Published online Jul 25, 2022. doi: 10.5527/wjn.v11.i4.127
Peer-review started: June 19, 2021
First decision: July 31, 2021
Revised: September 19, 2021
Accepted: June 21, 2022
Article in press: June 21, 2022
Published online: July 25, 2022
Processing time: 395 Days and 21.4 Hours
The burden of chronic kidney disease (CKD) is rising rapidly globally. Fluid overload (FO), an independent predictor of mortality in CKD, should be accurately assessed to guide estimation of the volume of fluid to be removed during haemodialysis (HD). Clinical score (CS) and bio-impedance analysis (BIA) have been utilized in assessment of FO and BIA has demonstrated reproducibility and accuracy in determination of fluid status in patients on HD. There is need to determine the performance of locally-developed CSs in fluid status assessment when evaluated against BIA.
To assess the hydration status of patients on maintenance HD using BIA and a CS, as well as to evaluate the performance of that CS against BIA in fluid status assessment.
This was a single-centre, hospital-based cross-sectional study which recruited adult patients with CKD who were on maintenance HD at Kenyatta National Hospital. The patients were aged 18 years and above and had been on main
From 100 patients on maintenance HD screened for eligibility, 80 were recruited into this study. Seventy-one (88.75%) patients were fluid overloaded when evaluated using BIA with mean extracellular volume of 3.02 ± 1.79 L as opposed to the forty-seven (58.25%) patients who had FO when evaluated using the CS. The difference was significant, with a P value of < 0.0001 (95% confidence interval: 0.1758-0.4242). Using CS, values above 4 were indicative of FO while values less than or equal to 4 denoted the best cut-off for no FO. The sensitivity and specificity for the CS were 63% and 78% respectively. None of the factors evaluated for association with FO showed statistical significance on the multivariable logistic regression model.
FO is very prevalent in patients on chronic HD at the Kenyatta National Hospital. CS detects FO less frequently when compared with BIA. The sensitivity and specificity for the CS were 63% and 78% respectively. None of the factors evaluated for association with FO showed statistical significance on the multivariable logistic regression model.
Core Tip: Bio-impedance analysis (BIA) has been validated as an accurate and reliable tool for determining fluid status in chronic kidney disease (CKD) patients but is not widely available in low-income settings. In this study we assess how a clinical score (CS) compares with BIA in this population for possible use as a low-cost substitute where BIA is not available. Patients with a CS score greater than 4 were considered to have fluid overload (FO), and detected using this parameter in 58.75% of patients. CSs of ≤ 4 represented no FO, and represented 41.25% of patients. The CS had a sensitivity of 63% and a specificity of 78% in making a diagnosis of FO compared with BIA, which was used as the reference in patients with CKD on maintenance haemodialysis.
- Citation: Muchiri K, Kayima JK, Ogola EN, McLigeyo S, Ndung’u SW, Kabinga SK. Concordance between bio-impedance analysis and clinical score in fluid-status assessment of maintenance haemodialysis patients: A single centre experience. World J Nephrol 2022; 11(4): 127-138
- URL: https://www.wjgnet.com/2220-6124/full/v11/i4/127.htm
- DOI: https://dx.doi.org/10.5527/wjn.v11.i4.127
Chronic kidney disease (CKD) refers to the abnormalities in kidney structure and function with effects on the individual’s health for more than 3 mo. The indicators for kidney damage include reduced estimated glomerular filtration rate, abnormal urinalysis findings, or abnormal histologic findings on kidney biopsy[1]. The risk factors for CKD include diabetes mellitus (DM), high blood pressure, and glomerulonephritides[2,3]. Advanced CKD is heralded by general ill-health, symptomatic anaemia, signs and symptoms of uraemia, and fluid overload (FO). End stage kidney disease (ESKD) is characterised by reduced ability to excrete enough sodium and the resultant water retention, which presents as a FO state. In this state, the patient requires kidney replacement therapy (KRT) for sustenance of bodily functions. The KRT modalities include dialysis and kidney allograft transplantation. Dialytic modalities include haemodialysis (HD) and peritoneal dialysis (PD). In Africa, less than 3% of those who require KRT receive it, with HD as the most popular modality[4].
The extracellular volume (ECV) varies by ± 1 L in healthy individuals and is dependent on salt intake. The ECV is severely affected by kidney disease. FO is defined as the ECV in excess of that observed in healthy individuals with normal kidney function[5]. Chronic FO increases mortality from arterial hypertension, left ventricular dilatation and hypertrophy, and congestive heart failure[6]. Oedema predisposes the patient to skin infections, especially in diabetic patients, and can result in sepsis, limb amputations, and high mortality. Oedema in the gut leads to malabsorption of nutrients and, in the lungs, it increases risk of bronchitis and pneumonia[7]. Among the patients with CKD, the state of hydration comes only second to the presence of DM in predicting mortality[8]. Greater than 15% of relative overhydration corresponds to > 2.5 L FO and is independently associated with high mortality[9]. This degree of over hydration is associated with an 8.5% increase in deaths even in stable CKD group of patients on dialysis[8]. HD removes waste products and fluids. Conversely, dehydration is associated with muscular cramps, low blood pressure, cardiac stunning, and loss of residual kidney function[6].
Several methods have been employed in assessment of fluid status in CKD patients. Varied signs and symptoms have been put together to comprise a clinical scoring system for assessment of state of hydration among patients. Clinical scores (CSs) are easy to document and can be recorded consistently and regularly. These make the scores appealing for utilization in assessment of hydration status. However, clinical scoring systems have inherent weaknesses of incompleteness, subjectivity of the observer, and lack of specificity. The specificity of CSs can be increased by scoring symptoms that manifest de novo and clear with correction of hydration status. Wizemann et al[10] utilized this approach in a study whereby signs and symptoms of dehydration and FO were grouped and scored.
There are other methods which have been employed in assessment of the fluid status. These include imaging like chest X-rays and ultrasonographic scanning, monitoring of plasma volume by dilution methods, clinical methods, and bio-impedance analysis (BIA). The BIA is based on the principle that in a cylinder, the electrical impedance varies directly with length and inversely to the product of cross-sectional area and specific sensitivity[9]. In BIA, an alternating current is passed through the body and the current passes extra- or intracellularly depending on whether it is low or high. High frequency current passes through extra- and intracellular spaces. Bio-impedance-defined overhydration (OH) independently predicts mortality due to its ability to discriminate absolute and relative extracellular fluid (ECF) volume[9].
Employment of various techniques simultaneously can achieve better results in evaluation of hydra
This was a single-centre hospital-based cross-sectional analytic study carried out between March and April 2019. It was performed in the Renal Department at the Kenyatta National Hospital, which is a national teaching and referral hospital located in Nairobi-Kenya. The study recruited ESKD who had been on maintenance HD. Those included were adult patients aged ≥ 18 years who had been on HD for at least 3 mo. We excluded patients who had undergone bilateral limbs amputation, had implanted metallic devices, pacemakers, or metallic intravascular devices, or who were very sick.
The sample size was estimated using the sample size formula for comparing paired proportions (McNemar’s Z test, 2-sided equality)[11]. Using the prevalence of FO using BIA by Bajaber et al[12] (69%), prevalence of FO using a CS by Wizemann et al[10] (35%), α of 5% and β of 20%, the calculated sample size after adjusting upwards by 15% to account for non-response was 80 patients. The study employed systematic random sampling without replacement. Structured history and physical examinations were performed by one clinician. Weight was measured to the nearest 0.1 kg using a digital scale placed on a firm flat surface after the participants had removed heavy outer garments, shoes and emptied their pockets. The weighing scale was calibrated daily. The height was taken using a stadiometer and employed a standard protocol. Two measurements were taken and the average of the two readings recorded to the nearest centimetre. Oedema was assessed using a standard scoring system for uniformity[13].
Chest radiographs were obtained to assess findings of FO. The findings assessed included dilated veins in the upper lung zones and cardiomegaly that were classified as stage 1 hypervolemia. Stage 2 hypervolemia was marked by interstitial oedema evidenced by Kerley B lines, while stage 3 was evidenced by alveolar oedema or pleural effusion as reported by two radiologists at the University of Nairobi who were blinded to study procedures. A CS that had not been previously validated was developed for the study. It entailed eliciting signs and symptoms for hypovolemia like intradialytic hypotension, muscle cramps, dizziness, or fatigue during HD session and the need for treatment of hypotension with normal saline infusion, which were scored at -1 each. Signs and symptoms scored as hypervolemia included hypertension, hypoxia noted by oxygen saturation < 90%, presence of ascites, pleural effusion, or pulmonary oedema, which were scored at +1 each. The interdialytic weight gain was determined and scored as +1 for each kilogram gained since the last session of HD. Presence of gallop rhythm was scored at +2, dyspnoea classification by New York Heart Association was scored from 0 to +3, chest radiograph features of FO scored from +1 to +3 based on stages described above, and oedema of ankles and tibia was scored from 0 to +4 as shown in Table 1.
| Symptoms | Score | |
| Scored as dehydration | Intradialytic hypotension | -1 |
| Muscle cramps, dizziness or fatigue during current session of dialysis | -1 | |
| Symptomatic dialysis hypotension treated by NaCl (0.9%) infusion | -1 | |
| Scored as normohydration | Absence of symptoms given in this table | 0 |
| Scored as fluid overload | Hypertension | +1 |
| SPO2 less than 90% | +1 | |
| Presence of ascites | +1 | |
| Presence of pleural effusion or pulmonary oedema on clinical examination | +1 | |
| Inter dialytic weight gain - per 1 kg gained | +1 | |
| Presence of gallop rhythm | +2 | |
| Dyspnoea based on NYHA class | 0 to +3 | |
| Chest radiography features based on stage | +1 to +3 | |
| Oedema (ankles, tibial, graded) | 0 to +4 | |
BIA was done by placing electrodes on one side of the body either left or right upper and lower limbs after lying supine for 10 min. For patients who used arteriovenous fistulae (AVF) for HD vascular access, the side without AVF was used. Measurement of resistance and reactance were then determined based on the manufacturer’s guidelines. The machine used was the Quantum® II bio-impedance analyser manufactured by RJL Systems, Inc., Clinton Township, Michigan, United States, together with the BC 4® software from the same manufacturer. Hydration status was based on Wabel et al[14], which classified fluid status into three categories based on ECF estimation by BIA. These included dehydration where the ECF is estimated to be less than of -1.1 L, normal hydration with ECF ± 1.1 L, and FO where ECF is > 1.1 L. FO was further stratified as mild FO, where ECF was 1.1-2.5 L, and gross FO, where ECF was > 2.5 L.
The target variable was FO, as diagnosed by the newly developed CS and BIA. The predictor variables included BMI in kg/m2, blood pressure in mmHg, antihypertensive medications used, fluid intake, salt intake, number of HD sessions per week, adherence to HD treatment, missed HD sessions during the 2 wk preceding the study period, HD vintage, antihypertensive medications, and fluid restriction. An adherent patient was one who had not missed any HD sessions in the 2 wk that preceded the study or any doses of scheduled antihypertensive medications in the week prior to evaluation and had received education on fluid and salt restriction which the patient was following, all based on the patients’ self-report.
Data were analysed using STATA® software. Continuous variables included age, duration of CKD, HD vintage, systolic and diastolic blood pressure, and BMI. Normally distributed continuous data had their means and standard deviations computed. For skewed continuous data, medians and inter-quartile ranges (IQR) were computed. Categorical variables like sex, co-morbidities, hydration status by both CS and BIA, and HD vascular access, had frequencies calculated and were presented as counts and percentages. The result for each participant’s CS (positive or negative for FO) was compared to its paired BIA result. Using BIA as the gold standard test for diagnosing FO, the CS sensitivity and specificity measures together with the false positive rate (FPR) at each of the CS values obtained by the participants were computed using the formulae: Se = TP/(TP + FN), Sp = FP/(FP + TN), FPR = 1 – SP. Where Se was sensitivity, Sp was specificity, TP was true positive, FN was false negative, FP was false positive, TN was true negative.
A receiver operating characteristic (ROC) curve (graph of sensitivity vs FPR) was plotted for scores obtained in order to establish the best cut-off point for determining FO using the CS[15]. The point which gave the greatest area under the ROC and in which the differential positive rate (DPR) value was highest was interpreted as the optimal cut-off point for the CS. The DPR was calculated using the formula: DPR = (Se + Sp) – 1. Values of the CS that were above and below the cut-off point were established as optimal on the ROC were interpreted as positive and negative for FO respectively.
The McNemar’s chi square test was used to assess any statistically significant differences in proportions of patients diagnosed as having FO by both CS and BIA. Stepwise logistic regression analysis was conducted in order to assess whether the duration of dialysis, number of missed dialysis sessions, advise on fluid intake, actual fluid intake, advise on salt intake, number of anti-hypertensives used, and BMI were significant predictors of FO in this study population. Univariable logistic regression models between each of the predictor variables and FO was conducted at a liberal P value of 0.20. The variables having a P value of < 0.20 in the univariable models were added to the multivariable model where their association with the odds of FO was tested at a 5% significance level. Non-significant variables were eliminated from the multivariable model if they did not result in > 30% change in the coefficient of the significant variables[16]. The Hosmer-Lemeshow goodness of fit was computed to evaluate how well the final logistic regression model fit the data with a P value > 0.05, indicating a well-fitting model[15].
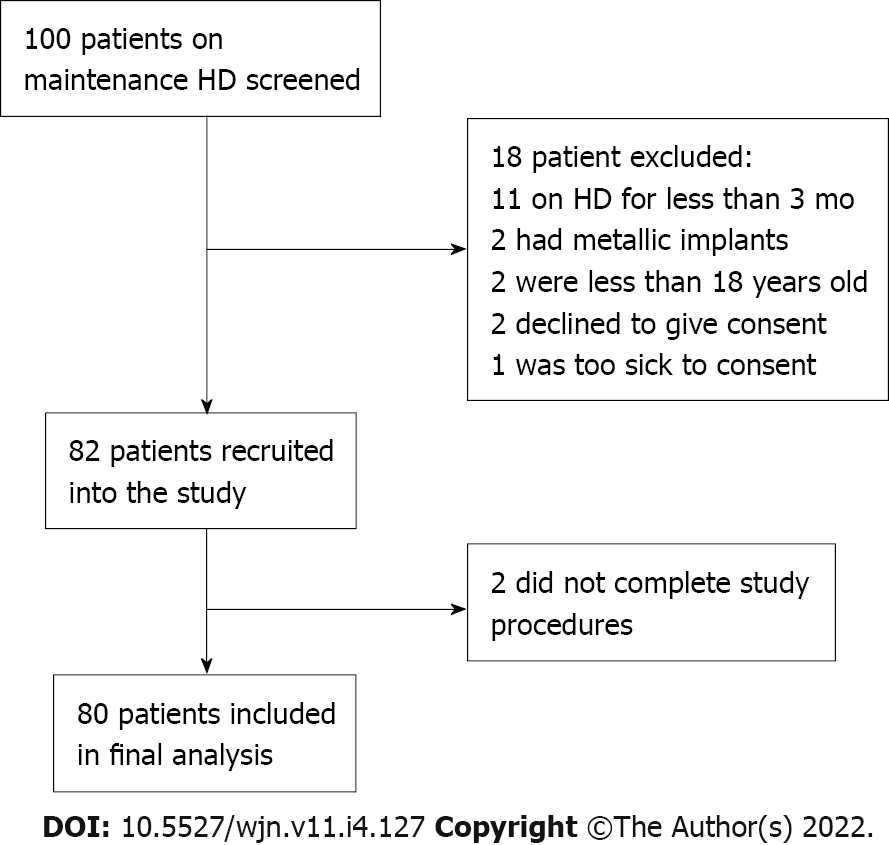
Altogether, there were about 100 patients who were on maintenance HD at Kenyatta National Hospital renal unit between March and April 2019. All 100 patients were screened for eligibility. Eleven patients were excluded because they had been on HD for less than 3 mo, two had metallic implants, two declined to participate, two were below 18 years of age, and one patient was critically ill. The excluded patients were aged between 15 years and 70 years with a median age of 40 years and were predominantly male (60%). Eighty-two patients met the inclusion criteria and were recruited into the study. However, at the time of analysis, it was noted that two participants had incomplete data (missing chest radiographs) and were excluded from the final analysis. The two excluded were a male and female patient, aged 53 years and 27 years, on HD for 28 and 3 mo, respectively. By BIA, their hydration statuses were normohydrated and gross OH, respectively. The final analyses included 80 participants (Figure 1).
Table 2 summarizes demographic and clinical characteristics of the study participants. The study participants were aged between 18 years and 75 years with a median age of 45 years with an IQR of 20.5 years. Forty-six (57.5%) were males. Most (63.75%) of the patients had secondary level education or higher. Fifty-seven (71.25%) of the participants were married and the majority (93.75%) had medical insurance that covered the costs of their HD, for a maximum of two HD sessions per week.
| Characteristic | Statistic | |
| Age (yr) | Median (IQR) | 45 (20.5) |
| Sex | Male, n (%) | 46 (57.5) |
| CKD duration (mo) | Median (IQR) | 12.5 (24.5) |
| Dialysis vintage | Median (IQR) | 9 (15) |
| Blood pressure (mmHg) | Systolic mean ± SD | 150 ± 31 |
| Diastolic mean ± SD | 91 ± 19 | |
| Body mass index (kg/m2) | Median (IQR) | 21.94 (5.13) |
| Comorbidities | Hypertension, n (%) | 41 (51.25) |
| Glomerulonephritis, n (%) | 22 (27.5) | |
| Diabetes mellitus, n (%) | 14 (17.5) | |
| Obstructive uropathy, n (%) | 8 (10) | |
| HIV, n (%) | 7 (8.75) | |
| Malignancy, n (%) | 2 (2.5) | |
| Graft failure post-transplant, n (%) | 2 (2.5) | |
| Cystic kidney disease, n (%) | 1 (1.25) | |
| Dialysis access | Arterio-venous fistula, n (%) | 26 (32.5) |
| Clinical score | Hypovolemia, n (%) | 0 (0.00) |
| Normovolemia, n (%) | 33 (41.25) | |
| Hypervolemia, n (%) | 47 (58.25) | |
| BIA | Dehydration, n (%) | 0 (0.00) |
| Normal hydration, n (%) | 9 (11.25) | |
| Fluid overload, n (%) | 71 (88.75) | |
| Mild FO, n (%) | 25 (31.25) | |
| Gross FO, n (%) | 46 (57.50) |
The median duration since diagnosis of CKD was 12.5 mo (IQR 24.5) and the median dialysis duration was 9 mo (IQR 15). Twenty-six (32.5%) patients had AVF while 54 (67.5%) were using venous catheters for HD vascular access. Seventy-seven (96.25%) patients had some residual kidney function while three participants were anuric. The comorbidities that preceded CKD as per their medical records included hypertension in 41 (51.25%) patients, glomerulonephritis in 22 (27.5%) patients, DM in 14 (17.5%) patients, obstructive uropathy in 8 (10%) patients, human immunodeficiency virus infection in 7 (8.75%) patients, kidney allograft failure in 2 (2.5%) patients, and cystic kidney disease in 1 (1.25%) patient. Seventy-six (95%) patients attended HD sessions twice per week as per institutional protocol with one of the patients on daily dialysis because he was scheduled for a kidney allograft transplantation during the week of assessment. Three (3.75%) patients were on once weekly HD. Sixty-six (82.5%) patients reported full adherence to attendance of their HD sessions and had not missed any sessions in the 2 wk preceding the study. Seventy-two (90%) patients had received education on fluid intake with the average actual fluid intake being 1010 mL in the interdialytic period with a range of 200-2800 mL. Seventy (87.5%) patients had received education on salt intake.
Fifty (62.5%) of the patients had systolic blood pressure (SBP) > 140 mmHg with mean SBP of 150 ± 31 mmHg. The mean diastolic blood pressure (DBP) was 91 ± 19 mmHg with 41 (51.25%) patients having DBP > 90 mmHg. Four patients (5%) were hypotensive with either SBP less than 90 mmHg or DBP less than 60 mmHg. Forty-four (55%) patients were on two or three antihypertensive agents. The median BMI was 21.94 kg/m2 (19.50-25.63). Seventy (87.5%) patients had received health education on salt intake as part of their management prior to enrolment on the study. Sixty-seven (83.75%) patients self-reported strict adherence to their anti-hypertensive medications and had not missed any of the prescribed dose in the week prior to evaluation. Forty-eight (60%) patients reported complete adherence to all the specific aspects ESKD management, which this study sought. The patients had received all the prescribed number of HD sessions in the previous 2 wk and had adhered to dietary, salt, and fluid intake restrictions, as well as having not skipped any of the prescribed doses of anti-hypertensive medications in the week that preceded this study.
The participants had volumes in the range of -0.53 L to 8.23 L and a median of 2.76 L (IQR 2.22 L). Fluid overload was found in 71 (88.75%) patients of which 46 (57.50%) were grossly overloaded with ECV of 2.5 L above the normal volume. Nine (11.25%) patients had normal volume, and none were dehydrated according to evaluation by BIA. On average, the study participants had 3.02 ± 1.79 L of ECV.
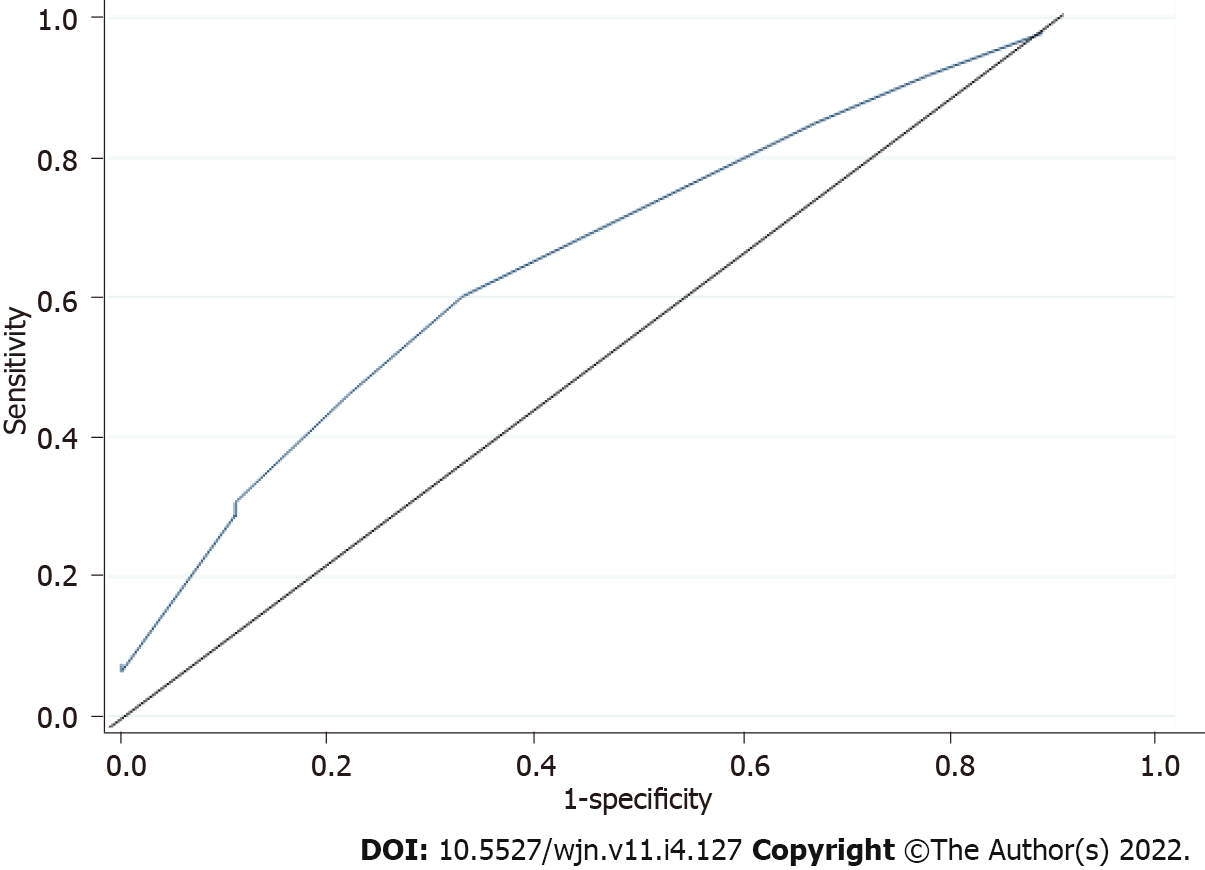
Symptoms scored as dehydration were scored in the negative. There were 2 (2.5%) with intradialytic hypotension, 1 (1.25%) with muscle cramps, dizziness, or fatigue during current session of dialysis, and 1 (1.25%) with symptomatic intradialytic hypotension treated by normal saline infusion. Absence of the above symptoms were scored as normohydration at zero score. Signs and symptoms of FO scored from +1 to +4. There were 54 (67.5%) with hypertension, 8 (10%) with oxygen saturation less than 90% by digital pulse oximeter, 26 (32.5%) with ascites, and 15 (18.75%) with pleural effusion or pulmonary oedema, each scored at +1. Interdialtyic weight gain was scored at +1 per kilogram gained. Five (6.25%) of the patients had gallop rhythm, which was scored at +2. Dyspnoea was scored using New York Heart Association classification with 54 (67.5%) patients at class I, scored at zero, 17 (21.25%) at class II scored at +1, 9 (11.25%) at class III scored at +2, while none were at class IV, which would have scored at +3. Chest radiography features were scored between +1 and +3 based on the stages. Oedema was graded and scored between 0 and +4. CSs obtained varied from -2 to 16 with a mean of 5.46 ± 3.68. Each of the results obtained from the CS was used to generate sensitivity and FPR that were used to plot a ROC curve. As shown in Figure 2, the optimal cut-off point for the CS obtained from the ROC was 4. This gave the score 63% and 78% sensitivity and specificity respectively. At this cut-off point, fluid overload was picked in 58.75% while no overload was in 41.25% of the patients.
BIA showed that 9 (11.25%) of the patients had normal hydration, 71 (88.75%) were fluid overloaded, and none was dehydrated. The CS showed that 33 (41.25%) had normal hydration, 47 (58.25%) were fluid overloaded, and none was dehydrated (Table 2). Fifty-two (65%) patients had similar results by both BIA and CS, and consisted of 45 patients with FO and 7 patients without FO. Twenty-eight (35%) patients had differing results by the 2 methods. Bio-impedance diagnosed 26 patients to have FO, but these same patients were not picked by CS as having FO. CS picked two patients as having FO who were picked by BIA as not having FO. The calculated McNemar’s chi-squared was 20.57 (P < 0.0001, 95% confidence interval: 0.1758-0.4242). The BIA detected significantly more patients with FO than CS. The true difference of the percentage of patients on HD picked with FO by CS and BIA was 17.58%-42.42% (Table 3).
| Positive | Negative | Total | P value | 95%CI | ||
| Clinical score | Positive | 45 | 2 | 47 | ||
| Negative | 26 | 7 | 33 | |||
| Total | 71 | 9 | 80 | |||
| McNemar’s Chi Square (20.57) | < 0.0001 | 0.1758-0.4242 | ||||
Duration of HD, number of missed HD sessions, whether the advice on fluid and salt restrictions against the fluid and salt was taken by the patient, as well as the number of antihypertensives medications each patient was using, and BMI were assessed for association with FO. Univariable logistic regression model of these factors associated with FO status was assessed. Using a liberal P value of 0.20, HD vintage in months, BMI and fluid intake were significantly associated with FO diagnosed by BIA (Table 4). However, from the multivariable model, using a significance level of 0.05, all these factors were not significantly associated with FO (Table 5).
| Variable | Values | FO+ (n = 71) | FO- (n = 9) | OR | 95%CI | P value |
| Duration of dialysis1 (mo) | 3-76 | 71 | 9 | 1.05 | 0.967-1.147 | 0.13 |
| No of missed dialysis sessions | 0 | 59 | 7 | |||
| ≥ 1 | 12 | 2 | 0.712 | 0.131-3.856 | 0.70 | |
| Advised on fluid restriction | No | 6 | 2 | |||
| Yes | 65 | 7 | 3.095 | 0.522-18.357 | 0.25 | |
| Actual fluid intake1 (mL) | 200-2800 | 71 | 9 | 0.998 | 0.997-1.000 | 0.082 |
| Advised on salt intake | No | 9 | 1 | |||
| Yes | 62 | 8 | 0.8611 | 0.096-7.719 | 0.89 | |
| Number of anti-hypertensives used | 0 | 11 | 0 | |||
| 1 | 13 | 2 | ||||
| 2 | 23 | 3 | ||||
| 3 | 14 | 4 | ||||
| 4 | 7 | 0 | ||||
| 5 | 2 | 0 | ||||
| 6 | 1 | 0 | 0.903 | 0.537-1.517 | 0.70 | |
| Patient’s BMI1 (kg/m2) | 15.82-32.53 | 71 | 9 | 1.196 | 0.942-1.520 | 0.11 |
The majority of the patients in this study were relatively young with a mean age of 45.6 years when compared with studies done in Europe, where the mean age was greater than 60 years[17]. The median duration of CKD was about 1 year and the median HD vintage was less than 1 year. There was a slight male predominance. In a study performed elsewhere in Kenya[12], the age distribution, male predominance, and BMI were comparable to those of our study. The majority of the patients in this study also suffered from hypertension (67.5%). One of the contributors to sustained hypertension in this population is likely to be FO. The majority of the patients reported to have been counselled on diet and fluid and salt intake as a way of controlling blood pressure and FO, and they reported adherence to the recommendations. Educating patients on dietary salt and fluid restrictions are important components of the management of ESKD. Previous studies have shown that patients who fail to adhere to dietary salt and fluid intake restrictions are more likely to have FO[18]. Less than a fifth of the patients suffered from DM (17.5%). This is a lower proportion than that reported in other parts of the world, where prevalence rates of 29.7%[19] and as high as 50%[17] have been reported in some studies in Europe.
Almost all the patients (95%) were receiving 4-h HD sessions twice a week as per the Kenyatta National Hospital Renal Department protocol, partially because the health insurance could only reimburse two HD sessions per week and the patients could not afford to pay for an extra sessions out of pocket. This treatment frequency is less than the recommended thrice weekly[20]. The longer interdialytic period could lead to fluid accumulation and may contribute to the higher proportion of FO in our population. Assessment of fluid status by CS and BIA revealed that the majority of the patients were fluid overloaded. Slightly more than a half of the patients were fluid overloaded when accessed by CS while almost 90% of them were fluid overloaded when assessed by BIA. The BIA was more sensitive in picking FO status. More patients were noted to be fluid overloaded in this study compared to studies done elsewhere in South Africa and Europe[17,21,22]. A study from South Africa among patients on dialysis reported almost two in every three patients as being fluid overloaded[21]. In Germany, Passauer et al[17] reported similar proportion of patients on dialysis as being fluid overloaded. Almost 60% of patients in this study had more than 2.5 L of excess ECV; this is in contrast to an analysis of 1500 European HD patients of whom only 25% had gross FO[22]. A plausible explanation why studies done elsewhere have reported lower proportions of FO could be due to the fact that elsewhere, patients have HD sessions more than twice per week. The longer interdialytic period could result in more interdialytic fluid accumulation.
The proportion of patients with FO in our study was higher than that reported in a study at Moi Teaching and Referral Hospital in Kenya, where 7 in every 10 patients were reported to have FO[12]. This study excluded patients who had not attained dry weight, and this could explain why the proportion with FO was lower than that in our study. The study assessed the level of agreement between the FO status diagnosed by CS and by BIA. The difference between the numbers of patients picked as having FO by the two methods was significant. BIA was more sensitive in detecting FO when compared with CS. Similar observations had been made by Kalainy et al[23], and were attributed to an inherent low sensitivity and specificity of clinical parameters in picking the fluid volume before dialysis. In contrast, Wizemann et al[10] reported a better concordance between symptom score and BIA, with more agreement towards over hydration than dehydration. Vasko et al[24] compared BIA with history, signs and symptoms, laboratory evaluation, and routine imaging with chest X-rays, lung ultrasound scanning, and cardiac evaluation with echocardiograph. They concluded that clinical judgment was the most important in assessing pre-dialysis OH. Use of patient history and examination, as well as chest radiograph data, compared favourably with BIA in guiding clinical decisions. At a cut-off point of 4, the CS resulted in 63% and 78% sensitivity and specificity respectively. The BIA was more sensitive in picking patients with FO than CS and the true difference of the percentage of patients on HD picked with FO by CS and BIA was 17.58%-42.42%.
Our study was limited by the small sample size and being done in a single centre. Some tests, which could aid in assessing FO, like echocardiography, were not performed in our study population due to financial constraints. The study relied on the recall by the patients and was subject to recall bias, especially on adherence to diet and fluid intake. Some aspects, which were purely self-reported by the patients, were not verifiable.
FO is very prevalent in patients on chronic HD at the Kenyatta National Hospital. CS picks less FO when compared with BIA. However, CS could still pick more than 6 in 10 patients with FO as picked by BIA with a specificity of almost 80%. In settings where BIA is not available, CS can be utilized as a low-cost alternative to assess fluid status of patients on HD and interpreted with the knowledge that CS identifies fewer patients with FO than does BIA.
Use of BIA should be incorporated into the routine care of patients on maintenance HD. CS should also be utilized in assessment of FO, especially in places where BIA is not available. Further studies are needed to evaluate how CS compares with BIA in bigger and heterogeneous populations. It is plausible to try and increase the HD sessions to thrice per week in attempt to reduce the proportion of patients who present with FO in our setting. In addition, future studies can evaluate the validity of the CS where patients have attained their dry weight at the baseline since this may improve both the sensitivity and the specificity.
Assessment of fluid status in patients with chronic kidney disease (CKD) on haemodialysis (HD) is important to guide treatment. Objective methods of assessment fluid status in this population of patients are needed. In CKD patients on HD, bio-impedance analysis (BIA) is reliable in assessment of fluid status though not available in many clinical situations. Clinical assessments for fluid overload (FO) are more popular in practice, though the individual elements are imprecise and may underestimate FO. There is need to determine the performance of a locally-developed clinical score (CS) in fluid status assessment when evaluated against BIA.
This study was motivated by the need to derive a local method of assessing fluid status in patients on HD and determine how this method compares with the BIA.
The objectives of this study were to assess the hydration status of patients on maintenance HD using BIA and a CS, as well as to evaluate the performance of that CS against BIA in fluid status assessment.
This was a single-centre, hospital-based cross-sectional study which recruited adult patients with CKD who were on maintenance HD. The patients were aged 18 years and above and had been on main
From 100 patients on maintenance HD screened for eligibility, 80 were recruited into this study. Seventy-one (88.75%) patients were fluid overloaded when evaluated using BIA with mean extracellular volume of 3.02 ± 1.79 L as opposed to the forty-seven (58.25%) patients who had FO when evaluated using the CS (P < 0.0001, 95% confidence interval: 0.1758-0.4242). The best cut-off point identified for the CS was four with values > 4 indicating FO and values 4 indicating no FO. At this cut-off point, the CS had 63% and 78% sensitivity and specificity respectively. None of the factors evaluated for association with FO showed statistical significance on the multivariable logistic regression model.
Fluid overload is very prevalent in patients on chronic HD at the Kenyatta National Hospital Clinical score detects less FO when compared with BIA. The sensitivity and specificity for the CS were 63% and 78% respectively. None of the factors evaluated for association with FO showed statistical significance on the multivariable logistic regression model.
Almost 90% of the patients had FO by BIA, and 57.5% had gross FO. BIA diagnosed significantly more patients with FO than the CS. The CS had a sensitivity if 63% and a specificity of 78% at a cut-off of 4.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Kenya
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheungpasitporn W, United States; Wang F, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Levin A, Stevens PE, Bilious RW, Coresh J, De Francisco ALM, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ, Levey AS, Riella MC, Shlipak MG, Wang H, White CT, Winearls CG. Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Supp. 2013;3:1-150. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1438] [Cited by in RCA: 1665] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 2. | Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1535] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 3. | GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4683] [Cited by in RCA: 4356] [Article Influence: 484.0] [Reference Citation Analysis (1)] |
| 4. | Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1485] [Article Influence: 148.5] [Reference Citation Analysis (0)] |
| 5. | Dou Y, Zhu F, Kotanko P. Assessment of extracellular fluid volume and fluid status in hemodialysis patients: current status and technical advances. Semin Dial. 2012;25:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Moissl U, Arias-Guillén M, Wabel P, Fontseré N, Carrera M, Campistol JM, Maduell F. Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:1575-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Kraemer M, Rode C, Wizemann V. Detection limit of methods to assess fluid status changes in dialysis patients. Kidney Int. 2006;69:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 471] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 9. | Tabinor M, Elphick E, Dudson M, Kwok CS, Lambie M, Davies SJ. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): systematic review and subgroup meta-analysis. Sci Rep. 2018;8:4441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Wizemann V, Schilling M. Dilemma of assessing volume state--the use and the limitations of a clinical score. Nephrol Dial Transplant. 1995;10:2114-2117. [PubMed] |
| 11. | Connor RJ. Sample size for testing differences in proportions for the paired-sample design. Biometrics. 1987;43:207-211. [PubMed] |
| 12. | Bajaber AA. Body water distribution and nutrition status of end stage renal disease patients undergoing haemodialysis at Moi teaching and referral hospital. Kenya: Moi University, 2014. |
| 13. | Nieman J, Patten A, Chung ES. A Standardized Method for Assessing Edema. J Card Fail. 2013;19:S86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, Taborsky P, Tetta C, Velasco N, Vlasak J, Zaluska W, Wizemann V. Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant. 2008;23:2965-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | Hosmer DW, Lemeshow S. Multiple Logistic Regression. In: Shewhart WA, Wilks SS. Applied Logistic Regression. 2nd ed. New York: John Wiley & Sons, 2000: 31-46. |
| 16. | Dohoo IR, Martin SW, Stryhn H. Methods in epidemiologic research. 3rd ed. Florida: VER Inc, 2012: 201-222. |
| 17. | Passauer J, Petrov H, Schleser A, Leicht J, Pucalka K. Evaluation of clinical dry weight assessment in haemodialysis patients using bioimpedance spectroscopy: a cross-sectional study. Nephrol Dial Transplant. 2010;25:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Kugler C, Vlaminck H, Haverich A, Maes B. Nonadherence with diet and fluid restrictions among adults having hemodialysis. J Nurs Scholarsh. 2005;37:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Cheng HT, Xu X, Lim PS, Hung KY. Worldwide Epidemiology of Diabetes-Related End-Stage Renal Disease, 2000-2015. Diabetes Care. 2021;44:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 20. | National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis. 2015;66:884-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 762] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 21. | Hassan MO, Duarte R, Dix-Peek T, Vachiat A, Dickens C, Grinter S, Naidoo S, Manga P, Naicker S. Volume overload and its risk factors in South African chronic kidney disease patients: an appraisal of bioimpedance spectroscopy and inferior vena cava measurements. Clin Nephrol. 2016;86:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 23. | Kalainy S, Reid R, Jindal K, Pannu N, Braam B. Fluid volume expansion and depletion in hemodialysis patients lack association with clinical parameters. Can J Kidney Health Dis. 2015;2:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Vasko R, Müller GA, Ratliff BB, Jung K, Gauczinski S, Koziolek MJ. Clinical judgment is the most important element in overhydration assessment of chronic hemodialysis patients. Clin Exp Nephrol. 2013;17:563-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |