.0) | 0.572 | 17 (1.2) | 13 (0.7) | 0.140 |
| RAS blocker | 360 (64.7) | 1623 (60.7) | 0.074 | 839 (42.3) | 1144 (57.7) | 0.124 |
| Dual RAS blockers | 24 (4.3) | 163 (6.1) | 0.102 | 70 (37.4) | 117 (62.6) | 0.091 |
| Spironolactone/eplerenone | 56 (10.1) | 56 (2.1) | < 0.001 | 80 (5.7) | 32 (1.7) | < 0.001 |
There were no significant differences in antihypertensive prescription patterns over the 12-mo and 24-mo follow-up period (Tables 6 and 7). Overall, there were more patients achieving BP < 140/90 mmHg at 12 mo (52% vs 48%, P = 0.008) and at 24 mo (51.7% vs 48%, P = 0.015) compared to baseline. However, when only patients on three or more agents over the 24-mo follow-up period were considered, there were no statistically significant results as to whether more patients achieved BP < 140/90 mmHg (Tables 8 and 9).
| Antihypertensive class | Baseline, n = 22561 | 12-mo follow-up, n = 22561 | P value1 |
| Diuretic (thiazide and loop) | 1131 (50.1) | 1107 (49.1) | 0.475 |
| CCB (dihydropyridine) | 905 (40.1) | 930 (41.2) | 0.449 |
| CCB (non- dihydropyridine) | 102 (4.5) | 90 (4.0) | 0.376 |
| Beta blocker | 783 (34.7) | 767 (34.0) | 0.616 |
| Alpha blocker | 544 (24.1) | 558 (24.7) | 0.628 |
| Central agent | 111 (4.9) | 117 (5.2) | 0.683 |
| Vasodilator | 19 (0.8) | 15 (0.7) | 0.491 |
| RAS blocker | 1416 (62.8) | 1383 (61.3) | 0.311 |
| Dual RAS blockers | 145 (6.4) | 163 (7.2) | 0.288 |
| Spironolactone/eplerenone | 84 (3.7) | 73 (3.2) | 0.372 |
| Antihypertensive class | Baseline, n = 17081 | 12-mo follow-up, n = 17081 | 24-mo follow-up, n = 17081 | P value1 |
| Diuretic (thiazide and loop) | 895 (52.4) | 874 (51.2) | 839 (49.1) | 0.153 |
| CCB (dihydropyridine) | 696 (40.7) | 710 (41.6) | 646 (37.8) | 0.063 |
| CCB (non- dihydropyridine) | 83 (4.9) | 74 (4.3) | 75 (4.4) | 0.719 |
| Beta blocker | 588 (34.4) | 581 (34.0) | 560 (32.8) | 0.573 |
| Alpha blocker | 421 (24.6) | 433 (25.4) | 394 (23.1) | 0.281 |
| Central agent | 81 (4.7) | 90 (5.3) | 66 (5.0) | 0.779 |
| Vasodilator | 18 (1.1) | 14 (0.8) | 18 (1.1) | 0.724 |
| RAS blocker | 1076 (63.0) | 1077 (63.1) | 1056 (61.8) | 0.417 |
| Dual RAS blockers | 99 (5.8) | 127 (7.4) | 122 (7.1) | 0.127 |
| Spironolactone/eplerenone | 58 (3.4) | 49 (2.9) | 43 (2.5) | 0.309 |
In Cox regression models, the prescription of three or more antihypertensive agents was strongly associated with all-cause mortality (multivariate model 3: hazard ratio: 1.14; 95% confidence interval: 1.03-1.26, P = 0.008) (Table 10). A similar association was observed when the outcome considered was progression to ESKD (multivariate model 3: hazard ratio: 1.47; 95% confidence interval: 1.25-1.72, P < 0.001) (Table 11).
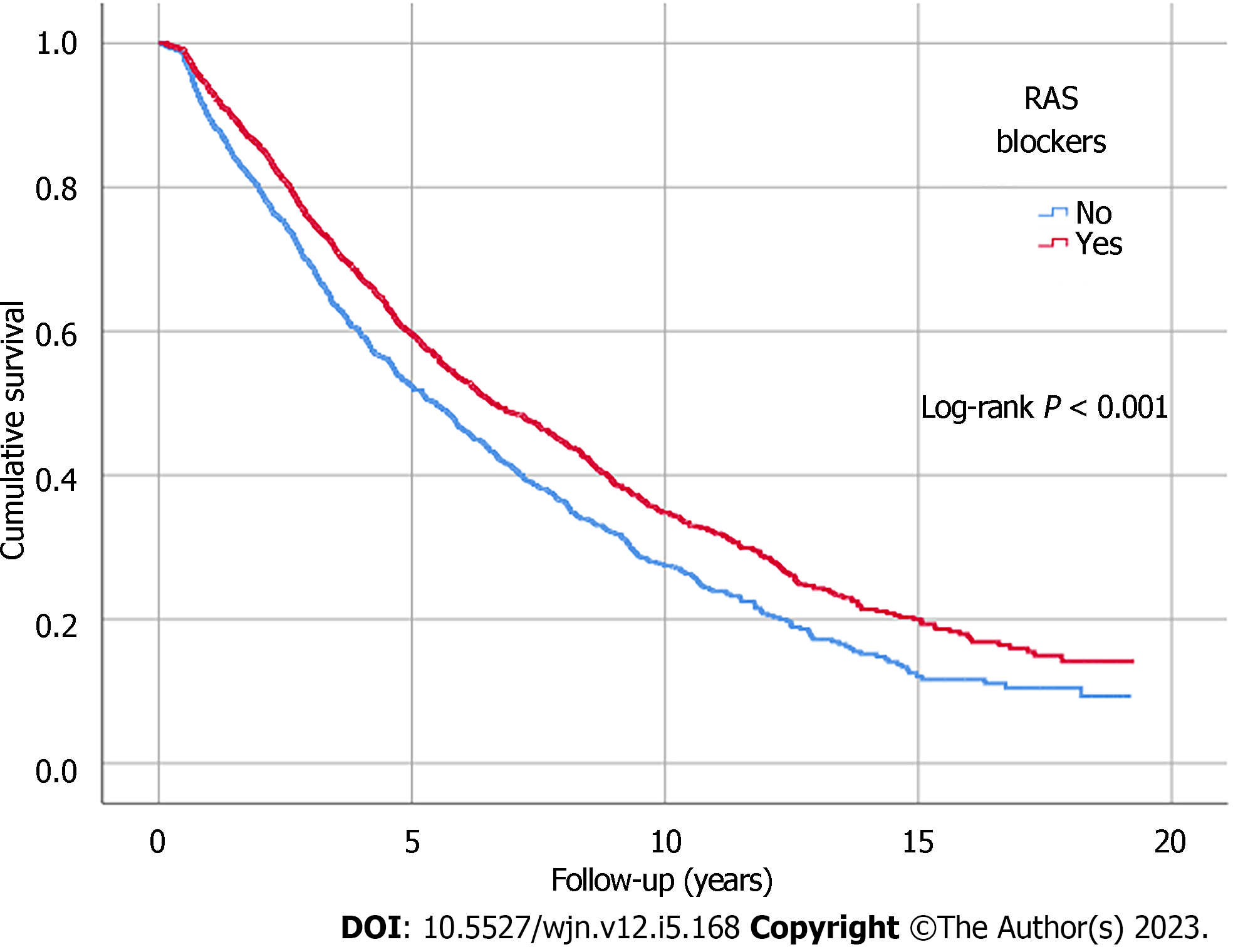
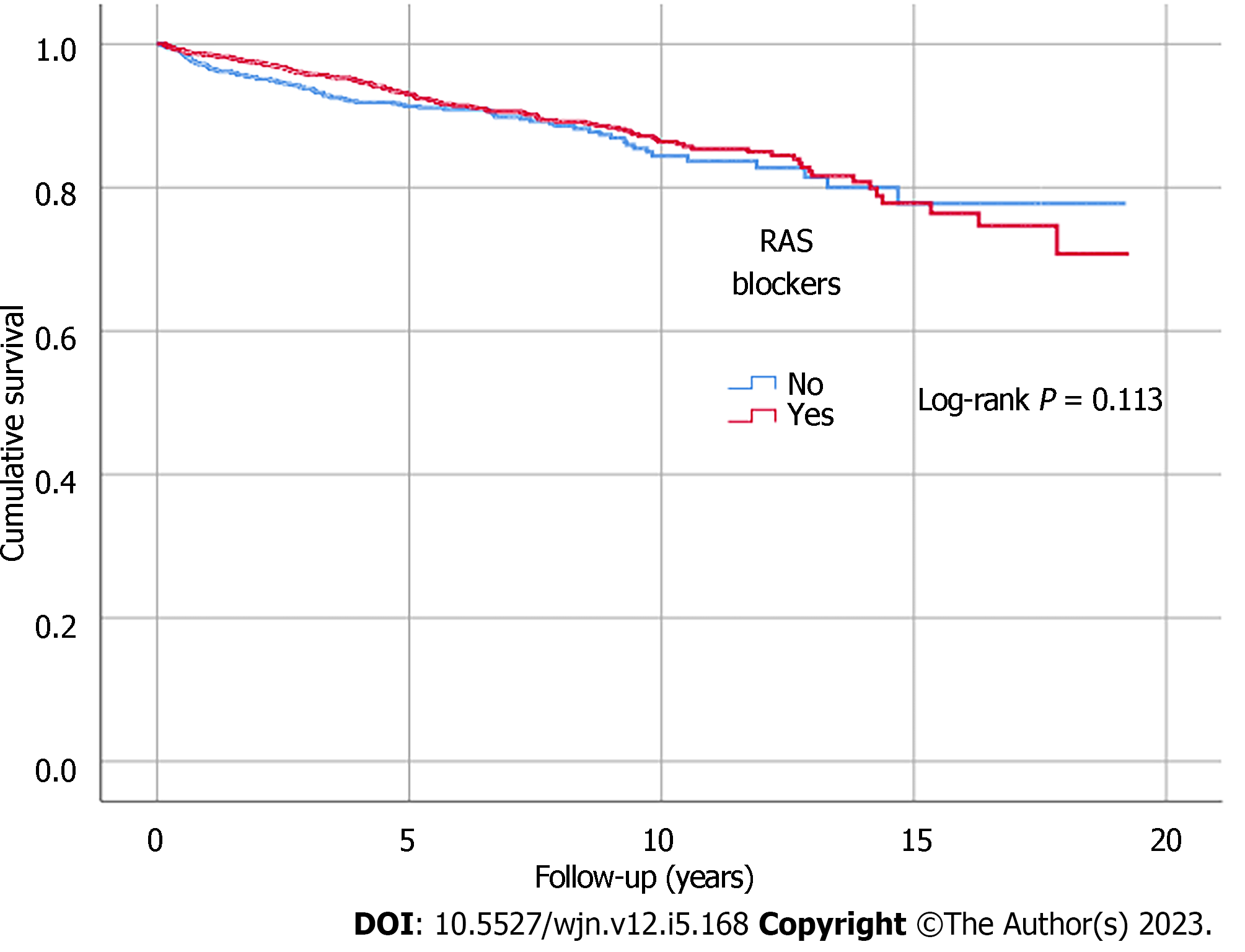
Kaplan-Meier analysis illustrated significant differences in survival outcomes (all-cause mortality and RRT-free survival) between patients receiving three or more compared to those with less than three antihypertensive agents prescribed (log-rank, P < 0.001) (Figures 1 and 2). Being on RAS blockers was associated with a higher survival (log-rank, P < 0.001) but demonstrated no differences in terms of reaching ESKD and requiring RRT (log-rank, P = 0.113) (Figures 3 and 4).
Linear regression analysis concluded that the annual rate of decline in eGFR was significantly higher in patients receiving three or more antihypertensive agents (-1.79 vs -1.07 mL/min/1.73 m2/year, P < 0.001) compared to those receiving less (Table 12).
In this study, we evaluated antihypertensive prescription patterns in patients from the SKS-CKD database, corresponding this to baseline eGFR, uACR, primary kidney disease aetiology and the presence of cardiovascular morbidities, and monitored prescription patterns over a 24-mo follow-up period. The number of antihypertensive agents prescribed for each patient was correlated with clinical outcomes, namely all-cause mortality and progression to ESKD.
Across the SKS-CKD cohort at baseline, RAS blockers were the most commonly prescribed agents for management of hypertension, followed by diuretics, dihydropyridine CCBs, and beta blockers. RAS blocker prescriptions decreased in patients with CKD stage 5 at baseline, whereas diuretics, dihydropyridine CCBs, and beta blockers were prescribed more frequently for patients with lower eGFR. Surprisingly, although it has not been widely advocated for use as an antihypertensive in current guidelines, alpha blockers were amongst the more commonly prescribed antihypertensive agents amongst patients in SKS-CKD, especially for those in the lower eGFR ranges. A major adverse effect of alpha blockers is orthostatic hypotension in patients with kidney function impairment[20-22]. The purposes of prescribing alpha blockers may not be solely for lowering of BP, as a substantial proportion of patients with benign prostatic hyperplasia are routinely initiated on them to relieve symptoms and improve urinary flow[23].
RAS blockers, diuretics, and dihydropyridine CCBs were prescribed with increasing frequency in patients with a greater degree of uACR. Ultimately, with an increase in the uACR category, a greater proportion of patients were prescribed more antihypertensive agents. RAS blockers remain the primary antihypertensive agents prescribed for patients with all forms of primary kidney disease. For CKD patients with CCF, the prescriptions of diuretics, beta blockers, and potassium sparing diuretics (spironolactone and eplerenone) were significantly higher compared to those without. This pattern was similar when comparing antihypertensive prescribing patterns between CKD patients with pre-existing cardiovascular events and those without.
Our analysis demonstrated that antihypertensive prescribing patterns at 12 mo and 24 mo had only minimally changed compared to baseline, but this was most likely because most patients had been enrolled in the renal service well before entry into the SKS. When determining associations between the number of antihypertensive agents prescribed and clinical outcomes, patients receiving a higher number of antihypertensive agents had worsened outcomes, namely increased all-cause mortality and reaching ESKD. Such associations remained following adjustment of baseline demographic factors (i.e. age, sex, ethnicity, smoking status, and alcohol intake), plus diabetes, cardiovascular comorbidities, baseline eGFR, and uACR.
Consensus recommendation to commence ACEI or angiotensin receptor ARB as a first-line antihypertensive treatment option for CKD patients, particularly for those with proteinuria and/or reduced eGFR defined by eGFR < 60 mL/min/1.73 m2 has been reached across the major international societies in cardiology and nephrology such as the American College of Cardiology, the American Heart Association, and KDIGO[8,24]. The updated 2021 KDIGO clinical practice guideline for BP management in CKD continues to advocate this approach in patients with hypertension and CKD, with or without diabetes, and not receiving dialysis[24]. Where an adult patient has a transplanted kidney, commencing an ARB or dihydropyridine CCB has been recommended[24]. In the United Kingdom, the National Institute of Health and Care Excellence (NICE) guideline defines BP targets (clinic measured) for patients with CKD according to the patient’s uACR[25]. Adult CKD patients are divided into 2 groups: those with a uACR < 70 mg/mmol (618.8 mg/g); and those with a uACR > 70 mg/mmol (618.8 mg/g). In patients with a uACR < 70 mg/mmol (618.8 mg/g), the BP target is below 140/90 mmHg, whereas in patients with a uACR > 70 mg/mmol (618.8 mg/g), the BP target is below 130/80 mmHg.
An ACEI or ARB is first-line treatment for hypertension in CKD patients with uACR > 30mg/mmol (265.2 mg/g). A thiazide diuretic or CCB is to be used as second-line medications. While both dihydropyridine CCB and non-dihydropyridine CCB have been shown to have similar effects in terms of BP control, non-dihydropyridine CCBs such as verapamil and diltiazem have been shown to reduce proteinuria to a greater extent. However, prescribing non-dihydropyridine CCBs over dihydropyridine CCBs would generally appear to be less popular in actual clinical practice, mainly due to concerns of increased risk of cardiac adverse effects such as bradycardia that could be potentially life-threatening in severe cases[26]. A potassium sparing agent such as spironolactone can also be added, but due to the increased risk of hyperkalaemia, this is recommended only if there is persistent poor BP control following the addition of a thiazide diuretic. Whilst these are the main antihypertensive options as per NICE guidelines, other antihypertensive classes exist, such as alpha blockers, direct renin inhibitors, vasodilators, and centrally acting antihypertensive agents. These medications are not currently recommended under the NICE and other international guidelines for various reasons, such as the presence of adverse effects as well as the lack of evidence that they offer a strong clinical benefit for CKD patients with hypertension.
A number of studies have been conducted reviewing antihypertensive prescribing patterns in patients with CKD. Amongst the more recent studies that have followed the introduction of updated hypertension guidelines, a study conducted by Magvanjav et al[12] utilized electronic health record data from 5658 CKD patients with hypertension to examine their antihypertensive drug prescribing patterns, BP control, and risk factors for resistant hypertension. As found in our study and in observational data stated from recent hypertension guidelines, Magvanjav et al[12] noted that 64% of patients were prescribed an ACEI or ARB. They also concluded that BP was better controlled in patients who were prescribed a combination of medications that included a diuretic and beta blocker. Another study by Alencar de Pinho et al[13] compared antihypertensive prescribing patterns in CKD patients internationally and similarly found that ACEI or ARB was the most commonly prescribed antihypertensive class. However, the investigators noted significant variations in antihypertensive medication prescribing practices globally for all antihypertensive agents across different stages of CKD. Taking ACEI or ARB for example, Alencar de Pinho et al[13] observed that the prevalence of an ACEI or ARB prescription varied between 54% and 91% across different countries. This emphasizes that significant variations remain regarding clinicians’ approaches to antihypertensive treatment prescription for their CKD patients within the real-world setting, whether they follow a guideline-recommended algorithm or basing their approach from personal clinical experiences and preferences.
Indeed there are numerous patient-specific and clinical challenges when treating hypertension in CKD, of which clinician variation in antihypertensive prescription practices is only one issue. This conundrum may be explained by the variability in national and international guideline recommendations at present. Areas where a global consensus has not been reached are the BP thresholds that determine when treatment initiation is indicated, for instance. There also remains no unified agreement on the BP targets to be achieved amongst CKD patients. More importantly, there is continuous debate and discussion on how best to optimize antihypertensive therapy for BP control and cardiorenal protection. These are avenues of research where further work is required.
There is now an increased indication for adding sodium glucose cotransport (SGLT2) inhibitors to the current portfolio of recommended medications for hypertension management in CKD, given their emergence as a therapeutic option for cardiorenal protection in people with and without diabetes[27]. Large, randomized, placebo-controlled trials have pointed to the potential of SGLT2 inhibitors as having positive effects on BP control in both office and out-of-office contexts. The SACRA, EMPA-REG BP, and CREDENCE trials were amongst the clinical trials that have made these conclusions[28-30]. The post hoc analysis of the CREDENCE trial demonstrated the BP-lowering effect of canagliflozin for patients with resistant hypertension, which is novel and encouraging[29]. Additional studies are needed to validate the role of SGLT2 inhibitors in optimizing BP control and reducing adverse cardiovascular outcomes amongst patients living with CKD and hypertension, particularly those with resistant hypertension.
Despite the main strength of our study being inclusion of an ethnically and socioeconomically diverse CKD patient group, as well as being conducted over a 20-year period, there are limitations to acknowledge. One limitation was an inability to clearly correlate details relating to the indication(s) for antihypertensive medication prescription and any adjustments during the follow-up period due to multiple clinicians being involved in a patient’s management. Furthermore, it is unclear if diuretics were prescribed as an antihypertensive agent within this context, or intended for other clinical purposes (e.g., for peripheral oedema). Understanding the indications for prescription of particular antihypertensive medication(s) would have been useful in determining the true patterns of antihypertensive prescribing practices in this study. Also, as our centre does not complete urinary antihypertensive screens routinely, there is always the confounding impact of drug non-compliance amongst patients receiving three or more antihypertensive agents. This contributes to poor BP control and its associated morbidity and mortality outcomes. Finally, there was incomplete data on determining the rate of decline in eGFR when comparing between CKD patients with three or more antihypertensive agents vs less than three antihypertensive agents prescribed due to delta eGFR data being unavailable for 162 patients (5% of entire cohort).
In summary, RAS blockers were found to be the most commonly prescribed antihypertensive agents, followed by diuretics and CCBs, which are recommended as second-line antihypertensive treatment options. Diuretics, beta blockers, and mineralocorticoid antagonists were found to be more commonly prescribed in CKD patients with cardiovascular comorbidities. Whilst our study results aligned with that of expectations from the current NICE guideline algorithm, further work determining optimal strategies in approaching antihypertensive prescription for CKD patients at both an individual and policy level is needed to reduce the variations currently observed in clinical practice. The opportunity to introduce newer and potentially more cost-effective therapies in the form of SGLT2 inhibitors for hypertension management in CKD is attractive and could be revolutionary in addressing these challenges, and continued research in this area is anticipated.
Hypertension is a major contributor towards the progression of chronic kidney disease (CKD) and a leading consequence of CKD. Despite standard guidelines, clinician practices on managing hypertension in CKD patients remain variable.
It is important to explore the factors relating to CKD patients that influences a clinician’s decision to use specific antihypertensive agents with the aim to better standardize current antihypertensive prescription practices.
To investigate hypertension management practices in CKD patients within a real-world setting.
We retrospectively analysed patients recruited into the Salford Kidney Study database. Data including patient demographic information, comorbidities, and a detailed antihypertensive medication history were reviewed. Prescription patterns of antihypertensive agents were explored based on estimated glomerular filtration rate expressed as mL/min/1.73 m2, urine albumin-creatinine ratio, primary kidney disease aetiology, and cardiovascular disease. The association between being prescribed three or more antihypertensive agents and clinical outcomes (i.e. all-cause mortality and reaching end stage kidney disease) was also studied.
A total of 3230 non-dialysis dependent CKD patients with data collected between October 2002 and December 2019 were included. The most frequently prescribed antihypertensive agents were renin angiotensin system blockers (61%), followed by diuretics (47%), dihydropyridine calcium channel blockers (39%), and beta blockers (34%). A greater proportion of patients were taking three or more antihypertensive agents with advancing CKD stages (53% of CKD stage 5 patients vs 26% of CKD stage 2 patients) and as the urine albumin-creatinine ratio increased (category A3: 62% vs category A1: 40%, P < 0.001). The prescription of three or more antihypertensive agents was associated with all-cause mortality, independent of blood pressure control (hazard ratio: 1.15; 95% confidence interval: 1.04-1.27, P = 0.006).
Renin angiotensin system blockers were found to be the most prescribed antihypertensive agents, followed by diuretics and calcium channel blockers. Outcomes were poorer in CKD patients with poor blood pressure control despite being on multiple antihypertensive agents.
Our study results aligned with expectations from the current National Institute of Health and Care Excellence guideline algorithm; further work determining optimal strategies in approaching antihypertensive prescriptions for CKD patients at both an individual and policy level is needed to reduce the variations currently observed in clinical practice.
The authors would like to acknowledge the National Institute of Health Research Manchester Biomedical Research Centre for their funding support in the SKS (NIHR203308). The views expressed are those of the author(s) and not necessarily those of the National Institute of Health Research or the Department of Health and Social Care, United Kingdom.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali A, Iraq S-Editor: Lin C L-Editor: Filipodia P-Editor: Chen YX
| 1. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-50. |
| 2. | Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, Brown JC, Friedman J, He J, Heuton KR, Holmberg M, Patel DJ, Reidy P, Carter A, Cercy K, Chapin A, Douwes-Schultz D, Frank T, Goettsch F, Liu PY, Nandakumar V, Reitsma MB, Reuter V, Sadat N, Sorensen RJD, Srinivasan V, Updike RL, York H, Lopez AD, Lozano R, Lim SS, Mokdad AH, Vollset SE, Murray CJL. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052-2090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1036] [Cited by in RCA: 1546] [Article Influence: 220.9] [Reference Citation Analysis (0)] |
| 3. | Ku E, Johansen KL, McCulloch CE. Time-Centered Approach to Understanding Risk Factors for the Progression of CKD. Clin J Am Soc Nephrol. 2018;13:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 744] [Article Influence: 186.0] [Reference Citation Analysis (1)] |
| 5. | Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 497] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 6. | Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: Core Curriculum 2019. Am J Kidney Dis. 2019;74:120-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 7. | Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O'Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT Jr; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2525] [Article Influence: 360.7] [Reference Citation Analysis (0)] |
| 9. | Lv J, Ehteshami P, Sarnak MJ, Tighiouart H, Jun M, Ninomiya T, Foote C, Rodgers A, Zhang H, Wang H, Strippoli GF, Perkovic V. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 10. | Pugh D, Gallacher PJ, Dhaun N. Management of Hypertension in Chronic Kidney Disease. Drugs. 2019;79:365-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 11. | Fraser SD, Roderick PJ, McIntyre NJ, Harris S, McIntyre CW, Fluck RJ, Taal MW. Suboptimal blood pressure control in chronic kidney disease stage 3: baseline data from a cohort study in primary care. BMC Fam Pract. 2013;14:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Magvanjav O, Cooper-DeHoff RM, McDonough CW, Gong Y, Segal MS, Hogan WR, Johnson JA. Antihypertensive therapy prescribing patterns and correlates of blood pressure control among hypertensive patients with chronic kidney disease. J Clin Hypertens (Greenwich). 2019;21:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Alencar de Pinho N, Levin A, Fukagawa M, Hoy WE, Pecoits-Filho R, Reichel H, Robinson B, Kitiyakara C, Wang J, Eckardt KU, Jha V, Oh KH, Sola L, Eder S, de Borst M, Taal M, Feldman HI, Stengel B; International Network of Chronic Kidney Disease cohort studies (iNET-CKD). Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019;96:983-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Imaizumi T, Hamano T, Fujii N, Huang J, Xie D, Ricardo AC, He J, Soliman EZ, Kusek JW, Nessel L, Yang W, Maruyama S, Fukagawa M, Feldman HI; CRIC Study Investigators. Cardiovascular disease history and β-blocker prescription patterns among Japanese and American patients with CKD: a cross-sectional study of the CRIC and CKD-JAC studies. Hypertens Res. 2021;44:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Prasad N, Yadav AK, Kundu M, Sethi J, Jaryal A, Sircar D, Modi GK, Kamboj K, Sahay M, Gopalakrishnan N, Kaur P, Vikrant S, Varughese S, Baid-Agrawal S, Singh S, Gang S, Parameswaran S, Kumar V, Ghosh A, Jha V. Prescription Practices in Patients With Mild to Moderate CKD in India. Kidney Int Rep. 2021;6:2455-2462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Thomas R, Sam S, Neelaphar P, Shabeeb P, Vishwanath BA. A study on prescribing pattern of antihypertensive in chronic kidney disease patients. J Drug Deliv Ther. 2020;10:75-81. [DOI] [Full Text] |
| 17. | Raman M, Green D, Middleton RJ, Kalra PA. Comparing the impact of older age on outcome in chronic kidney disease of different etiologies: a prospective cohort study. J Nephrol. 2018;31:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Ali I, Chinnadurai R, Ibrahim ST, Kalra PA. Adverse outcomes associated with rapid linear and non-linear patterns of chronic kidney disease progression. BMC Nephrol. 2021;22:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Mertens B, Verhofstede S, Abramowicz D, Couttenye MM. A surprising journey into the conversion of urinary protein creatinine ratio to urinary albumin creatinine ratio as needed in the Kidney Failure Risk Equation. Clin Kidney J. 2021;14:1481-1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Hundemer GL, Knoll GA, Petrcich W, Hiremath S, Ruzicka M, Burns KD, Edwards C, Bugeja A, Rhodes E, Sood MM. Kidney, Cardiac, and Safety Outcomes Associated With α-Blockers in Patients With CKD: A Population-Based Cohort Study. Am J Kidney Dis. 2021;77:178-189.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Aytaş G, Kazancıoğlu R, Elçioğlu ÖC, Gürsu M, Artan AS, Yabacı A, Soysal P, Bilgi K, Özçelik S. Comparative Evaluation of Orthostatic Hypotension in Patients with Diabetic Nephropathy. Kidney Blood Press Res. 2021;46:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Biaggioni I. Orthostatic Hypotension in the Hypertensive Patient. Am J Hypertens. 2018;31:1255-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9:181-190. [PubMed] |
| 24. | Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, Knoll GA, Muntner P, Pecoits-Filho R, Sarnak MJ, Tobe SW, Tomson CRV, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli M, Cheung M, Earley A, Mann JFE. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 25. | National Institute of Clinical Excellence. Chronic kidney disease: assessment and management. Nov 24, 2021. [Cited 30 June 2023]. Available from: https://www.nice.org.uk/guidance/ng203. |
| 26. | Buckley N, Dawson A, Whyte I. Calcium channel blockers. Medicine. 2007;35:599-602. [DOI] [Full Text] |
| 27. | Kario K, Ferdinand KC, Vongpatanasin W. Are SGLT2 Inhibitors New Hypertension Drugs? Circulation. 2021;143:1750-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, Uchiyama K, Niijima Y, Katsuya T, Urata H, Osuga JI, Fujiwara T, Yamazaki S, Tomitani N, Kanegae H. Twenty-Four-Hour Blood Pressure-Lowering Effect of a Sodium-Glucose Cotransporter 2 Inhibitor in Patients With Diabetes and Uncontrolled Nocturnal Hypertension: Results From the Randomized, Placebo-Controlled SACRA Study. Circulation. 2019;139:2089-2097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 29. | Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 30. | Ye N, Jardine MJ, Oshima M, Hockham C, Heerspink HJL, Agarwal R, Bakris G, Schutte AE, Arnott C, Chang TI, Górriz JL, Cannon CP, Charytan DM, de Zeeuw D, Levin A, Mahaffey KW, Neal B, Pollock C, Wheeler DC, Luca Di Tanna G, Cheng H, Perkovic V, Neuen BL. Blood Pressure Effects of Canagliflozin and Clinical Outcomes in Type 2 Diabetes and Chronic Kidney Disease: Insights From the CREDENCE Trial. Circulation. 2021;143:1735-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |