Published online Dec 25, 2023. doi: 10.5527/wjn.v12.i5.159
Peer-review started: August 5, 2023
First decision: September 19, 2023
Revised: September 21, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: December 25, 2023
Processing time: 138 Days and 22.3 Hours
Proteinuria is an important and well-known biomarker of many forms of kidney injury. Its quantitation is of particular importance in the diagnosis and ma
To investigate the correlation of spot uPCR with 24-h urinary protein estimation in patients suffering from different forms of glomerulopathies at a single large-volume nephrological center in Pakistan.
This cross-sectional, observational study was conducted at the Department of Nephrology, Sindh Institute of Urology and Transplantation, Karachi, Pakistan from September 2017 to March 2018. All newly presenting adult patients with proteinuria who were being investigated for suspected glomerulonephritis and persistent proteinuria with ages between 18 to 60 years were enrolled. All patients were given detailed advice regarding 24-h urine collection starting at 7:00 AM for total protein and creatinine excretion estimations. A spot urine sample was collected the next day at the time of submission of a 24-h urine sample for measuring uPCR along with a blood sample. The data of patients were collected in a proforma. SPSS version 20.0 was used for statistical analysis.
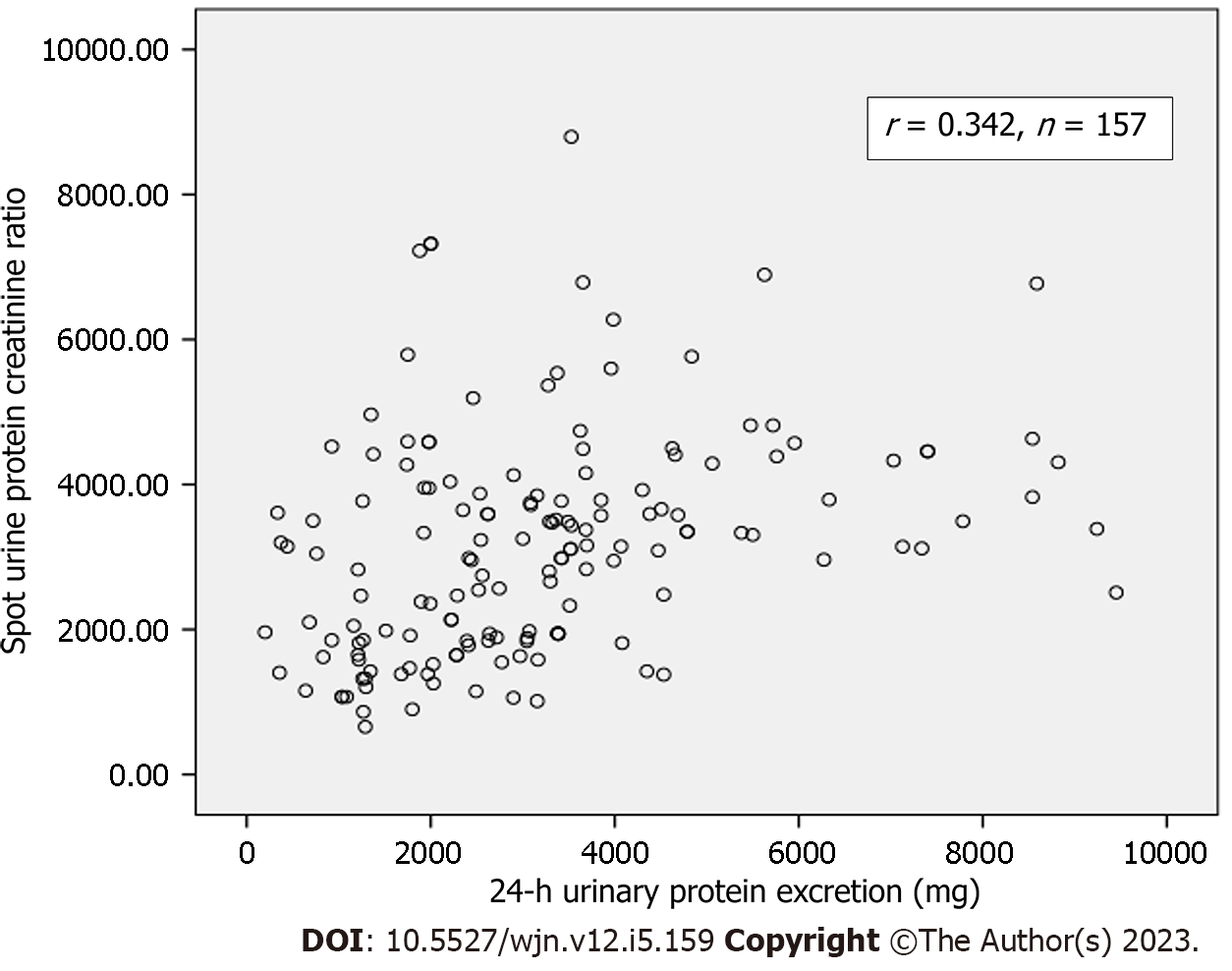
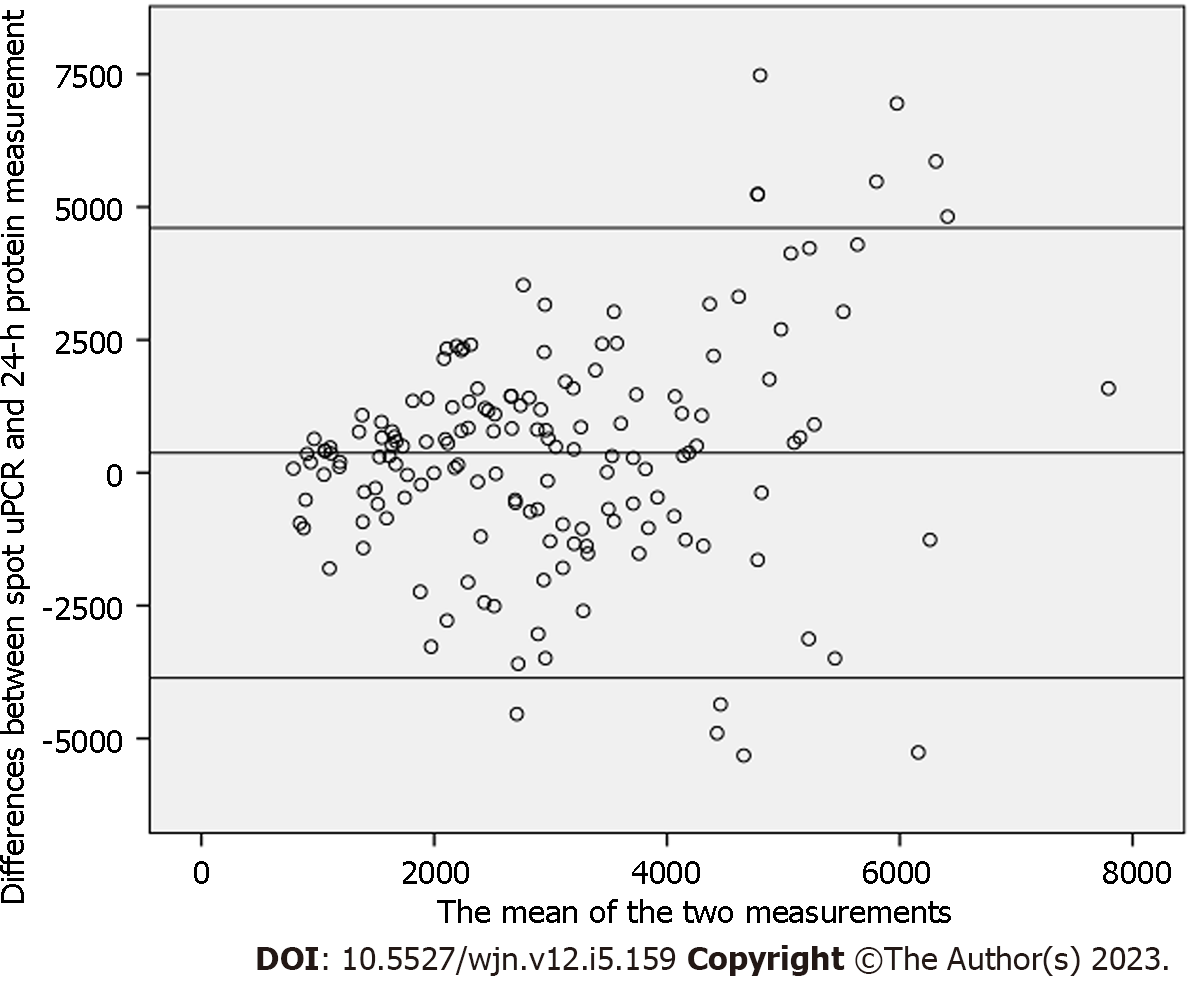
A total of 157 patients were included. Their mean age was 30.45 ± 12.11 years. There were 94 (59.8%) males and 63 (40.2%) females. The mean 24-h urinary protein excretion was 3192.78 ± 1959.79 mg and the mean spot uPCR was 3.16 ± 1.52 in all patients. A weak but significant correlation was observed between spot uPCR and 24-h urinary protein excretion (r = 0.342, P = 0.01) among all patients. On subgroup analysis, a slightly better correlation was found in patients older than 47 years (r = 0.78), and those with body mass index > 25 kg/m2 (r = 0.45). The Bland and Altman's plot analysis comparing the differences between spot uPCR and 24-h protein measurement also showed a wide range of the limits of agreement between the two methods.
Overall, the results from this study showed a significant and weakly positive correlation between spot uPCR and 24-h urinary protein estimation in different forms of glomerulopathies. The agreement between the two methods was also poor. Hence, there is a need for careful interpretation of the ratio in an unselected group of patients with kidney disease.
Core Tip: The quantitation of proteinuria is of particular importance in the diagnosis and management of glomerulonephritides. The measurement of 24-h urinary protein excretion is the gold standard. However, it is cumbersome, time-consuming, and inconvenient for patients. The measurement of urine protein-to-creatinine ratio (uPCR) is the most popular alternative. Numerous studies have been conducted on the correlation of these two methods with conflicting results. We assessed the correlation and the degree of agreement between the two methods. We conclude that uPCR shows poor correlation and poor agreement with 24-h proteinuria. It must be interpreted with caution in an unselected group of glomerulopathies.
- Citation: Raza A, Nawaz SH, Rashid R, Ahmed E, Mubarak M. The correlation of spot urinary protein-to-creatinine ratio with 24-h urinary protein excretion in various glomerulopathies. World J Nephrol 2023; 12(5): 159-167
- URL: https://www.wjgnet.com/2220-6124/full/v12/i5/159.htm
- DOI: https://dx.doi.org/10.5527/wjn.v12.i5.159
Proteinuria is an early sign of glomerular diseases and its quantification for the initial evaluation and follow-up of patients with glomerulonephritis (GN) is routine in clinical practice. It is not only indispensable in making a diagnosis but is also used in monitoring the treatment response of kidney diseases. In fact, remission of proteinuria in some glomerular diseases represents the most powerful predictive factor for ultimate clinical outcomes[1,2]. In some clinical settings, such as nephrotic syndrome, its magnitude directly reflects the disease activity. As protein excretion varies during the course of a day, its estimation in 24-h urine collection is the gold standard method for the evaluation of proteinuria, but this method is cumbersome, time-consuming, more expensive, uncomfortable to the patient, and prone to errors due to under-collection or over-collection of multiple voided samples in a time-dependent manner. Moreover, it cannot be performed in some groups of patients such as children, the elderly, and physically and mentally disabled patients[3-5]. Since 1983, when Ginsberg et al[6] used the ratio as an alternative to 24-h proteinuria, many studies have been carried out using protein-to-creatinine ratios (PCRs) in spot or single-voided urine samples in different clinical settings to correlate these results with 24-h proteinuria, with a correlation ranging from 0.6-0.9 in different studies[7-10]. However, urinary PCR (uPCR) is also influenced by certain features like age, sex, race, muscle mass, and the timing of the urine sample. A sole reliance on uPCR to start or defer specific immunosuppressive treatment without considering these features may be inappropriate. Low muscle mass in the South Asian population has been shown to be an important determinant of low creatinine excretion[11-17]. This is particularly true in elderly, females, and malnourished patients. All these conditions will cause a relatively high uPCR for the same degree of proteinuria.
The correlation between the above two methods of estimation of proteinuria is also influenced by types of renal disease, degree of deterioration in kidney function and degree of proteinuria. Weak correlation is observed in cases of severe kidney failure, interstitial nephritis or severe proteinuria[3-5].
There are very few studies from Pakistan comparing uPCR with 24-h urine protein excretion and only one was done in patients with normal glomerular filtration rate[13,14]. An excellent correlation (r = 0.96) was found between random uPCR and standard 24-h urinary protein excretion in these patients (P < 0.001)[13]. However, the difference between uPCR and 24-h protein excretion was not reported.
The objective of this study was to compare estimated protein excretion by uPCR with measured 24-h urine protein excretion in patients with different types of glomerulopathies at a single center in Pakistan.
This cross-sectional and observational study was conducted at the outpatient clinic of the Department of Nephrology, Sindh Institute of Urology and Transplantation between 28th September 2017 and 28th March 2018. Eligibility for the study were adult subjects with the suspected glomerular disease with dipstick-positive proteinuria on the day of recruitment. Consecutive patients who agreed to submit a 24-h urine sample, who were more than 18 years old, and who were not diabetic were included. Exclusion criteria were chronic kidney disease stage 5 based on the Cockcroft-Gault formula, urinary tract infection, and those deemed incapable of collecting 24-h urine.
All patients were informed about the study’s purpose and written consent was obtained. Patients were given detailed advice regarding 24-h urine collection from morning to the next morning. A wide-mouthed container was provided to every patient for collecting the 24-h urine. Patients were asked to collect the 24-h urine of their most convenient day and bring it on the day of completion. A spot urine sample was taken on the submission day for measuring the uPCR. A blood sample was taken at the same time. All samples were transported to the laboratory immediately. Serum creatinine and urinary creatinine (mg/dL) concentrations were determined using the modified Jaffe’s method on an auto-analyzer. Creatinine clearance was calculated by the standard formula. Urinary protein concentration was determined with the colorimetric method using pyrogallol red. To assess the completeness of the collection, creatinine excretion in a 24-h urine sample was used. Specimens with creatinine excretion of 15-25 mg/kg in males, and 12-20 mg/kg in females, were considered adequate. Patients with creatinine excretion outside these ranges were excluded from the study.
The statistical analysis for this research was done by using SPSS version 22.0 (IBM Corp, Armonk, NY, United States). mean ± SD were evaluated for continuous data such as age, weight, height, body mass index (BMI), 24-h urinary volume, urinary protein excretion, serum creatinine, creatinine clearance, and spot uPCR. For categorical data such as gender, the frequency and percentages were calculated. Correlation between spot uPCR and 24-h urinary protein excretion was carried out using Pearson’s correlation coefficient (r). P-value ≤ 0.05 was taken as significant. Bland and Altman plot was drawn using the average of protein excretion by both the methods on the X-axis and the difference between 24-h urinary protein and spot uPCR on the Y-axis. Mean bias and 95% confidence limits for the degree of agreement between the two methods were also calculated.
The main demographic, clinical and laboratory characteristics of the study population (n = 157) are shown in Table 1. The mean age of all patients was 30.45 ± 12.11 years. There were 94 (59.8%) males and 63 (40.2%) females. The majority of the patients (n = 133, 74.53 %) had a BMI of < 25 kg/m2. The mean values of urinary protein excretion by both methods were similar to each other, with maximum values of protein excretion by both being less than 10 g/24 h. The mean 24-h urinary creatinine excretion was 834.96 ± 391.43 mg/24 h. The histopathological results of kidney biopsies in these patients are also shown in Table 1. It is apparent that focal segmental glomerulosclerosis and membranous GN were the two most common lesions followed by a variety of less common other pathological lesions. A positive, fair, and significant correlation between spot uPCR and 24-h urinary protein excretion (r = 0.342, P-value < 0.001) in the entire group (Figure 1). On subgroup analysis, a slightly better correlation was found in patients older than 47 years, and those with BMI > 25 kg/m2 (Table 2).
| Parameters | Values |
| Age (yr) | 30.45 ± 12.108 |
| Weight (kg) | 59.70 ± 15.067 |
| Height (cm) | 163.98 ± 11.476 |
| Body mass index (kg/m2) | 22.30 ± 5.65 |
| Serum creatinine (mg/dL) | 1.00 ± 0.54 |
| 24-h urinary creatinine (mg/24-h) | 834.12 ± 391.43 |
| Creatinine clearance (ml/min) | 75.05 ± 33.55 |
| Spot urine protein (mg/dL) | 296.04 ± 159.65 |
| Spot urine creatinine (mg/dL) | 108.83 ± 58.01 |
| 24-h urine protein excretion (mg/24-h) | 3192.78 ± 1959.79 |
| Spot urine protein to creatinine ratio (mg/mg) | 3.15 ± 1.52 |
| 24-h urine volume | 1711.40 ± 882.03 |
| Histopathological diagnosis, n (%) | |
| Focal segmental glomerulosclerosis | 52 (33.12) |
| Membranous glomerulonephritis | 44 (28) |
| Mesangiocapillary glomerulonephritis | 28 (17.83) |
| IgM nephropathy | 11 (7) |
| Lupus nephritis | 10 (6.36) |
| Minimal change disease | 4 (2.54) |
| Others | 8 (5.09) |
| Parameters | No of patients (n) | Correlation coefficient (r) | P value |
| Age (yr) groups | |||
| 34-46 | 35 | 0.475 | 0.004 |
| > 47 | 21 | 0.780 | 0.001 |
| BMI (kg/m2) ranges | |||
| 23-25 | 24 | 0.411 | 0.046 |
| > 25 | 40 | 0.459 | 0.00 |
| Proteinuria | |||
| < 3000 mg/d | 79 | 0.021 | 0.28 |
| ≥ 3000 mg/d | 78 | 0.216 | 0.058 |
Bland and Altman's plot comparing the differences between spot uPCR and 24-h protein measurement is depicted in Figure 2. The mean bias was 373 mg; however, the limits of agreement were fairly wide (from -3682 to 4069 mg). The scatter of differences increased as the amount of proteinuria increased. The mean bias and limits of agreement in the groups with less than and more than 3000 mg protein excretion are shown in Figures 3 and 4, respectively. Briefly, the mean bias was -93.64 mg and the limits of agreement were -2861.53 to 2674.25 mg in the group of patients with less than 3000 mg of proteinuria. These values were 158.36 and -4738.46 to 5035.18 mg in the group of patients with more than 3000 mg of proteinuria. As is obvious, the limits are wider with increasing levels of proteinuria.
The quantification of proteinuria is an important investigation in patients with various glomerular diseases. It not only helps in making a diagnosis but also helps in follow-up to monitor disease progression, and often, important therapeutic decisions are made based on its exact value. The traditional reference method, 24-h urine protein measurement, is a cumbersome and tedious test, and therefore spot uPCR has replaced it in clinical practice due to its simplicity, convenience, and presumed accuracy. Initial studies focused more on the correlation between the two methods and not surprisingly a moderate to strong correlation (0.57 to 0.9) was reported in different studies[1-5].
In the present study, a fair correlation of spot uPCR with 24-h urinary protein measurement was observed. Although this was statistically significant, it is lower than the previously described correlation in many other studies[1-5,7-10,18-21]. These results are not entirely explained on the basis of extremes of proteinuria in our population. The median protein excretion in our population was 2970 mg in 24 h, and we, therefore, stratified them according to proteinuria above and below 3000 mg to see if the correlation changes, as most of the therapeutic decisions are taken when proteinuria is above 3000 mg. There was an equal number of patients in the two groups (79 in < 3000 mg group, and 78 in ≥ 3000 mg group). The correlation was much weaker in the group with protein excretion of less than 3000 mg and failed to reach statistical significance. In the group with protein excretion of more than 3000 mg in 24 h, the correlation with spot uPCR was just significant (P = 0.058). There were 13 patients in our group who had protein excretion in excess of 6000 mg and after excluding them from the group of 78 patients with protein excretion ≥ 3000 mg, the correlation with spot uPCR (r = 0.295) became significant (P = 0.02). Similarly, there were 12 patients in the group with protein excretion < 3000 mg who had proteinuria of less than 1000 mg, and after excluding them from this group, when we recalculated the correlation with spot uPCR it remained weak (r = 0.21).
As is well known, uPCR is also influenced by gender, as females have lower creatinine excretion due to lower muscle mass and this can give rise to a higher ratio compared to males for a similar degree of proteinuria. However, the correlation was not much different when we stratified the entire group according to gender. Besides the degree of proteinuria and gender, uPCR is also influenced by renal function, the timing of random (spot) urine specimens, and the handling of urine samples[10,11]. The mean creatinine clearance in this study population was 75 mL/minute, and 75% of the population had serum creatinine less than 1.23 mg/dL; therefore it is unlikely that the ratio was influenced by compromised renal functions. Patients were asked to submit spot urine samples on the following day of 24-h urine collection, but this sample was taken at different times of day in individual patients and physical activity may have influenced protein excretion in some patients who had come late for submitting their specimens.
One possible explanation for the poor correlation between uPCR and 24-h urinary protein measurement is that the latter assumes a creatinine excretion of 1000 mg/d which may be incorrect in some populations[6]. Around 50% of our population had a BMI of < 21.5 kg/m2 suggesting low muscle mass and hence low creatinine excretion. Indeed, when we analyzed the correlation between spot uPCR and 24-h urine protein excretion in the group of patients with BMI >25 kg/m2, we observed a much stronger correlation than patients with lower BMI (r = 0.45, P = 0.003).
More important than correlation from the clinical perspective is the degree of agreement or the difference between two measurements. To replace 24-h measurement with uPCR in spot urine samples for clinical decision-making, it is prudent to see whether the two techniques agree sufficiently. In this study, the limits of agreement were fairly wide, more so with higher grades of proteinuria. Irrespective of the fact that whether proteinuria was sub-nephrotic or in the nephrotic range, these differences are unacceptable. In an earlier study of 170 proteinuric patients, good agreement between the two methods was reported in the range between 200 mg and 3.5 g protein excretion[22]. A meta-analysis of 13 studies in patients with systemic lupus erythematosus showed poor agreement between the two methods[23]. In a study using NEPTUNE cohort of patients, there was modest correlation between the two methods, and both correlation and predictability improved on Log10 transformation of values[24]. Only 25% of patients in NEPTUNE cohort had nephrotic syndrome, making it difficult to generalize it for higher grades of proteinuria. Lately, in a single center study of 142 proteinuric patients, a poor agreement was reported between 24-h urine protein excretion and uPCR from different timed spot samples. The limits of agreement were widest when protein excretion exceeded 3.5 g in 24 h (-3.2 to 8.2)[25].
Our study has certain limitations. It is a single center and single laboratory-based study. We used uPCR rather than albumin: Creatinine ratio, which is more reliable compared to uPCR. Spot urine samples were taken at different times of day in individual patients which may have affected protein excretion and hence its concentration. Some patients were already on steroids, which causes sarcopenia and decreases creatinine excretion resulting in higher uPCR. Moreover, specific disease diagnoses were not recorded in this study. Some of the patients might have had non-glomerular pathology. The main strength of our study is the broad range of proteinuria used for comparison, with a nearly equal number of patients below and above the nephrotic threshold of proteinuria. The spot urine sample was collected on the very next day of 24-h urine collection so that both represented similar pathophysiology. Although 24-h urine protein excretion is considered the gold standard test for accurate estimation of proteinuria, over-collection and under-collection of urine sample affects its accuracy[26-28]. We tried to mitigate this issue by excluding patients who had creatinine excretion outside the expected range based on their weight.
In conclusion, considering the overall poor correlation and the wide limits of agreement between 24-h urine protein excretion and uPCR, the latter should be used with great caution to predict protein excretion in patients with glomerular disease. This is more so when important therapeutic decisions are being made based on the degree of proteinuria.
There are a number of methods by which the quantification of protein excretion in urine is done to inform clinical decisions. Among these, the estimation of protein excretion in the 24-h urinary sample is the traditional and gold standard method. However, it is cumbersome, time-consuming, and prone to errors. The alternative method of measuring urine protein-to-creatinine ratio (uPCR) is used widely in clinical practice as it is quick, patient-friendly, and reliable. The available data on the correlation between the above two methods is controversial.
We also heavily rely on uPCR for our routine patient care. However, we do not know how it correlates with 24-h urinary protein excretion. This motivated us to determine the correlation and degree of agreement between the two tests, so that we should use uPCR results accordingly.
The objectives of this study were to determine the correlation of spot uPCR with 24-h urinary protein excretion test and in particular, the degree of agreement between the two tests, in patients suffering from various forms of glomerulopathies so that we may use this test with caution in future.
This was a cross-sectional, observational study conducted on all newly presenting adult patients (age: 18 to 60 years) with proteinuria who were being investigated for suspected glomerulonephritis (GN). All patients were counseled regarding 24-h urine collection. A spot urine sample was collected the next day at the time of submission of a 24-h urine sample for measuring uPCR along with a blood sample. SPSS version 20.0 was used for statistical analysis.
A total of 157 patients with a mean age of 30.45 ± 12.11 years were included. There were 94 (59.8%) males and 63 (40.2%) females. The mean 24-h urinary protein excretion was 3192.78 ± 1959.79 mg and the mean spot uPCR was 3.16 ± 1.52 in all patients. A significant but poor correlation was observed between spot uPCR and 24-h urinary protein excretion (r = 0.342, P = 0.01) among all patients. On subgroup analysis, a slightly better correlation was found in patients older than 47 years (r = 0.78), and those with body mass index > 25 kg/m2 (r = 0.45). Bland and Altman's plot analysis of the two tests also showed a wide range of the limits of agreement between the two methods.
The results from this study show a significant, positive but poor correlation between spot uPCR and 24-h urinary protein estimation in various types of glomerular diseases. The agreement between the two methods was also poor. Hence, there is a need for careful interpretation of the ratio in an unselected group of patients with glomerular diseases.
There is a need to conduct a well-planned, international, multi-center study to resolve the controversy of correlation and agreement between the two most widely used methods of proteinuria estimation in clinical practice.
We greatly acknowledge the help of Ms. Maham Iqbal in collecting and interpreting the data on the urinary and serum parameters of all patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vlachopanos G, Greece S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Ahmed PI, Islam MN, Alam MB, Bhuiya FK, Noman MU, Chowdhury MN. Comparison of 24-Hour Urinary Protein and Spot Urinary Protein- Creatinine Ratio in the Assessment of Proteinuria in Patients with Glomerulonephritis. J Dhaka Med Coll. 2015;23:194-202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Antunes VV, Veronese FJ, Morales JV. Diagnostic accuracy of the protein/creatinine ratio in urine samples to estimate 24-h proteinuria in patients with primary glomerulopathies: a longitudinal study. Nephrol Dial Transplant. 2008;23:2242-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Patil P, Shah V, Shah B. Comparison of spot urine protein creatinine ratio with 24 hour urine protein for estimation of proteinuria. J Assoc Physicians India. 2014;62:406-410. [PubMed] |
| 4. | Wahbeh AM, Ewais MH, Elsharif ME. Comparison of 24-hour urinary protein and protein-to-creatinine ratio in the assessment of proteinuria. Saudi J Kidney Dis Transpl. 2009;20:443-447. [PubMed] |
| 5. | Kamińska J, Dymicka-Piekarska V, Tomaszewska J, Matowicka-Karna J, Koper-Lenkiewicz OM. Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice. Crit Rev Clin Lab Sci. 2020;57:345-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 485] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Montero N, Soler MJ, Pascual MJ, Barrios C, Márquez E, Rodríguez E, Berrada A, Riera M, Coca L, Orfila MA, Pascual J. Correlation between the protein/creatinine ratio in spot urine and 24-hour urine protein. Nefrologia. 2012;32:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Yang CY, Chen FA, Chen CF, Liu WS, Shih CJ, Ou SM, Yang WC, Lin CC, Yang AH. Diagnostic Accuracy of Urine Protein/Creatinine Ratio Is Influenced by Urine Concentration. PLoS One. 2015;10:e0137460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Chen CF, Yang WC, Yang CY, Li SY, Ou SM, Chen YT, Shih CJ, Chien CC, Chen MC, Wang YJ, Lin CC. Urinary protein/creatinine ratio weighted by estimated urinary creatinine improves the accuracy of predicting daily proteinuria. Am J Med Sci. 2015;349:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Methven S, MacGregor MS. Empiricism or rationalism: how should we measure proteinuria? Ann Clin Biochem. 2013;50:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Naresh CN, Hayen A, Craig JC, Chadban SJ. Day-to-day variability in spot urine protein-creatinine ratio measurements. Am J Kidney Dis. 2012;60:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Price CP, Newall RG, Boyd JC. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem. 2005;51:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Ali A, Asif N, Yaqub S, Kashif W, Merchant D, Yazdant I. Spot urine protein: creatinine ratio versus 24 hour urine protein at various levels of GFR patients referred to a tertiary care hospital of Pakistan. J Pak Med Assoc. 2008;58:476-479. [PubMed] |
| 14. | Khan DA, Ahmad TM, Qureshil AH, Halim A, Ahmad M, Afzal S. Assessment of proteinuria by using protein: creatinine index in random urine sample. J Pak Med Assoc. 2005;55:428-431. [PubMed] |
| 15. | Jafar TH, Chaturvedi N, Hatcher J, Levey AS. Use of albumin creatinine ratio and urine albumin concentration as a screening test for albuminuria in an Indo-Asian population. Nephrol Dial Transplant. 2007;22:2194-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Jafar TH, Chaturvedi N, Gul A, Khan AQ, Schmid CH, Levey AS. Ethnic differences and determinants of proteinuria among South Asian subgroups in Pakistan. Kidney Int. 2003;64:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Schultz CJ, Dalton RN, Turner C, Neil HA, Dunger DB. Freezing method affects the concentration and variability of urine proteins and the interpretation of data on microalbuminuria. The Oxford Regional Prospective Study Group. Diabet Med. 2000;17:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Ruggenenti P, Gaspari F, Perna A, Remuzzi G. Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ. 1998;316:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines--application to the individual patient. Kidney Int. 2012;82:840-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 21. | Methven S, MacGregor MS, Traynor JP, O'Reilly DS, Deighan CJ. Assessing proteinuria in chronic kidney disease: protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant. 2010;25:2991-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Chitalia VC, Kothari J, Wells EJ, Livesey JH, Robson RA, Searle M, Lynn KL. Cost-benefit analysis and prediction of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin Nephrol. 2001;55:436-447. [PubMed] |
| 23. | Medina-Rosas J, Yap KS, Anderson M, Su J, Touma Z. Utility of Urinary Protein-Creatinine Ratio and Protein Content in a 24-Hour Urine Collection in Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken). 2016;68:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Hogan MC, Reich HN, Nelson PJ, Adler SG, Cattran DC, Appel GB, Gipson DS, Kretzler M, Troost JP, Lieske JC. The relatively poor correlation between random and 24-hour urine protein excretion in patients with biopsy-proven glomerular diseases. Kidney Int. 2016;90:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Rydzewska-Rosołowska A, Kakareko K, Naumnik B, Hryszko T. Comparison of Different Methods of Urinary Protein Excretion Measurement: Is the King Really Dead? Kidney Blood Press Res. 2019;44:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Selvarajah V, Flynn R, Isles C. Comparison of estimated protein output and urine protein: creatinine ratio in first and second voids with 24-hour urine protein. Nephron Extra. 2011;1:235-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Morales JV, Weber R, Wagner MB, Barros EJ. Is morning urinary protein/creatinine ratio a reliable estimator of 24-hour proteinuria in patients with glomerulonephritis and different levels of renal function? J Nephrol. 2004;17:666-672. [PubMed] |
| 28. | Akin D, Ozmen S. An unresolved issue: The relationship between spot urine protein-to-creatinine ratio and 24-hour proteinuria. J Int Med Res. 2019;47:1179-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |