Published online Mar 25, 2025. doi: 10.5501/wjv.v14.i1.99923
Revised: September 27, 2024
Accepted: October 29, 2024
Published online: March 25, 2025
Processing time: 116 Days and 6.6 Hours
Cytomegalovirus (CMV) infections can cause significant morbidity and mortality in immunocompromised individuals. CMV targets dysfunctional lymphocytes. Chronic rituximab (RTX) therapy can cause B-lymphocyte dysfunction, increasing CMV risk. Rarely, CMV infections present with critical illness such as septic shock.
A 64-year-old African American woman presented with generalized weakness and non-bloody watery diarrhea of 4-6 weeks duration. She did not have nausea, vomiting or, abdominal pain. She had been on monthly RTX infusions for neu
This case illustrates an extremely rare case of CMV colitis associated with RTX use presenting with septic shock. High suspicion for rare opportunistic infections is imperative in individuals with long-term RTX use.
Core Tip: With the increasing use of biologics in medicine, there has been an emergence of opportunistic infections. Cytomegalovirus (CMV) infections remain asymptomatic or cause only mild symptoms in most immunocompetent adults. However, it can cause infectious complications in immunocompromised individuals-especially those with defective T-lymphocyte function. Rituximab (RTX) use is associated with B-lymphocyte depletion and increased risk of infections. However, CMV infections after long-term RTX are uncommon. Our case describes septic shock due to CMV colitis associated with RTX use which is an extremely rare entity. It highlights the need for consideration of rare opportunistic infections in all patients on biologics.
- Citation: Patel S, Jay J, Pathak P, Antony MA, Thiriveedi M. Septic shock due to cytomegalovirus colitis associated with rituximab use: A case report. World J Virol 2025; 14(1): 99923
- URL: https://www.wjgnet.com/2220-3249/full/v14/i1/99923.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i1.99923
Most adults (40%-100%) have been infected and are carriers of cytomegalovirus (CMV)[1-3]. In the immunocompetent, CMV’s acute presentation is asymptomatic. Once infected, CMV remains latent in the host’s cells indefinitely[1-3]. If an infected person becomes immunocompromised, their latent CMV infection can reactivate and present itself in a variety of manifestations including pneumonia, esophagitis, retinitis, hepatitis, and colitis[1,2].
Rituximab (RTX)- an anti-CD20 antibody, is used to treat patients with various autoimmune conditions[3]. Its use can lead to adverse effects, the most notable being increased infection risk due to its effect on humoral immunity[3-5]. A rare complication of long-term RTX use can be the development of opportunistic infections, like in our case, CMV. Some studies have found an association between RTX use and the development of low levels of immunoglobulins, increasing susceptibility for opportunistic infections[2,3,6].
A 64-year-old African American woman presented to the emergency room with generalized weakness for 24 hours. She complained of non-bloody, watery diarrhea of 4-6 times per day, for 4-6 weeks duration.
She denied nausea, vomiting, abdominal pain, fever, chills, or rash, as well as recent travel, consumption of contaminated food, or sick contacts.
She had a history of neuromyelitis optica (on RTX), hyperlipidemia (on atorvastatin), and diabetes mellitus type II (on metformin and glipizide). Colonoscopy 10 years ago was unremarkable.
Past and family history were negative for neoplasm or autoimmune disease. She denied tobacco, alcohol, or illicit drug use.
On initial assessment, the patient was tachycardic (119/min) and hypotensive (81/47 mmHg) without fever or hypoxia on room air. Physical examination was remarkable for lethargy, dry oral cavity, and conjunctival pallor. Abdomen was soft, non-tender, and non-distended with bowel sounds in all four quadrants.
Laboratory investigations were significant for pancytopenia, hypoglycemia, acute kidney injury, and lactic acidosis (Table 1).
| Patient values | Normal values | |
| White blood cell count (× 103 cells/mL) | 1.35 | 4.8-10.8 |
| Red blood cell count (million cells/mL) | 2.59 | 4.2-5.4 |
| Hemoglobin (mmol/L) | 4.96 | 8.7-11.2 |
| Hematocrit (%) | 25.6 | 37.0-47.0 |
| Mean corpuscular volume (FL) | 98.8 | 81-99 |
| Mean corpuscular hemoglobin (PG) | 30.9 | 27-31 |
| Mean corpuscular hemoglobin concentration (g/dL) | 31.3 | 33-37 |
| Red cell distribution width (%) | 14.9 | 11.5-14.5 |
| Platelet count (× 1000 cells/mL) | 16 | 130-400 |
| Neutrophils (%) | 37.1 | 42.2-75.2 |
| Lymphocytes (%) | 57.0 | 20.5-51.5 |
| Monocytes (%) | 3.7 | 1.7-9.3 |
| Percentage reticulocyte count (%) | 0.57 | 0.8-2.1 |
| Sodium (mmol/L) | 137 | 135-145 |
| Potassium (mmol/L) | 4.7 | 3.5-5 |
| Chloride (mmol/L) | 98 | 98-105 |
| Blood urea nitrogen | 5.36 | 3.6-7.1 |
| Serum creatinine (μmol/L) | 97.26 | 44-97 |
| Aspartate transaminase (U/L) | 74 | 10-30 |
| Alanine transaminase (U/L) | 18 | 10-36 |
| Alkaline phosphatase (U/L) | 53 | 32-104 |
| Erythrocyte sedimentation rate (mm/hour) | 56 | 0-20 |
| C- reactive protein (mg/L) | 160.98 | 0-5 |
| Cytomegalovirus DNA by polymerase chain reaction | 14800 IU/mL | Undetected |
| Immunoglobulin M (g/L) | 10 | 40-230 |
| Immunoglobulin A (g/L) | 239 | 70-400 |
| Immunoglobulin G (g/L) | 1200 | 700-1600 |
| Blood culture | Negative | Negative |
| Stool tests: Clostridioides difficile, Escherichia coli, Shiga toxin, Cryptosporidium, Cyclospora, Adenovirus, Norovirus, Rotavirus, Giardia, Entamoeba | Negative | Negative |
| Peripheral smear | Normocytic normochromic anemia, thrombocytopenia | No anemia or thrombocytopenia |
| Bone marrow and flow cytometry | Hypocellular (10%) bone marrow with mild erythroid hyperplasia. Negative for monoclonal plasma cell or blast cell population | Normal cellularity without any monoclonal or blast cell population |
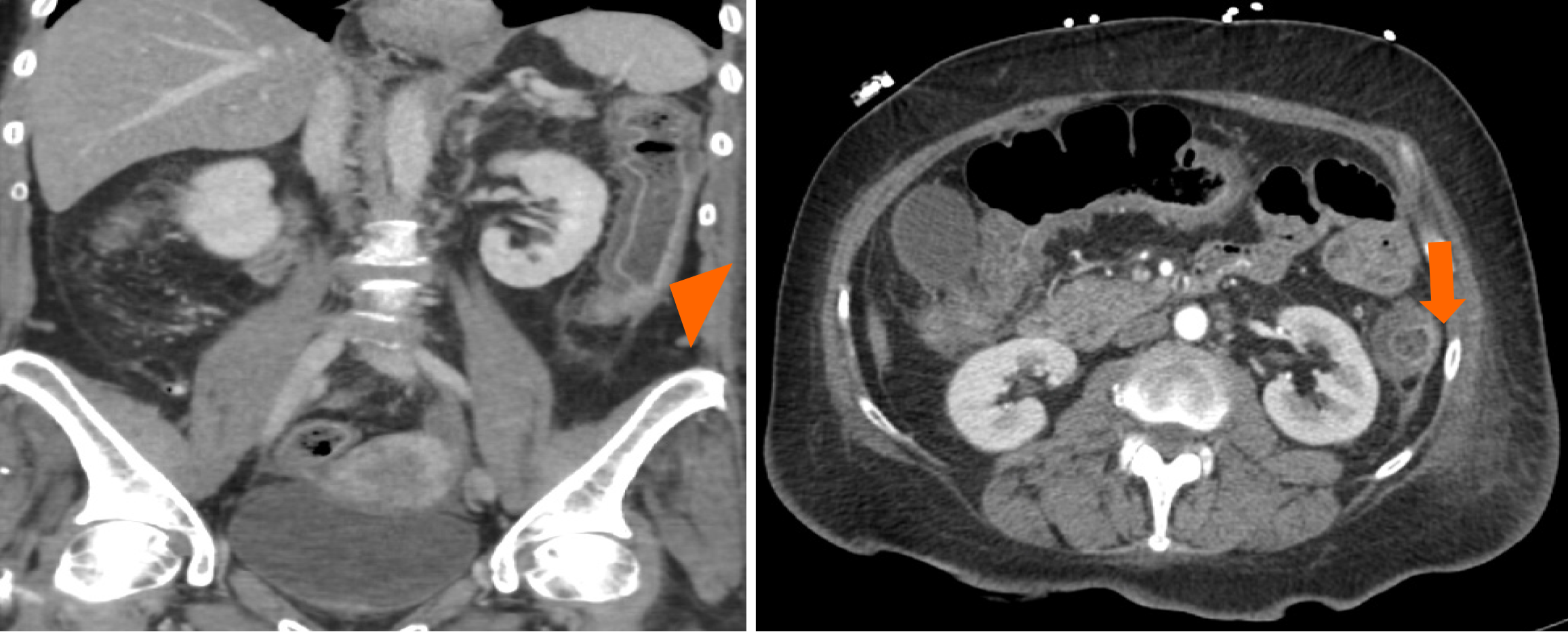
Computed tomography scan of the abdomen and pelvis with contrast detected diffuse thickening of the colonic wall with peri-colonic stranding consistent with pancolitis (Figure 1).
She was diagnosed with septic shock due to acute colitis and admitted to the critical care unit with intravenous fluids, vasopressor, and antibiotics.
Stool studies ruled out Clostridioides difficile, Escherichia coli, Shigella, and Salmonella among other infections (Table 1). Blood cultures and hepatitis panel were negative. Filgrastim injections were started to address pancytopenia. CMV serology demonstrated elevated CMV DNA. The patient could not undergo colonoscopy for tissue biopsy due to thrombocytopenia. Hence, the diagnosis of CMV colitis due to RTX was made based on clinical presentation and serology.
Valganciclovir was started. Her diarrhea and pancytopenia resolved over the next 7 days. She was discharged home in stable condition.
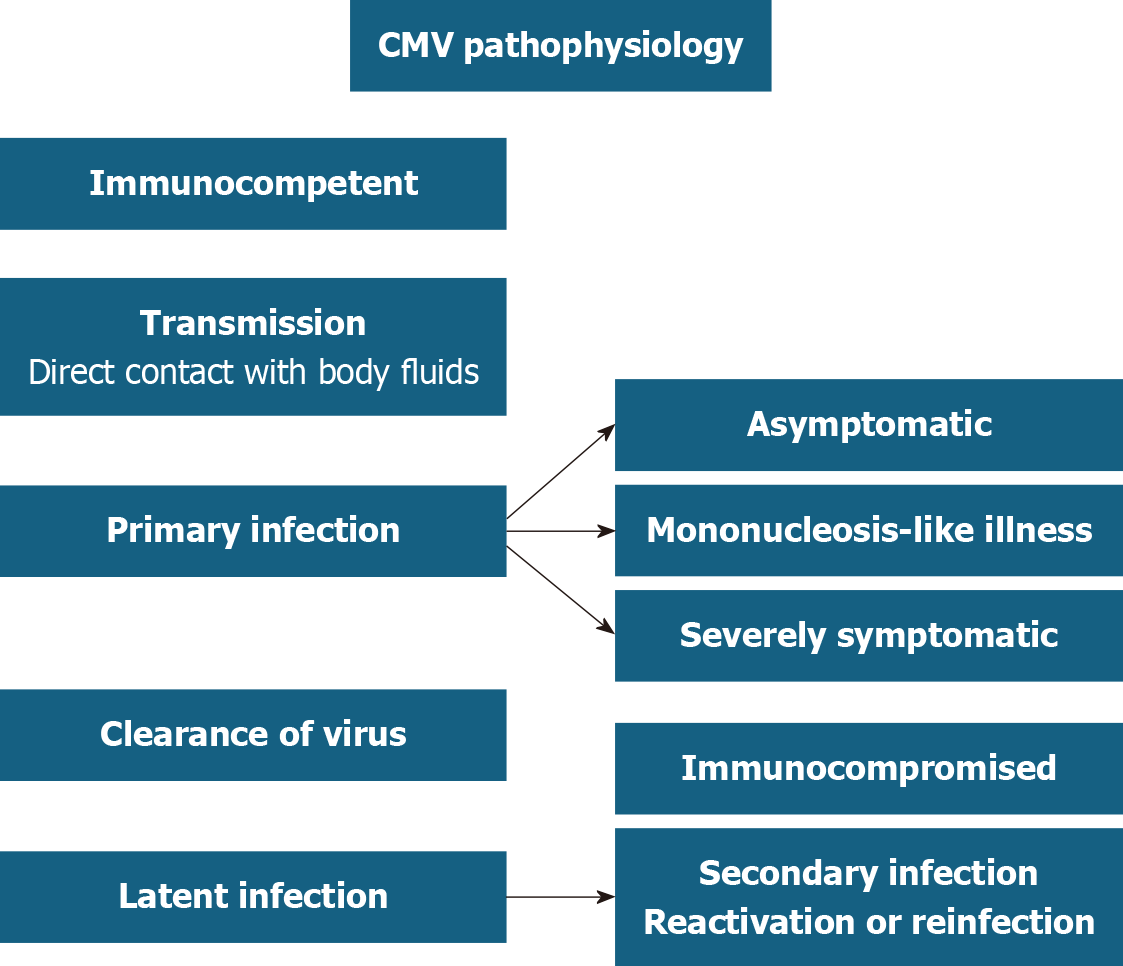
CMV infects about 40%-100% of the population by the adult life[1,2]. In the immunocompetent, it is mostly asymptomatic or presents as a mild mononucleosis-like syndrome (Figure 2)[1-3]. Once the primary infection resolves, CMV remains latent in its host indefinitely[1]. Most CMV disease manifestations are due to viral reactivation which commonly occurs when an infected individual becomes immunocompromised[1,2]. For example in solid organ and bone marrow transplant recipients, and HIV patients, reactivation of CMV can manifest as pneumonia, esophagitis, retinitis, hepatitis, and colitis[1,3].
CMV disease in immunocompromised individuals occurs through one of three different pathways: Acute infection, latent viral reactivation, or reinfection with a new viral strain[1]. Most commonly, reactivation is due to dysfunctional or inadequate numbers of T lymphocytes and natural killer cells[1,2]. Disease severity is closely related to the patient’s degree of immunodeficiency[1,2].
RTX is an anti-CD20 antibody used for the treatment of various conditions including non-Hodgkin’s lymphoma, rheumatoid arthritis, and neuromyelitis optica[3-6]. The most notable adverse effect of its use is an increased risk of infection[3-6] which is primarily related to B lymphocyte depletion[1,2]. One study found that 30% of patients reported side effects were infectious in nature[3]. Of these, 63% were bacterial infections, 33% viral and 3.3% fungal[3]. Despite an increased risk of infection, opportunistic infections are uncommon, highlighting the uniqueness of this case.
A rare complication of RTX is CMV reactivation. Although CMV primarily affects T lymphocytes, humoral immunity plays a critical role in suppressing CMV[2,3,6]. A literature review explored the association between CMV and RTX.
We queried PubMed and Google Scholar for articles related to CMV colitis induced by chronic RTX use. Key words included: Cytomegalovirus, CMV, colitis and rituximab. Articles were case reports or series written in English involving adult patients. The articles needed to include RTX use and definitive CMV diagnosis via biopsy or serology. Only one case of CMV colitis associated with RTX use was found[3]. All other cases demonstrated either non-colitis CMV manifestations- encephalitis, pneumonitis, retinitis, gastritis, and death[7,8]; or were precipitated by concomitant use of multiple immunosuppressing drugs such as bendamustine or fludarabine[8-10]. Unfortunately, these multi-drug regimens com
Only Vallet et al[3] case report demonstrated RTX induced CMV-colitis. Like our report, their patient was a middle-aged woman who developed CMV colitis after 2 cycles of RTX, although our patient was on RTX for > 3 years. The patient in our case had neuromyelitis optica while theirs suffered from rheumatoid arthritis. Both experienced hypogammaglobulinemia, hypo-IgM in our patient while hypo-IgG in theirs. There was no description of the severity of the symptoms in Vallet et al’s report whereas our case had septic shock requiring critical care management. Both patients eventually improved with valganciclovir[3].
Other reports have found that hypogammaglobulinemia increases the risk of CMV reactivation[3-6]. One paper reported CMV in five patients with hypo-IgG[3]. Repeated RTX treatments increase patients’ risk of developing hypo-IgG[4]. Our case is unique because our patient had hypo-IgM and normal IgG. Studies have found an association between RTX use and hypo-IgM leading to an increased risk of infections; however, there are no cases of RTX induced hypo-IgM leading to CMV colitis[4,5]. Low IgM levels were strongly associated with sepsis for those on RTX[3-5]. Similarly, our patient suffered from colitis and sepsis.
Overall, this case illustrates a rare case of CMV colitis associated with RTX use presenting with septic shock. High suspicion for rare opportunistic infections is imperative in individuals with RTX use. Immunocompromised patients with gastrointestinal signs and symptoms should be evaluated for possible opportunistic infections such as CMV.
| 1. | Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 305] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990;12 Suppl 7:S701-S710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 222] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Vallet H, Houitte R, Azria A, Mariette X. Cytomegalovirus colitis and hypo-IgG after rituximab therapy for rheumatoid arthritis. J Rheumatol. 2011;38:965-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 2018;57:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Ezhuthachan I, Shapero M, Fishbein J, Kaplan B. Lower IgM levels after rituximab use is associated with sepsis. J Allergy Clin Immunol. 2021;147:AB73. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Aggarwal A, Levoy E, Desimone M, Bhattacharya K. Rituximab-Associated Colitis: An Unusual Case Report. Am J Gastroenterol. 2018;113:S849. [DOI] [Full Text] |
| 7. | Eckmann JD, Chedid V, Quinn KP, Bonthu N, Nehra V, Raffals LE. De Novo Colitis Associated With Rituximab in 21 Patients at a Tertiary Center. Clin Gastroenterol Hepatol. 2020;18:252-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Libby E, Movva S, Quintana D, Abdul-Jaleel M, Das A. Cytomegalovirus retinitis during chemotherapy with rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone. J Clin Oncol. 2010;28:e661-e662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Magliano G, Cuccaro A, d'Alo' F, Maiolo E, Bellesi S, Hohaus S, Bacigalupo A, Pagano L. Severe CMV Infection after Chemo-Immunotherapy with Dose-Reduced Bendamustine and Rituximab in a Mantle Cell Lymphoma Old Patient. Mediterr J Hematol Infect Dis. 2021;13:e2021054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chan TS, Cheung CY, Yeung IY, Hwang YY, Gill H, Wong IY, Kwong YL. Cytomegalovirus retinitis complicating combination therapy with rituximab and fludarabine. Ann Hematol. 2015;94:1043-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |