Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.362
Peer-review started: April 13, 2022
First decision: June 16, 2022
Revised: June 24, 2022
Accepted: August 12, 2022
Article in press: August 12, 2022
Published online: September 25, 2022
Processing time: 163 Days and 17.2 Hours
There are numerous conflicting discussions about the outbreak of the new coronavirus 2019 (COVID-19).
To present some anatomical and physiological considerations about two of the symptoms reported by patients: The loss or reduction of smell and taste.
The loss or reduction of smell and taste is presented in a peculiar way, with some cases of persistence even after COVID-19. For this, it was searched in three databases, PubMed/MEDLINE, Web of Science, and Scopus, using the following keywords: "Smell", "Taste", "Smell AND COVID-19", "Taste AND COVID-19", with no publication time restriction, only in English with full text available, excluding also brief communications, letters to the editor, editorials, reviews, comments, and conference abstracts.
The search found 776 articles in the PubMed/MEDLINE database, 1018 in the Web of Science database, and 552 in the Scopus database, from which duplicates were removed (104 articles). Finally, 17 studies were selected for detailed analysis within the eligibility criteria, with titles and abstracts related to central nervous system lesions responsible for smell and taste. This review suggests that viral mechanisms of action may be related to lesions both at the local level and at the level of the central nervous system, lasting up to 3 to 4 wk. It is considered persistent if it exceeds this period, as reported in one case in this review. There are still few studies about the treatment, and among those addressed in this review, only two studies reported possible treatments and emphasized the scarcity of data, with the best option being treatments that do not cause harm, such as gustatory and olfactory physiotherapy
Given the scarcity of data, this review emphasizes the importance of prevention, through the correct use of personal protective equipment by health professionals and respect for local behavioral indications. It is also emphasized, through five studies, that there is a predominance of such symptoms in patients with COVID-19, which can be a tool to control dissemination, through the early isolation of patients until the results are ready.
Core Tip: We discuss the anatomical and physiological considerations about two of the symptoms reported by patients: The loss or reduction of smell and taste. There are still few studies about the treatment, and among those addressed in this review, only two studies reported possible treatments and emphasized the scarcity of data, with the best option being treatments that do not cause harm, such as gustatory and olfactory physiotherapy. Given the scarcity of data, this review emphasizes the importance of prevention, through the correct use of personal protective equipment by health professionals and respect for local behavioral indications. It is also emphasized, through five studies, that there is a predominance of such symptoms in patients with coronavirus disease 2019, which can be a tool to control dissemination, through the early isolation of patients until the results are ready.
- Citation: Vigliar MFR, Pomini KT, Buchaim DV, Buchaim RL. Anatomophysiological relationships and clinical considerations of taste and smell loss in patients with COVID-19. World J Virol 2022; 11(5): 362-374
- URL: https://www.wjgnet.com/2220-3249/full/v11/i5/362.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i5.362
In December 2019 in Wuhan (China), the emergence of an acute respiratory syndrome caused by severe acute respiratory syndrome coronavirus 2 CoV (SARS-CoV-2), with a peculiar and highly contagious behavior, was reported[1]. Thus, the World Health Organization decreed on March 11, 2020 a state of pandemic, considering the condition of community transmission of human infection by the virus[2]. In the current world scenario, there are already approximately 493 million infected and 6.1 million dead people. In Brazil, there are approximately 30.1 million infected and 661 thousand dead ones[2]. Such global and national data are alarming and may be related to the high speed of dissemination, high mortality rate in people with comorbidity, and the coping strategies of each country, as well as socioeconomic and health conditions[3,4].
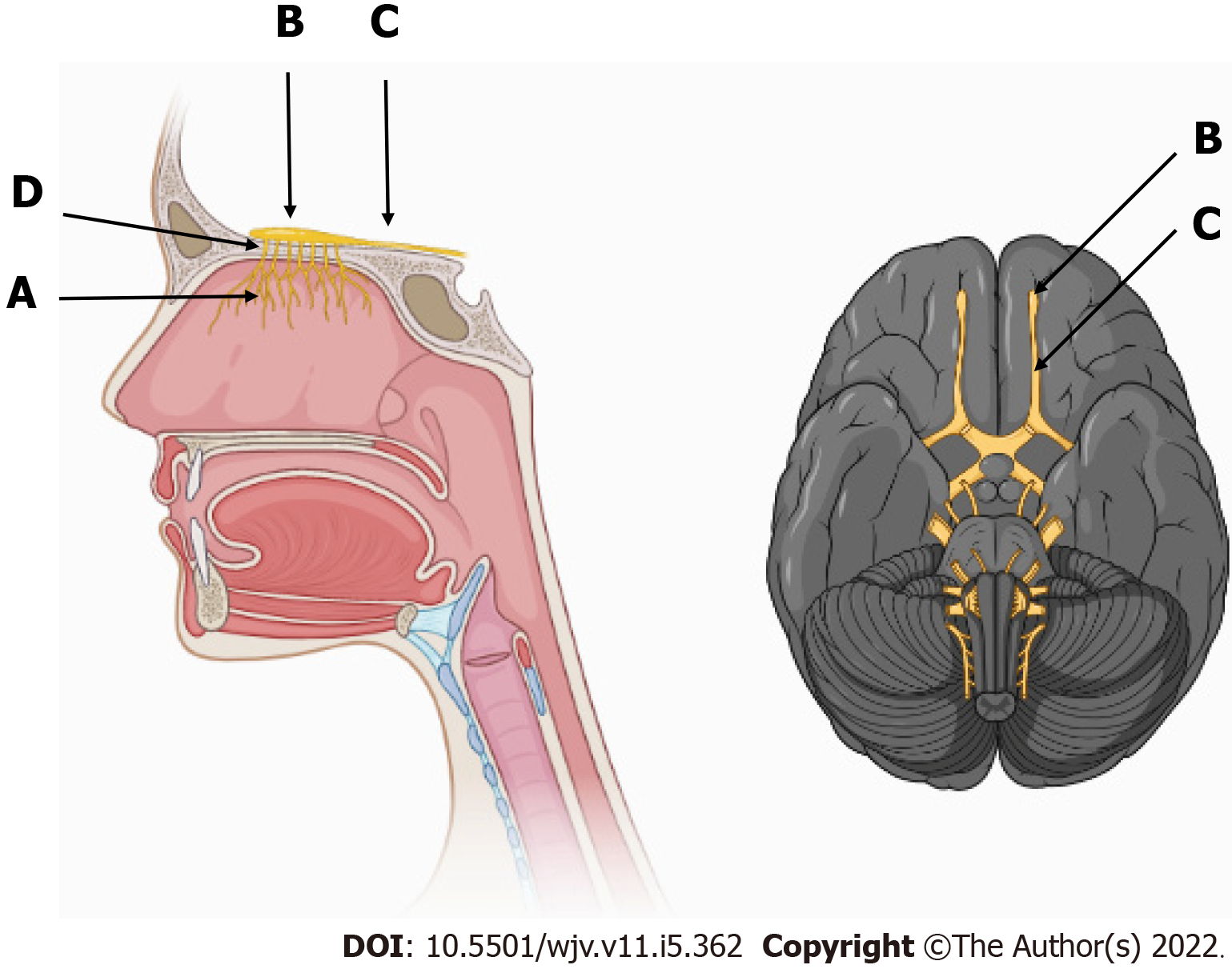
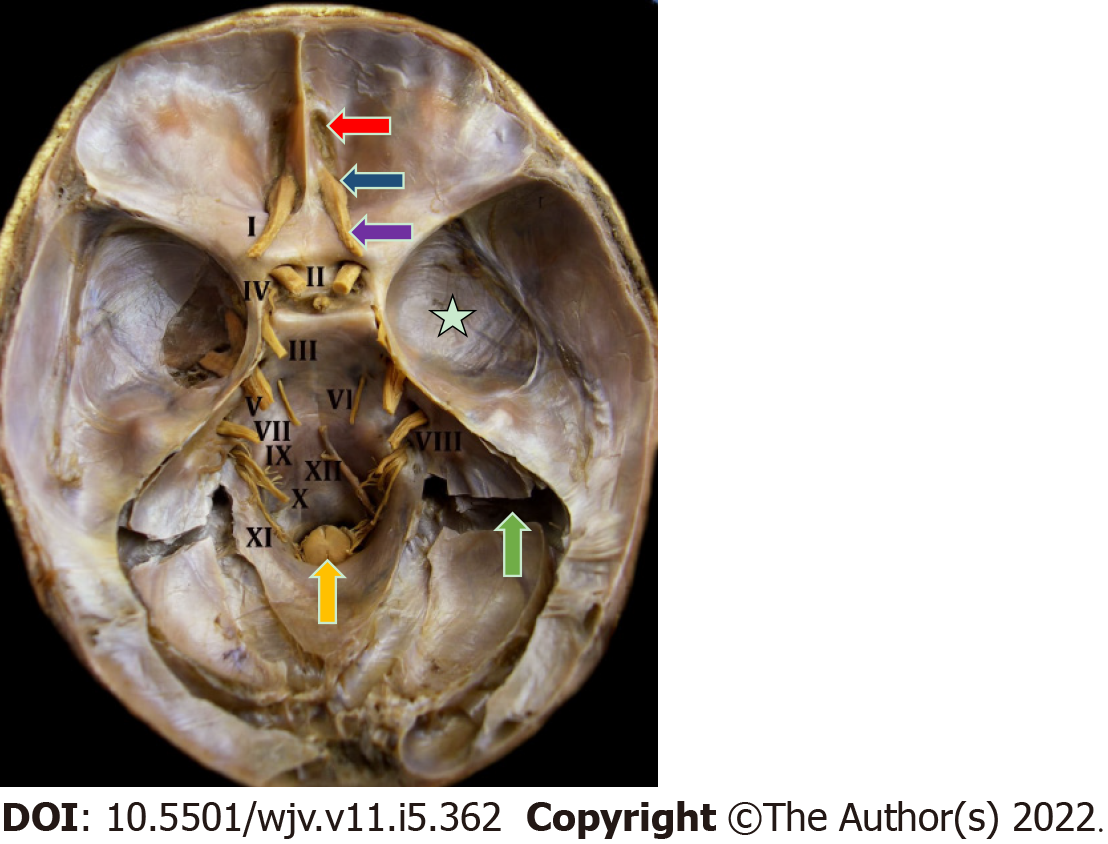
The clinical manifestations of the new coronavirus (COVID-19) are very varied, with the most common symptoms being fever (74%), cough (79%), fatigue (75%), headache (78%), gastrointestinal disorders (57%), and loss of smell (63%) and taste (65%). These symptoms, when present, lead to the diagnostic suspicion of COVID-19, until confirmed by examinations[5,6]. The sensory pathway of smell is initially given by the nerve endings of the olfactory nerve, the primary olfactory neuron, located in the upper third of the nasal cavity and nasal septum, stimulated by chemical substances from the air that are transformed into action potential[7-9]. This stimulus travels through the primary neuron crossing the cribriform plate of the ethmoid bone through its foramina[8]. Upon accessing the anterior cavity of the skull, they synapse with the secondary neuron. The olfactory nerve impulses terminate in the primary cortical projection area and travel to the thalamus, which proceeds to the orbitofrontal and rectus olfactory gyrus (Figure 1). There is also an association of some odors with the limbic system, causing reactions of pleasure or aversion[8-11] (Figures 2 and 3).
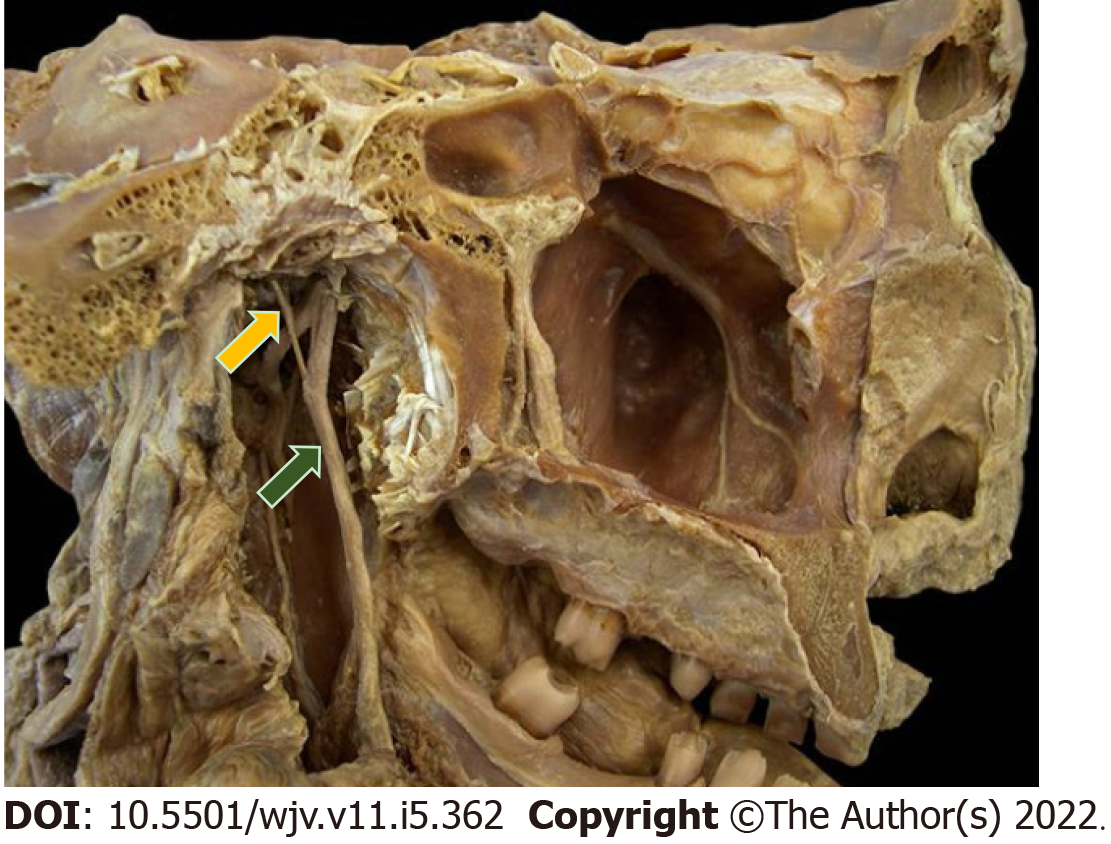
Taste is provided by the specialized sensitivity of the tongue, via the glossopharyngeal nerve (IX cranial nerve) in the posterior third, vagus nerve (X cranial nerve) with few branches at the base of the tongue and epiglottis, and chorda tympani nerve (branch of the VII cranial nerve, the facial-intermediate nerve) in the anterior two thirds of the tongue[7,8]. They receive the stimulus through taste buds that are made up of epithelial cells that have different receptors for each type of flavor, distributed throughout the tongue[12]. From there, the stimuli travel through the primary afferent fibers of the respective gustatory sensory nerves to the solitary tract in the medulla, then to the thalamus, passing to the cortex[8] (Figures 4 and 5).
Given this context, research has sought strategies in order to clarify the sensory alterations of loss of smell and taste, the possible mechanism of action of the virus in these nerves, and its treatment. Thus, the present study aimed to review the anatomy and physiology of the olfactory and gustatory pathways, and their relationship with symptomatology in patients with COVID-19.
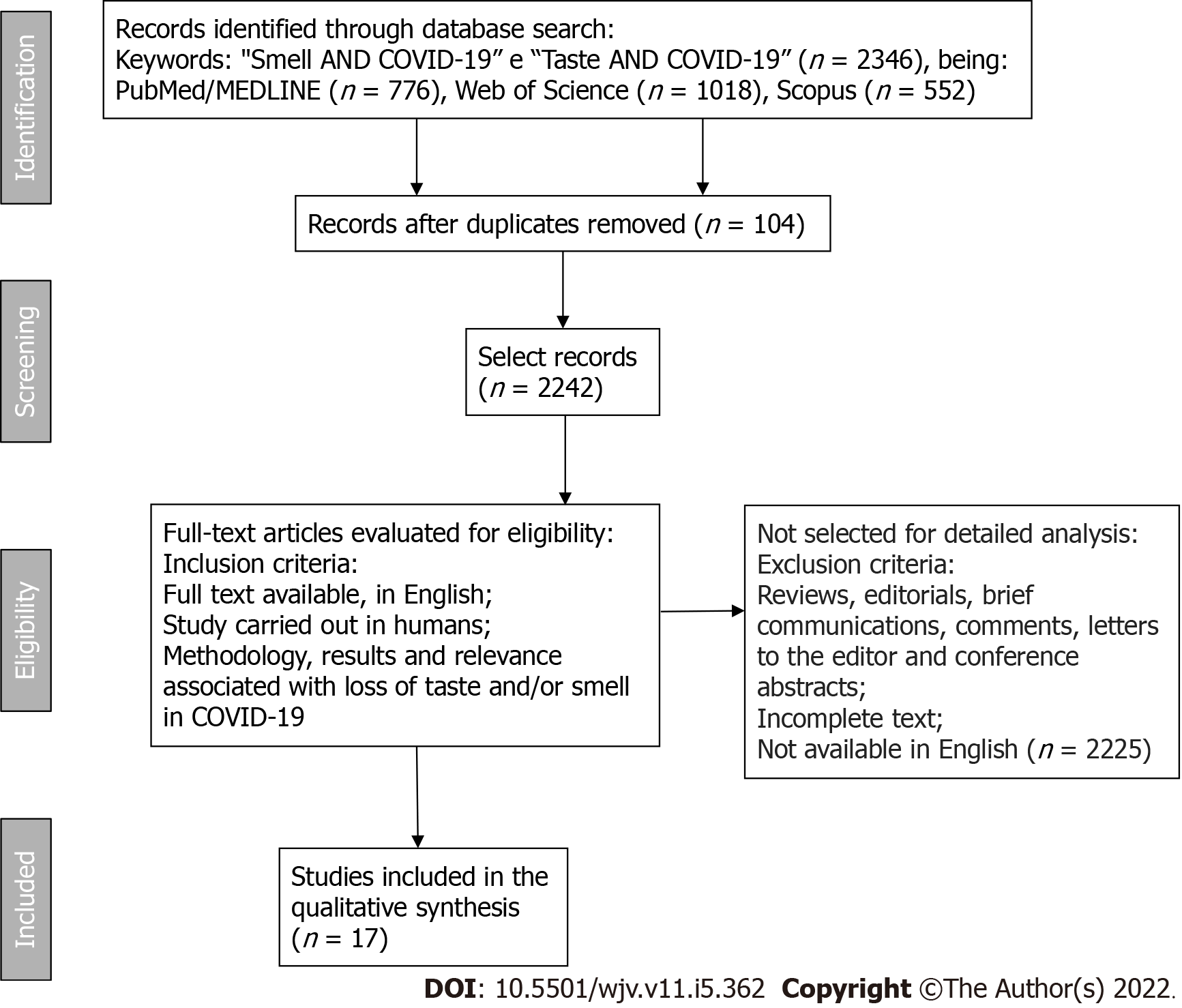
For this study, PubMed/MEDLINE, Web of Science, and Scopus databases were searched, using the following terms as keywords: "Smell", "Taste", "Smell AND COVID-19", "Taste AND COVID -19", without publication time restriction and only in the English language. Works that present titles and abstracts related to the topic of the initial research were verified, using the variables taste and/or smell and COVID-19. Subsequently, the text was evaluated of the articles previously selected by the abstract. The methodology, results, and relevance were considered to list the choice of articles. For inclusion in the research, the articles must necessarily be accessed in their full content (Figure 1).
The inclusion criteria were: Description of changes in smell and taste due to COVID-19; Human studies; Publications in English only; Publications that allow full access to the text. The exclusion criteria were: Articles that have been duplicated; Animal studies; The title was not related to the objective; There was no loss of taste; There was no loss of smell; Other languages (except English); Access to the full text has not been obtained; Brief communications, letters to the editor, editorials, reviews, comments, and conference abstracts.
The search found 776 articles in the PubMed/MEDLINE database, 1018 in the Web of Science database, and 552 in the Scopus database, from which duplicates were removed (104 articles), then editorials, review articles, brief communications, letters to the editor, comments, conference abstracts, and articles without full text available or not produced in English were excluded, as they are outside the eligibility criteria. Seventeen studies that met the eligibility criteria were selected (Table 1).
| Database | Title | Ref. | Sample/study type | Conclusion | Anatomophysiological relationship |
| PubMed/MEDLINE and Web of Science | A structural equation model to examine the clinical features of mild to moderate coronavirus disease 2019 (COVID-19): An Italian multicenter study | Barillari et al[15], 2021 | 294 patients/multicenter study | It has been reported that anosmia should be considered as a specific symptom for COVID-19, especially when the patient is "suspected" or untested | Inflammation in the olfactory epithelium or damage to olfactory receptor neurons, since the cells that make up this tissue have high expression of ACE2 and TMPRSS2, which have a strong capacity to bind the virus, being particularly susceptible to infection |
| PubMed/MEDLINE and Web of Science | Acute loss of smell and taste among patients with symptoms compatible with COVID-19 | Bodnia and Katzeinstein[20], 2020 | 95 patients/cross-sectional study | There was persistence of mild olfactory and/or taste changes even after the other symptoms of COVID-19 had disappeared. In addition, loss of smell and taste was reported in 50% of patients with COVID-19, especially in adults | Human angiotensin-converting enzyme 2 is the main receptor of the SARS-CoV-2 host cell, present in nasal and olfactory respiratory epithelial cells. In addition, ACE2 is expressed in the oral cavity |
| PubMed/MEDLINE | Anosmia in COVID-19 associated with olfactory bulb lesion evidenced on MRI | Aragão et al[19], 2020 | 5 patients/retrospective study | This study documented for the first time, through neuroimaging, a type of lesion of the olfactory bulb in patients with COVID-19, demonstrating that the possible mechanism of action that causes olfactory dysfunction, either through the olfactory bulbs or intracranially, by a microvascular phenomenon | Intracranial olfactory bulb lesion, studied and documented by magnetic resonance imaging |
| PubMed/MEDLINE | COVID-19 viral load in the severity and recovery of olfactory and gustatory dysfunction | Cho et al[21], 2020 | 143 patients/Prospective cross-sectional cohort study | Symptom severity is not correlated with SARS-CoV-2 viral load, and there is a high prevalence of olfactory and gustatory dysfunction in COVID-19 | The virus has affinity for ACE2 receptors that are found in the nasal and olfactory epithelium, causing peripheral neuropathy, which affects the functions of smell and taste. The virus is also able to invade the central nervous system through the olfactory bulb |
| PubMed/MEDLINE and Scopus | Evolution of olfactory disorders in patients with COVID-19 | Gorzkowski et al[14], 2020 | 229 patients/Cross-sectional study | Olfactory and taste disturbances can be an isolated symptom of COVID-19, being reported in two-thirds of COVID-19 patients. Knowledge of these symptoms and their evolution can be useful in creating therapeutic strategies for cases of persistence even after the resolution of other symptoms of COVID-19 | Mechanisms of olfactory disorders related to SARS-CoV-2 infection are still unknown, but it is likely to be associated with the outcomes of various patterns, such as nasal mucosa edema, olfactory epithelial damage (including neural and non-neural epithelium), and even involvement of olfactory pathways |
| PubMed/MEDLINE and Web of Science | Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19 | Jalessi et al[22], 2020 | 100 patients/prospective descriptive study | In patients with COVID-19, there is a high prevalence of sudden temporary olfactory loss and upper airway infection symptoms. However, among all these symptoms, there was a predominance of olfactory loss, showing that this symptom is not associated with the generalized mucosal edema that occurs during an upper respiratory infection with common coronaviruses | Binding between ACE2 receptors and SARS-CoV-2 spike protein on target cells. In addition, infected cells secrete pro-inflammatory cytokines and chemokines, which can generate localized edema |
| PubMed/MEDLINE | Importance of anosmia in SARS-CoV-2: from phenomenology for neurobiology | Pallanti[13], 2020 | 2 patients/descriptive study | Anosmia and hypogeusia among respiratory symptoms can be considered a symptom of COVID-19 infection, if confirmed; these symptoms could represent early markers or signs of SARS-CoV-2 infection to trigger quarantine. These symptoms go beyond sensory aspects, involving extensive neural circuits | The neuroinvasive potential of SARS-CoV-2 was highlighted: When penetrated transnasally, it may access the brain, possibly via the olfactory torsion nerves, and from there rapidly spread to some specific brain areas, including the thalamus and brainstem |
| PubMed/MEDLINE and Web of Science | Loss of smell in COVID-19 patients: MRI data reveal transient swelling of the olfactory clefts | Eliezer et al[16], 2020 | 20 patients/prospective, mono-centric, case-controlled study | Olfactory clefts were evaluated, as well as olfactory function in a cohort study of patients with SARS-CoV-2 infection with loss of olfactory function, which was present in the initial phase of the disease, with improvement at 1-mo follow-up, supporting the hypothesis that this loss, in patients infected with SARS-CoV-2, is caused, at least in part, by reversible inflammatory changes in the olfactory epithelium | SARS-CoV-2 infects cells through interactions between its S protein and ACE2 protein on target cells. Furthermore, it is suggested that SARS-CoV-2 could invade the brain through the cribriform plate near the medulla and olfactory epithelium, causing some structural changes in the olfactory bulb |
| PubMed/MEDLINE and Web of Science | Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, time and associated characteristics | Speth et al[17], 2020 | 103 patients/prospective, cross-sectional | Olfactory dysfunction is very prevalent during COVID-19, often in conjunction with loss of taste. This dysfunction is negatively associated with advanced age and positively associated with female sex | SARS-CoV-2 has great affinity with the host cell surface receptor, ACE2, located in the nasal mucosa, in particular in the ciliated epithelium and goblet cells. In addition, the virus appears to have neurotropism in which olfactory neurons are susceptible to infection |
| PubMed/MEDLINE | Histopathological findings of the olfactory epithelium reported anosmia due to long-term coronavirus disease 2019 | Vaira et al[18], 2020 | 1 patient/case report | 3 mo after the onset of COVID-19 anosmia, a biopsy was performed, which showed massive rupture of the olfactory epithelium, changing the focus of invasion of the olfactory bulb, encouraging further studies of treatments aimed at the superficial epithelium | The epithelium showed thinning with loss of the characteristic three-layer structure, and reduction in the number of olfactory receptor cells, while those that were present had no cilia. There was also an irregular regeneration of the olfactory epithelium interspersed with the respiratory epithelium and, in some cases, the olfactory epithelium was replaced by metaplastic squamous epithelium |
| PubMed/MEDLINE, Web of Science and Scopus | Psychophysical assessment of chemosensory functions after 5 weeks of olfactory loss due to COVID-19: a prospective cohort study in 72 patients | Le Bon et al[23], 2021 | 72 patients/prospective cohort study | Possibly, SARS-CoV-2 mainly affected odor thresholds, suggesting that the main cause of the loss of smell is at the level of the olfactory neuroepithelium rather than the central nervous system | The loss of taste may be related to a direct injury to the taste organ, and ACE2 receptors have been identified in the mouth and, in particular, on the tongue. ACE-2 receptors are also found in olfactory tissue, inducing olfactory loss at the peripheral rather than the more central nervous level. There is thickening of the olfactory cleft mucosa during COVID-19, reporting olfactory neuritis during COVID-19. There may also be viral spread to the central nervous system that started in the olfactory neuroepithelium |
| PubMed/MEDLINE | Head and neck symptomatology in coronavirus disease (COVID-19): A possible neuroinvasive action of SARS-CoV-2 | Freni et al[24], 2020 | 50 patients/ prospective descriptive study | The authors tried to confirm the theories about the neuroinvasiveness of the virus, from a clinical point of view, so the coronaviruses are neurotropic since the neural cells express the ACE2 entry protein, being able to enter the CNS by several routes, mainly by intranasal inoculation and by peripheral nerve pathway using trans-synaptic pathways. In addition, anosmia, dysgeusia, and xerostomia are the first symptoms of COVID-19, which can be exploited for early quarantine and a limitation of viral contagion | SARS-CoV-2 has neuroinvasive and neurotropic properties. First, there is infection of the neuronal olfactory receptor in the olfactory mucosa, then the virus is transported antegrade to the olfactory bulb, and then there is diffusion through channels formed by cells of the olfactory envelope, which form an open connection with the central nervous system |
| PubMed/MEDLINE | Trends in olfactory and gustatory dysfunction in quarantined COVID-19 patients | Seo et al[25], 2020 | 62 patients/prospective surveillance study | The prevalence of olfactory and gustatory dysfunction was 24.2% in patients with mild COVID-19, which may be characteristic indicators in these cases. All patients had hyposmia due to sensorineural olfactory dysfunction, confirmed by validated methods of olfactory and gustatory assessment and endoscopic examinations | It may involve olfactory neurons related to the central nervous system or non-neuronal olfactory epithelial cells. When viral infection occurs in olfactory neurons, permanent olfactory dysfunction may occur, and even if there is recovery, it may take a long time. Therefore, the location of olfactory neurons with sensorineural olfactory dysfunction can be inferred from the clinical course |
| Web of Science | Taste and smell disorders in COVID-19 patients: role of interleukin-6 | Cazzolla et al[26], 2020 | 125 patients/observational study | This study based on clinical evidence and laboratory data highlighted the importance of IL-6 in the pathogenesis of chemosensitive disorders | Action of local inflammatory phenomena on the receptors of olfactory and gustatory cells, rather than permanent cell damage linked to the action of the virus. The dysfunctions may be linked to the peripheral action of IL-6 at the level of cell receptors infected by the virus and to the central action of IL-6 at the level of intermediate taste stations and olfactory pathways, especially in the thalamus |
| Scopus | Brain metabolic correlates with persistent olfactory dysfunction after SARS-Cov-2 infection | Donegani et al[27], 2021 | 22 patients/cross-sectional study | The study provided a group analysis on brain metabolism of patients with persistent olfactory dysfunction after infection with SARS-CoV-2 for the first time proven by olfactory test. It highlighted the confusion of the subtle sequelae of SARS-COV-2 infection and its reflection on PET and other biomarkers | The virus can enter the central nervous system through the first neurons of the olfactory pathway located in the olfactory mucosa. Post-infectious olfactory dysfunction is thought to be caused by damage to the olfactory epithelium or central olfactory processing pathways, with current evidence that hypometabolism in two symmetrical and similar regions within the limbic cortex may support the occurrence of distal olfactory pathway involvement |
| Scopus | Olfactory function and chest CT findings in COVID-19: is there any correlation? | Mangia et al[28], 2021 | 57 patients/cohort-nested cross-sectional study | Olfactory dysfunction does not correlate with radiological lung involvement in hospitalized patients with COVID-19 | The nasal mucosa is an important entry site for SARS-COV-2, as it has a predilection for this neuroepithelium, in addition to having neurotrophic properties. The smell disorder in COVID-19 would not arise from local edema and nasal secretion, preventing odor molecules from reaching the olfactory neuroepithelium |
| Scopus | Structural and metabolic brain abnormalities in patients with sudden loss of smell with COVID-19 | Niesen et al[29], 2021 | 12 patients/ prospective descriptive study | This PET-MR study suggests that the sudden loss of smell in COVID-19 is not related to central involvement due to SARS-CoV-2 neuroinvasiveness. Loss of smell is associated with subtle brain metabolic changes in high-order central and cortical olfactory areas, likely related to combined processes of deaeration and active functional reorganization secondary to lack of olfactory stimulation | Considering that the metabolic abnormalities were not associated with any MRI signal abnormalities, they likely do not represent neuroimaging evidence supporting the neuroinvasive potential of SARS-CoV-2, but rather functional brain markers of olfactory deficit |
The present study aimed to select and evaluate articles that elucidate the anatomophysiological relationships of the olfactory and gustatory pathways with the loss of smell and taste as the main symptoms in patients with COVID-19[13,14]. This knowledge can help in the therapeutic approach during infection and in persistent post-infection cases. In addition, by giving due relevance to this symptomatology, which has a high incidence in patients with COVID-19, isolation is possible of patients with this symptomatology, even before the test results, thus decreasing the transmissibility of SARS-CoV-2[13,14].
Among all the articles selected, through the methodology used, the presence of a very variable number of patient samples was found, in addition, there was a variability in the anatomical structure placed as the focus of the discussion, which were studied and evaluated by different parameters. Six articles[15,18,20,22,23,28] focused on the olfactory and gustatory epithelium as the main responsible for the loss of smell and taste, due to the fact that they have angiotensin-converting enzyme 2 (ACE2) receptors, which at the time of the entry of SARS-CoV-2 alter and damage this mucosa, making it unable to act as local chemoreceptors. Eleven articles[13,14,16,17,19,21,24-27,29] in addition to highlighting ACE2 receptors as a gateway, indicate the olfactory pathways as the access pathways to the central nervous system by SARS-CoV-2, due to its neurotropic properties, which intracranially, are capable of injuring regions responsible for these senses.
Some studies concluded that there is a high prevalence of loss of smell and taste in patients with COVID-19, there was a variation in the ages studied and the degree of severity of the patient's disease[13-17], but due to the strong correlation of the symptomatology with the COVID-19 is considered a specific symptom of the disease[13,14]. These symptoms usually resolve in approximately 1 mo[17]; however, there are cases of persistent loss of smell and taste[18], which was observed by Vaira et al[18]. After biopsy of the nasal mucosa, there was alteration of the olfactory epithelium, which in some places, instead of forming normal tissues, formed metaplastic tissue, which is a possible explanation for the persistence in some cases.
Most articles[13,14,16,17,19,21,24-27,29] highlighted the neurotropic activity of SARS-CoV-2, allowing access and changes in the central nervous system. In the study carried out by Aragão et al[19], a microvascular lesion in the olfactory bulb in a patient with COVID-19 and loss of smell and taste were documented through magnetic resonance imaging (MRI), demonstrating a possible mechanism of action of the virus in addition to its action on the olfactory epithelium.
The study by Izquierdo-Dominguez et al[30], not included in this study because it is a systematic review, confirms the change in smell and taste due to the presence of ACE2 receptors in the respective mucosa and the fact that SARS-CoV-2 has affinity for these receptors. In addition to these sites, ACE2 can be found in various types of tissues, such as those of the central nervous system, which may also represent one of the causes of loss of smell and taste, if damaged. Studies that performed the autopsy of patients with COVID-19 and found hyperemic, swollen brain tissue and some sites with degenerated neurons were also discussed in this article, and also detected the presence of SARS-CoV-2 nucleic acid in the cerebrospinal fluid[30].
Among the main questions on the subject, in addition to the cause of the loss of smell and taste, as well as its anatomophysiological relationships, there are also discussions on whether there are possible preventions and what therapeutic measures can be carried out in the treatment in cases of persistent losses. Among the selected articles, there are no reports of possible preventive measures for loss of smell and taste, but Xu et al[31] raised the hypothesis that the use of vitamin D, as it has several independent neuroprotective mechanisms, can generate protection of central and peripheral nervous tissues, through neurotrophins. The authors hypothesized that the neuroprotective potential could prevent the neurological complications of COVID-19[31].
Regarding therapeutic measures, we selected the study by Vaira et al[18] who cited the existence of evidence of the use of steroid rinses and a pilot study with submucosal injection of platelet-rich plasma into the epithelium, obtaining relevant improvement, but they need more studies to reach significant conclusions and be indicated as clinical treatments for lesions[18]. The study by Kanjanaumpor et al[32], not addressed in our study because it is a systematic review, argued that there is still no significant evidence to recommend any type of pharmacological treatment; however, olfactory training, without contraindications but with low cost and evidence of improvement, is an interesting therapy in patients with persistent loss of smell and taste with COVID-19[32].
About prognosis, Kanjanaumpor et al[32] revealed that in about 32-66% of patients, there is spontaneous recovery and that a US study reported improvement in the loss of smell and taste in 74% of infected patients correlating with the overall resolution of clinical symptoms. Jalessi et al[22] mentioned recovery of smell in 44.0% of patients in the short term (2 wk) and Vaira et al[18] reported that about 66% of patients achieved complete recovery in an average of 19.3 d from the onset of symptoms.
Regarding preventive measures against COVID-19 and its symptoms, such as loss of smell and taste, the importance of using personal protective equipment (PPE) is found in the literature, as in the study by Kim et al[34]. Limited access to this equipment (mask, lab coat, new gloves, and face shield) was significantly associated with a higher risk of developing symptoms of COVID-19, in addition to being associated with more severe disease, with moderate or severe symptoms[34].
Adequate access to PPE by health professionals, especially those on the front line, is associated with a lower chance of contracting the disease, and even if PPE fails, there is an association with less severe and shorter forms[34].
There are numerous studies in progress, including a study by da Fonseca Orcina et al[36], who proposed the therapeutic use of a phthalocyanine-derived mouthwash, which is able to reduce the severity of the disease locally, the viral load in the oral cavity, and consequently the clinical symptoms, such as sore throat, cough, and mouth ulcers. It can thus also reduce the severity of the general disease by reducing the viral load and dissemination, since the oral cavity and oropharynx are an important means of dissemination of SARS-CoV-2[35,36]. The authors emphasized the need for more randomized clinical trials for further conclusions[36].
Regarding therapeutic measures in the loss of smell and taste, there are ongoing research testing several drugs; among them, the therapy with sprays and topical rinses based on corticosteroids has obtained good results, in addition to presenting a high safety profile, being appropriate for post-infection patients with persistent loss of these senses[37]. However, smell and taste training is the only specific therapy with proven efficacy. Although the exact mechanism of action is not known, it is believed that through repeated stimulation, there is an increase in the neuroplastic and regenerative capacity of the brain. Thus, it is an important therapy indicated at first in patients with a persistent condition[37-39].
There are difficulties in quantifying the prevalence and incidence of gustatory and olfactory dysfunction in the general population, due to causes such as analysis and evaluation methods, sample size and area, and the correct definitions of dysfunctions[40]. Multicentric research from Europe, in the year 2020, showed interesting data: 85.6% of patients with COVID-19 reported olfactory loss. It was also one of the pioneering studies in the identification of taste loss, which at the time was 88.0% in patients with COVID-19. In addition, that study described that infected patients could experience this loss in the absence of other significant symptoms[40].
With the emergence of coronavirus variants, infections caused by Omicron can currently be highlighted, which resulted in mild disease, mainly due to the discovery and use of vaccines. Compared to other strains such as Delta, Omicron infections were more often associated with symptomatology and upper respiratory tract infections, and have lower viral loads, less dysregulated immune cell profiles, and lower levels of pro-inflammatory cytokines[41].
A study, through questionnaires, evaluated the clinical profile of patients who developed COVID-19 after full vaccination, in symptomatic patients. The most frequent symptoms were asthenia (82.4%), chemosensory dysfunction (63.4%), headache (59.5%), coryza (58.2%), muscle pain (54.9%), loss of appetite (54.3%), and nasal obstruction (51.6%). However, 62.3% and 53.6% of survey participants reported olfactory and gustatory dysfunction, respectively. Symptom severity was mild or moderate in almost all cases. Chemosensory dysfunction is still a frequent symptom, even in people who contracted the infection after full vaccination. In this way, the sudden loss of smell and taste may continue to represent a useful and specific diagnostic aid in suspected COVID-19, even in vaccinated individuals[42].
As limitations of this study, one can consider the rapid change in the literature on COVID-19, as well as the emergence of new variants, with different symptoms from the initial versions.
Most of the articles studied reported that possible anatomophysiological mechanisms related to the loss of smell and taste, are local lesions in the olfactory and gustatory tissue due to having ACE-2 receptors, with the SARS-CoV-2 gateway being the oral and nasal cavity. In addition to local lesions, there are central changes in the tissues of the nervous system related to taste and smell, which are also damaged by the neurotropic capacity of SARS-CoV-2. The duration, in most cases, can extend from 3 to 4 wk, and it is considered persistent after 1 mo.
Therapeutic conducts in persistent cases with better initial results, which could be indicated by the doctor, are the use of steroid-based sprays and rinses and, mainly, the training of the senses of smell and taste. Likewise, the best measure to be taken is prevention, with the correct use of PPE by health professionals, and respect for local health recommendations determined in order to reduce viral spread.
There are numerous conflicting discussions about the outbreak of the new coronavirus (COVID-19).
Describe the anatomy and physiology relationships of taste and smell losses due to COVID-19
To present some anatomical and physiological considerations about two of the symptoms reported by patients: the loss or reduction of smell and taste.
Since, these symptoms are presented in a peculiar way, with some cases of persistence even after COVID-19. For this, it was searched in three databases, PubMed/MEDLINE, Web of Science and Scopus, using the following keywords: "Smell", "Taste", "Smell AND COVID-19", "Taste AND COVID-19", no publication time restriction, only in English with full text available, excluding also brief communications, letters to the editor, editorials, reviews, comments and conference abstracts.
The search found 776 articles in the database PubMed/MEDLINE, 1018 in the Web of Science database, and 552 in the Scopus database, from which duplicates were removed (104 articles). Finally, 17 studies were selected for detailed analysis within the eligibility criteria, with titles and abstracts related to central nervous system lesions responsible for smell and taste. This review suggests that viral mechanisms of action may be related to lesions both at the local level and at the level of the central nervous system, lasting up to 3 to 4 wk. It is considered persistent if it exceeds this period, as reported in one case in this review. There are still few studies about the treatment, and among those addressed in this review, only two studies reported possible treatments and emphasized the scarcity of data, with the best option being treatments that do not cause harm, such as gustatory and olfactory physiotherapy
Most of the articles studied reported that possible anatomophysiological mechanisms related to the loss of smell and taste, are local lesions in the olfactory and gustatory tissue due to having ACE-2 receptors, with the SARS-CoV-2 gateway being the oral and nasal cavity. In addition to local lesions, there are central changes in the tissues of the nervous system related to taste and smell, which are also damaged by the neurotropic capacity of SARS-CoV-2. The duration, in most cases, can extend from 3 to 4 wk, and it is considered persistent after 1 mo. Therapeutic conducts in persistent cases with better initial results, which could be indicated by the doctor, are the use of steroid-based sprays and rinses and, mainly, the training of the senses of smell and taste. Likewise, the best measure to be taken is prevention, with the correct use of PPE by health professionals, and respect for local health recommendations determined in order to reduce viral spread.
Future studies should further describe the relationships between the anatomy and physiology of taste and smell losses due to COVID-19.
The authors thank Mr. Junqueira JAG from the Faculty of Dentistry of Araçatuba for providing the anatomical images.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu D, China; Yuan H, China S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1284] [Cited by in RCA: 1522] [Article Influence: 304.4] [Reference Citation Analysis (0)] |
| 2. | Pan American Health Organization (PAHO). [cited 20 April 2022]. Available from: https://www.paho.org/pt/covid19. |
| 3. | Shenoy R, D'Souza V, Roma M. The safety of dental care for older adults during COVID-19 pandemic era. Patient Saf Surg. 2021;15:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Lima DLF, Dias AA, Rabelo RS, Cruz IDD, Costa SC, Nigri FMN, Neri JR. Covid-19 in the State of Ceará: behaviors and beliefs in the arrival of the pandemic. Cien Saude Colet. 2020;25:1575-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Zayet S, Klopfenstein T, Mercier J, Kadiane-Oussou NJ, Lan Cheong Wah L, Royer PY, Toko L, Gendrin V. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection. 2021;49:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Mullol J, Alobid I, Mariño-Sánchez F, Izquierdo-Domínguez A, Marin C, Klimek L, Wang DY, Liu Z. The Loss of Smell and Taste in the COVID-19 Outbreak: a Tale of Many Countries. Curr Allergy Asthma Rep. 2020;20:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 7. | Doty RL, Cometto-Muñiz JE, Jalowayski AA, Dalton P, Kendal-Reed M, Hodgson M. Assessment of upper respiratory tract and ocular irritative effects of volatile chemicals in humans. Crit Rev Toxicol. 2004;34:85-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Buchaim RL, Issa JPM. Manual de Anatomia Odontologica (Book). 1o edição ed. São Paulo: Ed. Manole, 2018. |
| 9. | Martin JH. Neuroanatomia Texto e Atlas (Book). 4o edição ed. Porto Alegre: Ed. Artmed, 2013. |
| 10. | Williams PL et al Gray Anatomia (Book). 37o ed. Ed. Rio de Janeiro: Guanabara Koogan, 1995. |
| 11. | Machado A, Haertel LM. Neuroanatomia Funcional (Book). 3° ed. São Paulo: Ed. Atheneu, 2013. |
| 12. | Negri R, Di Feola M, Di Domenico S, Scala MG, Artesi G, Valente S, Smarrazzo A, Turco F, Morini G, Greco L. Taste perception and food choices. J Pediatr Gastroenterol Nutr. 2012;54:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Pallanti S. Importance of SARs-Cov-2 anosmia: From phenomenology to neurobiology. Compr Psychiatry. 2020;100:152184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Gorzkowski V, Bevilacqua S, Charmillon A, Jankowski R, Gallet P, Rumeau C, Nguyen DT. Evolution of Olfactory Disorders in COVID-19 Patients. Laryngoscope. 2020;130:2667-2673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Barillari MR, Bastiani L, Lechien JR, Mannelli G, Molteni G, Cantarella G, Coppola N, Costa G, Trecca EMC, Grillo C, La Mantia I, Chiesa-Estomba CM, Vicini C, Saussez S, Nacci A, Cammaroto G. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: A multicenter Italian study. J Med Virol. 2021;93:983-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Eliezer M, Hamel AL, Houdart E, Herman P, Housset J, Jourdaine C, Eloit C, Verillaud B, Hautefort C. Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology. 2020;95:e3145-e3152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 17. | Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol Head Neck Surg. 2020;163:114-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 18. | Vaira LA, Hopkins C, Sandison A, Manca A, Machouchas N, Turilli D, Lechien JR, Barillari MR, Salzano G, Cossu A, Saussez S, De Riu G. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J Laryngol Otol. 2020;134:1123-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Aragão MFVV, Leal MC, Cartaxo Filho OQ, Fonseca TM, Valença MM. Anosmia in COVID-19 Associated with Injury to the Olfactory Bulbs Evident on MRI. AJNR Am J Neuroradiol. 2020;41:1703-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Bodnia NC, Katzenstein TL. Acute loss of smell and taste among patients with symptoms compatible with COVID-19. Dan Med J. 2020;67:A05200370. [PubMed] |
| 21. | Cho RHW, To ZWH, Yeung ZWC, Tso EYK, Fung KSC, Chau SKY, Leung EYL, Hui TSC, Tsang SWC, Kung KN, Chow EYD, Abdullah V, van Hasselt A, Tong MCF, Ku PKM. COVID-19 Viral Load in the Severity of and Recovery From Olfactory and Gustatory Dysfunction. Laryngoscope. 2020;130:2680-2685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Jalessi M, Barati M, Rohani M, Amini E, Ourang A, Azad Z, Hosseinzadeh F, Cavallieri F, Ghadirpour R, Valzania F, Iaccarino C, Ahmadzadeh A, Farhadi M. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci. 2020;41:2331-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Le Bon SD, Pisarski N, Verbeke J, Prunier L, Cavelier G, Thill MP, Rodriguez A, Dequanter D, Lechien JR, Le Bon O, Hummel T, Horoi M. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. 2021;278:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Freni F, Meduri A, Gazia F, Nicastro V, Galletti C, Aragona P, Galletti B, Galletti F. Symptomatology in head and neck district in coronavirus disease (COVID-19): A possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020;41:102612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | Seo MY, Seok H, Hwang SJ, Choi HK, Jeon JH, Sohn JW, Park DW, Lee SH, Choi WS. Trend of Olfactory and Gustatory Dysfunction in COVID-19 Patients in a Quarantine Facility. J Korean Med Sci. 2020;35:e375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Cazzolla AP, Lovero R, Lo Muzio L, Testa NF, Schirinzi A, Palmieri G, Pozzessere P, Procacci V, Di Comite M, Ciavarella D, Pepe M, De Ruvo C, Crincoli V, Di Serio F, Santacroce L. Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS Chem Neurosci. 2020;11:2774-2781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 27. | Donegani MI, Miceli A, Pardini M, Bauckneht M, Chiola S, Pennone M, Marini C, Massa F, Raffa S, Ferrarazzo G, Arnaldi D, Sambuceti G, Nobili F, Morbelli S. Brain Metabolic Correlates of Persistent Olfactory Dysfunction after SARS-Cov2 Infection. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Mangia LRL, Soares MB, de Souza TSC, Scarabotto PC, De Masi RDJ, Salvador GLO, Hamerschmidt R. Olfactory function and findings on chest computed tomography in COVID-19: is there any correlation? Acta Otolaryngol. 2021;141:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Niesen M, Trotta N, Noel A, Coolen T, Fayad G, Leurkin-Sterk G, Delpierre I, Henrard S, Sadeghi N, Goffard JC, Goldman S, De Tiège X. Structural and metabolic brain abnormalities in COVID-19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging. 2021;48:1890-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 30. | Izquierdo-Dominguez A, Rojas-Lechuga MJ, Mullol J, Alobid I. Olfactory Dysfunction in the COVID-19 Outbreak. J Investig Allergol Clin Immunol. 2020;30:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Xu Y, Baylink DJ, Chen CS, Reeves ME, Xiao J, Lacy C, Lau E, Cao H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J Transl Med. 2020;18:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 32. | Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S. Smell and taste dysfunction in patients with SARS-CoV-2 infection: A review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol. 2020;38:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Villani FA, Aiuto R, Paglia L, Re D. COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Kim H, Hegde S, LaFiura C, Raghavan M, Sun N, Cheng S, Rebholz CM, Seidelmann SB. Access to personal protective equipment in exposed healthcare workers and COVID-19 illness, severity, symptoms and duration: a population-based case-control study in six countries. BMJ Glob Health. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | de Toledo Telles-Araujo G, Caminha RDG, Kallás MS, Sipahi AM, da Silva Santos PS. Potential mouth rinses and nasal sprays that reduce SARS-CoV-2 viral load: What we know so far? Clinics (Sao Paulo). 2020;75:e2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | da Fonseca Orcina B, Vilhena FV, Cardoso de Oliveira R, Marques da Costa Alves L, Araki K, Toma SH, Ragghianti Zangrando MS, da Silva Santos PS. A Phthalocyanine Derivate Mouthwash to Gargling/Rinsing as an Option to Reduce Clinical Symptoms of COVID-19: Case Series. Clin Cosmet Investig Dent. 2021;13:47-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Cavazzana A, Larsson M, Münch M, Hähner A, Hummel T. Postinfectious olfactory loss: A retrospective study on 791 patients. Laryngoscope. 2018;128:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Buchaim RL, Barbalho SM, Hamzé AL, de Alvares Goulart R, Rocha KTP, Reis CHB. Loss of smell and COVID-19: Anatomical and physiological considerations. Int J Adv Eng Res Sci. 2020;7:278-280. [DOI] [Full Text] |
| 39. | Buchaim RL. Bioengineering applied to Covid-19 pandemic: from bench to bedside. AIMS Bioengineering. 2021;8:14-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1729] [Cited by in RCA: 1738] [Article Influence: 347.6] [Reference Citation Analysis (0)] |
| 41. | Young B, Fong SW, Chang Z, Tan KS, Rouers A, Goh YS, Tay DJW, Ong SWX, Hao Y, Chua SL. Comparison of the Clinical Features, viral Shedding and Immune Response in Vaccine Breakthrough Infection by the Omicron and Delta Variants. Research Square. 2022;. [DOI] [Full Text] |
| 42. | Vaira LA, De Vito A, Lechien JR, Chiesa-Estomba CM, Mayo-Yàñez M, Calvo-Henrìquez C, Saussez S, Madeddu G, Babudieri S, Boscolo-Rizzo P, Hopkins C, De Riu G. New Onset of Smell and Taste Loss Are Common Findings Also in Patients With Symptomatic COVID-19 After Complete Vaccination. Laryngoscope. 2022;132:419-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |