Published online Mar 25, 2025. doi: 10.5501/wjv.v14.i1.97482
Revised: August 29, 2024
Accepted: October 15, 2024
Published online: March 25, 2025
Processing time: 180 Days and 8.7 Hours
For decades, hepatitis A virus (HAV) has been a leading cause of acute hepatitis among children and was less prevalent among adults. However, recently a paradigm shift has been observed in the epidemiology of HAV, as evident by cases of acute hepatitis due to HAV among adults.
To estimate frequency of HAV in acute viral hepatitis and compare characteristics in HAV and hepatitis E virus (HEV) infection.
This was a trend analysis conducted at Aga Khan University Hospital Karachi (Sindh, Pakistan) from February 2024 to May 2024. Individuals aged 18 years and older diagnosed with acute viral hepatitis attributed to hepatotropic viruses in 2024 were reviewed. To compare the trend patients admitted with acute hepatitis during 2019-2023 were also reviewed. Data regarding clinical and laboratory parameters were recorded. The yearly trend of acute hepatitis due to HAV and HEV was analyzed, and comparative analysis was done between HAV and HEV cases among adults.
A total of 396 patients were found to have acute hepatitis during our study duration. HAV was diagnosed in 234 patients (59%) while 157 patients (39.6%) were found to have acute HEV infection. Additionally, acute hepatitis B virus infection was identified in 3 patients (0.7%), whereas acute hepatitis C virus infection was found in 2 (0.5%) cases of acute hepatitis. Yearly trends showed increasing occurrence of HAV infection among adults over last 5 years. The patients with acute HAV were younger than patients with HEV (28 years ± 8 years vs 30 years ± 8 years; P < 0.01). Higher levels of total bilirubin were seen in HEV infection, while higher levels of alanine transaminase were seen in HAV infection. However, a higher proportion of acute liver failure (ALF), coagulopathy, and mortality were observed in HEV.
An increase in acute hepatitis A cases among adults shows less severity than hepatitis E, highlighting the need for better sanitation, hygiene, and adult hepatitis A vaccination programs.
Core Tip: In recent years, there has been a notable shift in the prevalence of viruses causing acute hepatitis. The hepatitis A virus (HAV) is increasingly affecting adults, contrary to its previous predominance among children, while the incidence of hepatitis E virus (HEV) among adults appears to remain unchanged. This change highlights a significant epidemiological transition. Although cases of HEV infection are associated with higher mortality rates, acute liver failure, and coagulopathy compared to those of HAV, signaling distinct impacts between the two viruses in adult populations.
- Citation: Shahid Y, Butt AS, Jamali I, Ismail FW. Rising incidence of acute hepatitis A among adults and clinical characteristics in a tertiary care center of Pakistan. World J Virol 2025; 14(1): 97482
- URL: https://www.wjgnet.com/2220-3249/full/v14/i1/97482.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i1.97482
Affecting approximately 1.4 million individuals annually worldwide, hepatitis A virus (HAV) is attributed to an increasing burden of disease especially in the developing world and individuals with underprivileged conditions[1]. HAV is a single-stranded, non-enveloped RNA virus, which belongs to the Picornaviridae family and the hepatovirus genus. HAV spreads primarily through the fecal-oral route, typically through direct person-to-person contact or consumption of contaminated food or water[2,3]. Acute hepatitis A infection typically leads to a self-limiting illness. Nonetheless, instances of fulminant liver failure have been documented, with advanced age identified as the primary risk factor for developing symptomatic disease[4]. Numerous studies have emphasized a shift in the epidemiology of HAV among adults. While children were predominantly affected by HAV in the past, there has been a noticeable increase in cases among the adult population in recent years. This trend necessitates further investigation to understand the underlying reasons for this shift. The Korea Centers for Disease Control and Prevention noted an exponential rise in symptomatic HAV infections since 2006 among adults, reflecting a shifting pattern of HAV which has also been observed in other Asian countries as well. A study conducted in Egypt also noted a significant rise in acute HAV infection rates, increasing from 2.1% in 1983 to 34% in 2002 among adult population[5,6]. Some studies have shown higher HEV prevalence compared with HAV, indicating variations in epidemiological trend[7]. HAV transmission varies globally, influenced by socioeconomic factors, with higher endemicity in resource-poor regions where early childhood infections are common and often mild, conferring lifelong immunity. By contrast, low endemicity in high-income regions results in fewer childhood exposures, leading to susceptibility in adults and the potential for severe outbreaks among high-risk groups. Improved sanitation in some low- and middle-income countries has reduced transmission but paradoxically increased morbidity and mortality by shifting the age of infection to older populations[8].
Exposure to HAV during early childhood typically leads to asymptomatic infection and confers lifelong immunity. However, with advancements in hygiene and the resulting reduction in HAV exposure, a segment of the population remains susceptible to acquiring the infection during adulthood. At this age, HAV infection carries a higher chance of morbidity and mortality. A review from the Indian subcontinent suggests that the lower prevalence of anti-HAV antibodies may be one of the reasons for the higher frequency of hepatitis A infection among adults[9]. The older studies from Pakistan reported HAV to be responsible for 50%-60% of cases of acute hepatitis among children and 3.5% among adults[10,11]. However, in Pakistan, a shift in HAV epidemiology has been observed but the data are very limited. There is one study from Pakistan where cases of HAV have been reported among adults (beyond 18 years), but this was based on a study conducted on individuals during two outbreaks of hepatitis A[12]. In 2009, the Pakistan Field Epidemiology and Laboratory Training Program collaborated with the Ministry of Health, Pakistan, and the Centers for Disease Prevention and Control, United States, to establish the Hepatitis Sentinel Surveillance System. This initiative identified five public sector tertiary care hospitals across Pakistan to collect relevant data. Analysis of the sentinel site data from June 2010 to March 2011 revealed a total of 712 cases of viral hepatitis, with acute hepatitis A accounting for 19.8% of the cases. Notably, males were more affected, comprising 69.5% of the cases. A noteworthy observation in this study was the shift in the age distribution pattern, with the highest prevalence of HAV observed in the 20-29 age group (41.2%), followed by the 30-39 age group (16.3%), and the 6-19 age group (12.8%)[13].
HAV can lead to significant morbidity and mortality among adults if not treated in a timely manner. Very limited data were available from Pakistan on the burden of HAV among adults in Pakistan. Hence, the evaluation of HAV among adult patients with acute hepatitis will help to assess the disease burden among the adult population in Pakistan and the need for immunization against HAV in adults.
In this study, we established the increasing incidence of HAV among adults and compared the clinical characteristics, severity, and mortality rates of acute hepatitis among adults caused by HAV vs hepatitis E virus (HEV).
This was a trend analysis conducted at Aga Khan University Hospital Karachi (Sindh, Pakistan). Data were collected during February 2024 to May 2024 for patients admitted with acute hepatitis during February 2024 to May 2024, as well as patients admitted with acute hepatitis during 2019 to 2023 to compare the trend. Inclusion criteria comprised individuals aged 18 years and older, who presented to the outpatient and inpatient departments and were diagnosed with acute viral hepatitis attributed to hepatotropic viruses. Exclusion criteria encompassed cases of acute hepatitis unrelated to hepatotropic viruses, drug induced, alcoholic hepatitis, and other metabolic etiologies (including hemochromatosis, autoimmune hepatitis, Wilson’s disease, alpha 1 anti-trypsin deficiency, metabolic dysfunction-associated steatotic liver disease). Acute hepatitis due to hepatotropic viruses was considered if hepatitis was due to HAV, hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV) and HEV. These viruses specifically affect liver hence the term “hepatotropic.” Non-hepatotropic viruses encompass all other viral agents capable of inducing acute hepatitis, such as cytomegalovirus, Epstein-Barr virus, dengue, and herpes simplex virus[14,15].
The diagnosis of acute hepatitis was established based on symptoms such as jaundice, fever, or vomiting lasting for less than 6 months recognized by elevation of liver enzymes, specifically alanine transaminase (ALT) and aspartate transaminase (AST) more than five times the upper limit of normal. Acute hepatitis A and hepatitis E were defined as patients with acute hepatitis found to have immunoglobulin M (IgM) antibody to HAV (anti-HAV) and HEV (anti-HEV), respectively[16]. Acute liver failure (ALF) was characterized by a severe, sudden liver injury lasting less than 26 weeks, accompanied by encephalopathy and impaired synthetic function (indicated by an international normalized ratio of 1.5 or higher), in individuals without preexisting liver disease or cirrhosis. Deranged coagulopathy and altered mentation are two important criteria of ALF. It is associated with high rates of complications and death, often necessitating intensive care, and in many cases, an emergency liver transplant[17].
Data pertaining to demographic characteristics, clinical and laboratory parameters, duration of hospitalization, disease severity, and mortality were retrieved by reviewing their medical charts and electronic patient medical records. The Aga Khan University hospital is a Joint Commission International accredited hospital currently using “MypatientsAku,” a locally developed electronic medical record data base application with availability of data for all laboratory tests including radiology, access to medication records, diagnosis, details about hospital encounters, and discharge summaries, etc. This is a validated application and accessible to credentialed house staff. The records of daily follow-ups are maintained in patients’ charts.
Informed consent was not required by ethic review board since methodology involved review of patient charts. Ethics approval was obtained from Ethics Review Committee Aga Khan University Pakistan (No. 2024-9479-28215).
Data were analyzed using Statistical Package for the Social Sciences version 22 (IBM, Armonk, NY, United States). Continuous variables are presented as the mean ± standard deviation or median (minimum-maximum), while categorical variables are expressed as the frequency and percentages. The normality of quantitative data was checked using histogram and Shapiro–Wilk test. In cases where the data did not meet the assumption of normality (P < 0.05), non-parametric alternatives were employed. Specifically, for continuous variables that were not normally distributed, the Mann-Whitney U test was used in place of the independent t-test, and the Wilcoxon signed-rank test was used instead of the paired t-test. Additionally, transformations of the data or bootstrapping techniques were considered when necessary to meet normality assumptions. These approaches ensured robust analysis even in the presence of deviations from normality.
Comparative analysis was performed using the independent samples t-test, while categorical variables were analyzed using the χ² or Fischer exact test wherever appropriate. P ≤ 0.05 was considered statistically significant. The effect size estimation was done using Cohen's d test for independent samples t-test and Cramér's for χ². The Cohen’s d = 0.2 d was considered a small effect, medium effect: D = 0.5 d and large effect: D = 0.8 d. For Cramér's v = 0.1, V = 0.3 and V = 0.5 were considered as small, medium, and large effect, respectively.
A total of 396 patients fulfilled the eligibility criteria and were found to have acute hepatitis during our study. A total of 234 (59%) patients were diagnosed with acute HAV infection, 157 (39.6%) patients were found to have acute HEV infection. Additionally, HBV infection was identified as the cause in 3 patients (0.7%), whereas HCV infection was found in only 2 (0.5%) cases of acute hepatitis. No case of acute HDV infection was identified. The mean age of overall patients was 29 years ± 8 years. Overall frequencies of baseline characteristics are presented in Table 1.
| Baseline characteristics | mean ± SD |
| Age in years | 29 ± 8 |
| Male | 221 (55.8) |
| Female | 175 (44.2) |
| Hemoglobin in g/dL | 12.5 ± 2 |
| White cell count as × 109/L | 8 ± 5 |
| Platelet as × 109/L | 237 ± 127 |
| Total bilirubin in mg/dL | 9.2 ± 9 |
| Direct bilirubin in mg/dL | 7 ± 7 |
| Gamma-glutamyl transferase in IU/L | 171 ± 142 |
| Alanine aminotransferase in IU/L | 2626 ± 1925 |
| Alkaline phosphatase | 198 ± 92 |
| Aspartate aminotransferase in IU/L | 2229 ± 2036 |
| International normalized ratio | 1.6 ± 0.8 |
| Coagulopathy | 169 (42.7) |
| Acute liver failure | 32 (8.1) |
| Pregnancy | 39 (9.8) |
| Chronic liver disease | 15 (3.8) |
| Mortality | 13 (3.3) |
After excluding HBV and HCV cases from dataset, comparative analysis was done between HAV and HEV cases. The mean age of patients with HAV was 28 years ± 8 years, while those with HEV were 30 years ± 8 years with a P value of 0.017 showing that older adults were affected with HEV infection. An elevated total leukocyte count of 11 ± 7 was observed among patients with HEV, whereas individuals with HAV exhibited a comparatively lower leukocyte count of 6 ± 3, with a significant P value (P < 0.001). Total bilirubin (TB) was noted to be raised among HEV, which is 13 ± 11 while in HAV, TB was found to be 6 ± 7 with P < 0.001. Increased ALT, gamma-glutamyl transferase (GGT), and AST were seen among HAV compared with HEV. Laboratory parameters of HAV and HEV are shown in detail in Table 2.
| Baseline characteristics | Hepatitis A virus | Hepatitis E virus | Effect size | P value |
| Age in years | 28 ± 8 | 30 ± 8 | 0.23 | 0.017 |
| Sex | ||||
| Male | 124 (53) | 93 (59.2) | 0.09 | 0.223 |
| Female | 110 (47) | 64 (40.8) | ||
| Hemoglobin in g/dL | 12.8 ± 1 | 12.1 ± 2 | 0.32 | 0.002 |
| White cell count as × 109/L | 6 ± 3 | 11 ± 7 | 0.87 | < 0.001 |
| Platelet as × 109/L | 236 ± 140 | 239 ± 106 | 0.03 | 0.80 |
| Total bilirubin in mg/dL | 6 ± 7 | 13 ± 11 | 0.76 | < 0.001 |
| Direct bilirubin in mg/dL | 5 ± 5 | 10 ± 9 | 0.72 | < 0.001 |
| Gamma-glutamylcysteine in IU/L | 215 ± 138 | 110 ± 126 | 0.79 | < 0.001 |
| Alanine aminotransferase in IU/L | 3274 ± 1937 | 1760 ± 1517 | 0.87 | < 0.001 |
| Alkaline phosphatase | 181 ± 83 | 224 ± 100 | 0.46 | < 0.001 |
| Aspartate aminotransferase in IU/L | 2820 ± 2255 | 1445 ± 1338 | 0.74 | < 0.001 |
| International normalized ratio | 1.5 ± 0.7 | 1.8 ± 1 | 0.36 | < 0.001 |
| Coagulopathy | 80 (34.2) | 87 (55.4) | 0.21 | < 0.001 |
| Acute liver failure | 8 (3.4) | 24 (15.3) | 0.22 | < 0.001 |
| Pregnancy | 3 (1.3) | 36 (23) | 0.36 | < 0.001 |
| Chronic liver disease | 1 (0.4) | 13 (8.3) | 0.26 | < 0.001 |
| Mortality | 2 (0.9) | 11 (7) | 0.17 | < 0.001 |
| Hospital stay | 2.8 ± 3 | 4 ± 3.6 | 0.26 | 0.002 |
The effect size for the difference between patients with HAV vs HEV, i.e. Cohen's d ≥ 0.6 for white cell count, total and direct bilirubin, GGT, ALT, and AST indicating a moderate to large effect. This suggests that the observed difference between the two groups is not only statistically significant but also substantial in magnitude. Although the difference between HAV and HEV for hospital stay was statistically significant but the effect size for difference was small reflecting early recovering for both HAV and HAV. Likewise, a significantly higher proportion of patients with HEV infection develop coagulopathy, ALF with higher mortality but the effect size for this difference ranged between medium-small. However, the observed difference and higher proportion of pregnancy in HEV group as compared to HAV was moderate.
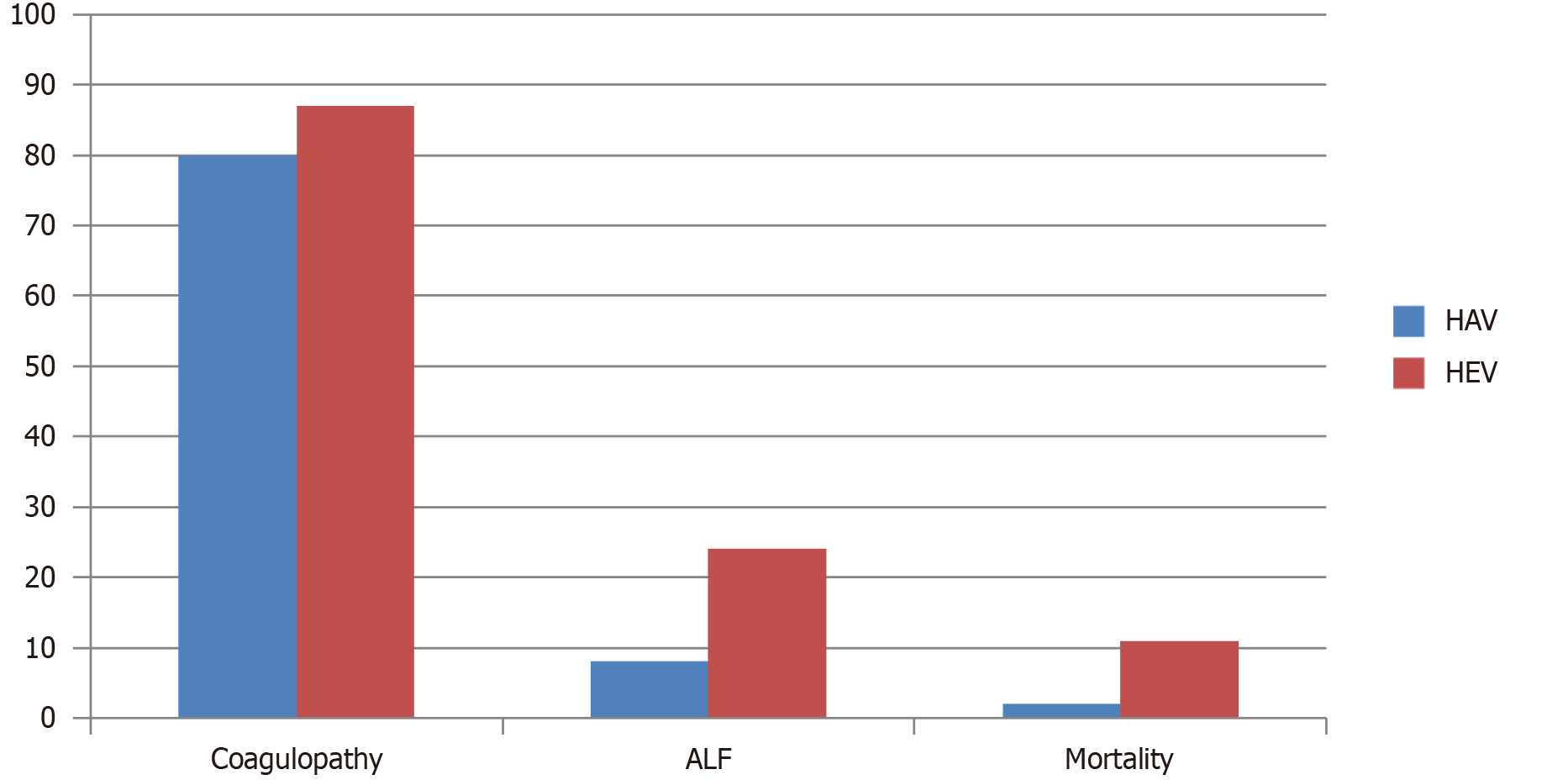
Extended hospitalization for a duration of 4 days was observed among patients diagnosed with HEV, as opposed to a 3-day stay in the HAV cohort. A total of 87 instances of coagulopathy were documented in the HEV group, while 80 cases were identified among those with HAV infection. ALF manifested in 24 patients within the HEV cohort, in contrast to a mere 8 cases observed in the HAV group. Regarding mortality, HEV resulted in 11 fatalities, contrasting with two mortalities attributed to HAV infection (Table 2). These data represent that HEV causes more severe disease and complications like coagulopathy and ALF (Figure 1).
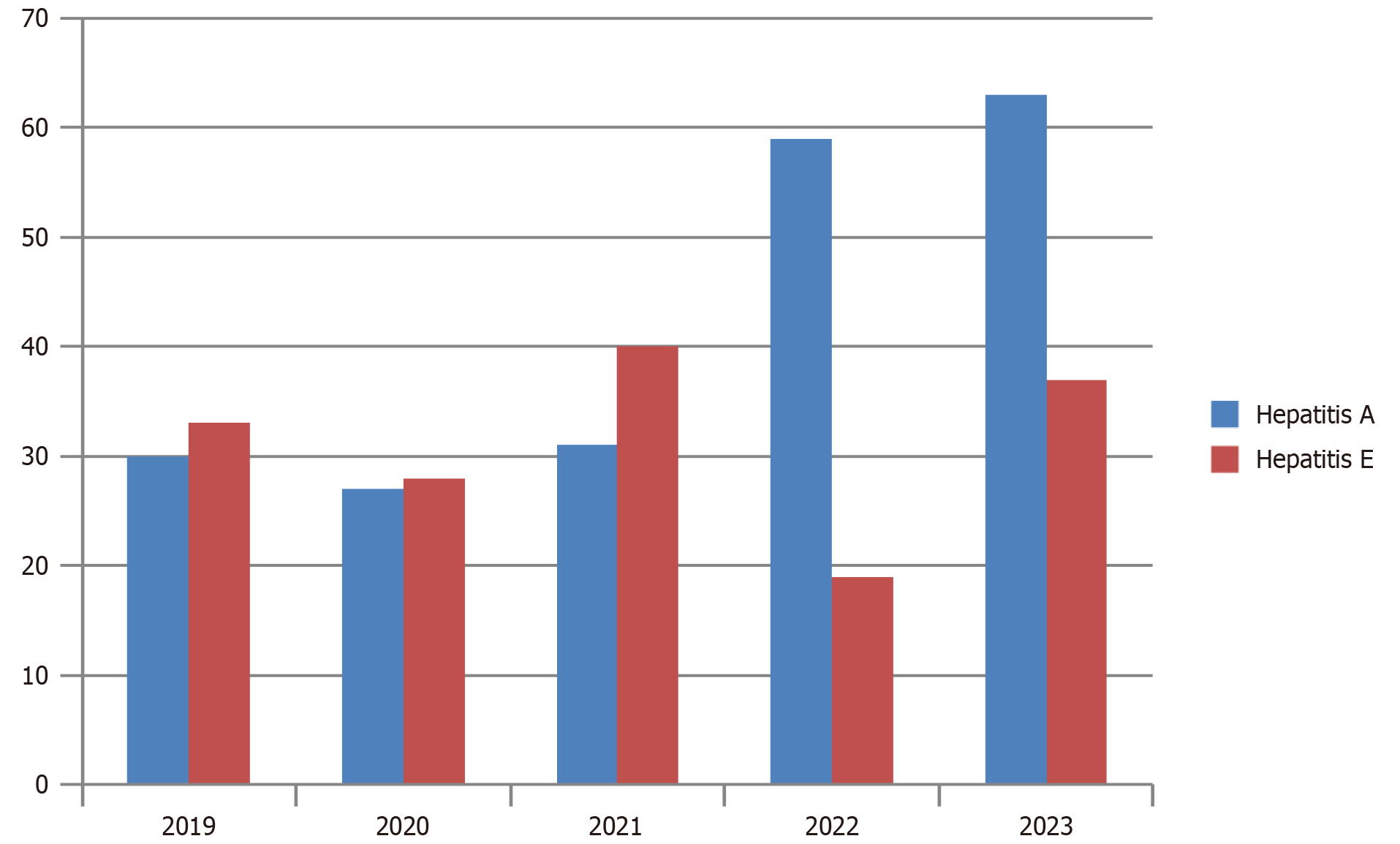
Yearly trends revealed a notable increase in the incidence of HAV, with 30 cases reported in 2019, 27 cases in 2020, 31 cases in 2021, a substantial surge to 59 cases in 2022, and a further escalation to 63 cases in 2023. By contrast, the prevalence of HEV remained comparatively lower. HEV accounted for 33 cases in 2019, 28 cases in 2020, 40 cases in 2021, experienced a decline to 19 cases in 2022, and slightly rose to 28 cases in 2023. The cases from 2024 were not included in the bar chart because the data only cover January to May. Therefore, it does not accurately represent the yearly trend for 2024 (Figure 2).
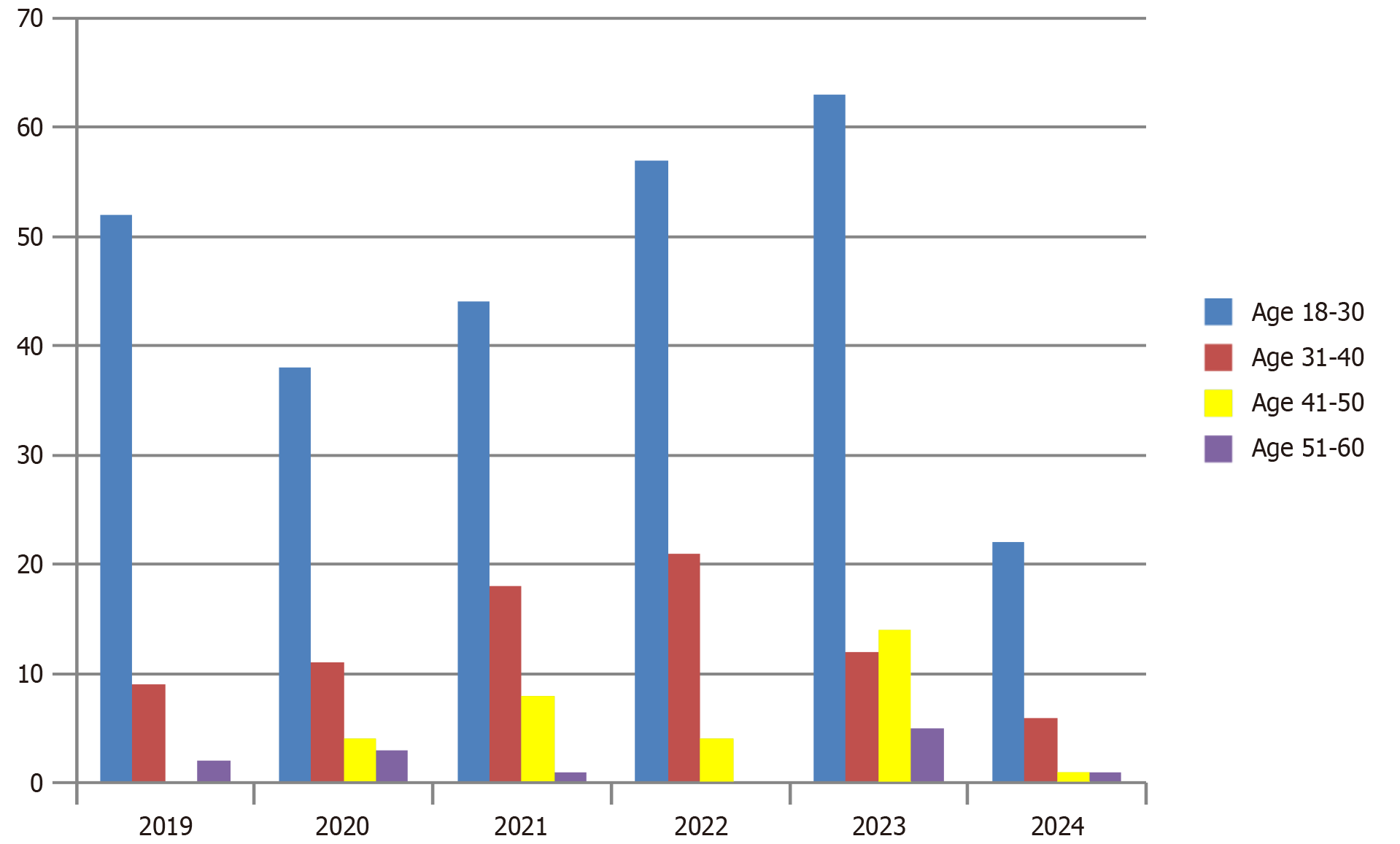
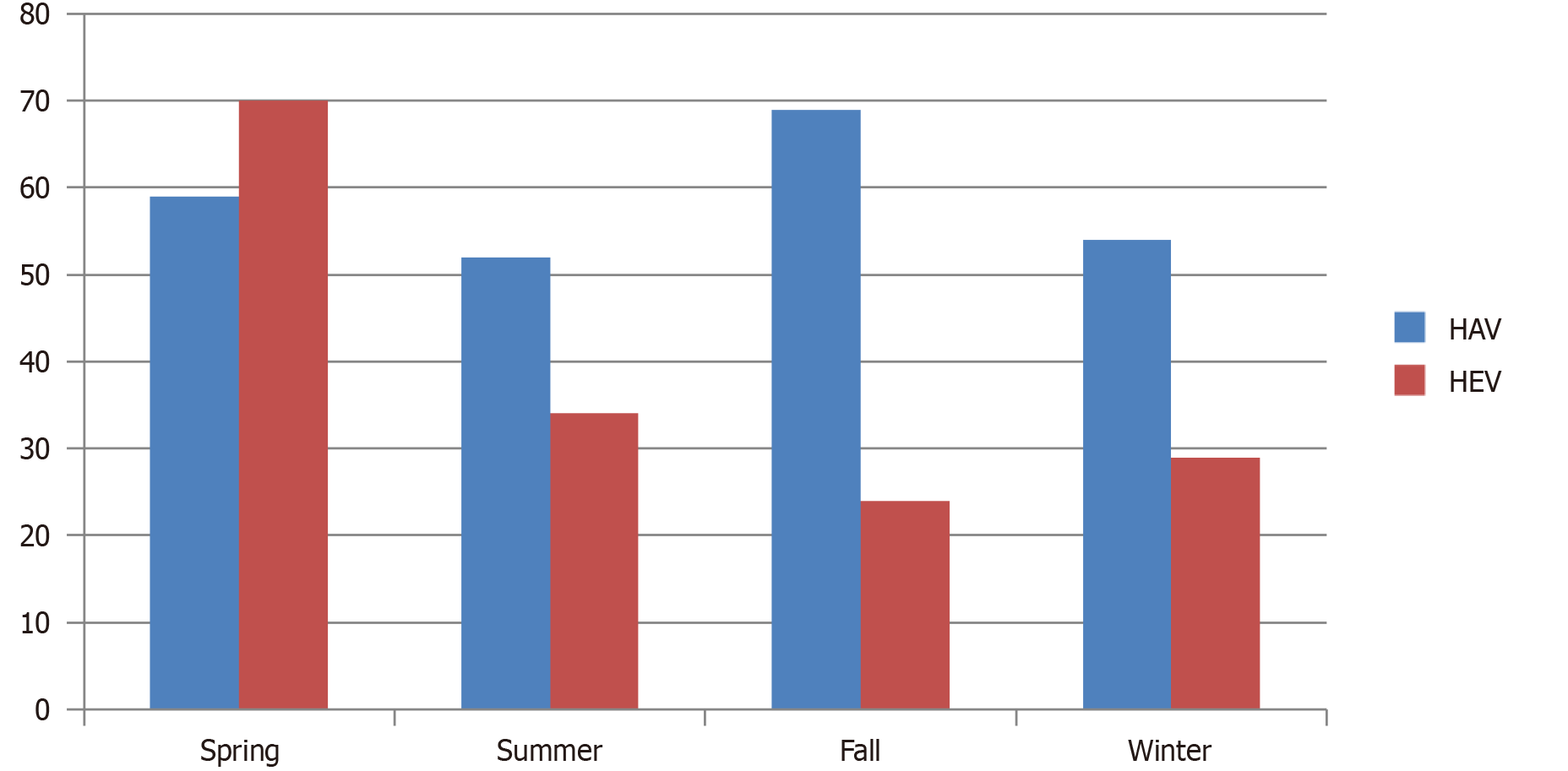
Young adults (age 18-30 years) are the most common group affected by acute hepatitis (Figure 3). HEV was more prevalent in summers and spring while HAV was seen throughout the year (Figure 4).
In this study, we evaluated clinical spectrum of acute hepatitis, changing trends of HAV occurrence and compared the disease activity of HAV and HEV, which are the most common causes of acute hepatitis worldwide[16]. Our study revealed that HAV constituted the predominant etiology of acute hepatitis among adults, accounting for 59% of cases. Furthermore, our findings indicate a notable upward trend in HAV incidence over successive years, consistent with findings reported in recent literature. For instance, a Brazilian study has evaluated temporal trend analysis on hepatitis A cases reported from 2007 to 2018, which showed a fall in the incidence of hepatitis A among people under 20 years from 2007 to 2016, whereas after 2016 a rising trend in hepatitis A was observed among males aged 20-39 years[18]. Zakaria et al[6] similarly observed a parallel trend in their study, where the prevalence of HAV cases among adults increased substantially from 2.1% in 1983 to 34% in 2002. Additionally, the incidence of non-A non-B acute hepatitis demonstrated a decline from 38.7% to 31% over the same period[6]. In our study, we meticulously examined the annual patterns of HAV and HEV from 2019 to 2023 and found similar results. Our findings revealed a consistent upward trajectory in HAV cases, escalating from 30 cases in 2019 to 85 cases in 2023. Conversely, no significant fluctuation was observed in HEV cases over the same period (Figure 2).
Overall in our study, we discovered that the most common age group affected with acute hepatitis was 18-30 years, while older adults (51-60 years) were affected more in 2023 compared with previous years (Figure 3). Sun et al[19] studied the epidemiology of hepatitis A in three different regions of China. In 2004–2007, the median ages of HAV cases were 38 years, 29 years, and 21 years in the eastern, central, and western regions, respectively. Subsequently, during 2008–2011, the median ages rose to 40 years, 36 years, and 24 years. Lastly from 2012 to 2017, the median ages further increased to 43 years, 47 years, and 33 years in the respective regions[19]. Another study from China reported a decrease in the incidence of HAV cases among individuals aged ≤ 19 years. Their study showed a declining pattern among the younger age group (< 19 years) from 1.68 cases per 100000 individuals in 2008 to 0.22 cases per 100000 individuals in 2014. They noticed an increase in the mean age during the study period from 36.8 years in 2005 to 47.2 years in 2014[20]. Globalization has also contributed to the change in the epidemiology of hepatitis A. In low socioeconomic countries, improved sanitation has reduced the incidence of HAV but high-income countries are facing foodborne outbreaks which are mainly affecting the adult population. Rural-to-urban migration is one of the most important factors that is likely contributing to this shift in epidemiology[21]. Recent research indicates that the prevalence of anti-HAV antibodies is notably lower among individuals in their twenties and has remained relatively stable over the past decade. Conversely, the seropositivity of anti-HAV among adults in their thirties has exhibited a consistent decline, dropping from 69.6% in 2005 to 32.4% in 2014. This suggests that many young adults who have not yet encountered hepatitis A and have not received vaccination are susceptible to the infection. Consequently, there is a pressing need for effective strategies to control and prevent acute hepatitis A, particularly targeting individuals in their twenties and thirties[22].
Upon comparing patients diagnosed with HAV to those with HEV, notable differences emerged in their laboratory parameters and clinical outcomes. Specifically, individuals with HAV infection exhibited elevated aminotransferase levels (ALT = 3274 U/L; P < 0.001), whereas those with HEV infection demonstrated higher levels of TB (TB = 13 mg/dL ± 11 mg/dL; P < 0.001). Moreover, the HEV group displayed a higher incidence of mortality and ALF (Figure 2). Interestingly, these findings contrast with those reported in a study conducted in Thailand, where HAV-infected patients presented with higher aminotransferase and TB levels. Additionally, both cohorts exhibited comparable outcomes in terms of mortality, ALF, and hospitalization rates[23]. In another study, patients diagnosed with acute hepatitis E displayed markedly lower median levels of ALT (798 U/L) and TB (1.8 mg/dL) compared to those with acute hepatitis A (2326 U/L, P < 0.001 and 5.2 mg/dL; P < 0.001), indicating a relatively milder form of hepatitis. These findings diverge from our study's observations[24]. Acute-on-chronic liver failure (ACLF) represents a potentially reversible syndrome that manifests in individuals with cirrhosis or underlying chronic liver disease (CLD), marked by acute decompensation, organ failure, and elevated short-term mortality rates. Notably, HAV and HEV are prominent etiological factors contributing to ACLF[25]. Our study findings highlight the distinct impact of HEV compared to HAV on ACLF development. Among the observed cases, HEV infection was associated with a higher prevalence of underlying CLD, suggesting a greater propensity for inducing ACLF compared to HAV. Furthermore, HEV infection was characterized by a more severe disease phenotype, heightened coagulopathy, prolonged hospitalization, and a predilection for affecting pregnant individuals. Conversely, HAV infection often presented as a benign, self-limiting illness in the majority of cases.
Although more mortalities were caused by HEV (n = 11; P < 0.001) compared to HAV (n = 2; P < 0.001), a considerable number of complications such as coagulopathy and ALF were associated with HAV (Figure 2). If we compare seasonal occurrence, most of the acute hepatitis cases in our cohort occurred in summers and spring, while HAV was consistent throughout the year (Figure 4). Another study from Pakistan also showed that the majority of HAV and HEV cases were seen in June to July[26]. Although evidence indicates a tendency for increased incidence during the spring and summer months, there is no definite and consistent seasonal pattern for acute viral hepatitis[27].
Over the years, frequency of HAV has been increasing among adults. Therefore it is imperative to implement routine vaccination programs and enhance sanitation and hygiene awareness, aligning with recommendations from previous studies. HAV immunization should be made mandatory for adult population to reduce its incidence[28,29]. The HAV vaccine was first approved in 1992. Both inactivated and live attenuated vaccines are highly effective and well tolerated, providing immune protection for at least 20 years. HAV vaccination is effective for both preexposure and postexposure prophylaxis, particularly for children and young adults. Vaccination strategies for HAV differ across countries and generally include targeting high-risk populations, regional childhood vaccination programs, and universal childhood vaccination. Over the past 30 years, the incidence of hepatitis A has significantly decreased in many countries. However, outbreaks still frequently occur among high-risk groups and individuals not covered by universal vaccination programs[30]. High-risk groups for hepatitis A vaccination include travelers to or workers in areas with high or intermediate infection rates, men who have sex with men, and users of illicit drugs, whether injected or not. It is also recommended for individuals with CLD, including those with HBV or HCV infections, cirrhosis, liver fibrosis, immunocompromised or those awaiting or recovering from liver transplantation. Additionally, people with clotting factor disorders and those working with HAV-infected primates or handling HAV in research laboratories should receive the vaccine[31].
It is recommended to organize public awareness programs and provide counseling to high-risk groups, particularly in low socioeconomic countries such as Pakistan. These initiatives should encourage vaccination and promote good handwashing and hygiene practices.
The limitations of this study are important to acknowledge, but each arises from practical considerations inherent to the study design. The single-center design was chosen due to logistical and resource constraints, allowing for more controlled data collection and analysis within a specific context. While this may limit generalizability, the findings still provide valuable insights within the study population, laying the groundwork for future multicenter studies that can validate and extend these results across diverse settings. Additionally, the focus on a single ethnic group was necessary to reduce variability and enhance the internal validity of the findings, though it is recognized that broader ethnic representation would increase the external relevance of the data. Lastly, the absence of seroprevalence testing for anti-HAV immunoglobulin G antibodies was due to resource limitations, but the combination of symptoms, pattern of LFTS and reactive IgM still confirms hepatitis A diagnosis. Future studies incorporating seroprevalence testing will allow for a more accurate assessment of hepatitis A incidence and burden, addressing this gap.
A rising trend has been observed in acute hepatitis due to HAV among adults. However, cases due to HAV were less severe and had lower proportion of ALF, coagulopathy and mortality than HEV. The escalating incidence of HAV among adults highlights a concerning public health challenge, potentially stemming from reduced vaccination rates in this demographic. To address this trend, it is imperative to enhance awareness regarding the importance of vaccination and promote rigorous adherence to good hygiene practices. Mitigating the risk of foodborne outbreaks necessitates prudent choices such as minimizing dining out. Furthermore, given the global implications of this epidemiological shift, further research on a broader scale is essential to elucidate and effectively address this emerging trend.
| 1. | The World Health Organization. Immunization, Vaccines and Biologicals: Hepatitis A. Available from: https://www.who.int/immunization/diseases/hepatitisA/en/. |
| 2. | Coulepis AG, Locarnini SA, Westaway EG, Tannock GA, Gust ID. Biophysical and biochemical characterization of hepatitis A virus. Intervirology. 1982;18:107-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (4)] |
| 3. | Coulepis AG, Anderson BN, Gust ID. Hepatitis A. Adv Virus Res. 1987;32:129-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Rezende G, Roque-Afonso AM, Samuel D, Gigou M, Nicand E, Ferre V, Dussaix E, Bismuth H, Féray C. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 5. | Kim YJ, Lee HS. Increasing incidence of hepatitis A in Korean adults. Intervirology. 2010;53:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Zakaria S, Fouad R, Shaker O, Zaki S, Hashem A, El-Kamary SS, Esmat G, Zakaria S. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis. 2007;44:e30-e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Palewar MS, Joshi S, Choudhary G, Das R, Sadafale A, Karyakarte R. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in patients presenting with acute viral hepatitis: A 3-year retrospective study at a tertiary care Hospital in Western India. J Family Med Prim Care. 2022;11:2437-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Webb GW, Kelly S, Dalton HR. Hepatitis A and Hepatitis E: Clinical and Epidemiological Features, Diagnosis, Treatment, and Prevention. Clin Microbiol Newsl. 2020;42:171-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 9. | Agrawal A, Singh S, Kolhapure S, Hoet B, Arankalle V, Mitra M. Increasing Burden of Hepatitis A in Adolescents and Adults and the Need for Long-Term Protection: A Review from the Indian Subcontinent. Infect Dis Ther. 2019;8:483-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Bosan A, Qureshi H, Bile KM, Ahmad I, Hafiz R. A review of hepatitis viral infections in Pakistan. J Pak Med Assoc. 2010;60:1045-1058. [PubMed] |
| 11. | Ahmed W, Qureshi H, Arif A, Alam SE. Changing trend of viral hepatitis--"A twenty one year report from Pakistan Medical Research Council Research Centre, Jinnah Postgraduate Medical Centre, Karachi". J Pak Med Assoc. 2010;60:86-89. [PubMed] |
| 12. | Waheed-uz-Zaman Tariq, Hussain AB, Hussain T, Anwar M, Ghani E, Asad-ullah. Hepatitis A virus infection -- shifting epidemiology. J Coll Physicians Surg Pak. 2006;16:15-18. [PubMed] |
| 13. | Centers for Disease Control and Prevention (CDC). Establishment of a viral hepatitis surveillance system-Pakistan, 2009-2011. MMWR Morb Mortal Wkly Rep. 2021;60:1385-1390. [PubMed] |
| 14. | Lanini S, Ustianowski A, Pisapia R, Zumla A, Ippolito G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect Dis Clin North Am. 2019;33:1045-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Gupta E, Ballani N, Kumar M, Sarin SK. Role of non-hepatotropic viruses in acute sporadic viral hepatitis and acute-on-chronic liver failure in adults. Indian J Gastroenterol. 2015;34:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Schaefer TJ, John S. Acute Hepatitis. Updated 2022 Jul 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023. [PubMed] |
| 17. | Shah NJ, Royer A, John S. Acute Liver Failure. Updated 2023 Apr 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 18. | Grandi G, Lopez LF, Burattini MN. Temporal Trends of Acute Hepatitis A in Brazil and Its Regions. Viruses. 2022;14:2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Sun XJ, Zhang GM, Zhou RJ, Zheng H, Miao N, Yin ZD, Wang FZ. Changes in the epidemiology of hepatitis A in three socio-economic regions of China, 1990-2017. Infect Dis Poverty. 2019;8:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Wang Z, Chen Y, Xie S, Lv H. Changing Epidemiological Characteristics of Hepatitis A in Zhejiang Province, China: Increased Susceptibility in Adults. PLoS One. 2016;11:e0153804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Jacobsen KH. Globalization and the Changing Epidemiology of Hepatitis A Virus. Cold Spring Harb Perspect Med. 2018;8:a031716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 22. | Kang SH, Kim MY, Baik SK. Perspectives on Acute Hepatitis A Control in Korea. J Korean Med Sci. 2019;34:e230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Khongviwatsathien S, Thaweerat W, Atthakitmongkol T, Chotiyaputta W, Tanwandee T. A Comparison of Clinical Manifestations and Outcomes between Acute Sporadic Hepatitis A and Hepatitis E Infections in Thailand. Viruses. 2023;15:1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Brehm TT, Mazaheri O, Horvatits T, Lütgehetmann M, Schulze Zur Wiesch J, Lohse AW, Polywka S, Pischke S. Lower Levels of Transaminases but Higher Levels of Serum Creatinine in Patients with Acute Hepatitis E in Comparison to Patients with Hepatitis A. Pathogens. 2021;10:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Bhatti TK, Singal AK, Kwo PY. Viral Hepatitis and Acute-on-Chronic Liver Failure. Clin Liver Dis. 2023;27:617-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Khan MA. Comparative prevalence of different types of viral hepatitis in the district Dera Ismail Khan, Khyber Pakhtunkhwa, Pakistan. Egypt Liver J. 2022;12:40. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Fares A. Seasonality of hepatitis: a review update. J Family Med Prim Care. 2015;4:96-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Tekin R, Yolbas I, Dal T, Demirpençe Ö, Kaya S, Bozkurt F, Deveci Ö, Çelen MK, Tekin A. Evaluation of adults with acute viral hepatitis a and review of the literature. Clin Ter. 2013;164:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Bane A, Sultan A, Ahmed R. Increasing Burden of Acute Hepatitis A among Ethiopian Children, Adolescents, and Young adults: A Change in Epidemiological Pattern and Need for Hepatitis A Vaccine. Ethiop J Health Sci. 2022;32:255-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Zhang L. Hepatitis A vaccination. Hum Vaccin Immunother. 2020;16:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |