Published online Mar 25, 2025. doi: 10.5501/wjv.v14.i1.100003
Revised: October 11, 2024
Accepted: November 5, 2024
Published online: March 25, 2025
Processing time: 114 Days and 20.7 Hours
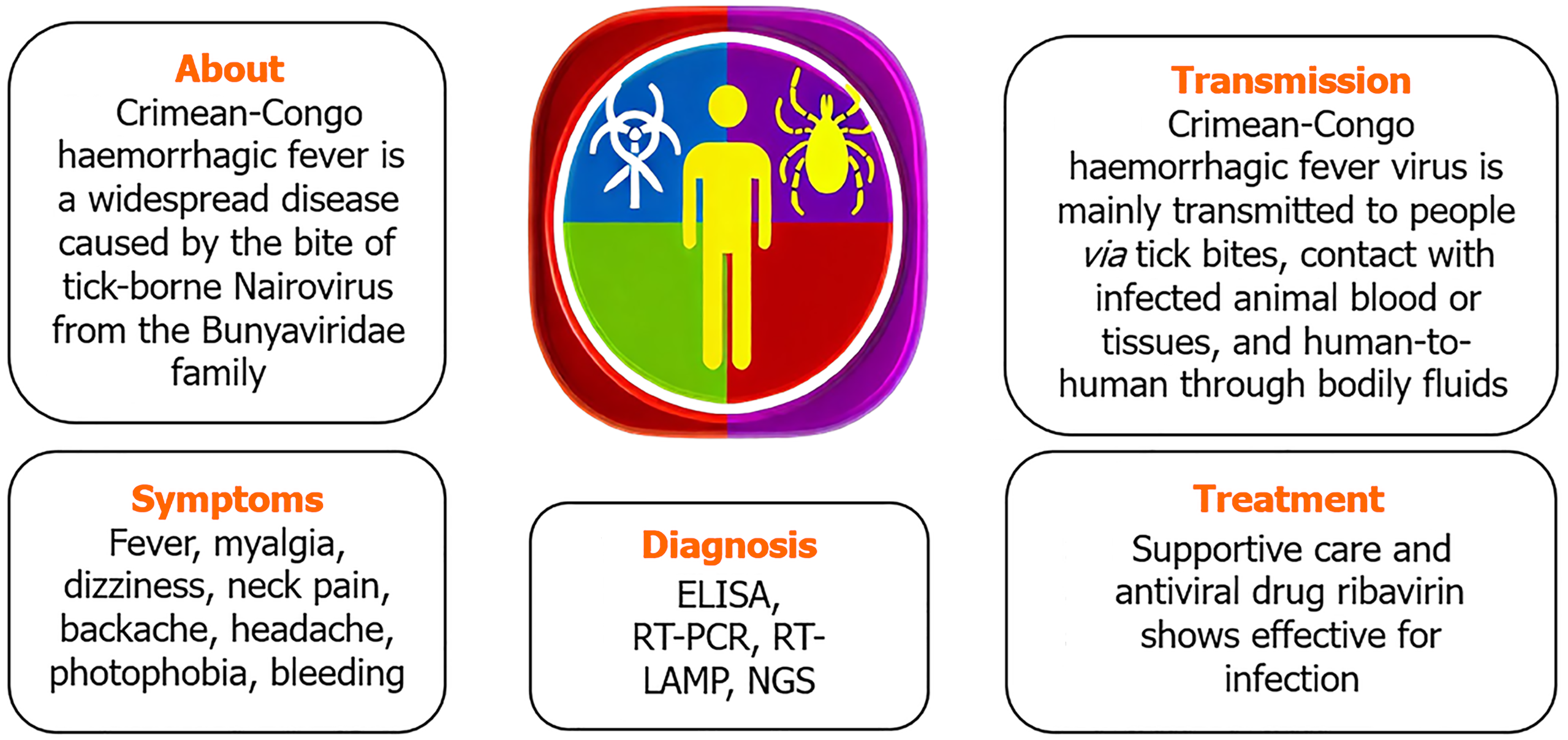
The dangerous Crimean-Congo hemorrhagic fever virus (CCHFV), an encapsu
Core Tip: This review provides a comprehensive overview of Crimean-Congo hemorrhagic fever (CCHF), a severe tick-borne viral disease with significant public health implications. The article discusses the virus's transmission dynamics, clinical manifestations, current diagnostic techniques, and available treatments, including the use of antiviral therapy. It emphasizes the urgent need for vaccine development, better diagnostic tools, and efficient therapies. By addressing gaps in knowledge and highlighting the importance of a one health approach, this review serves as a critical resource for researchers and healthcare professionals seeking to improve CCHF control and prevention strategies.
- Citation: Karanam SK, Nagvishnu K, Uppala PK, Edhi S, Varri SR. Crimean-Congo hemorrhagic fever: Pathogenesis, transmission and public health challenges. World J Virol 2025; 14(1): 100003
- URL: https://www.wjgnet.com/2220-3249/full/v14/i1/100003.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i1.100003
Between 10%-40% of infected people die during Crimean-Congo hemorrhagic fever virus (CCHFV) outbreaks of severe viral hemorrhagic fever. As a febrile sickness, the disease was first known as Crimean haemorrhagic fever when it was initially discovered in 1944 among troops on the Crimean Peninsula (near the Black Sea)[1]. The present name of the disease was given to it in 1969 when the same bacterium was identified as the cause of febrile sickness in the Congo Basin. The main vector and reservoir of CCHFV are ticks of the species Hyalomma[2]. The species of Hyalomma tick that causes Crimean-Congo hemorrhagic fever (CCHF) depends on the geographic region. Hyalomma marginatum is the primary vector of CCHF in Europe and the Balkans where as Hyalomma anatolicum in Iran, Pakistan, Turkmenistan, and Tajikistan, Hyalomma asiaticum in Central Asia and China and Hyalomma rufipes in Africa.
Many non-domesticated animal species, including ostriches, buffalo, tiny rodents, hares, and rhinoceroses, may be infected with CCHFV, according to serological investigations. Important amplifying hosts for the transmission of tick-borne diseases include animals that ingest infected ticks or co-feed with them[3]. Tick bites or coming into contact with sick animals during slaughter are the most common ways for humans to get the disease. In humans, an infection usually manifests as a fever, which may later develop into a hemorrhagic condition and could be deadly. Particularly in areas without access to high-containment facilities, molecular testing have allowed for safe and quick diagnosis. Sheep, goats, and cattle are just a few of the many domestic and wild animal species that may harbour the CCHFV[4].
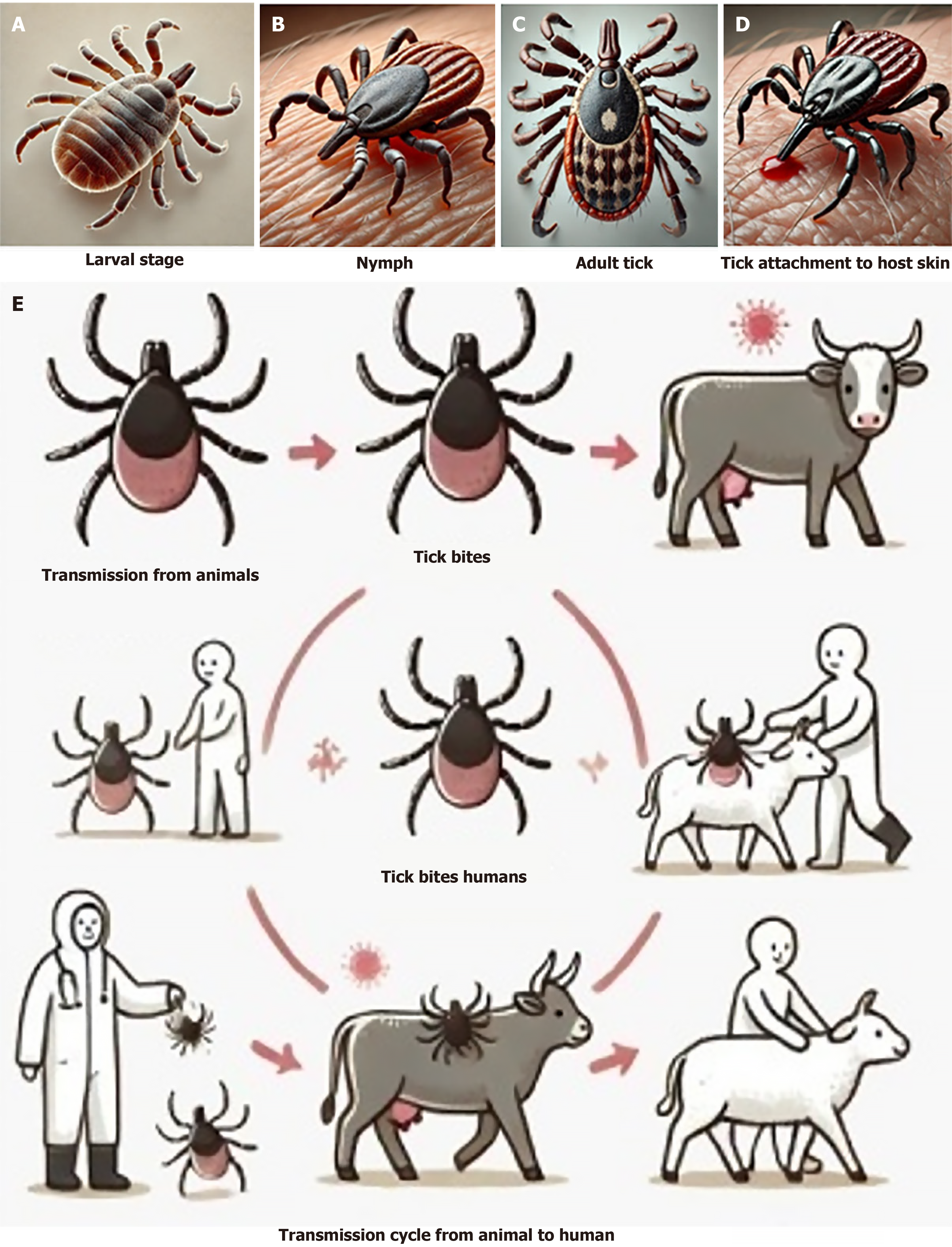
Although most birds can withstand infections, ostriches aren't immune and may have a high infection rate in regions where it is common. The tick-borne disease stays in an infected animal's bloodstream for around seven days after infection[5]. When another tick bites the sick animal, the tick-animal-tick cycle might resume. Hyalomma ticks are the most common vectors of the CCHFV (Figure 1), however infections may occur in other tick genera as well.
Although CCHF is common in many regions of the globe, including the Indian subcontinent, northwest China, Africa, the Balkans, and Eastern Europe, there is no vaccine that can protect people or animals against this disease at this time[6].
The four families of viruses known to cause viral hemorrhagic fevers are Arenaviridae, Bunyaviridae, Filoviridae, and Flaviviridae which cause severe systemic febrile diseases (Table 1). The arenaviridae family of viruses includes both Old World and New World Strains, and they are known to cause infections transmitted by rodents. Europe, Asia, and Africa are just a few of the numerous regions where rats are susceptible to viral infections[7]. Urine or droppings from rodents are a common vector for infection. A 50% case mortality rate has been reported in West African epidemics caused by the arenavirus Lassa. Insects and rodents may transmit viruses that belong to the Bunyaviridae family, which can cause mild to severe illness[8]. Rift Valley fever, hemorrhagic fever in the Crimean-Congo region, and hantavirus infections are among the most prominent ailments. The Ixodid tick is the vector for the virus. The family Filoviridae includes the Ebola virus and Marburg hemorrhagic sickness, both of which have been found in African bats. The danger of transmission from person to person is considerable for human infections, particularly among those in care takers[9]. In low-income nations, the case fatality rate for Marburg hemorrhagic fever may reach 82%, while in the Democratic Republic of Congo, it can reach 80% to 90%. Various forms of infections are carried by the flavivirus family, which is carried by arthropods[10]. Dengue fever affects more than a hundred nations throughout Europe, Asia, Australia, Africa, and the Pacific Islands. It is a global health crisis. The mosquitoes Aedes aegypti and Aedes albopictus are vectors for the flavivirus that causes dengue fever. The disease can progress through three stages: (1) Mild; (2) Moderate; and (3) Severe[11].
| Family | Causative virus | Disease | Symptoms | Treatment |
| Arenaviridae | Lassa virus | Lassa fever | Fever, weakness, and haemorrhage | Supportive care and ribavirin |
| Junin virus | Argentine haemorrhagic fever | Fever, malaise, and haemorrhage | Supportive care | |
| Chapare virus | Chapare hemorrhagic fever | Fever, malaise, headache, vomiting and diarrhoea | Supportive care and early diagnosis | |
| Guanarito virus | Venezuelan hemorrhagic fever | Confusion, convulsions, coma, and bleeding from body orifices | No specific anti-viral treatment is available | |
| Lujo virus | Lujo hemorrhagic fever | Fever, headache, vomiting, diarrhea, arthralgia, and myalgia | Supportive care | |
| Lymphocytic choriomeningitis virus | Lymphocytic choriomeningitis | Fever (38.5 °C to 40 °C), malaise, myalgia, retro-orbital headache, photophobia, and anorexia | Supportive care and ribavirin | |
| Machupo virus | Bolivian hemorrhagic fever | Fever, malaise, fatigue headache, dizziness, myalgias, and severe lower back pain | Supportive care | |
| Sabia virus | Brazilian hemorrhagic fever | High fever, fatigue, maculopapular/petechial rash bleeding and haemorrhage | Supportive care and ribavirin antiviral drug | |
| Bunyaviridae | Crimean-Congo haemorrhagic fever virus | Crimean-Congo haemorrhagic fever | Fever, myalgia, and haemorrhage | Supportive care and ribavirin |
| Hantan virus | Hantavirus pulmonary syndrome | Fever, muscle pain, and pulmonary edema | Supportive care | |
| Dobrava-Belgrade virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, and blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin | |
| Seoul virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, and blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin | |
| Puumalavirus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, and blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin | |
| Rift Valley fever virus | Rift Valley fever | Transient fever, headache, severe muscle and joint pain, photophobia and anorexia | Drugs like Ibuprofen or Acetaminophen | |
| Saaremaa virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, and blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin | |
| Sin nombre virus | Hantavirus pulmonary syndrome | Fever, muscle pain, and pulmonary edema | Intubation and oxygen therapy, fluid replacement and use of medications to support blood pressure | |
| Severe fever and thrombocytopenia syndrome virus | Severe fever and thrombocytopenia syndrome | Fever, vomiting, diarrhoea, multiple organ failure, thrombocytopenia, and leucopoenia elevated liver enzyme levels | Intravenous ribavirin | |
| Tula virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, and blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin | |
| Filoviridae | Bundibugyo ebola virus | Ebola virus disease | Fever, severe hemorrhage, and organ failure | Supportive care and experimental treatments |
| Marburg marburg virus | Marburg haemorrhagic fever | Fever, severe hemorrhage, and organ failure | Supportive care and experimental treatments | |
| Sudan ebola virus | Ebola virus disease | Sudden onset of fever, fatigue, muscle pain, headaches, sore throat, vomiting, diarrhoea, rash, impaired kidney, and liver functions | Monoclonal antibodies like Inmazeb and Ebanga | |
| Tai forest ebola virus | Ebola virus disease | Sudden onset of fever, fatigue, muscle pain, headaches, sore throat, vomiting, diarrhoea, rash, impaired kidney, and liver | Monoclonal antibodies like Inmazeb and Ebanga | |
| Zaire ebola virus | Ebola virus disease | Sudden onset of fever, fatigue, muscle pain, headaches, sore throat, vomiting, diarrhoea, rash, impaired kidney, and liver functions | Monoclonal antibodies like Inmazeb and Ebanga | |
| Flaviviridae | Dengue virus | Dengue fever | Fever, rash, and haemorrhage | Supportive care and fluids |
| Kyasanur forest disease virus | Kyasanur forest disease | Sudden onset of chills, fever, and headache | Supportive treatment with maintenance of proper hydration and circulation by transfusion of IV fluids | |
| Omsk hemorrhagic fever virus | Omsk hemorrhagic fever | Fever, headache, myalgia, cough, petechial rash or bruises | Supportive care | |
| Yellow fever virus | Yellow fever | Fever, chills, headache, back pain, vomiting, and fatigue | Rest, hydration and seek medical advice |
Tick bites or coming into touch with infected animal blood or tissues during or just after slaughter are the main routes of transmission for the CCHF virus to humans”. Direct contact with infected blood, saliva, organs, or other bodily fluids is the most typical way infectious illnesses are transmitted from one person to another (Figure 2)[12]. Additional factors that may lead to nosocomial infections include reusing needles, contaminated medical supplies, and insufficient sterilisation of medical equipment[13].
On June 29, a male patient suffering from CCHF passed away in a private hospital in Ahmedabad. He was 51 years old and lived in the hamlet of Lakhapar in the Anjar taluka of Kutch. According to health officials, this is the first case of CCHF that has been documented in Gujarat this year. The state of Gujarat recorded five instances of confirmed CCHF in 2022. Since 2011, when the state first recorded a case of CCHF, Gujarat has been the reporting centre for the vast majority of CCHF cases in India[14]. Gujarat reported verified cases of CCHF from 2011 to 2019, with Rajasthan reporting extra cases in 2014, 2015, and 2019. A 39-year-old guy who survived after testing positive for the virus in March 2022 was a cattle rearer. One of them was a 55-year-old housewife who died of CCHF after a tick bit her while she was tending to her cattle. One case of CCHF was recorded in the Sabarkantha district in 2021 by the state[15].
There were 494 CCHF cases (115 fatal) recorded in Africa between January 1, 1956, and July 25, 2020. Over the last decade, nine nations Kenya, Mali, Mozambique, Nigeria, Senegal, Sierra Leone, South Sudan, Sudan, and Tunisia have reported the first cases of CCHF[16].
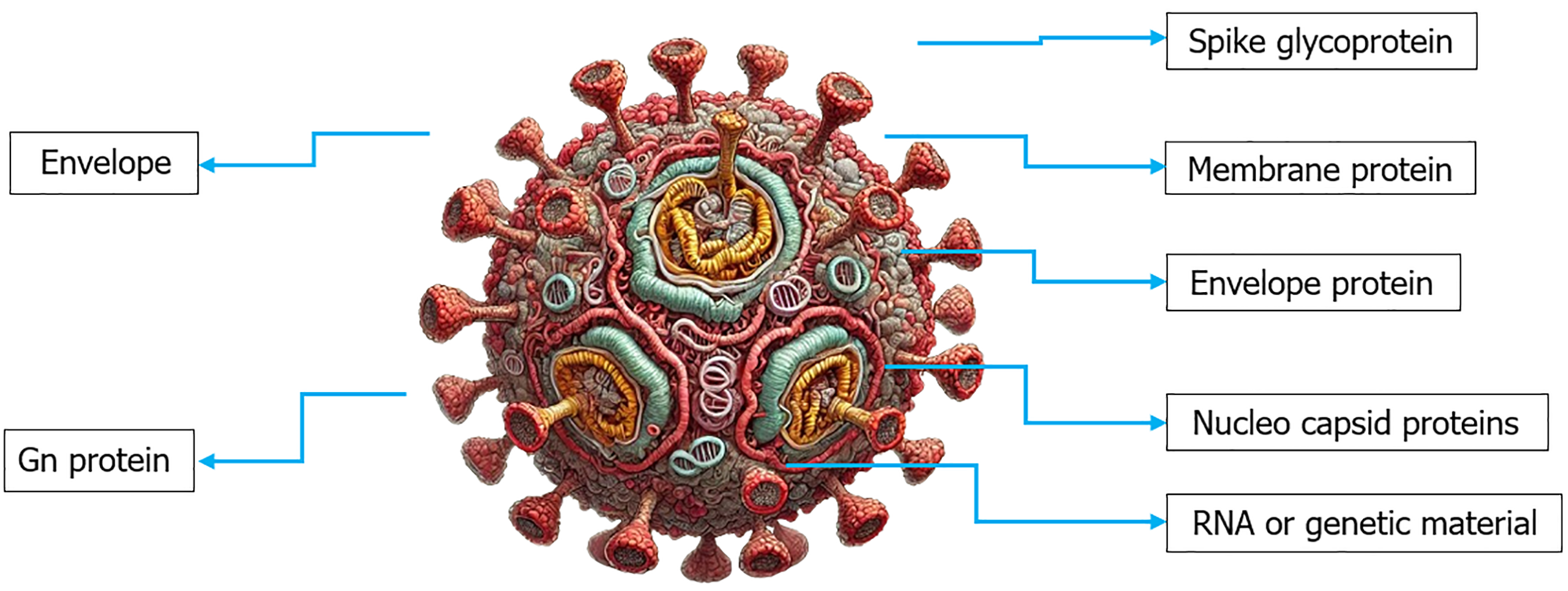
Within the Bunyavirales order, the Nairoviridae family, and the Orthonairo virus genus, there will be enveloped, negative-sense RNA virus known as CCHFV. One crucial component of the virus is the envelope Gn glycoprotein, which, at its C-terminus, possesses a cytoplasmic tail. The genetic code of CCHFV consists of S, M, and L segments of RNA. Segment L encodes an RNA-dependent RNA polymerase, segment M viral glycoproteins, and segment S the nucleocapsid protein (N) (Figure 3)[17].
The duration of incubation is usually less than a week, falling anywhere from one to nine days (depending on the way the virus was exposed to and the dosage). It lasts the shortest time after a tick bite (about 1-3 days) and the longest time after coming into contact with contaminated human or cattle blood, tissue, or secretions (5-6 days).
Begins suddenly with vague symptoms and lasts an average of two to four days (range: One to seven days). Signs and symptoms may manifest as a high temperature (39–41 °C), neck discomfort or stiffness, dizziness, headache, myalgia, backache, eye pain, or photophobia. Nausea, vomiting, diarrhoea, stomach ache, and a sore throat are possible side effects. Jaundice, conjunctivitis, congested sclera, and hyperaemia of the chest, neck, and face are some possible symptoms. Hepatomegaly and splenomegaly, as well as changes in mood and sensory perception (such as somnolence supplanting agitation), may be seen in extreme instances.
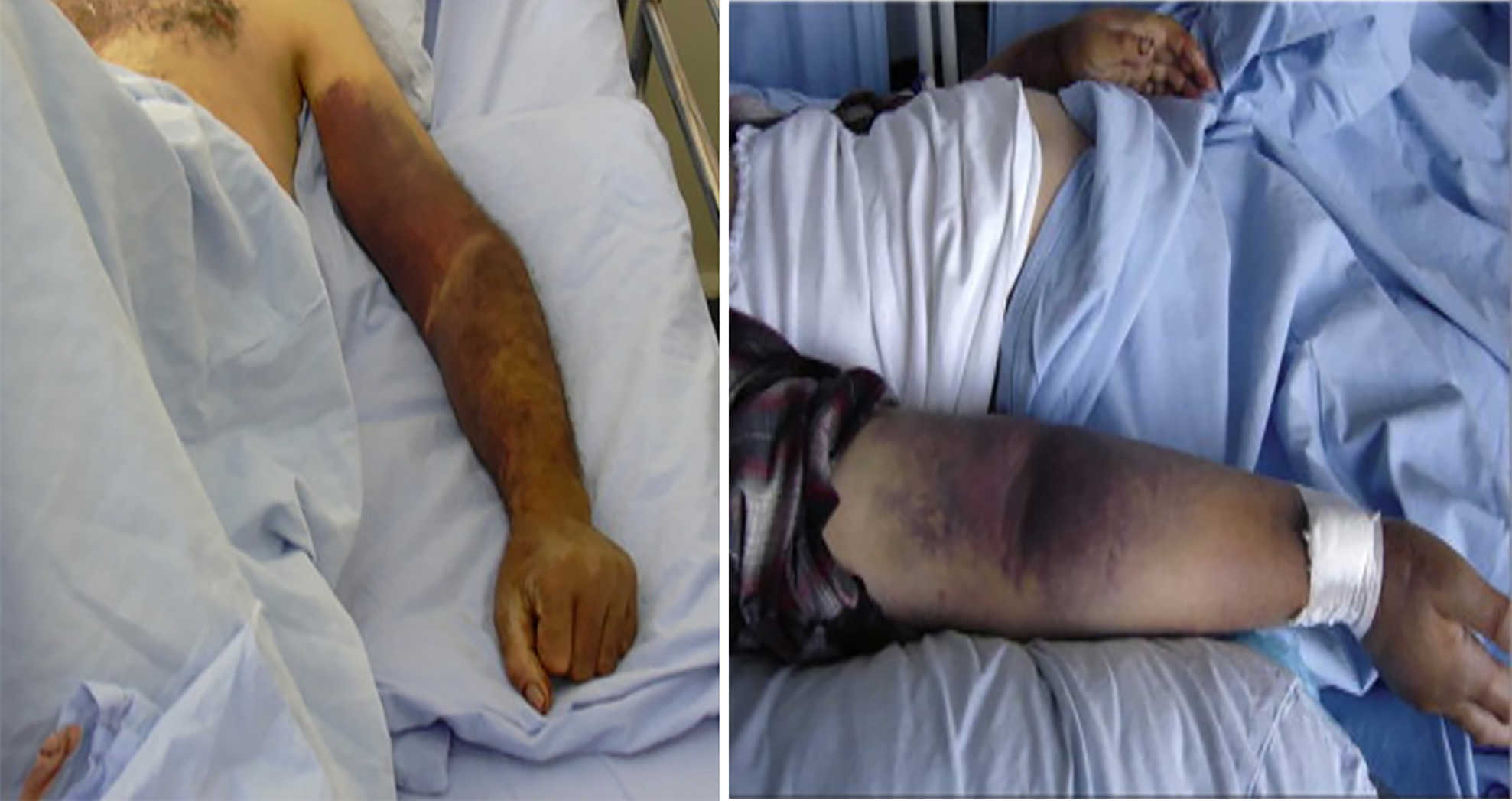
Usually brief (around two or three days), but may last up to two weeks. One distinctive aspect of CCHF compared to other viral haemorrhagic fevers is the wide variety of hemorrhagic symptoms it may cause, which can vary from little bleeding patches (peteziae) to widespread discolouration of the skin (ecchymosis) on both the skin and mucosal membranes. Some common symptoms may include injection site bleeding, epistaxis, melena, haematuria, haemoptysis, and haematemesis.
Within 9–10 days after the beginning of the disease (within a range of 9–20 days), convalescence often occurs in survivors, in the same vein as when laboratory measurements were back to normal. Symptoms such as hypotension, bradycardia or tachycardia, polyneuritis, difficulty breathing, xerostomia, impaired vision or hearing, hair loss, memory loss, and other difficulties might manifest during this protracted period. Although long-term consequences have not been thoroughly investigated, there is no solid proof of recurrence or a biphasic progression of the illness[18-21].
Depending on how the virus is acquired, the incubation time for CCHF might vary in duration. The incubation phase, which begins three to nine days following a tick bite, is when the disease is usually spread. Contact with infected blood or tissues may spread viruses, and the incubation period for these viruses can be anywhere from 5 days to 6 days, up to 13 days[22]. A broad variety of symptoms, including myalgia, vertigo, headache, neck pain, backache, and photophobia, all manifest suddenly. Petechiae, or red patches on the palate, red eyes, heated cheeks, and a red throat are other common early signs (Figure 4). In the beginning, you can have a sore throat, nausea, vomiting, diarrhoea, stomach ache, and confusion[23]. In the two to four days after the start of symptoms, restlessness, melancholy, and lassitude may take the place of agitation. Additionally, the stomach discomfort may shift to the upper right quadrant and be accompanied by noticeable hepatomegaly. About 30% of those with CCHF will pass away throughout the course of their disease, usually during the second week. On the ninth or tenth day after the start of symptoms, individuals who are able to recover often start to feel better. Symptoms such as extensive bruising, nosebleeds, and uncontrolled bleeding at injection sites become apparent on day four of sickness and last for around two weeks. The reported mortality rates of CCHF patients in hospitals range from 9% to 50%[24].
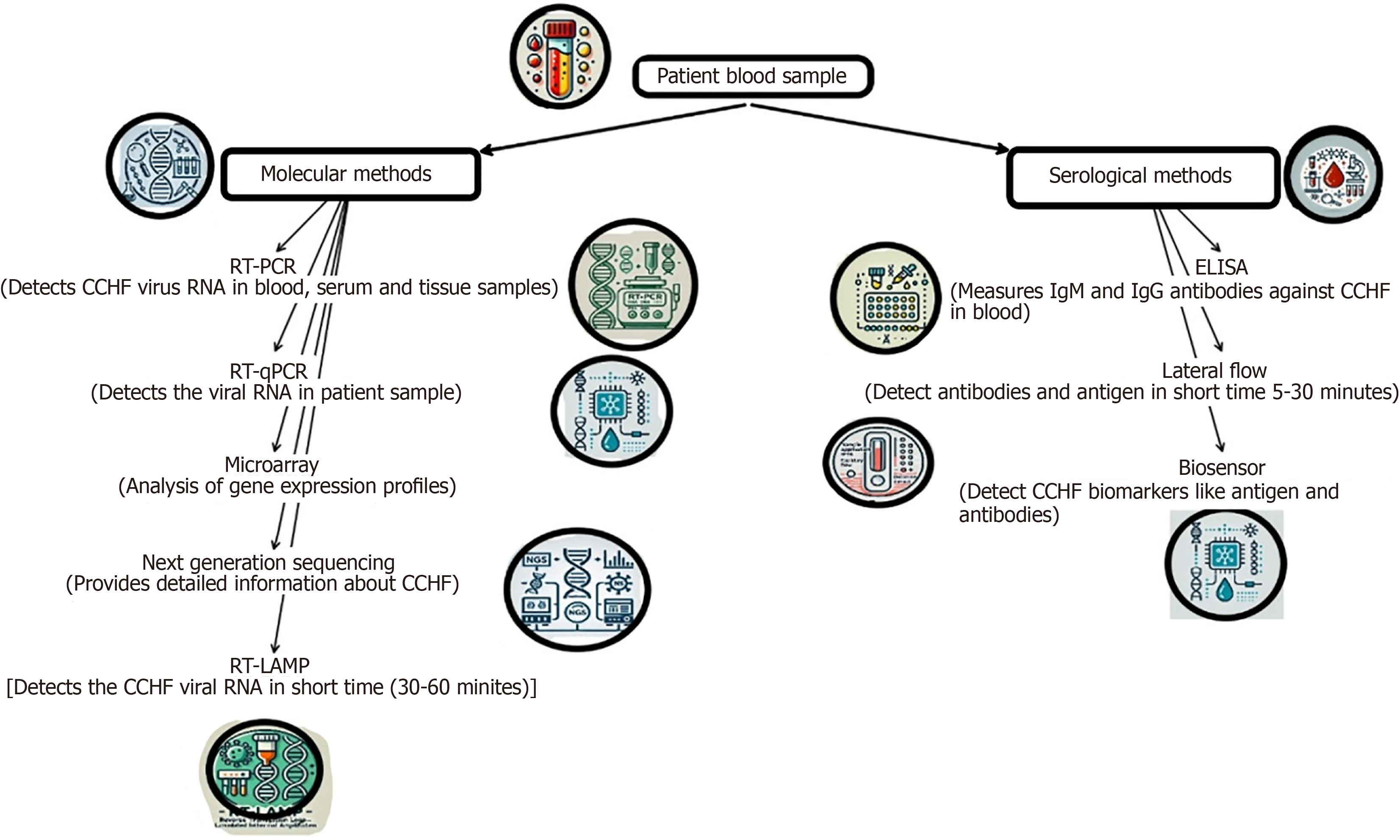
A numberof laboratory techniques may be used to diagnose CCHF. By integrating enzyme-linked immunosorbent assay antigen capture for viral antigen detection with RT-PCR in blood or tissues collected from a dead patient and virus isolation, it is feasible to detect CCHF in its acute phase in individuals with a proper medical history. Tissues treated with formalin may also be stained immune-histo-chemically to reveal viral antigens (Figure 5)[25].
The main strategy is to provide general supportive care with an emphasis on symptom treatment. Both the oral and injectable forms of the antiviral medication ribavirin have shown efficacy in treating CCHF infection. Fluid balance, electrolyte imbalances, oxygenation, haemodynamic support, and secondary infection therapy should all be part of the supportive care plan[26]. At this time, neither humans nor animals have access to any immunizations that have proven successful. Without a vaccine, informing the public about the virus and its dangers and encouraging them to take precautions against exposure is the only method to lessen the likelihood of infection. Everyone who works with animals or in agriculture should wear insect repellent and stay away from potentially infectious blood and other body fluids[27]. Control and prevention The Unnoticed tick-animal-tick cycle poses a significant challenge to tick-borne disease prevention and management. Ticks are abundant, and only properly supervised livestock farms are allowed to use acaricides. There is now no widely accessible, safe, and effective vaccination against CCHF for humans, despite the development and limited use of an inactivated vaccine produced from the mouse brain in Eastern Europe[28]. Public health advice several factors should be at the centre of public health recommendations.
Reducing the risk of tick-to-human transmission: Put on protective clothing, such as long pants and sleeves. To make ticks easier to see, dress in bright colours. Approved acaricides should be applied on garments. To protect one's skin and clothes, use an authorised repellant. Keep an eye out for ticks on a frequent basis, and gently remove them if you discover any. Get out of areas where ticks are common and stay away from areas where ticks are active.
Reducing the risk of animal-to-human transmission: When dealing with animals or their tissues in endemic regions, it is important to use protective clothing, such as gloves, whether you are in an abattoir or at home. This is particularly true while butchering, culling or slaughtering. Either regularly treat animals with pesticides two weeks before slaughter or quarantine them before they reach slaughterhouses.
Reducing the risk of human-to-human transmission in the community: Avoid physical contact with someone who seems to be sick with CCHF. When caring for sick persons, always wear protective clothing, including gloves. After touching or visiting someone who is sick, be sure to wash your hands often.
Controlling infection in health-care workers: All healthcare personnel who come into contact with patients who have CCHF, whether it's suspected or proven, or who handle specimens from these patients, should follow basic procedures for infection control. Basic hand hygiene, personal protective equipment usage, safe injection procedures, and proper burial procedures are all included in this. Samples collected from patients suspected of having CCHF should only be handled by qualified personnel in labs with the proper equipment[29-31].
Health concept: A health concept, emphasizing the interconnectedness of human, animal, and environmental health in the context of CCHF. The zoonotic nature of CCHFV and the role of livestock, wildlife, and tick habitats in the transmission cycle make this approach particularly relevant. By addressing these links, the review highlights the importance of integrated efforts across human, veterinary, and environmental health sectors to effectively manage and control the disease.
Conclusion despite the vast number of individuals who could be affected and the virus's extensive circulation, much remains unclear about the viral and host variables that contribute to CCHFV pathogenesis. We will gain greater mechanistic insights into the mechanisms by which CCHFV causes illness when molecular virology techniques and better small-animal models are developed. It is probable that novel viral protein functions will yet be found. Educating the public, limiting tick contact, treating livestock to minimise infestations, quarantining animals, and safeguarding people involved in high-risk activities are all necessary preventative actions for communities at risk in endemic regions. Limiting the impact of CCHF on patients and public health systems requires effective vaccinations and antivirals, as well as prompt and accurate diagnostics. Future perspective research one hope for the future of CCHF therapy is the availability of immunoglobulin products and other alternative medicines. In order to create targeted treatments, researchers need a deeper knowledge of the CCHF pathophysiology. Developing a vaccine against CCHF is an important objective, despite the fact that it is difficult and is not nearing practical application. People in endemic locations are eagerly awaiting the development of a CCHF vaccination since it is the most effective way to decrease the mortality and morbidity caused by CCHF. This review discussed not only the development of new antiviral drugs and vaccines but also basic research aimed at better understanding the mechanisms of the virus, improving tick control strategies, and addressing the human-animal-environment interface. The review aims to serve as a foundation for future studies and interventions in these critical areas.
I acknowledgement my sincere thanks to Maharajah’s College of Pharmacy, Vizianagaram for continuous support and cooperation for completion of this work.
| 1. | Huang HF, Huang I, Matschke J. Treatment strategy for venous congestion in digit replantations. J Plast Reconstr Aesthet Surg. 2023;83:80-83. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Ergonul O, Whitehouse CA. Crimean-Congo Hemorrhagic Fever. Germany: Springer, 2007. [DOI] [Full Text] |
| 3. | Ono K, Kuroda H. [Pannus Formation Two Years after Bioprosthetic Aortic Valve Implantation;Report of a Case]. Kyobu Geka. 2015;68:785-787. [PubMed] |
| 4. | Akıncı E, Bodur H, Leblebicioglu H. Pathogenesis of Crimean-Congo hemorrhagic fever. Vector Borne Zoonotic Dis. 2013;13:429-437. [RCA] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Bains RS, Wells S, Sillito RR, Armstrong JD, Cater HL, Banks G, Nolan PM. Assessing mouse behaviour throughout the light/dark cycle using automated in-cage analysis tools. J Neurosci Methods. 2018;300:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64:145-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 463] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 7. | Frank MG, Weaver G, Raabe V; State of the Clinical Science Working Group of the National Emerging Pathogens Training and Education Center’s Special Pathogens Research Network. Crimean Congo Hemorrhagic Fever Virus for Clinicians-Virology, Pathogenesis, and Pathology. Emerg Infect Dis. 2024;30:847-853. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Vorou R, Pierroutsakos IN, Maltezou HC. Crimean-Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Verreault J, Villa RA, Gabrielsen GW, Skaare JU, Letcher RJ. Maternal transfer of organohalogen contaminants and metabolites to eggs of Arctic-breeding glaucous gulls. Environ Pollut. 2006;144:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Chiappelli F, Fotovat L. Post acute CoViD-19 syndrome (PACS)-Long CoViD. Bioinformation. 2022;18:908-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 11. | Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. Report of two cases. Dermatologica. 1978;156:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | History of sports medicine in The Netherlands. Br J Sports Med. 1989;23:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Martin PA, Robins HI, Dennis WH. Monitoring body site temperatures during systemic hyperthermia. Crit Care Med. 1987;15:163-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Ahmed AM, Miyoshi SI, Shinoda S, Shimamoto T. Molecular characterization of a multidrug-resistant strain of enteroinvasive Escherichia coli O164 isolated in Japan. J Med Microbiol. 2005;54:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Lietard J, Abou Assi H, Gómez-Pinto I, González C, Somoza MM, Damha MJ. Mapping the affinity landscape of Thrombin-binding aptamers on 2΄F-ANA/DNA chimeric G-Quadruplex microarrays. Nucleic Acids Res. 2017;45:1619-1632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Ergönül O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 706] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 17. | Yan C, Li HR, Chen X, Zhang XQ, Cheng XB, Xu R, Huang JQ, Zhang Q. Regulating the Inner Helmholtz Plane for Stable Solid Electrolyte Interphase on Lithium Metal Anodes. J Am Chem Soc. 2019;141:9422-9429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 18. | Marín-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res. 2012;227:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Geier DA, Kern JK, Geier MR. Increased risk for an atypical autism diagnosis following Thimerosal-containing vaccine exposure in the United States: A prospective longitudinal case-control study in the Vaccine Safety Datalink. J Trace Elem Med Biol. 2017;42:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Shi L, Li Z, Chen M, Zhu T, Wu L. Ultrasensitive and Ultraprecise Pressure Sensors for Soft Systems. Adv Mater. 2023;35:e2210091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Atif QAA. An audit of operative notes in general surgery at Pakistan Institute of Medical Sciences (P.I.M.S.), Pakistan. Do we follow the Royal College of Surgeons (England) guidelines? J Pak Med Assoc. 2020;70:491-493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Travassos TC, De Oliveira JMI, Selegatto IB, Reis LO. COVID-19 impact on bladder cancer-orientations for diagnosing, decision making, and treatment. Am J Clin Exp Urol. 2021;9:132-139. [PubMed] |
| 23. | Xu C, Li H, Zhang K, Binzel DW, Yin H, Chiu W, Guo P. Photo-controlled release of paclitaxel and model drugs from RNA pyramids. Nano Res. 2019;12:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Pasteur Institute. Crimean-Congo Hemorrhagic Fever. Available from: https://www.pasteur.fr/en/medical-center/disease-sheets/crimean-congo-hemorrhagic-fever. |
| 25. | Leblebicioglu A, Eroglu O, Ergonul O. Crimean-Congo Hemorrhagic Fever: A Systematic Review and Meta-Analysis of the Global Distribution of the Virus. J Infect Dev Ctries. 2017;11:359-367. |
| 26. | Keshtkar-Jahromi M, Kuhn JH, Christova I, Bradfute SB, Jahrling PB, Bavari S. Crimean-Congo hemorrhagic fever: Current and future prospects of vaccines and therapies. Antivir Res. 2011;90:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 27. | Al-Abri SS, Abaidani IA, Fazlalipour M, Mostafavi E, Leblebicioglu H, Pshenichnaya N, Memish ZA, Hewson R, Petersen E, Mala P, Nhu Nguyen TM, Rahman Malik M, Formenty P, Jeffries R. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization Eastern Mediterranean Region: issues, challenges, and future directions. Int J Infect Dis. 2017;58:82-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | Tabassum S, Naeem A, Khan MZ, Mumtaz N, Gill S, Ohadi L. Crimean-Congo hemorrhagic fever outbreak in Pakistan, 2022: A warning bell amidst unprecedented floods and COVID-19 pandemic. Health Sci Rep. 2023;6:e1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Zohaib A, Saqib M, Athar MA, Hussain MH, Sial AU, Tayyab MH, Batool M, Sadia H, Taj Z, Tahir U, Jakhrani MY, Tayyab J, Kakar MA, Shahid MF, Yaqub T, Zhang J, Wu Q, Deng F, Corman VM, Shen S, Khan I, Shi ZL. Crimean-Congo hemorrhagic fever virus in humans and livestock, Pakistan, 2015‐2017. Emerg Infect Dis. 2020;26:773-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Fanelli A, Buonavoglia D. Risk of Crimean Congo haemorrhagic fever virus (CCHFV) introduction and spread in CCHF-free countries in southern and Western Europe: A semi-quantitative risk assessment. One Health. 2021;13:100290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Mazzola LT, Kelly-Cirino C. Diagnostic tests for Crimean-Congo haemorrhagic fever: a widespread tickborne disease. BMJ Global Health. 2019;4:e001114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |