Published online Mar 25, 2024. doi: 10.5501/wjv.v13.i1.91457
Peer-review started: December 28, 2023
First decision: January 16, 2024
Revised: January 19, 2024
Accepted: February 20, 2024
Article in press: February 20, 2024
Published online: March 25, 2024
Processing time: 73 Days and 19.7 Hours
Dengue fever is the most common cause of viral hemorrhagic fever, with more than 400 million cases being reported annually, worldwide. Even though hepatic involvement is common, acute liver failure (ALF) is a rare complication of dengue fever.
To analyze the demographic profile, symptomology, hospital course and outcomes of patients presenting with ALF secondary to dengue infection by reviewing the published case reports.
A systematic search was performed from multiple databases including PubMed, Reference Citation Analysis, Science Direct, and Google Scholar. The search terms used were "dengue" OR "severe dengue" OR "dengue shock syndrome" OR "dengue haemorrhagic syndrome" OR "dengue fever" AND "acute liver failure" OR "hepatic failure" OR "liver injury". The inclusion criteria were: (1) Case reports or case series with individual patient details; (2) Reported acute liver failure secondary to dengue infection; and (3) Published in English language and on adult humans. The data were extracted for patient demographics, clinical symptomatology, clinical interventions, hospital and intensive care unit course, need for organ support and clinical outcomes.
Data from 19 case reports fulfilling the predefined inclusion criteria were included. The median age of patients was 38 years (inter quartile range: Q3-Q1 26.5 years) with a female preponderance (52.6%). The median days from diagnosis of dengue to development of ALF was 4.5 d. The increase in aspartate aminotransferase was higher than that in alanine aminotransferase (median 4625 U/L vs 3100 U/L). All the patients had one or more organ failure, with neurological failure present in 73.7% cases. 42.1% patients required vasopressor support and hepatic encephalopathy was the most reported complication in 13 (68.4%) cases. Most of the patients were managed conservatively and 2 patients were taken up for liver transplantation. Only 1 death was reported (5.3%).
Dengue infection may rarely lead to ALF. These patients may frequently require intensive care and organ support. Even though most of these patients may improve with supportive care, liver transplantation may be a therapeutic option in refractory cases.
Core Tip: Dengue infection frequently affects liver function but, in most cases, it exhibits transient and mild increase of transaminases. Rarely, it may lead to severe liver injury and development of acute liver failure (ALF). As there is no specific therapy, most of these patients are managed conservatively and provided with organ support. N-acetyl cysteine is increasingly been used in the management of non-paracetamol induced ALF. However, its utility in ALF secondary to dengue is still limited to small case series and case reports. Even liver transplantation has been rarely attempted in these patients because of high incidence of underlying multi-organ failure and increased risk of bleeding. However, clinical outcomes in these patients may be improved with early recognition and timely supportive care.
- Citation: Juneja D, Jain R, Nasa P. Dengue induced acute liver failure: A meta summary of case reports. World J Virol 2024; 13(1): 91457
- URL: https://www.wjgnet.com/2220-3249/full/v13/i1/91457.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i1.91457
Dengue is the most common cause of viral hemorrhagic fever, globally. It is endemic in many tropical countries, but in the last few years, cases have also been frequently reported from non-endemic regions[1,2]. As per the current estimates, worldwide, more than 5 billion people are at risk of getting affected with dengue, and more than 400 million cases are being reported annually[2].
Traditionally, dengue was classified as non-classical dengue fever (DF), classical DF, dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS)[3]. However, the modified dengue classification by the World Health Organisation divides it into three categories: Dengue without warning signs, dengue with warning signs and severe dengue. Patients with severe capillary leak, hypotension, severe bleeding or severe organ involvement are all classified as severe dengue[4].
Classically, dengue patients present with fever and rash and in severe cases with bleeding and shock. Liver injury is commonly reported in patients with dengue, and various phases of liver dysfunction have been described as secondary to dengue infection. In most patients, the liver enzymes have transient mild elevation[5]. Marked elevation of transaminases by more than ten times has also been described, and termed as dengue–induced severe hepatitis (DISH), which may occur in 4%-15% of the dengue cases[5-7]. However, the progression of DISH to acute liver failure (ALF) is rare and is reported in less than 1% of cases[5].
Patients with liver involvement generally present with gastrointestinal symptoms like nausea, vomiting, abdominal pain and anorexia, along with yellowish discoloration of the eyes and skin. Hepatomegaly has been reported to be present in 4%-79% of the patients[7-10]. However, in patients with severe disease, the presence of complications or multiple organ involvement may complicate the clinical picture. Patients with severe dengue may frequently require intensive care unit (ICU) admission and usually a multi-organ support[11]. Such patients have significant morbidity and mortality. Further, there is a substantial difference in mortality rates between DISH and ALF secondary to dengue, making it essential to recognize it early and institute supportive care[5].
As ALF secondary to dengue is rare and there is a dearth of data from large trials, we aimed to analyze the demographic profile, symptomology, hospital course and outcomes of patients presenting with ALF secondary to dengue infection by reviewing the published case reports and case series.
For the present meta-summary, a systematic search was performed from multiple databases including, PubMed, Reference Citation Analysis, Science Direct, and Google Scholar. The search terms used were "dengue" OR "severe dengue" OR "dengue shock syndrome" OR "dengue haemorrhagic syndrome" OR "dengue fever" AND "acute liver failure" OR "hepatic failure" OR "liver injury". The inclusion criteria were: (1) Case reports or case series with individual patient details; and (2) reported acute liver failure secondary to dengue infection. Further, it was filtered for the literature published in English and on adult (> 18 years) humans. We excluded: (1) Conference abstracts; and (2) case reports or series that did not have individual biochemical data. All reports published till September 30, 2023 were included. The authors manually screened the results to include only the relevant literature and removed the duplicate articles.
All the selected case reports and case series were evaluated. The data were extracted for patient demographics, clinical symptomatology, clinical interventions, hospital and ICU course, need for organ support and clinical outcomes. A datasheet for evaluation was further prepared.
Excel and Microsoft Office 2019 were used to analyze the prepared datasheet. Categorical variables were presented as frequency and percentage. For continuous variables, mean [standard deviation (SD)] or median [interquartile range (IQR)] were calculated, as appropriate. SPSS (version 25.0, IBM SPSS Inc., Chicago, IL, United States) was used for statistical analysis. For tabulation and final documentation, Microsoft (MS) Office software (MS Office 2019, Microsoft Corp, WA, United States) was used.
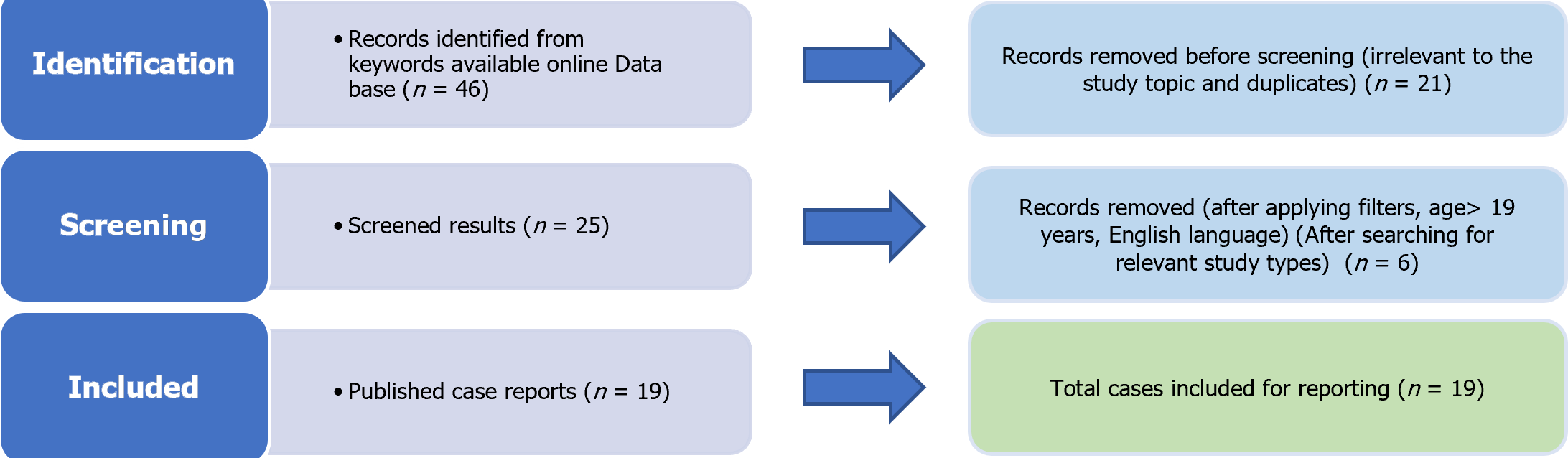
The present meta-summary was performed using the PRISMA 2009 checklist (Figure 1). Eventually, data from 19 case reports fulfilling the predefined inclusion criteria were included in the analysis (Table 1)[12-30]. The median age of patients was 38 years (IQR: Q3-Q1 26.5 years) with a female preponderance (52.6%), as shown in Table 2. Most cases were reported from India (7, 36.8%) and Sri Lanka (5, 26.3%). The median days from diagnosis of dengue to development of ALF was 4.5 d. The baseline laboratory reports and hospital course are given in Table 3. The increase in aspartate aminotransferase (AST) was higher than that in alanine aminotransferase (ALT) (median 4625 U/L vs 3100 U/L). All the patients had one or more organ failure, with neurological dysfunction being most commonly reported in 14 patients (74%). The most common organ support required was cardiac, with 42.1% requiring vasopressors to maintain blood pressure. Most patients recovered with supportive therapy and two patients had undergone liver transplantation. The patients reported to require ICU stay had a median ICU stay of 6 days. 42.1% of patients required vasopressor support. Hepatic encephalopathy was the most commonly reported complication in 13 (68.4%) cases. Only one death was reported (mortality rate 5.3%).
| Number | Ref. | Country | Age | Sex | Comorbidities | MELD score | Complications | Organ support | Outcome |
| 1 | Subramanian et al[28], 2005 | India | 35 | M | None | 17 | None | None | Alive |
| 2 | Vinodh et al[29], 2005 | India | 19 | M | None | 30 | Shock | Vasopressors | Alive |
| 3 | Penafiel et al[25], 2006 | Singapore | 69 | M | None | NA | GI bleed, HE | RRT, IMV, MARS | Alive |
| 4 | Ling et al[22], 2007 | Singapore | 55 | M | None | NA | None | None | Alive |
| 5 | Gasperino et al[19], 2007 | United States | 69 | F | CAD | 30 | HE | Vasopressors, RRT, IMV | Alive |
| 6 | Osorio et al[23], 2008 | Canada | 31 | F | None | NA | MODS | NA | Dead |
| 7 | Sedhain et al[27], 2011 | Nepal | 20 | F | None | 31 | ARF | None | Alive |
| 8 | Agarwal et al[13], 2011 | India | 33 | M | None | 25 | HE, seizures | None | Alive |
| 9 | Jhamb et al[21], 2011 | India | 19 | M | None | 27 | HE, shock, GI bleed | IMV | Alive |
| 10 | Abeysekera et al[12], 2012 | Sri Lanka | 52 | F | Hypertension | NA | HE | None | Alive |
| 11 | Arora et al[14], 2015 | India | 22 | M | None | 10 | Minor bleeding, HE | None | Alive |
| 12 | Dalugama et al[16], 2017 | Sri Lanka | 53 | M | None | 16 | None | None | Alive |
| 13 | Dalugama et al[17], 2018 | Sri Lanka | 43 | F | Diabetes, dyslipidaemia | NA | Shock, HE, AKI, GI bleed | RRT | Alive |
| 14 | Samarasekara et al[26], 2018 | Sri Lanka | 38 | F | None | 26 | DVT, AKI | None | Alive |
| 15 | Galante et al[18], 2019 | Canada | 49 | F | None | NA | Shock, AKI, HE | Vasopressors, RRT, IMV | Alive |
| 16 | Paul et al[24], 2020 | India | 50 | M | Alcoholic liver disease | 29 | HE | None | Alive |
| 17 | Lewis et al[30], 2020 | United States | 23 | F | None | NA | HE | None | Alive |
| 18 | Chikkala et al[15], 2021 | India | 29 | F | None | 38 | HE | Vasopressors, RRT, IMV | Alive |
| 19 | Gunasekera et al[20], 2022 | Sri Lanka | 54 | F | Hypertension, diabetes | 36 | Bleeding, HE, AKI | Vasopressors, RRT, IMV | Alive |
| Parameter of interest | Frequency (%) |
| Age (yr), median | 38 (IQR 26.5) |
| Sex | Females 10 (52.6%) |
| Country of origin | |
| India | 7 (36.8) |
| Sri Lanka | 5 (26.3) |
| Canada | 2 (10.5) |
| Singapore | 2 (10.5) |
| United States of America | 2 (10.5) |
| Nepal | 1 (5.3) |
| Diagnostic methodology | |
| Only serology (IgM) | 14 (73.7) |
| Only antigen (NS1 Ag) | 3 (15.8) |
| Both antigen (NS1 Ag) and serology (IgM) | 2 (10.5) |
| Capillary leak syndrome | 11 (57.9) |
| Dengue shock syndrome | 15 (78.9) |
| Diagnosis of dengue to ALF in days, median (IQR Q3-Q1) | 4.5 (1) |
| Parameter of interest | Frequency (%) |
| Hematocrit, median | 42.60 (IQR 15.15) |
| Total leucocyte count (per µL), median | 5800 (IQR 5800) |
| Platelet count (per µL), median | 25500 (IQR 28250) |
| AST (U/L), median | 4625 (IQR 11902.5) |
| ALT (U/L), median | 3100 (IQR 2607) |
| AST/ALT ratio, median | 2.45 (IQR 1.75) |
| Alkaline phosphatase (IU/L), median | 191 (IQR 117) |
| Total bilirubin (mg/dL), median | 5.5 (IQR 5.63) |
| Direct bilirubin (mg/dL), median | 2.55 (IQR 2.75) |
| INR, median | 2.13 (IQR 1.79) |
| Albumin (g/L), median | 2.85 (IQR 0.48) |
| Creatinine (mg/dL), median | 1.63 (IQR 2.12) |
| MELD score, median | 27.5 (IQR 5) |
| Days of ICU stay, median | 6 (IQR 2) |
| Specific therapies | |
| N-acetyl cysteine | 6 (31.6) |
| Types of organ support | |
| Vasopressors | 8 (42.1) |
| Renal replacement therapy | 6 (31.6) |
| Ventilation | 6 (31.6) |
| MARS | 1 (5.3) |
| Organ failure | 14 (73.7) |
| Neurological | 10 (52.6) |
| Cardiac | 8 (42.1) |
| Renal | 7 (36.8) |
| Respiratory | |
| Complications | |
| Hepatic encephalopathy | 13 (68.4) |
| Bleedings | 3 (15.8) |
| DVT | 1 (5.3) |
| Liver transplantation | 2 (10.5) |
| Death | 1 (5.3) |
Dengue infection may rarely lead to ALF. In the present meta-summary, all the patients had severe dengue, with 79% being diagnosed with DSS. The rise in AST was more than ALT, along with an increase in other liver function parameters. The median international normalized ratio (INR) value was 2.13, and the most common reported complication was hepatic encephalopathy (68.4%). Only 1 death was reported in our cohort of patients.
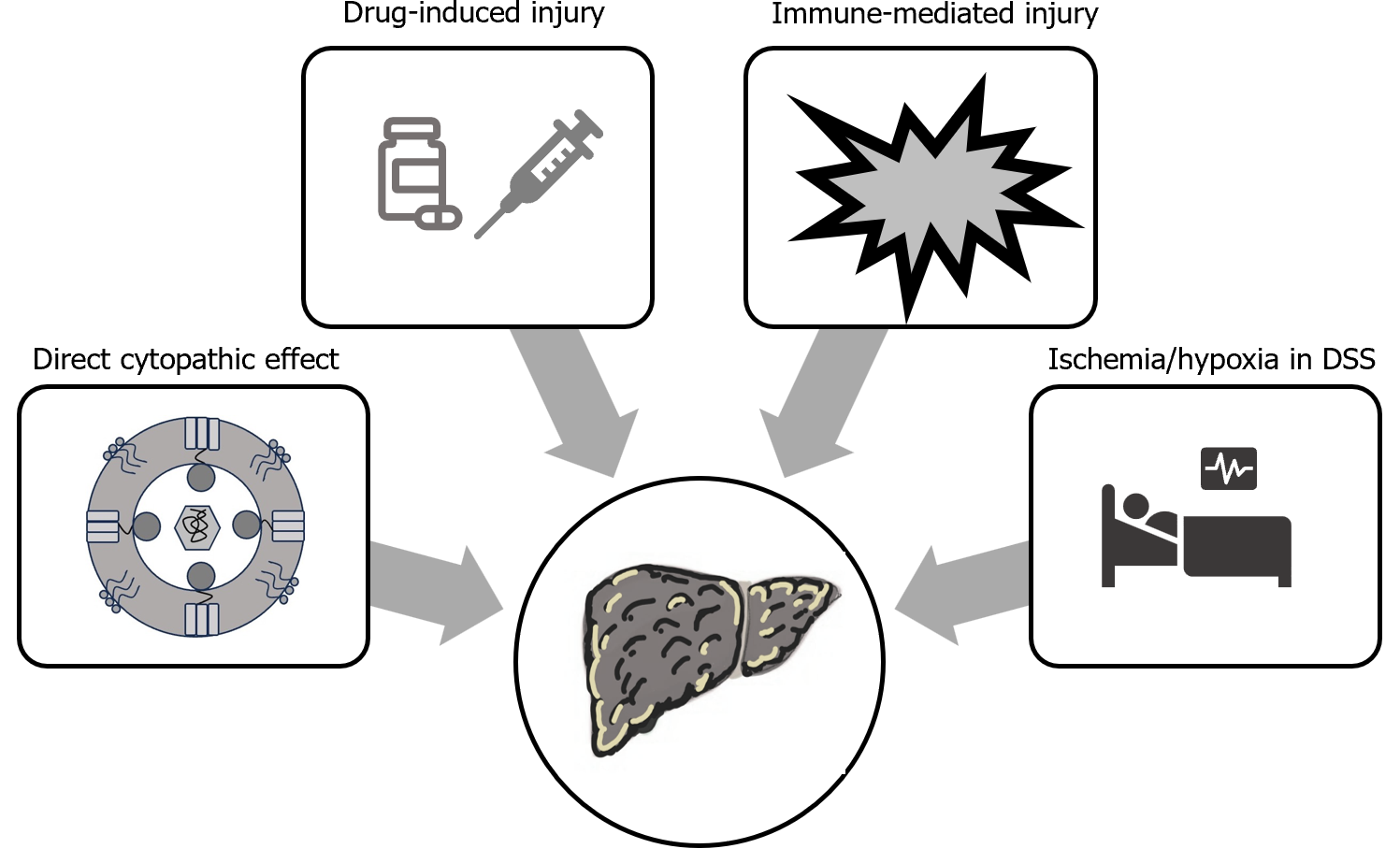
Dengue infection commonly leads to deranged liver functions, but ALF is rarely reported. Liver injury in patients with dengue may be multifactorial. The direct cytopathic effect of dengue virus may lead to liver injury. Further, the cytokine storm associated with severe dengue fever may cause immune-mediated hepatic injury and may progress to ALF. Severe hypotension associated with DSS, may also lead to hepatic hypoperfusion and contributes to liver injury. Additionally, frequent use of hepatotoxic drugs (paracetamol, nonsteroidal anti-inflammatory drugs, antibiotics) may contributes to liver injury (Figure 2)[5,6,31].
Even though DEN-1 and DEN-3 types of dengue virus have been shown to have more prominent liver tropism, all 4 serotypes (DEN-1 to DEN-4) have been shown to affect the liver and may cause fulminant hepatitis[32-34]. Most of the cases in our summary were reported from the Indian subcontinent (India 37% and Sri Lanka 26%). This is reasonable as these are tropical countries, where dengue is endemic and all four serotypes are prevalent. In India alone, more than 63000 dengue cases were reported in 2022[35].
The median days to develop ALF from the diagnosis of dengue was 4.5 d. This is consistent with the previous reports which have shown that there is a gradual increase in transaminase levels which peak around seven days of illness[5]. Even though the increase in transaminases is the most common liver function abnormality associated with dengue fever, there may be derangement of other parameters including, bilirubin, alkaline phosphatase and INR levels, especially in severe disease. This was also evidenced in our report, where we observed higher median levels of bilirubin (5.5 mg/dL), alkaline phosphatase (191 IU/L) and INR (2.13).
Transaminases are more frequently raised in patients with severe forms of dengue. Even the level of increase in transaminases depends on the severity of dengue[36,37]. As per a recent meta-analysis, AST may be raised in 75% of cases of DF as compared to 80% of patients with DHF. Similarly, ALT was raised in 52% of patients with DF and 54% of patients with DHF[38]. The increase in AST levels is greater as compared to ALT levels. It can partly be due to release of AST from the muscular injury secondary to dengue[39]. In most cases, the transaminase levels return to their baseline by day 21 of illness, with ALT levels taking longer duration to normalize due to their longer half-life[5]. Again, the coagulopathic derangement is also dependent on the severity of dengue. Greater increase in INR has been reported in patients with DSS (1.53) as compared to DHF (1.27), while it remained normal in patients with DF[9].
The treatment of ALF associated with dengue fever is largely supportive. Although no specific treatment is recommended, there is increasing interest in using intravenous N-acetylcysteine (NAC) for managing such cases. Even in our cohort, 6 (31.6%) patients were administered NAC for ALF, albeit in different doses and wide variation of duration[12,15,16,17,20,30]. NAC is the recommended antidote for managing ALF secondary to paracetamol overdose[40], but is increasingly been used in managing non-paracetamol related ALF[41]. In ALF secondary to dengue infection, small case series have shown improved survival with early NAC administration in patients with grade 1 and 2 encephalopathy[6,42]. The exact mechanism of action remains unknown, but it is postulated that NAC administration may help restore hepatic anti-oxidants, scavenge oxygen free radicals and improve oxygen delivery due to its vasodilatory effect[6,41,42].
As dengue induced ALF is rare, the data regarding utility of NAC has been extrapolated from studies in aceta-minophen and non-acetaminophen induced ALF. Earlier reports suggested that NAC may be more useful in preventing rather than treating hepatic injury and hence, it was recommended to start NAC early (within 8-12 h) of acetaminophen overdose[43]. However, it is difficult to determine the exact time of hepatic insult in patients with non-acetaminophen induced liver failure and hence, it is recommended to initiate NAC in patients with significant acute liver injury as soon as ALF is detected[44]. Further, it may not be beneficial in later stages of the disease, when liver injury is advanced[42,45]. Hence, NAC may be a useful adjunct in managing patients with severe liver injury, if initiated early. Further, large scale studies need to be performed to evaluate its efficacy, dose and duration of therapy in patients with ALF secondary to dengue infection.
Among the other therapies, corticosteroids have also been shown to be beneficial. Corticosteroids may improve outcomes in patients with severe dengue, but their role in ALF secondary to dengue has not been evaluated[46]. Further, most of these patients may require organ support in the form of renal replacement therapy, invasive mechanical ventilation or vasopressors. Therapies like cytokine filtration, plasma exchange and molecular adsorbent recirculating system have also been used in patients with severe ALF. However, large scale data is missing to recommend their routine use[20,25].
Although liver transplantation is considered as the ultimate therapeutic intervention in patients with ALF, it may be challenging in ALF secondary to dengue due to presence of hemodynamic instability, high risk of bleeding and underlying organ dysfunction. Hence, till date it has been successfully conducted in only a few cases[15,18].
Outcome of dengue patients with liver involvement depends on the severity of liver injury. Most patients with mild increase in transaminases show complete recovery and even those with DISH, have low reported mortality rates of less than 1%. However, ALF secondary to dengue is associated with high mortality rates ranging from 58.8%-66.7%[5,47]. In our cohort, only one death was reported, which may be attributed to selective reporting[23].
The present meta-summary compiled 19 case reports of ALF secondary to dengue infection from across the world, and is first of its kind. Moreover, we included those case reports and series which had individual patient details to compare patient demographics, clinical course, and outcomes. However, the included studies were only case reports without any control arm. As these reports were heterogeneous, they are prone to high risk of bias and missing data, which may affect the generalizability of the results.
Dengue infection may rarely lead to ALF. These patients may frequently require intensive care and organ support. There is no specific therapy, but intravenous NAC therapy, if initiated early, maybe beneficial. Even though most of these patients may improve with supportive care, liver transplantation may be a therapeutic option in refractory cases. Early recognition is important for institution of supportive care, prognostication and timely referral for liver transplantation.
This research sheds light on the complexities of dengue-induced acute liver failure (ALF) and provides a foundation for further investigations and targeted interventions.
ALF secondary to dengue infection is a rare but critical manifestation, requiring intensive care and organ support. Early recognition is vital for prognostication and timely referral for potential liver transplantation. Intravenous N-acetylcysteine shows promise as a supportive therapy, but large-scale studies are needed to validate its efficacy, dosage, and duration. Despite the challenges associated with liver transplantation in these cases, it remains a therapeutic option in refractory situations.
Nineteen case reports met the inclusion criteria, revealing a median age of 38 years, female preponderance (52.6%), and a median of 4.5 d from dengue diagnosis to ALF development. Most cases originated from India (36.8%) and Sri Lanka (26.3%). Elevated transaminases, neurological dysfunction, and cardiac support were common. Notably, only one death was reported (5.3% mortality), and most patients recovered with supportive therapy, while two underwent liver transplantation.
A systematic search of multiple databases, including PubMed, Reference Citation Analysis, Science Direct, and Google Scholar, was conducted using specific keywords. Inclusion criteria comprised case reports or series with individual patient details and acute liver failure secondary to dengue infection. Data extracted from selected reports included patient demographics, clinical interventions, organ support requirements, and clinical outcomes. Statistical analysis was performed using SPSS, and the PRISMA 2009 checklist guided the meta-summary.
This meta-summary aims to analyze the demographic profile, symptomatology, hospital course, and outcomes of patients with ALF secondary to dengue infection. By reviewing published case reports and case series, we seek to delineate the patterns of liver involvement, identify factors influencing disease severity, and explore potential therapeutic strategies.
The motivation behind this study arises from the scarcity of large-scale data on ALF secondary to dengue, its varied clinical presentations, and the need for tailored therapeutic interventions. Given the rising frequency of dengue cases in both endemic and non-endemic areas, insights into ALF dynamics become crucial for effective management and prognosis.
Dengue, a prevalent cause of viral hemorrhagic fever, has witnessed an increasing global impact, extending beyond tropical regions. With over five billion people at risk and 400 million annual cases, the spectrum of dengue manifestations has expanded. Though liver involvement in dengue is common, ALF is rare, necessitating a comprehensive under-standing of its demographics, clinical course, and outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: da Silva FAF, Brazil; Hashimoto N, Japan; van Leeuwen DJ, United States S-Editor: Liu JH L-Editor: A P-Editor: Chen YX
| 1. | Lee IK, Hsieh CJ, Lee CT, Liu JW. Diabetic patients suffering dengue are at risk for development of dengue shock syndrome/severe dengue: Emphasizing the impacts of co-existing comorbidity(ies) and glycemic control on dengue severity. J Microbiol Immunol Infect. 2020;53:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Parveen S, Riaz Z, Saeed S, Ishaque U, Sultana M, Faiz Z, Shafqat Z, Shabbir S, Ashraf S, Marium A. Dengue hemorrhagic fever: a growing global menace. J Water Health. 2023;21:1632-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar JJ. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | World Health Organization; Special Programme for Research and Training in Tropical Diseases Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization; 2009; 147. |
| 5. | Teerasarntipan T, Chaiteerakij R, Komolmit P, Tangkijvanich P, Treeprasertsuk S. Acute liver failure and death predictors in patients with dengue-induced severe hepatitis. World J Gastroenterol. 2020;26:4983-4995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (38)] |
| 6. | Treeprasertsuk S, Kittitrakul C. LIVER COMPLICATIONS IN ADULT DENGUE AND CURRENT MANAGEMENT. Southeast Asian J Trop Med Public Health. 2015;46 Suppl 1:99-107. [PubMed] [DOI] [Full Text] |
| 7. | Trung DT, Thao le TT, Hien TT, Hung NT, Vinh NN, Hien PT, Chinh NT, Simmons C, Wills B. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Karoli R, Fatima J, Siddiqi Z, Kazmi KI, Sultania AR. Clinical profile of dengue infection at a teaching hospital in North India. J Infect Dev Ctries. 2012;6:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Bandyopadhyay D, Chattaraj S, Hajra A, Mukhopadhyay S, Ganesan V. A Study on Spectrum of Hepatobiliary Dysfunctions and Pattern of Liver Involvement in Dengue Infection. J Clin Diagn Res. 2016;10:OC21-OC26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Saha AK, Maitra S, Hazra SCh. Spectrum of hepatic dysfunction in 2012 dengue epidemic in Kolkata, West Bengal. Indian J Gastroenterol. 2013;32:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Juneja D, Nasa P, Singh O, Javeri Y, Uniyal B, Dang R. Clinical profile, intensive care unit course, and outcome of patients admitted in intensive care unit with dengue. J Crit Care. 2011;26:449-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Abeysekera RA, Illangasekera U, Jayalath T, Sandeepana AG, Kularatne SA. Successful use of intravenous N-acetylcysteine in dengue haemorrhagic fever with acute liver failure. Ceylon Med J. 2012;57:166-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Agarwal MP, Giri S, Sharma V, Roy U, Gharsangi K. Dengue causing fulminant hepatitis in a hepatitis B virus carrier. Biosci Trends. 2011;5:44-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Arora S, Nathaniel SD, Paul JC, Hansdak SG. Acute liver failure in dengue haemorrhagic fever. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Chikkala BR, Pandey Y, Acharya R, Sreekumar S, Dey R, Agarwal S, Gupta S. Living Donor Liver Transplant for Dengue-Related Acute Liver Failure: A Case Report. Exp Clin Transplant. 2021;19:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Dalugama C, Gawarammana IB. Dengue hemorrhagic fever complicated with acute liver failure: a case report. J Med Case Rep. 2017;11:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Dalugama C, Gawarammana IB. Lessons learnt from managing a case of dengue hemorrhagic fever complicated with acute liver failure and acute kidney injury: a case report. J Med Case Rep. 2018;12:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Galante A, Adeyi O, Lau L, Humar A, Galvin Z, Selzner N, Lilly L, Sapisochin G, Bhat M. Liver Transplantation for Acute Liver Failure Due to Dengue Fever. Hepatology. 2019;70:1863-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 19. | Gasperino J, Yunen J, Guh A, Tanaka KE, Kvetan V, Doyle H. Fulminant liver failure secondary to haemorrhagic dengue in an international traveller. Liver Int. 2007;27:1148-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Gunasekera AM, Eranthaka U, Priyankara D, Kalupahana R. A rare case of acute liver failure with intrahepatic cholestasis due to dengue hemorrhagic fever: CytoSorb® and plasma exchange aided in the recovery: case report. BMC Infect Dis. 2022;22:938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Jhamb R, Kashyap B, Ranga GS, Kumar A. Dengue fever presenting as acute liver failure--a case report. Asian Pac J Trop Med. 2011;4:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Ling LM, Wilder-Smith A, Leo YS. Fulminant hepatitis in dengue haemorrhagic fever. J Clin Virol. 2007;38:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Osorio J, Carvajal C, Sussman O, Buitrago R, Franco-Paredes C. Acute liver failure due to dengue virus infection. Int J Infect Dis. 2008;12:444-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Paul J. Dengue Infection: A Hidden Cause of Acute Insult in a Case of Acute on Chronic Liver Failure in Endemic Area. Prague Med Rep. 2020;121:118-123. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Penafiel A, Devanand A, Tan HK, Eng P. Use of molecular adsorbent recirculating system in acute liver failure attributable to dengue hemorrhagic fever. J Intensive Care Med. 2006;21:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Samarasekara K, Munasinghe J. Dengue shock syndrome complicated with acute liver failure and kidney injury, infective endocarditis, and deep vein thrombosis: a case report. J Med Case Rep. 2018;12:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Sedhain A, Adhikari S, Regmi S, Chaudhari SK, Shah M, Shrestha B. Fulminant hepatic failure due to dengue. Kathmandu Univ Med J (KUMJ). 2011;9:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Subramanian V, Shenoy S, Joseph AJ. Dengue hemorrhagic fever and fulminant hepatic failure. Dig Dis Sci. 2005;50:1146-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Vinodh BN, Bammigatti C, Kumar A, Mittal V. Dengue fever with acute liver failure. J Postgrad Med. 2005;51:322-323. [PubMed] |
| 30. | Lewis J, Mitra A, Chang M. Acute Liver Failure in a Patient With Dengue Shock Syndrome. ACG Case Rep J. 2020;7:e00371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Dissanayake HA, Seneviratne SL. Liver involvement in dengue viral infections. Rev Med Virol. 2018;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Fabre A, Couvelard A, Degott C, Lagorce-Pagès C, Bruneel F, Bouvet E, Vachon F. Dengue virus induced hepatitis with chronic calcific changes. Gut. 2001;49:864-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Mourão MP, Lacerda MV, Bastos Mde S, Albuquerque BC, Alecrim WD. Dengue hemorrhagic fever and acute hepatitis: a case report. Braz J Infect Dis. 2004;8:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Singh N, Singh AK, Kumar A. Dengue outbreak update in India: 2022. Indian J Public Health. 2023;67:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Souza LJ, Alves JG, Nogueira RM, Gicovate Neto C, Bastos DA, Siqueira EW, Souto Filho JT, Cezário Tde A, Soares CE, Carneiro Rda C. Aminotransferase changes and acute hepatitis in patients with dengue fever: analysis of 1,585 cases. Braz J Infect Dis. 2004;8:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 37. | Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 335] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Wang XJ, Wei HX, Jiang SC, He C, Xu XJ, Peng HJ. Evaluation of aminotransferase abnormality in dengue patients: A meta analysis. Acta Trop. 2016;156:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 40. | Chiew AL, Reith D, Pomerleau A, Wong A, Isoardi KZ, Soderstrom J, Buckley NA. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust. 2020;212:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Sklar GE, Subramaniam M. Acetylcysteine treatment for non-acetaminophen-induced acute liver failure. Ann Pharmacother. 2004;38:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Kumarasena RS, Mananjala Senanayake S, Sivaraman K, de Silva AP, Dassanayake AS, Premaratna R, Wijesiriwardena B, de Silva HJ. Intravenous N-acetylcysteine in dengue-associated acute liver failure. Hepatol Int. 2010;4:533-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 43. | Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2:1097-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 577] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 44. | Pulley JM, Jerome R. Acetylcysteine (N-acetylcysteine, NAC) for the management of non-acetaminophen-induced acute liver failure. Assessed on 12 Jan 2024. Available from: https://cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-expert-committee/applications-for-new-indications-for-existing-listed-medicines/i.13_n-acetylcysteine.pdf?sfvrsn=6945b088_4. |
| 45. | Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ 2nd, Murray NG, McCashland T, Reisch JS, Robuck PR; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864, 864.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 46. | Bandara SMR, Herath HMMTB. Effectiveness of corticosteroid in the treatment of dengue - A systemic review. Heliyon. 2018;4:e00816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Parkash O, Almas A, Jafri SM, Hamid S, Akhtar J, Alishah H. Severity of acute hepatitis and its outcome in patients with dengue fever in a tertiary care hospital Karachi, Pakistan (South Asia). BMC Gastroenterol. 2010;10:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (35)] |