INTRODUCTION
Ischaemia reperfusion (IR) injury is typified by initial hypoperfusion and inadequate oxygen supply to end organs. This is followed by a secondary inflammatory reperfusion injury which impacts organ function and may affect distant organs[1]. IR injury can occur as part of a global hypoperfusion phenomenon such as that seen in trauma, sepsis and haemorrhage[1-4]. IR injury may also represent a local issue of poor perfusion that primarily affects a single organ or body region.
In the clinical setting, liver IR injury is commonly seen following liver resection and liver transplantation (LT)[5]. Liver IR injury following transplant is associated with major complications related to the liver injury, including early allograft dysfunction, primary nonfunction and ischaemic-type biliary complications[6,7]. In addition to liver specific outcomes, secondary organ injury may occur, which also increases the morbidity and mortality of liver transplantation and resection. Acute kidney injury (AKI) in particular, is very strongly linked to liver IR injury following liver transplantation[8,9]. 40% of liver transplant patients develop AKI, and 7% require renal replacement therapy (RRT)[10]. These patients have an increased mortality with a mortality odds ratio of 2.96, increasing to 8.15 in severe AKI with RRT requirement[10]. AKI post LT is also associated with graft failure, prolonged intensive care unit stay, delay to hospital discharge and subsequent development of chronic kidney disease (CKD)[10-14]. Post-transplant CKD is independently associated with an increase in late mortality and cardiovascular events[11].
Supportive treatment of AKI with renal replacement therapy does not resolve the excess mortality and poor outcomes associated with this condition[15,16]. This may be because AKI needing RRT is a marker of a more global injury affecting the function and viability of multiple organs[15].
There are no specific drug therapies that reverse AKI or block its development. This may in part be related to the overall lack of understanding of the mechanisms underlying the development of AKI following liver IR injury. An improved understanding of the underlying mechanisms of injury is likely to facilitate development of new strategies to avoid and downregulate injury, provide targets for new therapies and improve clinical outcomes post liver transplantation and resection. In the context of liver transplantation, effective therapeutic interventions for both liver IR injury and AKI would also allow expansion of the donor organ pool by inclusion of more marginal grafts, which are more susceptible to IR injury.
In recent years, the indications for liver transplantation have been expanded to include the treatment of primary hepatocellular carcinoma and carefully selected patient groups with some forms of metastatic disease[17,18]. Meeting this potential enormous expansion in transplant demand would necessitate the routine use of marginal grafts. Marginal grafts include those with background hepatic steatosis, grafts from donors following cardiac death and prolonged graft ischaemia times[19,20]. They are especially susceptible to IR injury and are associated with an increased incidence of AKI and higher mortality[19]. The lack of therapeutic interventions which either provide recipient renal protection from significant liver IR injury or downregulate liver IR injury continues to limit the use of marginal grafts in liver transplantation[21]. Addressing these issues has the potential to revolutionise the use of marginal grafts and meet the current deficit between graft supply and demand.
The clinical importance of both liver IR injury and resultant AKI is clear. Several recent reviews have addressed either mechanisms of liver IR injury or clinical aspects of liver IR injury and AKI. However, no prior review has explored the experimental and clinical evidence for the link between liver IR injury and AKI and the mechanisms mediating AKI after liver transplantation. With a recent expansion in the primary literature on this topic, we believe a review is now warranted to crystalise current understanding, identify unanswered questions and to prioritise future research. In this review we will pull together current evidence for the molecular and physiological mechanisms of kidney injury following liver IR injury.
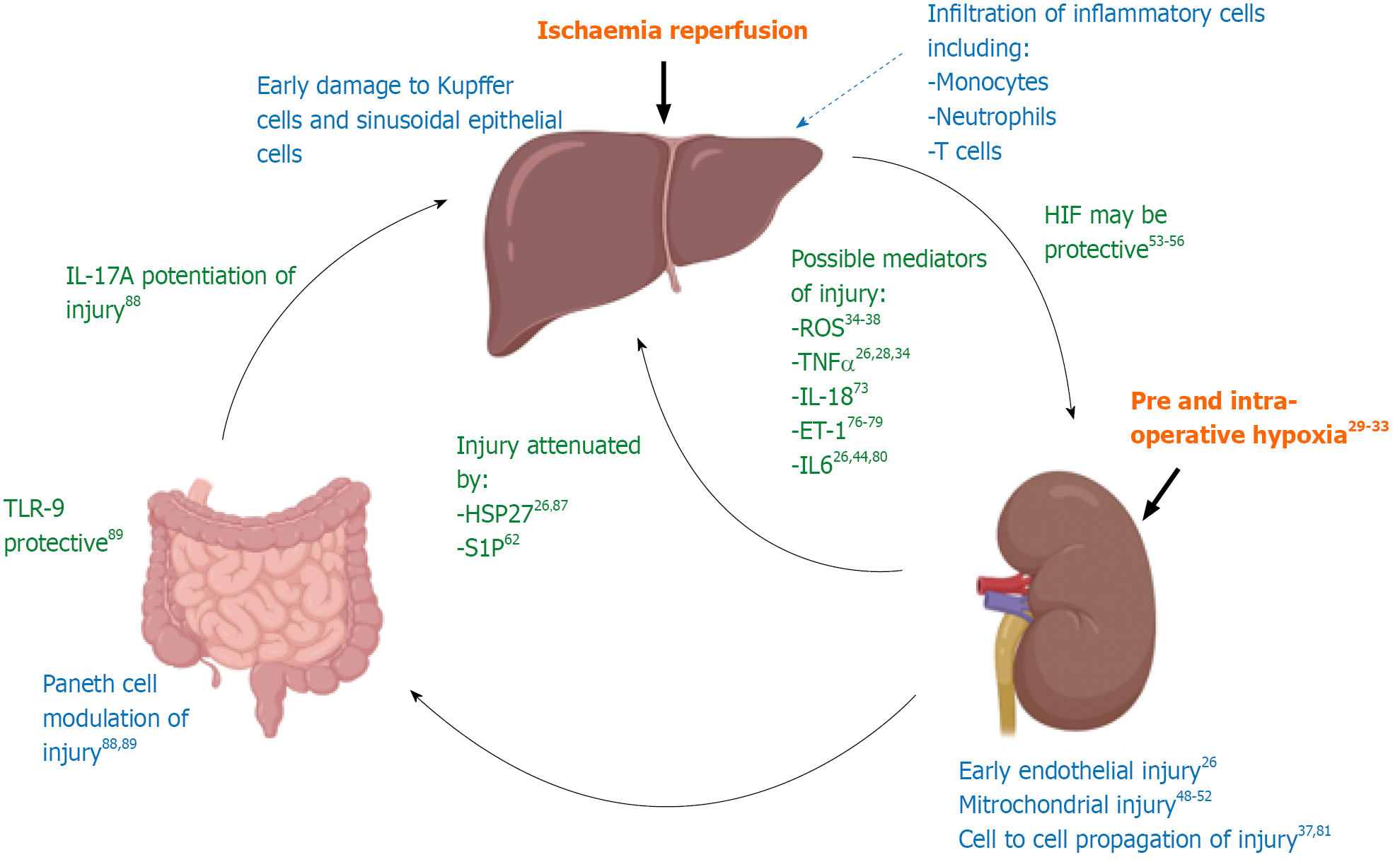
Figure 1 provides a schematic summary of the evidence for pathways mediating liver IR injury leading to kidney injury that will be discussed throughout this review.
Figure 1 Schematic representation of current evidence to support the mechanistic link between liver IR injury and resultant kidney injury.
The evidence for the possible mediators of injury detailed in this diagram will be discussed in more detail in the text of this review. A summary of the major studies discussed in this review can also be found in Supplementary Table 1.
RENAL INJURY IS DIRECTLY LINKED TO LIVER IR INJURY AND OCCURS EARLY FOLLOWING LIVER REPERFUSION
The link between liver IR injury and AKI in liver transplantation has been well established in multiple analyses. A retrospective study of 116 patients undergoing deceased donor liver transplant in our unit identified post transplant serum AST/ALT as the only independent predictor of early post-operative AKI[8], a finding also demonstrated by Jochmans et al[9] in their prospective analysis of 88 patients who received livers from donation after brainstem death donors. These clinical data are supported by findings from rodent models of liver IR injury, typified by Lee et al[22], who demonstrated a direct relationship between plasma ALT and severity of AKI at 4 h and 24 h in a mouse model of partial hepatic ischaemia (right lobe of liver spared).
Renal injury is not only linked to liver ischaemia injury, but occurs promptly after reperfusion, both in the clinical setting and in animal models. In human liver transplantation, Neutrophil Gelatinase Associated Lipocalin (NGAL), a biomarker of early renal injury, is elevated in urine as early as two hours post reperfusion[23].
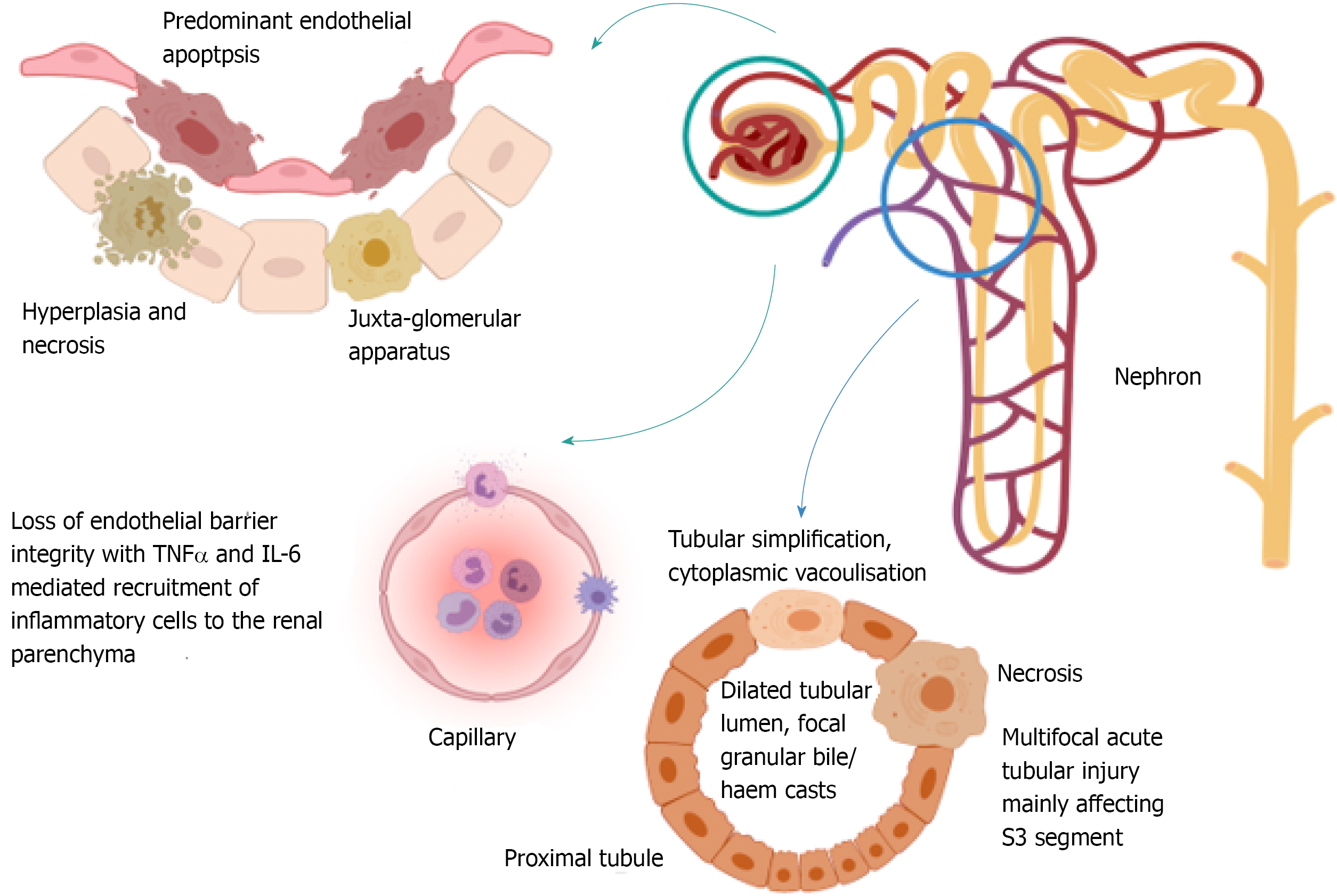
In rodent models of liver IR injury, histologically demonstrable renal injury is evident two to four hours post liver reperfusion[24]. Key histological features of renal injury in this context include hyperplasia and necrosis of the juxta-glomerular apparatus, endothelial apoptosis and multifocal acute tubular injury with disruption of F-actin cytoskeletal architecture, leading to S3 segment proximal tubule necrosis, focal tubular simplification (loss of brush border with cellular flattening), cytoplasmic vacuolisation, dilated tubular lumina and focal granular bile/haem casts[22,25,26], as depicted in Figure 2. A standardised grading system for severity of renal injury in rodent models of liver IR injury and AKI, including stratification of histological findings that are more associated with severe AKI, has not yet been developed. Additionally, both the sequence of injury and time frame for improvement in histological changes has not been fully defined.
Figure 2 Diagrammatic representation of the current understanding of histological changes within the kidney that accompany acute kidney injury following liver ischaemia reperfusion injury.
These data are obtained from animal studies. To date no objective grading system for histological severity of injury has been developed which means that only limited comparison of injury severity between studies is possible. Development of an objective scoring system across the first 48 h of renal injury would be of great benefit in this field of research.
The development of renal injury within a few hours of liver IR injury in both human clinical and animal experimental data hints at direct transmission of injury from liver to kidney. Liver derived molecules, washed out of the liver during organ reperfusion, may be critical mediators of AKI in this context. As the first cells to encounter haematologically transmitted mediators of injury, endothelial cells might be expected to bear the initial brunt of injury. In rodent models of liver IR injury and AKI, renal endothelial injury predominates[25], supporting this hypothesis. Human histological data is sparse and so we await verification that the rodent pattern of renal injury occurs in the human setting. An in vitro human model that permitted demonstration of haematological transmission of liver IR injury to the kidney would also be of huge experimental benefit. This has yet to be developed.
SETTING THE STAGE FOR RENAL INJURY POST LIVER IR INJURY: PRE-OPERATIVE AND INTRA-OPERATIVE PROMOTERS OF INJURY
Whilst molecular mediators released by the liver following IR injury are likely to play a key role in renal injury, evidence suggests that renal injury following liver IR injury is a two-hit phenomenon. Both pre-existing renal abnormalities and intra-operative fluctuations in arterial oxygen concentrations may render the kidney relatively chronically hypoxic and prime it for further damage by circulating mediators of reperfusion injury[27,28]. This seems to be a different phenomenon from controlled ischaemic pre-conditioning which appears to reduce liver and renal injury in a mouse model of liver IR (unpublished data).
Background liver cirrhosis is associated with chronic renal injury and poor renal perfusion which may predispose the kidney to further injury
Renal biopsies performed in the context of cirrhosis demonstrate pathological changes in the kidney, mainly centred around the glomerulus, in 70% of patients. These include mesangial expansion, thickening of capillary walls, a mild increase in the number and size of endothelial and epithelial cells and IgA deposition[28]. These changes may reflect the chronic release of pro-inflammatory mediators from ongoing chronic inflammation in the liver.
Cirrhosis also reduces systemic vascular resistance[28]. When the increased cardiac output can no longer compensate for the reduction in systemic resistance there is arterial hypoperfusion. This leads to activation of vasoconstrictor systems, including the sympathetic nervous system and the renin: Angiotensin: Aldosterone axis with hypersecretion of Anti-Diuretic Hormone. The net result is Na+ and water retention but with hypovolaemia, renal arterial hypoperfusion and renal vasoconstriction leading to renal failure[28]. This pre-existing inflammatory and hypoxic injury may prime the kidney for further injury during liver transplantation.
There may be intra-operative fluctuations in renal perfusion during liver transplantation leading to a primary kidney insult before liver IR injury
Liver transplantation results in huge fluctuations in mean arterial pressure (MAP) but there is conflicting evidence for an association between MAP and AKI. In a retrospective study of patients undergoing living donor liver transplantation, severe hypotension (MAP < 40) in the recipient for even less than 10 min was independently related to development of post-operative AKI[29]. In a rat model of liver transplantation with doppler assessment of renal artery flow, Kong et al[24] noted increased renal resistive index (RI) during the anhepatic phase and reduced renal RI (compared to background) immediately post reperfusion (although this normalised within 30 min). The findings indicate maldistribution of blood flow to the kidney during the anhepatic phase with increased renal vein pressure secondary to IVC clamping serving to increase renal RI and reduce renal perfusion. Reperfusion is associated with reduced renal arteriolar tone, which the authors suggest may be due to an imbalance between vasoconstrictive and vasodilative factors, disturbing the adaptive capacity of the renal vasculature (not measured in this study). RI did not correlate with the development of AKI at 30 min and 2 h post operatively with this animal model and so RI and renal perfusion may not be the most important factors influencing AKI development.
Kandil et al[30] demonstrated similar fluctuations in MAP between the anhepatic and post reperfusion phases of human liver transplantation, although these were not statistically evaluated. In this double-blinded trial, patients were randomised to intra- and post-operative terlipressin infusion or placebo. Terlipressin induces systemic arterial vasoconstriction with renal sparing. It was hypothesised that systemic vascular resistance support with terlipressin would improve renal perfusion and reduce post-operative renal injury. However, the authors demonstrated equivalent incidence of AKI in both the terlipressin and placebo groups which was subsequently supported by evidence from a meta-analysis on the subject[31]. Other causes of fluctuating MAP that may contribute to renal hypoperfusion in addition to systemic vascular resistance were not evaluated in this study.
Thus, whilst a short period of significant hypotension may promote the development of post-operative AKI, the relationship between renal perfusion and subsequent development of AKI requires further investigation and so far evidence suggests that renal perfusion may be less important than circulating factors for the development of AKI following liver IR injury.
Renal hypoxia in liver transplantation may promote development of liver IR induced AKI
In human liver transplantation, low arterial oxygen concentration at 5 min post reperfusion is independently associated with development of AKI (this study included assessment of hypotension)[32]. Arterial hypoxia may result in renal hypoxia, causing primary renal injury. However, only absolute oxygen concentrations rather than relative changes were evaluated in this study. It may be that the relative drop in arterial oxygen concentration at reperfusion reflects the degree of ischaemia and oxygen debt within the donor graft, with higher oxygen tension gradients between the recipient vasculature and more profoundly ischaemic grafts (although this has not yet been evaluated experimentally). Post reperfusion arterial hypoxiaemia may therefore be a surrogate measure of liver IR injury, rather than arterial hypoxia providing a direct contribution to renal injury.
That said, the kidney is highly susceptible to hypoxic injury. Under normal physiological conditions, 80% of the renal oxygen requirement is used to drive the Na+/K+/ATPase pump in the proximal tubule. To meet these demands, the kidney is rich in vascular endothelium and has an excellent blood supply[27]. This in turn may make the kidney particularly vulnerable to circulating cytokines which trigger endothelial injury, especially in the situation of mass dilation of capillary beds as can occur during reperfusion secondary to the imbalance of vasodilatory and vasoconstrictive factors discussed in section 1[24].
Put together, the data suggest that the kidney is vulnerable to hypoxic injury and that post reperfusion arterial hypoxia is linked to the severity of renal injury following liver IR injury. Clinically it would be difficult to tease out the relative contributions to AKI from primary renal hypoxia and the more severe liver IR injury that is suggested by arterial hypoxia. Use of in vitro human models of injury where renal hypoxia can be controlled independently of liver IR injury would help to resolve this question. Such models have not yet been reported in the literature.
WHAT ARE THE MOLECULAR MEDIATORS OF RENAL INJURY FOLLOWING LIVER IR INJURY?
Many inflammatory mediators have been implicated in liver IR injury and/or resultant AKI. The discussion below will focus on the major molecules (both injurious and protective) of current investigative interest and draw together the discussion in the literature to provide an overview of current understanding.
Reactive oxygen species may be critical in the early transmission of injury to the kidney following liver IR injury
Reactive oxygen species may originate from the liver and circulate to the kidney[33] or arise primarily in the kidney, where they may be generated following endothelial injury and poor capillary perfusion with resultant relative hypoxia. Hydrogen peroxide (H2O2), superoxide anion and hydroxyl radical have all been implicated in this process[34]. Oxidative stress is thought to be the main mediator of primary tissue damage during the first four hours of reperfusion. In a rat model of liver transplantation, oxidative stress in the kidney was shown to increase roughly 2.5 fold and peak at 8 h post reperfusion (measured as H2O2 normalised to sham laparotomy)[35,36]. Reactive oxygen species (ROS) bind to critical cellular biomolecules including proteins, DNA and membrane lipids, and cause oxidative modification, with resultant tissue injury[37].
The detrimental action of ROS may be potentiated by ongoing release of ROS from infiltrating inflammatory cells in the later phase of liver reperfusion injury. Activated neutrophils and macrophages release ROS, including superoxide anions and hydroxyl radicals which promote cell death[33]. However, in the longer term ROS may also be regenerative; late neutrophil release of ROS may play a key role in the development of reparative macrophages to orchestrate liver tissue repair following liver injury[38].
Albumin, which acts as a free radical scavenger and endothelium stabiliser is protective in this clinical context; low circulating levels of albumin as found in advanced liver disease are associated with an increased incidence of AKI post liver transplantation[39]. Likewise, administration of various antioxidants and free radical scavengers have been shown to reduce markers of renal oxidative stress and attenuate injury post liver IR in different animal models[40,41]. Iron free radicals may play an important role in the generation of ROS and ferroptosis[42]. Desferrioxamine (DFO), the iron chelator, blocks oxygen free radical production and lipid peroxidation. Administration of DFO was found to attenuate liver IR injury in pigs and was associated with no or subtle tubular injury. Pigs exposed to liver IR injury without DFO demonstrated extensive necrosis of tubular epithelial cells and dilatation of tubular lumina, indicating severe renal injury[43]. Notably the circulating serum iron concentration was not different between DFO-treated animals and controls, implying a specific function of DFO with reactive iron species. It is not known whether this function is separate from the iron binding capacity of DFO.
These findings have not been successfully translated to the clinical setting. Administration of N-acetylcysteine during major liver surgery, including transplantation, is associated with a modest improvement in transaminase levels without impacting either AKI, graft or patient survival[44-46]. Thus whilst ROS are likely to be critical in the early mediation of AKI following liver IR injury, further work is required to identify clinically useful targets that will downregulate injury following liver transplantation and hepatic resection.
Mitochondria are vulnerable to injury and may be the main site of ROS production following liver IR injury
Mitochondria are believed to play a key role in the pathogenesis of renal injury following a variety of insults, with reduced biogenesis (generation of new mitochondria in response to increased energy demand, mitochondrial stress or damage) resulting in attenuated capacity to meet the energy demand and ATP production necessary for injured cells. Mitochondria are also the key site of ROS generation within the cell[35] and ironically mitochondrial injury may also be mediated by ROS[47] or iron species, with DFO demonstrated to attenuate mitochondrial injury in other settings[48]. In a rat model of liver transplantation and AKI, Liu et al[49] demonstrated a reduction in key proteins (and mRNA) involved in or regulating mitochondrial biogenesis, fission and fusion including AS-B, ND3, PGC-1α, Tfam, Drp-1 and Fis-1. Mediators of mitophagy and autophagy (PINK-1 and LC3) were also upregulated with AKI in this model. Stimulation of mitochondrial biogenesis has also been demonstrated to reduce renal IR injury[50,51].
Taken together, the data on ROS suggest local involvement in the pathogenesis of both liver IR injury and subsequent renal injury, with mitochondrial involvement in both the generation of ROS and mediation of ROS effects. However, demonstration of direct haematological transmission of ROS from liver to kidney producing subsequent kidney injury has not been demonstrated.
Hypoxia Inducible Factors may be protective following liver IR injury
Hypoxia inducible factor 1 (HIF-1) is an important mediator of the cellular transcriptional response to hypoxia and plays a key role in the response to liver IR injury. HIF-1 comprises an oxygen destructible alpha subunit and an oxygen-indestructible beta subunit, which dimerise under hypoxic conditions.
HIF-1α silencing pre-injury promotes cellular damage in response to hypoxia, leading to increased serum levels of glucose, lipids, ALT and AST[52]. Conversely, pre-injury activation of HIF-1α attenuates hepatic IR injury by attenuating liver necrosis, the inflammatory response, oxidative stress and apoptosis[53]. HIF-1α stability is partially mediated by the oxygen sensing prolyl hydroxylase domain 1 (PHD1), which under normoxic conditions tags HIF-1α for proteosomal degradation. Interestingly PHD1 function is repressed by miR122, a target gene of HIF-1α, which is almost exclusively expressed in hepatocytes[54]. By this mechanism, HIF-1α enhances HIF mediated cellular responses through PHD1 repression.
Downstream actions of HIF-1 may be key in the attenuation of liver IR injury with subsequent downregulation of AKI but the exact involvement and mechanisms remain unclear. It may be that such effects are mediated by other microRNAs involved in the transcriptional response to HIF-1[54]. The concentration of microRNAs from donor liver perfusate (but not tissue) at the end of cold ischaemia has been linked to elevated AST and graft long term survival[56]. If present in perfusate, these microRNAs may be produced by damaged liver cells that are being flushed out of the liver. The role of such microRNAs in the mediation of kidney injury requires further investigation.
CYTOKINES
Cytokines released from the liver following IR injury
A multitude of cytokines are upregulated in response to liver IR injury. Bezinover et al[57] evaluated cytokine upregulation in response to the ischaemia and reperfusion phases of human liver IR injury in 11 extended criteria donor grafts and 6 standard criteria donor grafts for liver transplantation. They obtained samples from the portal vein (prior to reperfusion, thought to represent the ischaemic phase of IR injury), the hepatic veins (at the beginning and end of post implantation liver flush with recipient circulating blood, thought to represent the reperfusion phase of IR injury) and arterial samples (from recipient prior to reperfusion and at 10 min and 20 min post reperfusion). Samples were analysed for TNF, IL-1, IL-2, IL-6 and IL-8 with comparison between levels of individual cytokines at each location. The results suggest early hepatic release of IL-6 during the ischaemic phase. This is followed by TNFα release (without observed increase in systemic circulating TNFα). IL-2 was likewise released from the liver towards the end of reperfusion. IL-1 was released from the liver during the process of reperfusion, without elevated levels seen in systemic samples. IL-8 and TNF are both known to be released by various cells including activated Kupffer cells in response to IR injury[58,59]. IL-8 is chemotactic, leading to recruitment of neutrophils to injured tissues[59], whilst TNFα is important for cell signalling leading to apoptosis or necrosis and neutrophil recruitment[60]. Interestingly, no difference was noted in IL-8 and TNFα release from standard and extended criteria groups. This is significant; given that extended criteria grafts are strongly associated with IR injury[21], higher concentrations of IL-8 and TNFα would be expected from this cohort. Thus release of IL-8 and TNFα may be associated with, but potentially not mechanistic to, IR injury and AKI development.
To summarise, in contrast to most published studies which focus on animal models, Bezinover et al[57] attempted to provide real-time human data on liver IR injury and hinted at possible temporal relationships between different cytokines in this context including IL-6, TNFα, IL-2 and IL-1. However, the study made significant assumptions, with no independent experimental validation of their methodology which matched sampling from different liver sites to the various phases of IR injury (for example portal vein sampling was matched to pre-reperfusion phase of injury). Such assumptions may explain the lack of expected difference in cytokine levels between standard and extended criteria grafts. Additionally, the short period of reperfusion may explain the lack of correlation between liver flush samples and systemic samples. The “reperfusion phase” was only 20 min and therefore further changes within the liver during reperfusion injury may well have been missed in this data. Data from systemic blood samples over a longer time phase would have been interesting in this context.
A pilot study evaluating pre-conditioning in human liver transplantation performed in our unit investigated circulating cytokines at two hours post reperfusion. Levels of IL-6, IL-8, IL-10 and IL-17α were all significantly elevated, whilst plasma levels of IL-2, IFNγ and TNFα did not change during the peri-transplant period[61]. In addition, IL-10 was particularly associated with marginal grafts in this study, although small patient numbers mean that these data are not conclusive.
In a mouse model of 90 min partial hepatic IR injury (right lobe spared), Lee et al[62] demonstrated elevated serum IL-6, TNFα and MCP-1 at 6 h. These findings tallied with those from a previous study by the same authors that identified hepatic mRNA upregulation of TNFα, Intracellular Adhesion Molecule 1 (ICAM-1), Keratinocyte-derived Chemokine (KC), Monocyte Chemoattractant protein-1 (MCP-1) and Macrophage Inflammatory Protein-2 (MIP-2) following 60 min partial liver ischaemia[22]. This pattern of upregulation and protein expression is supported by other animal studies of hepatic IR injury[26].
In summary, investigation of liver cytokine release following IR injury has identified numerous molecules that may be present in serum and are capable of transmitting injury to the kidney. However, results between studies are conflicting and there is no clear evidence that the cytokines are responsible for AKI in this context. Further clinical studies that make use of targeted cytokine inhibition or specific rodent knockout models are required to link individual cytokines with AKI. Clarifying liver origin of the cytokine would also be important in establishing the pathway of injury. Additionally, single cell analysis of key liver cells in response to injury might help to identify new mediators of injury that have not been investigated to date.
Cytokines are primarily released from non-parenchymal cells in early liver IR injury
Non parenchymal cells (i.e., non-hepatocytes) seem to be key in the mediation of early liver IR injury[63]. Sinusoidal endothelial cells are damaged during ischaemia, whilst Kupffer cells appear to be activated in response to reperfusion injury, demonstrating five times the TNFα production of control animals[64] in addition to IL-1 and superoxide anions[63]. TNFα production in Kupffer cells may be primarily driven by ROS[65]. In a rat model of liver transplantation, ischaemia-reperfusion preconditioned livers demonstrated a reduction in Kupffer cell superoxide formation, reduced TNF production and reduced non-parenchymal cell death leading to improved recipient survival[66], again suggesting that Kupffer cells are key in the mediation of injury. Acute liver graft failure has been linked to loss of viability of sinusoidal cells and activation of Kupffer cells, further demonstrating the importance of these cell types in the mediation of IR injury[64].
The late phase of liver reperfusion injury is categorised by infiltration of neutrophils, T lymphocytes and monocytes[67-69]. These cells are recruited to the liver parenchyma by upregulation of ICAM-1, VCAM-1 and MCP-1 on damaged hepatocytes and SECs. The infiltrating cells secrete matrix metalloproteinases, other proteases and ROS which cause further liver damage[68,70].
In summary, activation of non-parenchymal cells in the liver is fundamental for the early stages of IR injury. Inflammatory cells are recruited to the liver parenchyma by damaged hepatocytes and SECs and drive ongoing inflammation. Single cell analysis of non-parenchymal cells following liver IR injury may identify key transmitters of renal injury and clarify existing data.
The key cytokine culprits implicated in the mediation of renal injury
Many cytokines have been proposed as mediators of kidney injury following hepatic IR injury. Pulitano et al[71] performed molecular profiling of liver biopsies in 65 patients undergoing full size liver graft transplantation. Wedge biopsies were taken from the liver following graft preservation and 90 min after reperfusion in addition to serum samples preoperatively, 30 min after liver reperfusion and on post-operative days 1, 2, 5 and 7. 32% of recipients developed AKI. The authors demonstrated mRNA upregulation in 23 vasoactive, inflammatory, adhesion molecule, apoptosis inducing and oxidation genes (including ET-1, TNFα, IL-6, IL-18 and ICAM-1). Upregulation of the gene was correlated with serum expression of the protein for ET-1, TNFα, IL-6, IL-18 and RANTES 30 min post liver reperfusion and on post-operative days 1, 2, 5 and 7. Of the studied cytokines, only serum levels of Endothelin-1 (ET-1) and IL-18 were independently associated with AKI development at post-operative day 1, suggesting a key role for ET-1 and IL-18 in the mediation of injury. Interestingly serum ET-1 also correlated with use of inotropes in donors and hepatic steatosis, both risk factors for liver IR injury, and so alternatively, ET-1 may be a surrogate marker for renal injury (which is related to severity of liver IR injury). Renal biopsies to evaluate local gene expression were not performed in this study and so the relationship between gene induction in the liver and effector genes for injury in the kidney cannot be established. Additionally, this study provides a limited look at 23 known mediators of inflammatory injury. Single cell analysis in this context would provide a more precise look at gene upregulation and potentially provide new targets for investigation.
At best, Pulitano et al[71] provides evidence for associations between liver mRNA upregulation, circulating IL-18 and ET-1 and kidney injury. However, causality is not established by these data and alternative explanations exist for the findings.
IL-18 may potentiate renal injury following liver IR injury with IL-18BP providing a protective effect
The IL-18-precursor is constitutively present in nearly all cells, where its activity is balanced by the high affinity IL-18 binding protein (IL-18BP). In its active form IL-18 is mostly secreted by macrophages, including Kupffer cells, although some disease processes lead to an imbalance of IL-18/IL-18BP with the liberation of free IL18 from other cell types. IL-18 is known to be an inducer of inflammatory cytokines[72]. Gonul et al[33] investigated the role of IL18 in renal injury post liver IR injury using a rat model of hepatic IR (clamping of portal triad for 1 hour followed by 4 h reperfusion) with administration of intraperitoneal IL-18BP 30 min before commencing the laparotomy for liver IR injury. There was no difference in liver IR injury (as measured by AST/ALT/LDH and histological damage) between the groups, but an almost 50% reduction in serum creatinine with administration of IL-18BP compared to controls. This was confirmed by a significant improvement in histological renal injury with a reduction in mononuclear cell infiltration, glomerular necrosis and tubular epithelial necrosis suggesting that IL-18BP does not modify the primary liver IR injury but is involved in the pathway for secondary renal injury. Findings in this study contrasted to a previous study by the same authors which demonstrated improvement in both liver-IR and renal injury with peritoneal administration of IL-18BP[72]. The authors attribute this difference to the higher dose of IL-18BP used in the first study (100μg versus 50μg in this study). This explanation is in keeping with an overall hypothesis of high IL-18 release in response to liver injury and subsequent haematological washout impacting secondary organs. Of note, both studies used human IL-18BP, which has limited homology with rat IL-18BP. This represents a fundamental flaw, and the studies would be better repeated with rat IL-18BP.
Overall IL-18 may be critical in the mediation of renal injury following liver IR injury. However, these data require validation with rat IL-18BP in the animal model, and successful translation of findings to the human setting.
ET-1 may contribute to renal injury post liver IR injury
In addition to the evidence regarding ET-1 provided above, circulating ET-1 has been demonstrated to correlate with both early reduction in GFR and long-term renal dysfunction in patients with normal renal function who are undergoing first Orthotopic Liver Transplantation (OLT)[74]. Patients with liver disease have background high circulating ET-1, due to increased synthesis and reduced clearance[75]. ET-1 is also significantly elevated at the end of the anhepatic phase of liver transplantation in clinical studies[76], although it may be cleared within 30 min by a functioning liver graft. The significance of this is unclear. ET-1 may contribute to renal injury or be a surrogate marker for MELD score and severity of liver disease, which is independently associated with worse outcomes post liver transplantation[77].
ET-1 has been demonstrated to promote Na+ retention and increase renal vascular resistance without a significant change in blood pressure in healthy volunteers[78]. This function of ET-1 appears contradictory to evidence presented earlier where a reduction in renal resistive index was seen with reperfusion[24] and may reflect differences between the rat model and human situation or differences between the healthy liver and background liver disease or a compensatory mechanism in response to chronically high ET-1. Additionally, evidence suggests that the oxidative status of the renal microvasculature can significantly influence renal microcirculatory responses to ET-1 which may account for different results in different experimental settings. The vasoactive functions of ET-1 in the kidney may be mediated by its action to increase superoxide accumulation in preglomerular smooth muscle cells. Apocynin (an NADPH oxidase inhibitor) has been demonstrated to attenuate ET-1’s ability to reduce renal blood flow[79].
Cytokines recruit inflammatory cells to the kidney with potentiation of injury
In addition to the role they play in the mediation of liver IR injury, IL-6 and TNFα are upregulated in the kidney in response to liver IR injury. TNFα triggers leukocyte-endothelium interactions and microcirculatory dysfunction and is known to impact renal microvascular oxygen distribution and promote organ damage[27]. It has also been demonstrated to promote migration of inflammatory cells into the renal parenchyma through upregulation of KC (rodent equivalent of IL-8), MCP-1 and MIP-2, with macrophage recruitment[25,33]. This is similar to the functions of TNFα seen in the liver following IR as in section “Cytokines released from the liver following IR injury”.
Likewise, IL-6 is a major pro-inflammatory cytokine that stimulates release of neutrophils from bone marrow, prevents neutrophil apoptosis and activates neutrophils to produce toxic enzymes. Additionally, IL-6 activates endothelial cells to express adhesion molecules and produce chemokines[43] which promote the recruitment of inflammatory cells to the renal parenchyma. Activated neutrophils release oxygen free radicals, neutrophil elastase and products of arachidonic acid metabolism, further potentiating renal injury[25,80].
Thus both IL-6 and TNFα are believed to be key for the potentiation of renal injury following liver IR injury by recruitment of inflammatory cells as part of the systemic inflammatory response to injury. Further investigation is required to establish other potentiators of injury in this context.
POTENTIATION OF INJURY WITHIN THE KIDNEY: THERE IS CELL TO CELL SIGNALLING OF DAMAGE
There is growing evidence for transmission of injury between cells in a variety of settings. Connexins are a big family of transmembrane proteins, expressed in all human organs and tissues, which form internal gap junctions between cells and manipulate small molecule (less than 1KDa), direct-transfer signalling[36]. Luo specifically investigated the role of Connexin-32 (Cx32), because this connexin is normally richly expressed in the kidney. Cx32 expression was found to increase following reperfusion in a rat model of liver transplantation, peaking in tandem with kidney damage and functional impairment at 8 h[36]. Treatment with 2-APB, a relatively specific inhibitor of Cx32 channels, reduced renal injury. This study only evaluated renal function and would have benefited from measurement of liver injury, both in response to IR and following addition of 2-ARB, to evaluate the specificity of the renal response.
Cx32 expression has been demonstrated to positively correlate to the degree of IR injury in liver biopsies from patients undergoing liver transplantation[81], but human evidence to support the role of Cx32 in subsequent kidney cell to cell transmission of injury is lacking. Such data is worth pursuing, along with supplementary evidence to further define cell to cell signalling in the kidney.
THE INJURED KIDNEY MAY MODULATE THE PROGRESSION OF LIVER IR INJURY
Accumulating evidence suggests that in addition to liver IR injury mediation of renal injury, the kidney itself plays a key role in the potentiation or amelioration of liver injury.
There is demonstrable liver injury after ischaemic renal injury, with derangement of AST/ALT and evidence of hepatocyte apoptosis (via activation of NFB-receptor)[82,83]. IL-10, IL-6 and TNFα are upregulated within the liver and multiple markers of oxidative stress have been identified following ischaemic AKI. It is not known whether this is related to systemic inflammation or targeted liver injury. Either way, the effect may be persistent; renal IR injury is associated with the development of hepatic steatosis in the longer term[80].
Human Heat Shock Protein 27 (HSP27) is a member of the chaperone protein family. These proteins are upregulated in response to a variety of cellular stresses. HSP27 is a key stabiliser of F-actin and a potent anti-apoptotic. In a genetically manipulated mouse model with demonstrated robust and widespread overexpression of HSP27, Park et al[25] demonstrated attenuation of both partial liver IR injury (left and middle liver lobe inflow clamped), and secondary renal injury. The hepatic protection was primarily mediated by the kidneys as the liver injury was abolished by unilateral and bilateral nephrectomy. The findings of this study contrast with a previous study by the same group, where HSP27 overexpression provided primary protection against liver IR injury (significantly less necrosis and apoptosis at 2 h post reperfusion)[84]. In that study the HSP27 protection was thought to be mediated by Kupffer cells; depletion of Kupffer cells obliterated protection in HSP27 over-expressing mice but did not impact IR injury in wild type mice. Such results are not in keeping with the previously discussed, known roles of Kupffer cells in liver IR injury. One would expect obliteration of Kupffer cells in wild type mice to downregulate IR injury. Further investigation of these controversies is required but these studies hint that it might be possible to “switch off” liver IR injury and AKI, given the right therapeutic targets.
The sphingosine-1-phosphate (S1P)/S1P1-receptor interaction on endothelial cells is known to be critical in the maintenance of endothelial barrier integrity in the kidney. In a mouse model of hepatic IR injury, pre-treatment with S1P did not significantly attenuate liver injury (ALT/histology) at 6 h but provided marked attenuation at 24 h[62]. Renal injury was reduced at 6 h (TUNEL assay), with significantly improved endothelial integrity and reduced expression of CD44+ cells (indicating a reduction in endothelial injury) compared to non S1P treated mice. Pre-treatment with the S1P1 antagonist, VPC 23019, partially reversed the protection afforded by S1P.
Together these studies support the hypothesis that renal injury is both triggered by early liver IR injury and modulates ongoing liver IR injury. The mechanisms by which this occurs remain unknown but may involve the systemic inflammatory response to renal injury. Further work is required to determine the “switches” that decide whether renal modulation is pro- or anti-inflammatory and to harness these for therapeutic intervention.
THERE MAY BE ADDITIONAL EXTRA-RENAL MODIFICATION OF LIVER IR AND RENAL INJURY
Some recent studies have focussed on the role of the intestinal immune system in primary renal injury leading to secondary liver injury. IL-17A released by Paneth cell degranulation in the small intestine in response to primary renal IR injury contributes to hepatic, renal and intestinal injury, with improvement in all three when IL-17A is depleted[85]. Contrastingly Paneth cell TLR-9 knockout mice demonstrate progression of hepatic, intestinal and renal injury in response to kidney IR injury[86]. These data are obtained from models of kidney IR injury and therefore do not directly relate to liver IR injury. However, future studies to investigate the role of Paneth cells in the mediation of renal and liver injury following IR insult to the liver may reveal similar intriguing findings and provide additional opportunities to modulate the potentiation of systemic and local response to injury.
LIMITATIONS OF THE CURRENT LITERATURE
The studies discussed within this review present some interesting data related to the mechanisms of renal injury secondary to liver IR injury. However, a clear understanding of the pathways mediating the transmission of injury from liver to kidney and back again is not yet within our grasp. Investigative work in this field has relied heavily upon small rodent models. Rodent models often lack applicability to the human setting and clinical interventions that show promise in rodents often fail upon translation to the human setting[44,61,87]. Rodent populations used for experimental work are inbred animals with relatively limited genetic diversity and so cannot fully represent human populations with polymorphic genetic backgrounds[88]. Liver injury often occurs in patients who do not have background “normal” liver (including transplantation, ALF and ACLF). Background altered liver function may prime the immune and/or renal systems to injury, potentiating the effects of an acute insult. This is not accounted for in rodent models and may also impact the applicability of any results to the human setting.
A second limitation with all studies in this field is the difficulty associated with defining AKI clinically. Most studies included here rely upon serum creatinine (+/-urea), with clinical studies applying the AKIN or KDIGO criteria. Both AKIN and KDIGO rely upon changes in serum creatinine or urine output. Serum creatinine is well known to be a relatively insensitive marker of renal injury. Patients with end-stage liver disease are often deplete in skeletal muscle and so have low circulating creatinine, which may mask underlying renal injury[89,90]. Changes in serum creatinine take time to reflect renal injury, often between 12 and 24 h. During this time, renal injury may be potentiated, with worse long-term outcomes.
A third limitation with studies in this field is the lack of an animal model that allows serial sampling to dynamically assess changes over time. Rodent models are too small to accommodate serial liver and kidney biopsies or blood samples. As demonstrated herein, renal injury and liver injury following liver IR is a dynamic and evolving process. Serial, in vivo sampling would be highly informative.
FUTURE DIRECTIONS
Whilst the limitations of rodent models may be here to stay, improved diagnostic methodology for acute kidney injury may be provided by one, or a combination of biomarkers. NGAL shows great promise in this respect[91], and is already being used as an alternative to serum creatinine for the diagnosis of renal injury in some studies. In a study of liver transplant patients, we found that urinary NGAL measured at the time of abdominal closure accurately predicted post-operative AKI[23]. This has been confirmed by other studies[92,93]. The site of release and role of NGAL in liver IR injury leading to renal injury is not currently known. NGAL has multiple functions[94] including iron transport[95]. Speculatively, NGAL could “mop up” iron free radicals which contribute to injury in the context of liver IR and resultant renal injury. An interesting recent study identified that NGAL is co-localised with Arl13b to the primary cilium of human renal tubular epithelial cells in chronic allograft nephropathy[96]. KIM-1, another potential biomarker for renal injury[97] is also expressed on primary cilia[98]. The primary cilium is a key organelle and performs a variety of functions including mechano- and chemo-sensitisation[99]. In liver IR injury, primary cilia are shed into the urine and are demonstrable as early as 1 h post injury[98]. Whether NGAL is co-incidentally shed with cilia, or promotes shedding of cilia, awaits clarification.
CONCLUSION
The mechanisms by which liver injury mediates renal injury require further clarification but it is likely that multiple circulating molecules are involved, including currently unidentified molecules. The kidney may be primed to injury by alterations in renal microcirculation with early endothelial and subsequent tubular injury. Renal injury in turn, may potentiate liver IR injury and this process may involve other organs with immune function, including the gut.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Xu XY S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ