Published online Jun 25, 2024. doi: 10.5501/wjv.v13.i2.92586
Revised: May 18, 2024
Accepted: June 7, 2024
Published online: June 25, 2024
Processing time: 145 Days and 20.4 Hours
Rotavirus is a highly contagious virus responsible for a significant burden of acute gastroenteritis, particularly among infants and young children worldwide, however, vaccination against this viral agent is available. Several studies have hypothesized that rotavirus vaccination has been linked to lower rates of anti
To assess the relationship between rotavirus vaccination and antibiotic resistance.
The present systematic review was tailored based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Several electronic databases (PubMed/MEDLINE, Scopus and Web of Science) were searched independently by two investigators in order to retrieve relevant publications published until April 2023 that investigated the aforementioned research question.
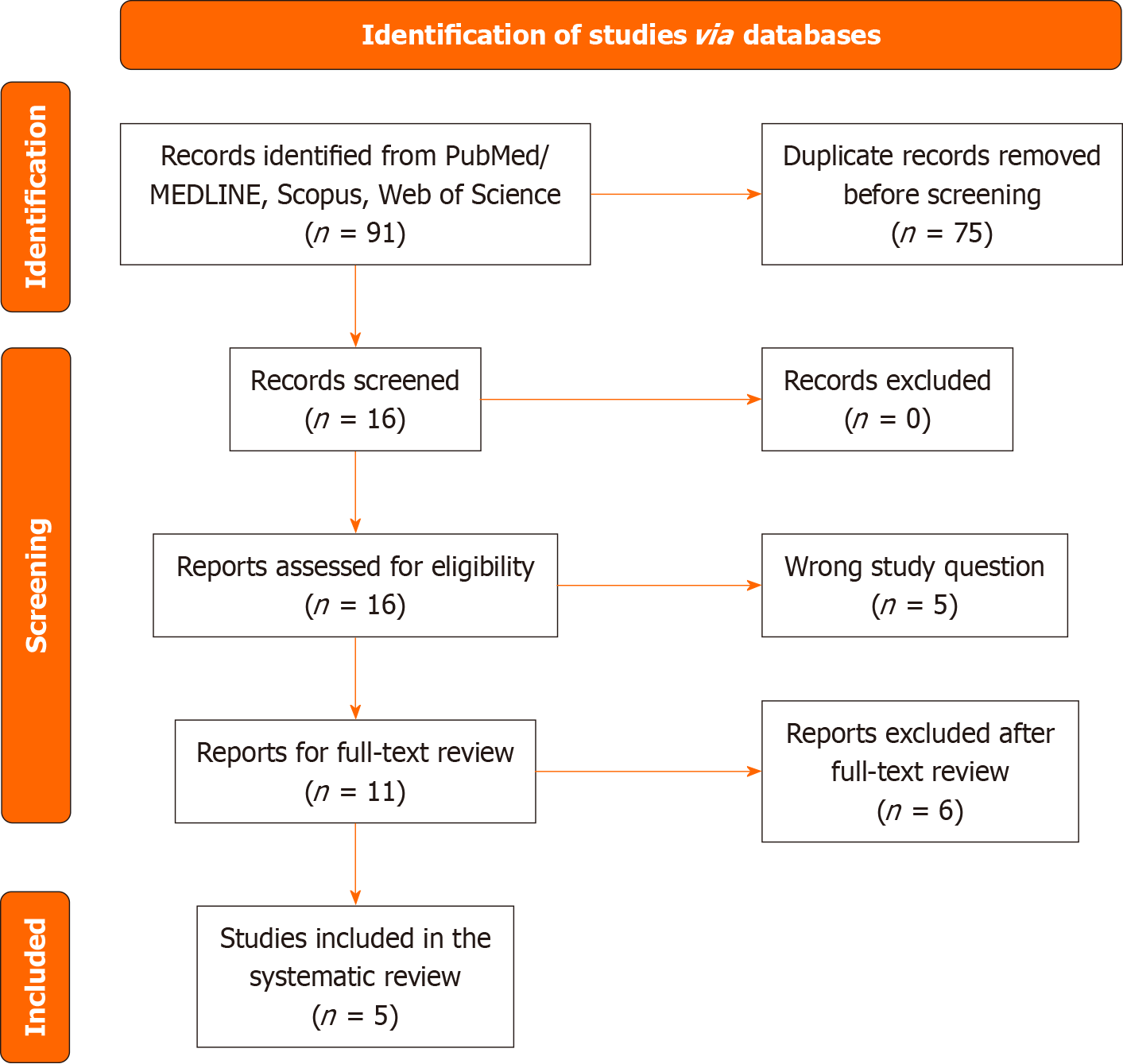
The comprehensive database search identified a total of 91 records. After the duplicates were removed (n = 75), we screened the titles and abstracts of 16 potentially eligible publications. After the irrelevant records were excluded (n = 5), we screened the full texts of 11 manuscripts. Finally, 5 studies were entered into the qualitative and quantitative analysis.
In conclusion, all the studies support the idea that vaccinations can reduce the need for antibiotic prescriptions which could potentially contribute to mitigating antibiotic resistance. However, to fully comprehend the mechanisms of antibiotic resistance, enhance treatment guidelines, and consider diverse demographic situations, further research is necessary to use evidence-based strategies to fight antibiotic misuse and resistance.
Core Tip: Vaccination against rotavirus has been hypothesized to reduce the need for antibiotic prescriptions. Herein, we conducted a systematic review to evaluate the relationship between antibiotic resistance and rotavirus vaccination. Our findings support the idea that vaccinations, including rotavirus vaccination, can reduce the need for antibiotic prescriptions which could potentially contribute to mitigating antibiotic resistance.
- Citation: Simhachalam Kutikuppala LV, Cozma MA, Maddineni G, Chorya HP, Tummala N, Godugu S, Chintala JS, Găman MA. Exploring the impact of rotavirus vaccination on antibiotic prescription and resistance: A comprehensive systematic review. World J Virol 2024; 13(2): 92586
- URL: https://www.wjgnet.com/2220-3249/full/v13/i2/92586.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i2.92586
Rotavirus is a highly contagious virus responsible for a significant burden of acute gastroenteritis (AGE), particularly among infants and young children worldwide[1]. It is estimated that over 25 million emergency department visits and more than 2 million hospital admissions are attributed to rotavirus infections annually. According to the World Health Organization, in developed nations, a significant proportion of children (approximately 75%) experience the initial occurrence of rotavirus diarrhea before the age of 12 months. Conversely, in developing countries, the onset of the first episode of rotavirus diarrhea is often postponed until the age range of 2–5 years. The prevalence of severe rotavirus gastroenteritis is primarily observed in children between 6 months and 24 months of age[2]. From 2013 to 2017, approximately 122000–215000 infant deaths due to diarrhea were attributed to rotavirus each year[3-5]. Rotavirus has been identified as the third most prevalent infection linked to childhood mortality among children under 5 years of age[5]. Children residing in low- and medium-income countries (LMICs) have a disproportionate burden of diarrheal mortality compared to their counterparts in high-income countries[3].
Public health experts widely acknowledge the importance of rotavirus vaccination as a vital intervention to decrease the disease burden. The implementation of rotavirus vaccines has been associated with significant reductions in hospitalizations and fatalities caused by rotavirus in numerous countries[6]. Despite these positive outcomes, vaccination coverage for rotavirus varies greatly across different countries. It is crucial to explore the broader implications of this intervention, including its potential influence on antibiotic resistance. One such implication is the potential impact on antibiotic resistance. Antibiotic resistance, a growing public health concern, has emerged as a global challenge, impacting the effectiveness of bacterial infection treatments and outcomes[7,8]. While the association between viral infections and antibiotic resistance may seem counterintuitive, there is a need to investigate the potential relationship between rotavirus and antibiotic resistance to comprehend the broader implications for patient management, public health, and antimicrobial stewardship. The misuse and overuse of antibiotics have contributed significantly to the emergence and spread of antibiotic-resistant bacteria[8]. While rotavirus is a viral infection for which antibiotics are not typically indicated, it is crucial to investigate if there are instances of antibiotic misuse in the management of rotavirus-related complications or co-infections.
Understanding the association between rotavirus and antibiotic resistance is essential due to the potential impact on patient outcomes[9]. There are several studies that show evidence of a decrease in antibiotic resistance with rotavirus vaccination. This potential effect is due to the decrease in the usage of antibiotics. One of the studies was a retrospective analysis that showed a decrease of the rotation of antibiotics needed in fully vaccinated children[7]. Other such studies showed decades worth of decrease in antibiotic resistance and usage in children who were vaccinated especially in low to middle-income countries[8-10]. Rotavirus infection can lead to compromised immune systems and damage to the intestinal lining, potentially increasing the susceptibility to secondary bacterial infections. In such cases, appropriate antibiotic therapy may be warranted, necessitating an assessment of potential antibiotic resistance patterns[10]. The association between rotavirus and antibiotic resistance may have implications for infection control strategies, especially in healthcare settings. Nosocomial infections pose a significant challenge, and if rotavirus-infected patients are more prone to acquiring bacterial infections, particularly those caused by antibiotic-resistant strains, it has implications for infection prevention and control measures[11].
This systematic review will provide a robust and evidence-based analysis of the existing literature on the association between rotavirus and antibiotic resistance. This systematic review aims to explore the association between rotavirus and antibiotic resistance, considering the implications for patient care, public health, and antimicrobial stewardship. By synthesizing the available evidence, we aim to shed light on this topic and provide insights that can inform clinical practice, policy-making, and future research endeavors in this field.
The present systematic review was tailored based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Table 1)[12]. The study protocol was registered in PROSPERO (International prospective register of systematic reviews) PROSPERO registration number CRD42023429481[13].
| Ref. | Country | Study type | Study period | Population | Intervention | Other vaccinations (PCV, etc.) | Comparison | Assessment for (history of infections) | History of antibiotic use | Any antibiotic prescription | Antibiotic prescription following AGE | Antimicrobial resistance |
| Hall et al[7], 2022 | Unite States | Retrospective cohort study | 2007-2018 | Children aged 5 born 2007-2018 | RV | PCV | Children with no RV | AGE | Aminoglycosides, cephalosporin, β-lactam, erythromycin and macrolide, penicillins, miscellaneous antibiotics, quinolones, sulfonamides and combination, sulfones | NS | 55.4% received antibiotics during the follow-up period; 1.5% of antibiotic prescriptions followed an AGE diagnosis | NS |
| Lewnard et al[16], 2020 | Gambia; Mali; Mozambique; Kenya; Bangladesh; India; Pakistan | Case-control study | 2007-2011 | Children aged 0–59 months with diarrhea | None | NS | Children without diarrhea | AGE caused by Salmonella, Shigella, Campylobacter, Aeromonas, and Vibrio spp Escherichia coli | NS | NS | NS | NS |
| Lewnard et al[17], 2020 | Kenya, Bangladesh, India | Case–control study | 2015-2019 | Children aged < 5 years with acute respiratory infections and diarrhea in the 2 wk prior to the study | RV | PCV10/13 | Children aged < 5 years unvaccinated for RV/PCV | Acute respiratory infection and diarrhea | NS | NS | NS | NS |
| At Thobari et al[15], 2020 | Indonesia | Phase IIb randomized, double-blinded, controlled trial | January 2013 through July 2016 | 0-18 months of age | RV3-BB RV | NS | Placebo | NS | 551 infants received ≥ 1 antibiotic in the 18-month observation period | 956 antibiotic courses, 1.74 antibiotic uses per infant, mean duration of antibiotic use per child was 4.92 (± 1.86) d; no significant association between sex or vaccination group and the duration of antibiotic courses | NS | NS |
| Perez-Scha et al[14], 1990 | Venezuela | Clinical/field trial | ≥ 1 year and 1 year follow-up; sequential vaccine administration in 4 periods: February-March 1985, June-July 1985, October 1985, and February 1986 | < 6 months of age | RV | NS | Placebo | The provided text does not mention a specific section on the history of infections (gastroenteritis) in the study | NS | NS | NS | NS |
Several electronic databases (PubMed/MEDLINE, Scopus and Web of Science) were searched independently by two investigators in order to retrieve relevant publications published until April 2023. The search strategy was based on the use of keywords, word combinations and Medical Subject Headings, including: “Rotavirus Vaccination”, “Rotaviral immunization”, “Rotavirus”, “Antibiotic resistance”, “Antibiotic Prescribing”, “Antimicrobial resistance”, “Antibiotic prescribing”, “Drug Resistance”, “Antibiotics”. In addition, we used the snowball strategy and checked the reference lists of all relevant manuscripts to avoid missing out any potentially eligible publications. No language restrictions were applied to the search.
We formulated the following Population Intervention Comparator Outcome (PICO) question: In children, does rotavirus vaccination reduce antibiotic prescribing from AGE or is it associated with a reduction in the growth of antibiotic resistance? The following PICO terms were used: (1) Population: Children who were born in areas with universal vaccination; (2) Intervention: Rotavirus vaccination; (3) Comparator: Non-vaccinated children; (4) Primary outcome: Reduction in antibiotic prescribing for AGE; and (5) Secondary outcomes: Reduction in growth of Antibiotic resistance.
We employed the following inclusion criteria: (1) Cross-sectional, cohort, and case-control, observational studies, as well as randomized and non-randomized clinical trials; (2) Studies conducted in children and individuals who were vaccinated against rotavirus; and (3) Papers published in languages spoken by the investigators (English, Hindi, French, Italian, Romanian). We excluded case reports, case series, narrative, scoping, and systematic reviews, meta-analyses, short communications (letters, commentaries), book chapters, study protocols, and conference abstracts, articles not published in languages spoken by the assessors, manuscripts with unavailable full-texts or published in non-peer reviewed journals, and investigations conducted in children with improper vaccination history and/or inadequately treated.
Rayyan software (Rayyan Systems Inc., Cambridge, MA) was employed to perform the screening of titles and abstracts. Relevant data was extracted in Google Sheets and analyzed using Microsoft Office Excel 2003. The following data were extracted and entered into a summarization table: Name of the first author of the study, year of publication of the study, country of execution, study type, study period, population, intervention, administration of other vaccinations, population of comparison, assessment for or history of infections, history of antibiotic use, antibiotic prescriptions, antibiotic prescription following AGE, and data on antimicrobial resistance. The quality of the included publications was evaluated using the Newcastle Ottawa Scale for observational studies and the Cochrane Risk of Bias (ROB) 2.0 tool for randomized controlled trials.
The systematic search of the databases identified a total of 91 records. After the duplicates were removed (n = 75), we screened the titles and abstracts of 16 potentially eligible publications. After the irrelevant records were excluded (n = 5), we screened the full-texts of 11 manuscripts. Finally, 5 studies were entered into the qualitative and quantitative analysis. The flowchart diagram of the literature search process is presented in Figure 1.
The characteristics of the publications entered in the systematic review are depicted in Table 1.
The studies were published between 1990 and 2022 and conducted between 1985 and 2019 either in North America (United States of America, n = 1)[7], South America (Venezuela, n = 1)[14], Asia (Indonesia, n = 1)[15] or as multi-country international collaborations (Africa-Asia multicentric studies, n = 2)[16,17]. The research was designed as retrospective cohort studies (n = 1)[7], case-control studies (n = 2)[16,17] or clinical trial studies (n = 2)[14,15] and recruited participants of both sexes. The subjects analyzed were children aged 0 month to 60 months who did/did not experience diarrhea. The intervention explored consisted in the administration of the rotavirus vaccine alone[14,15] or in combination with other vaccines (e.g., pneumococcal conjugate vaccine, n = 3)[7,16,17]. The comparator groups included children without diarrhea and/or who were not vaccinated against the rotavirus. Three studies assessed enrolled participants for medical history relevant for AGE and four manuscripts mentioned data on the antibiotic use of the analyzed children, however, the specific antibiotics/classes of antibiotics prescribed were not mentioned (Table 1). ROB analysis for the included studies showed high-quality of evidence for the majority (80%) of the evaluated observational studies (4 out of 5) (Table 2). Only one of the six included studies was randomized and its risk of bias was low according to the Cochrane ROB2 tool.
| Ref. | Selection | Comparability | Outcome | Total1 | |||||
| Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of the study | Comparability of cohorts based on basis of design or analysis | Assessments of outcomes | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | ||
| Hall et al[7], 2022 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Lewnard et al[16], 2020 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Lewnard et al[17], 2020 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| At Thobari et al[15], 2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Perez-Schae et al[14], 1990 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
This systematic review comprehensively analyzes the intricate relationship between rotavirus and pneumococcal vaccinations in children, focusing on their impact on antibiotic prescriptions and broader implications for antibiotic resistance mitigation and the prevention of antibiotic-treated illnesses. The systematic exploration of the association between rotavirus vaccination and antibiotic resistance represents a critical endeavor in understanding the intricate dynamics between viral infections, antibiotic use, and resistance patterns. Our review aimed to dissect this relationship through an in-depth analysis of available literature, shedding light on potential implications for healthcare, antimicrobial stewardship, and public health strategies.
The retrieved studies, encompassing diverse geographical locations and study designs, provided valuable insights into this complex interplay. The results of case-control studies in several countries and retrospective cohort studies in the United States of America have different views on how the rotavirus vaccine might affect the use of antibiotics and the development of antibiotic resistance[7,16].
Children vaccinated against rotavirus experienced a significant decrease in the number of antibiotic prescriptions required after an AGE diagnosis, according to the study by Hall et al[7]. Rotavirus-vaccinated children with AGE had fewer antibiotic prescriptions and a lower likelihood of switching antibiotics within 28 d of their first prescription. This suggests that antibiotic resistance may be slowing down. The observed effect magnified over a 5-year follow-up, especially during the rotavirus season, bolstering cumulative vaccine effectiveness. Extrapolating these findings to the wider United States child population estimates a substantial prevention of over 67000 initial antibiotic prescriptions since the inception of the rotavirus vaccination[7].
Conversely, Lewnard et al[16] investigated clinically attended, antibiotic-treated diarrhea across diverse age groups, identifying differential incidence rates among cohorts. However, this study did not explicitly delve into antibiotic switching or resistance. Notably, it encountered challenges in determining appropriate treatment for Shigella-associated diarrhea, highlighting the complexities in establishing suitable treatment strategies for certain causative agents. Lewnard et al[16] examined the impact of the pneumococcal conjugate vaccine (PCV) and rotavirus vaccines on illnesses treated with antibiotics in LMICs. Their study estimated a substantial annual prevention of millions of episodes, with potential additional prevention through universal vaccine coverage. Moreover, children who got at least three doses of PCV10/13 had lower chances of getting an acute respiratory infection that needed antibiotics. This was especially true for kids aged 24 months to 59 months , where the number of cases dropped by 19.7%[17].
Several studies, including the important randomized controlled trial by At Thobari et al[15], have highlighted the need for further investigation into antibiotic prescription practices in rotavirus-vaccinated populations. However, it is important to note that while this trial provided essential insights into antibiotic usage patterns, it did not explicitly investigate antibiotic resistance development. In the early '90s, Perez-Schael et al[14] conducted a historical clinical trial in Venezuela that contributed contextually to the landscape of rotavirus vaccination. However, this study lacked detailed assessments of infections, antibiotic usage, or resistance patterns, limiting its direct relevance to the primary focus of our review.
The synthesis of these studies highlights the complexity inherent in unraveling the association between rotavirus vaccination and antibiotic resistance. Therefore, there is an urgent need for stronger, more standardized methods that include full analyses of antibiotic use, microbial causes, and resistance profiles in children who have rotavirus. Furthermore, the absence of consistent reporting standards across studies poses a significant challenge to synthesizing conclusive evidence. To address these limitations, the implementation of robustly designed, multi-centered prospective studies is essential. Such studies should encompass detailed antibiotic histories, resistance profiles, and clinical outcomes among rotavirus-vaccinated vs non-vaccinated cohorts. This approach could effectively bridge existing gaps and provide a clearer understanding of the intricate relationship between rotavirus vaccination and antibiotic resistance.
These collective findings underscore a significant association between rotavirus and pneumococcal vaccinations in children, resulting in a subsequent reduction in antibiotic prescriptions. This synthesis corroborates the pivotal role of vaccinations in public health, particularly in averting antibiotic-treated illnesses, contributing invaluable insights to global vaccination strategies. However, further research is imperative to harness these findings effectively to untangle the complexities of antibiotic resistance, establish refined treatment protocols, and discern broader implications for child health across diverse populations.
The reviewed studies strengths lie in their diverse geographical locations and comprehensive evaluations of vaccination effects on antibiotic utilization. Hall et al’s[7] longitudinal analysis and extrapolation to a national scale provides robust evidence supporting the association between rotavirus vaccination and reduced antibiotic prescriptions. Lewnard et al’s[17] investigation in LMICs adds a global perspective, highlighting the potential impact of vaccination coverage on reducing antibiotic-treated illnesses. However, limitations include variations in study designs, with some studies lacking explicit analysis of antibiotic resistance patterns and challenges in determining appropriate treatments for specific pathogens.
In conclusion, our systematic review provides evidence supporting the notion that rotavirus vaccination is associated with a reduction in the need for antibiotic prescriptions, which could potentially contribute to mitigating antibiotic resistance. The studies included in our analysis consistently showed a decrease in antibiotic prescriptions among children vaccinated against rotavirus, particularly after an AGE diagnosis. This finding suggests a potential role for rotavirus vaccination in mitigating antibiotic resistance. However, to fully understand the mechanisms underlying this association and to develop evidence-based strategies for combating antibiotic misuse and resistance, further research is necessary.
| 1. | Leshem E, Tate JE, Steiner CA, Curns AT, Lopman BA, Parashar UD. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. JAMA. 2015;313:2282-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Rotavirus. [cited 27 May 2024]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/rotavirus. |
| 3. | Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Petri WA Jr, Riddle MS, Steele AD, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC Jr. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018;172:958-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 578] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 4. | Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, Regional, and National Estimates of Rotavirus Mortality in Children < 5 Years of Age, 2000-2013. Clin Infect Dis. 2016;2:S96-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 828] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 5. | Nguyen TV, Le Van P, Le Huy C, Weintraub A. Diarrhea caused by rotavirus in children less than 5 years of age in Hanoi, Vietnam. J Clin Microbiol. 2004;42:5745-5750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Burke RM, Tate JE, Parashar UD. Global Experience With Rotavirus Vaccines. J Infect Dis. 2021;224:S792-S800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Hall EW, Tippett A, Fridkin S, Anderson EJ, Lopman B, Benkeser D, Baker JM. Association Between Rotavirus Vaccination and Antibiotic Prescribing Among Commercially Insured US Children, 2007-2018. Open Forum Infect Dis. 2022;9:ofac276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization, 2014. |
| 9. | Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277-283. [PubMed] |
| 10. | Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, Jarlier V, Nathwani D, Goossens H; Global-PPS network. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6:e619-e629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 428] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 11. | Mack I, Bielicki J. What Can We Do About Antimicrobial Resistance? Pediatr Infect Dis J. 2019;38:S33-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40136] [Article Influence: 10034.0] [Reference Citation Analysis (2)] |
| 13. | Simhachalam Kutikuppala LV, Tummala N, Chintala JS, Maddineni G, Cozma MA, Gaman MA, Godugu SA, Chorya H. Association between Rotavirus Vaccination and Antibiotic Resistance: Systematic Review. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023429481. |
| 14. | Perez-Schael I, Garcia D, Gonzalez M, Gonzalez R, Daoud N, Perez M, Cunto W, Kapikian AZ, Flores J. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. J Med Virol. 1990;30:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | At Thobari J, Satria CD, Ridora Y, Watts E, Handley A, Standish J, Bachtiar NS, Buttery JP, Soenarto Y, Bines JE. Non-antibiotic medication use in an Indonesian community cohort 0-18 months of age. PLoS One. 2020;15:e0242410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Lewnard JA, Rogawski McQuade ET, Platts-Mills JA, Kotloff KL, Laxminarayan R. Incidence and etiology of clinically-attended, antibiotic-treated diarrhea among children under five years of age in low- and middle-income countries: Evidence from the Global Enteric Multicenter Study. PLoS Negl Trop Dis. 2020;14:e0008520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Lewnard JA, Lo NC, Arinaminpathy N, Frost I, Laxminarayan R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature. 2020;581:94-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |