Peer-review started: February 7, 2017
First decision: May 8, 2017
Revised: April 28, 2017
Accepted: June 6, 2017
Article in press: June 7, 2017
Published online: August 12, 2017
Processing time: 193 Days and 2.5 Hours
To investigate the mechanism(s) by which potential effects of multi-drug highly-active antiretroviral therapy contributes to lipodystrophy syndrome.
Preadipocytes from healthy donors were assessed for proliferation and differentiation in the presence of nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) individually and in combination. Effects on proliferation were assessed with a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide assay and effects on differentiation were assessed from glycerol-3-phosphate dehydrogenase (GP DH) activity and quantitation of Oil Red O staining for intracellular lipid. Data were analyzed with a randomized block ANOVA with post-hoc Fisher’s Least Significant Difference test.
Preadipocyte proliferation was inhibited by a combination of NNRTI + NRTI (14% at 48 h, P < 0.001) and PI + NRTI (19% at 48 h, P < 0.001) with additional suppression when ritonavir (RTV) was added (26% at 48 h). The drug combination of atazanavir (ATV) + RTV + emtricitabine (FTC) + tenofovir (TDF) had the greatest inhibitory effect on proliferation at 48 h. Preadipocyte differentiation was most significantly reduced by the efavirenz + FTC + TDF assessed either by GPDH activity (64%) or lipid accumulation (39%), P < 0.001. Combining NRTIs with a PI (ATV + FTC + TDF) significantly suppressed differentiation (GPDH activity reduced 29%, lipid accumulation reduced by 19%, P < 0.01). This effect was slightly greater when a boosting amount of RTV was added (ATV + FTC + TDF + RTV, P < 0.001).
Although combination antiretroviral therapy is clinically more efficacious than single drug regimens, it also has a much greater inhibitory effect on preadipocyte proliferation and differentiation.
Core tip: We demonstrated an in vitro system for evaluating potential antiretroviral regimens for adipose tissue toxicity. In general, combination regimens resulted in greater preadipocyte proliferation and differentiation inhibition than single therapies. The drug combination of atazanavir + emtricitabine + tenofovir had inhibitory effects on preadipocytes and adding ritonavir at levels equivalent to clinical boosting, increased toxicity still further.
- Citation: Jones E, Mazirka P, McNurlan MA, Darras F, Gelato MC, Caso G. Highly active antiretroviral therapy dysregulates proliferation and differentiation of human pre-adipocytes. World J Virol 2017; 6(3): 53-58
- URL: https://www.wjgnet.com/2220-3249/full/v6/i3/53.htm
- DOI: https://dx.doi.org/10.5501/wjv.v6.i3.53
A link between highly active antiretroviral therapy (HAART) and HAART-associated lipodystrophy (HALS) has been recognized for well over a decade. HALS is associated with abnormal changes in fat distribution throughout the body, insulin resistance and altered levels of triglycerides, cholesterol and lipoproteins[1]. These changes impact the health of an individual as well as their quality of life and have reduced the impact of anti-HIV therapy development[2,3]. HAART regimens with various combinations of protease inhibitors (PI), nucleoside reverse transcriptase inhibitors (NRTI) and nonnucleoside reverse transcriptase inhibitors (NNRTI) have been associated with HALS (e.g.[4-6]). Previously we reported on the in-vitro effects of two PIs, ritonavir (RTV) and atazanavir (ATV) on preadipocyte proliferation and adipogenesis[7]. In the present study, we report the effects of common first-line combination regimens used in HIV treatment; efavirenz (EFV) + emtricitabine (FTC) + tenofovir (TDF), ATV + FTC + TDF and ATV + RTV + FTC + TDF, as well as their individual components, on in-vitro preadipocyte proliferation and differentiation.
Preadipocytes were obtained from abdominal subcutaneous fat tissue from healthy kidney donors undergoing nephrectomy. The samples were collected from 10 kidney donors (6 females, 4 males) who gave a written informed consent. All participants were HIV-seronegative, had an average age of 37 ± 4 years and a BMI of 29 ± 1 kg/m2. Subjects were placed under standard general anesthesia and subcutaneous fat tissue was removed from the peri-umbilical area during nephrectomy. The specimens were then immediately placed in a sterile Hank’s Buffered Salt Solution (HBSS) at pH 7.4 containing antibiotics and amphotericin. All fat samples were processed within one hour.
Once isolated, preadipocytes were tested in vitro for their ability to replicate and differentiate in the presence of different classes of antiretroviral drugs, which were applied individually or in combination. Fat samples from each donor were processed individually and each of the test conditions (drug combinations) were repeated with each donor sample. The selected drug combinations are recommended antiretroviral regimens for (naïve) HIV patients[8]. These included a NNRTI-based regimen consisting of a NNRTI (EFV) and 2 NRTIs (TDF and FTC) (i.e., EFV + TDF + FTC); and a PI-based regimen consisting of a PI (ATV) and 2 NRTIs (TDF and FTC) (i.e., ATV + TDF + FTC). The PI-based combination was tested with or without the addition of another PI, RTV (i.e., ATV + RTV+ TDF + FTC), since this regimen is often recommended to boost the effects of other protease inhibitors[9]. The following drug concentrations were used in all the experiments: EFV, 20 μmol/L; FTC, 15 μmol/L; TDF, 1 μmol/L; ATV, 10 μmol/L; RTV, 2 μmol/L. These concentrations are in the range of those observed in the plasma of patients treated with the specific antiretroviral combination regimens[10-12]. The effects of antiretroviral medications were compared with control samples in which preadipocytes were cultured and stimulated to differentiate in the absence of antiretroviral medications.
Preadipocyte isolation and culture from subcutaneous fat biopsies were previously described[7]. In brief, fat tissue was digested with collagenase (3 mg/mL, type II, Worthington, Lakewood, NJ) to obtain stromal cells that were then separated from mature adipocytes by centrifugation and incubated in erythrocyte lysing buffer (154 mmol/L NH4Cl, 10 mmol/L K2HPO4, 1 mmol/L EDTA, pH 7.4) for 10 min at room temperature to eliminate red cells. Remaining debris was then removed by filtering cell suspension through a 70 μm nylon filter and then centrifuged. Pelleted preadipocytes were plated in a basal medium consisting of DMEM/F-12 (Gibco, Carlsbad, CA) supplemented with 10% Fetal Calf Serum (FCS), 2 mmol/L glutamine, 100 IU/mL penicillin and 100 μg/mL streptomycin (Gibco) and incubated for 16-18 h. After incubation, attached cells were extensively washed with warm PBS, removed from the plates with trypsin, suspended and counted. Preadipocytes were then seeded at a density of 5 × 103/cm2.
To assess cell replication, cultures were incubated in untreated growth medium for 48 h after which the medium was exchanged for fresh medium (control) or medium containing drugs. Cell numbers were assessed over a 72 h period. For the assessment of differentiation, cultures were grown to confluence and differentiation was induced with serum-free medium (control) or the same medium containing antiretroviral drugs as previously described[7,13]. Since some drugs (i.e., EFV) are tightly bound to plasma albumin, 2 g/L bovine serum albumin (Sigma, St. Loius, MO) was added to differentiation medium in all control and treated groups. Medium was replaced every 72 h and differentiation assessed after 12 d.
The effect of antiretroviral drugs on preadipocyte proliferation was assessed by measuring the cell number in cultures exposed to the drugs for 48 and 72 h. Viable cell number was assessed with a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide based assay, as previously described[7,14]. Since all cultures were plated at the same initial cell number, an increase or decrease in viable cells at 48 and 72 h was assumed to represents a change in the potential of cells to increase in number, i.e., proliferate.
The differentiation of preadipocytes into mature adipocytes was estimated after 12 d by measuring the activity of the lipogenic marker enzyme glycerol-3-phosphate dehydrogenase (GPDH) and by quantifying the intracellular lipid accumulation after staining with Oil Red O.
GPDH assay: Determination of GPDH activity was based on the oxidation of NADH and reported as mU where 1 mU corresponded to the amount of enzyme needed to oxidize 1 nmol of NADH/min as reported previously[7].
Oil Red O staining: Cells fixed in a 10% formaldehyde solution were stained with Oil Red O, extracted with isopropanol and assessed for absorbance at 500 nm[7].
Results are expressed as means ± SEM. Differences between control and treated groups and among the treated groups were analyzed with a randomized block ANOVA with post-hoc Fisher’s Least Significant Difference (LSD) test. P values < 0.05 were considered statistically significant.
The effect of anti-retroviral drugs, individually and in combination, was assessed for two distinct aspects of preadipocyte metabolism; namely the proliferation of preadipocytes and the ability of preadipocytes to differentiate into adipocytes.
All individual antiretroviral drugs inhibited the proliferation of preadipocytes incubated in concentrations of the drugs comparable to the levels seen in the plasma of treated patients compared with untreated cells (control). This effect was statistically significant at 72 h (Figure 1; P < 0.02). The inhibition of proliferation in FTC-treated cells was apparent even at 48 h of incubation (P < 0.01).
All drug combinations tested significantly suppressed preadipocyte proliferation. In the presence of EFV + FTC + TDF (NNRTI + NRTIs) preadipocyte proliferation was inhibited by 14% and 26% respectively after 48 and 72 h compared to controls. Similarly, therapeutic combinations with ATV + FTC + TDF (PI + NRTIs) showed a reduction in preadipocyte growth of 19 % at 48 h, and of 30% at 72 h (Figure 1, P < 0.001). When RTV was added to ATV + FTC + TDF, as it is clinically known to boost other PIs, the inhibitory effect was more noticeable, with a suppression in proliferation of 26% and 37% respectively after 48 and 72 h compared to controls (Figure 1, P < 0.001).
The inhibition of proliferative activity of preadipocytes in multi-drug combinations was more severe than the inhibition of proliferation observed with the individual component drugs in some cases, but not all. In the combination EFV + FTC + TDF, suppression of proliferation was greater than the suppression observed when EFV and TDF were used individually (P < 0.02), but there was not an additional effect when EFV + FTC + TDF combination was compared to FTC treatment alone (P = NS). Combining ATV + FTC + TDF or ATV + RTV + FTC + TDF increased the inhibition of adipocyte proliferation to a greater extent than treatment with the same concentration of the individual drugs both at 48 (P < 0.05) and 72 h (P < 0.01).
Of the three multi-drug regimens, the drug combination ATV + RTV + FTC + TDF had a more suppressive effect on proliferation of preadipocytes than the other multi-drug regimens at 48 h (P < 0.02). At 72 h, the combination, ATV + RTV + FTC + TDF, was not statistically significantly different from ATV + FTC + TDF but was significantly different from EFV + FTC + TDF (P = 0.05).
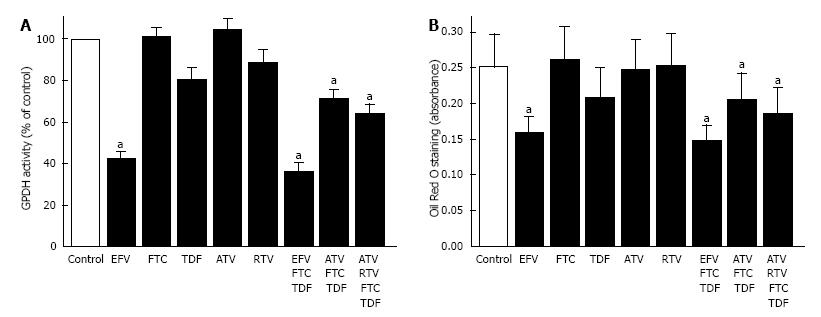
Preadipocyte differentiation, in the presence of anti-retroviral drugs, was assessed by 2 different techniques, one involved measuring the enzymatic activity of GPDH and the other involved quantifying intracellular lipid accumulation after staining with Oil Red O. The two techniques produced comparable results (Figure 2).
EFV had a profound inhibitory effect on preadipocyte differentiation (Figure 2). Both GPDH activity and lipid accumulation were greatly reduced in cells treated with EFV compared to controls (Figure 2, P < 0.001). Of the other anti-retroviral drugs tested, only TDF appeared to have an effect on intracellular lipid accumulation, which tended to be lower when cells were treated with TDF (P = 0.06). Preadipocyte differentiation in the presence of remaining individual drugs did not differ from controls (Figure 2).
Figure 2 demonstrates that, preadipocyte differentiation was significantly reduced when the anti-retroviral drugs were used in combination compared to untreated cultures. Compared to control cells, the EFV + FTC + TDF (NNRTI + NRTIs) combination showed the most suppressive effect on differentiation with GPDH activity and lipid accumulation 64% and 39% lower respectively (Figure 2, P < 0.001). Combining NRTIs with a PI (ATV + FTC + TDF) inhibited GPDH activity by 29% and lipid accumulation by 19% compared to controls (Figure 2, P < 0.01). This effect was slightly greater when a boosting amount of RTV was added (ATV + FTC + TDF + RTV, Figure 2, P < 0.001).
The inhibitory effect of a multi-drug combination NNRTI and NRTI (EFV + FTC + TDF) was also compared to the suppression in differentiation observed with the anti-retroviral medications individually. Suppression with the combination resulted in a greater reduction in preadipocyte differentiation than either FTC or TDF alone (P < 0.001). However, suppression of differentiation with the combination of EFV + FTC + TDF was comparable in magnitude to treatment with EFV alone suggesting EFV was accountable for most of the reduction in differentiation observed with the combination regimen (Figure 2). The two multi-drug regimens containing PI + NRTI were also examined relative to the incubations with the individual medications. The multi-drug combinations containing PIs had greater inhibitory effects on differentiation than treatment with the same concentration of each individual drug (Figure 2, P < 0.003), with the exception of TDF.
The multi-drug regimens have also been compared with each other. The combination with NNRT and NRTI (EFV, FTC, TDF) reduced differentiation to a greater extent than either of the two regimens with PI + NRTI, whether assessed as either GPDH activity (P < 0.001) or intracellular lipid accumulation (P < 0.02).
There is no doubt that the etiology of HIV/HAART-associated lipodystrophy syndrome is multi-factorial, but antiretroviral medications contribute to the condition. This in vitro technique, with primary cultures of preadipocytes isolated from healthy subjects, provides a way of assessing effects of single and combination drug regimens on preadipocyte proliferation and differentiation; and consequently, on the potential of drug regimens to contribute to HALS. The antiretroviral medications currently in use have profound effects on both preadipocyte proliferation and differentiation.
All of the antiretroviral agents tested inhibited preadipocyte proliferation. Individually, the NRTI, FTC, had a more pronounced effect on preadipocyte proliferation than the NRTI, TDF; the NNRTI, EFV; or the PIs ATV or RTV (Figure 1). While in general combinations of anti-retroviral drugs were more toxic than the individual drugs, this was not true for combinations containing FTC. The addition of TDF (another NRTI) and EFV (a NNRTI) to emtricitabine did not produce any greater toxicity than was observed with EFV alone. However, multidrug regimens containing PIs in combination with NRTI (ATV + FTC + TDF and ATV + RTV + FTC + TDF) resulted in further suppression of proliferation. Having previously demonstrated that RTV does not suppress preadipocyte proliferation at levels comparable to those used for boosting[7], this study indicates that adding RTV to a combination of ATV, TDF and FTC does increase toxicity (Figure 1). Clearly, the toxicity of individual antiretrovirals can be affected by concurrent antiretroviral administration.
In contrast to preadipocyte proliferation, it is the NNRT EFV that has the most profound effect on preadipocyte differentiation, an effect that has been reported previously[15,16]. Combining EFV with NRTIs (EFV + FTC + TDF) does not result in any greater suppression. Regimens containing PIs and NRTIs (ATV + FTC + TDF and ATV + RTV + FTC + TDF) are not as toxic as those containing EFV. However, the multi-drug combinations containing PIs suppressed differentiation to a greater extent than the use of any drug individually. This study highlights the importance of assessing both the effects on the proliferation of preadipocytes and the differentiation of preadipocytes into mature adipocytes since multi-drug regimens affect them differently.
In conclusion, antiretroviral medications affect not only the differentiation of preadipocyte into mature adipocytes, these drugs also affect the proliferation of preadipocytes and can, therefore, impact on the number of preadipocytes that are available. While FTC has the most profound effect on preadipocyte proliferation, it is EFV that has the greatest impact on differentiation. Combinations of antiretroviral medications, which have no impact when used individually, increase the toxicity for preadipocytes.
Highly active antiretroviral therapy (HAART) regimens with various combinations of protease inhibitors (PI), nucleoside reverse transcriptase inhibitors (NRTI) and nonnucleoside reverse transcriptase inhibitors (NNRTI) have long been linked to HAART-associated lipodystrophy syndrome (HALS). Once HALS is manifest, it is difficult to reverse. There is a need to develop ways of assessing drug combinations for the potential to contribute to HALS.
The effect of individual anti-retroviral drugs have been studied in vitro, but this is the first report of the effect of drug combinations assessed at clinically relevant concentrations. The differential effects on preadipocyte proliferation and differentiation were also assessed, providing important mechanistic information.
The study demonstrates that replacing ritonavir-based regimens with the clinically more acceptable protease inhibitor, atazanavir, does not eliminate the potential for toxic effects on adipose tissue. In addition, adding ritonavir at “boosting” levels to regimens containing nucleoside reverse transcriptase inhibitors and non-reverse transcriptase inhibitors also increases the lipo-toxic potential of these antiretroviral combinations.
Although combination antiretroviral therapy is clinically more efficacious than single drug regimens, the combination regimens also have the potential to contribute to adipose tissue toxicity through effects of preadipocyte replication and differentiation. The study also illustrates the value of an in vitro system for screening drug combinations for potential adipose tissue toxicity.
HALS is a condition that is characterized by loss of subcutaneous fat, particularly in the face, buttocks, arms and legs. Antiretroviral therapy typically includes a combination of drugs with different mechanisms of action including NRTIs, NNRTIs, and PIs. These drug classes have the potential for differential effects on adipose tissue and the effect of combination therapy may be greater than the individual drugs alone. This study also investigated the mechanisms by which antiretroviral drugs can contribute to the loss of adipose tissue; an inability of preadipocytes to proliferate reduces the potential number of precursor cells for the formation of adipose tissue, the inability of pre-adipocytes to differentiation reduces the formation of adipose tissue by arresting precursor cells in an undifferentiated state.
This manuscript is worth publishing, reporting the synergistic or cooperative effects of anti-HIV agents on inhibiting adipogenesis by performing in vitro experiments using human materials. It will help us to recognize the importance to take extra caution in executing HAART.
Manuscript source: Invited manuscript
Specialty type: Virology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen CJ, Saeki K, Shen WJ S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Guaraldi G, Stentarelli C, Zona S, Santoro A. HIV-associated lipodystrophy: impact of antiretroviral therapy. Drugs. 2013;73:1431-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Lake JE, Wohl D, Scherzer R, Grunfeld C, Tien PC, Sidney S, Currier JS. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011;23:929-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Quintas RC, de França ER, de Petribú KC, Ximenes RA, Quintas LF, Cavalcanti EL, Kitamura MA, Magalhães KA, Paiva KC, Filho DB. Treatment of facial lipoatrophy with polymethylmethacrylate among patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): impact on the quality of life. Int J Dermatol. 2014;53:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Domingo P, Estrada V, López-Aldeguer J, Villaroya F, Martínez E. Fat redistribution syndromes associated with HIV-1 infection and combination antiretroviral therapy. AIDS Rev. 2012;14:112-123. [PubMed] |
| 5. | Martin A, Moore CL, Mallon PW, Hoy JF, Emery S, Belloso WH, Phanuphak P, Ferret S, Cooper DA, Boyd MA; Second-Line Study Team. HIV lipodystrophy in participants randomised to lopinavir/ritonavir (LPV/r) +2-3 nucleoside/nucleotide reverse transcriptase inhibitors (N(t)RTI) or LPV/r + raltegravir as second-line antiretroviral therapy. PLoS One. 2013;8:e77138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Minami R, Yamamoto M, Takahama S, Ando H, Miyamura T, Suematsu E. Comparison of the influence of four classes of HIV antiretrovirals on adipogenic differentiation: the minimal effect of raltegravir and atazanavir. J Infect Chemother. 2011;17:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Caso G, Mileva I, McNurlan MA, Mynarcik DC, Darras F, Gelato MC. Effect of ritonavir and atazanavir on human subcutaneous preadipocyte proliferation and differentiation. Antiviral Res. 2010;86:137-143. [PubMed] |
| 8. | Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Section accessed on March 18. 2015;(Table 6) Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. |
| 9. | Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Clay PG, Taylor TA, Glaros AG, McRae M, Williams C, McCandless D, Oelklaus M. “One pill, once daily”: what clinicians need to know about Atriplatrade mark. Ther Clin Risk Manag. 2008;4:291-302. [PubMed] |
| 11. | Kiser JJ, Fletcher CV, Flynn PM, Cunningham CK, Wilson CM, Kapogiannis BG, Major-Wilson H, Viani RM, Liu NX, Muenz LR. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2008;52:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | von Hentig N, Dauer B, Haberl A, Klauke S, Lutz T, Staszewski S, Harder S. Tenofovir comedication does not impair the steady-state pharmacokinetics of ritonavir-boosted atazanavir in HIV-1-infected adults. Eur J Clin Pharmacol. 2007;63:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286-R1296. [PubMed] |
| 14. | Caso G, McNurlan MA, McMillan ND, Eremin O, Garlick PJ. Tumour cell growth in culture: dependence on arginine. Clin Sci (Lond). 2004;107:371-379. [PubMed] |
| 15. | El Hadri K, Glorian M, Monsempes C, Dieudonné MN, Pecquery R, Giudicelli Y, Andreani M, Dugail I, Fève B. In vitro suppression of the lipogenic pathway by the nonnucleoside reverse transcriptase inhibitor efavirenz in 3T3 and human preadipocytes or adipocytes. J Biol Chem. 2004;279:15130-15141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Gallego-Escuredo JM, Del Mar Gutierrez M, Diaz-Delfin J, Domingo JC, Mateo MG, Domingo P, Giralt M, Villarroya F. Differential effects of efavirenz and lopinavir/ritonavir on human adipocyte differentiation, gene expression and release of adipokines and pro-inflammatory cytokines. Curr HIV Res. 2010;8:545-553. [PubMed] |