Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.237
Peer-review started: March 13, 2022
First decision: April 13, 2022
Revised: April 25, 2022
Accepted: June 20, 2022
Article in press: June 20, 2022
Published online: September 25, 2022
Processing time: 195 Days and 0.9 Hours
The coronavirus disease 2019 (COVID-19) pandemic continues to be a global problem with over 438 million cases reported so far. Although it mostly affects the respiratory system, the involvement of extrapulmonary organs, including the liver, is not uncommon. Since the beginning of the pandemic, metabolic com-orbidities, such as obesity, diabetes, hypertension, and dyslipidemia, have been identified as poor prognostic indicators. Subsequent metabolic and lipidomic studies have identified several metabolic dysfunctions in patients with COVID-19. The metabolic alterations appear to be linked to the course of the disease and inflammatory reaction in the body. The liver is an important organ with high metabolic activity, and a significant proportion of COVID-19 patients have metabolic comorbidities; thus, this factor could play a key role in orchestrating systemic metabolic changes during infection. Evidence suggests that metabolic dysregulation in COVID-19 has both short- and long-term metabolic implications. Furthermore, COVID-19 has adverse associations with metabolic-associated fatty liver disease. Due to the ensuing effects on the renin-angiotensin-aldosterone system and ammonia metabolism, COVID-19 can have significant implications in patients with advanced chronic liver disease. A thorough understanding of COVID-19-associated metabolic dysfunction could lead to the identification of important plasma biomarkers and novel treatment targets. In this review, we discuss the current understanding of metabolic dysfunction in COVID-19, focusing on the liver and exploring the underlying mechanistic pathogenesis and clinical implications.
Core Tip: In coronavirus disease 2019 (COVID-19) patients, the virus induces a complex viral-host interaction that leads to metabolic reprogramming, altered immunological responses, and a variety of clinical consequences. In metabolomic and lipidomic studies, a variety of alterations in amino acids, lipids, carbohydrates, and energy metabolism have been identified in such patients. The liver is the primary metabolic organ; thus, these metabolic alterations may have a major impact on patients with liver diseases and metabolic comorbidities that are common in COVID-19 patients. Therefore, this review article discusses the pathophysiological aspects and clinical implications of metabolic dysfunction in COVID-19 patients with a focus on the liver.
- Citation: Kumar R, Kumar V, Arya R, Anand U, Priyadarshi RN. Association of COVID-19 with hepatic metabolic dysfunction. World J Virol 2022; 11(5): 237-251
- URL: https://www.wjgnet.com/2220-3249/full/v11/i5/237.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i5.237
Patients with metabolic disorders such as obesity, hypertension, diabetes mellitus (DM), and non-alcoholic fatty liver disease (NAFLD) are more likely to develop a severe case of coronavirus disease 2019 (COVID-19)[1-5]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection per se is linked to the changes in numerous metabolic pathways involving glucose, lipids, and amino acids[6-8]. The metabolic reprogramming that occurs in COVID-19 patients performs several roles, including providing energy and substrates for viral replication and modulating the immunological response. Pre-existing metabolic comorbidities may fire up metabolic reprogramming more strongly due to the varying amounts of metabolites and their influence on the immune response. Untargeted metabolomic and lipidomic methods provide new insight into the host’s response to COVID-19 infection. Hyper-glycemia, new-onset DM, dyslipidemia, and worsening of pre-existing metabolic abnormalities have all been described in COVID-19 patients[9-11]. As the liver is a primary metabolic hub, it is crucial in orchestrating systemic metabolic alterations during infection. The angiotensin-converting enzyme 2 (ACE2) that allows SARS-CoV-2 to enter the body is normally present in the liver and is overexpressed in patients with chronic liver disease (CLD)[12,13]. Additionally, ACE2 is an integral part of the renin-angiotensin-aldosterone system (RAAS), which plays a major role in the pathophysiology of liver cirrhosis[14].
Obesity and DM have been associated with a poor disease prognosis since the outset of the COVID-19 pandemic[15,16]. As these metabolic conditions are still among the world's most common public health issues, a sizable section of the population is at risk of severe COVID-19 infection. Compelling evidence suggests that patients with metabolic comorbidities also have a higher risk of post-infection sequels[17,18]. Furthermore, NAFLD is common in subjects with obesity and DM. When compared to non-NAFLD COVID-19 patients, those with NAFLD have a higher risk of disease progression (6.6% vs 44.7%), a higher likelihood of impaired liver function (70% vs 11.1%), and a longer viral shedding period (17.5 vs 12.1 d)[19]. Moreover, patients with COVID-19 frequently have elevated liver enzyme levels, and this has been linked to poor clinical outcomes[20,21]. Similarly, COVID-19 has been proven to have a negative impact on the complications and outcomes of CLD patients[21].
Infections trigger a wide range of responses in the host, including inflammation, tissue injury, and healing. In this context, evidence suggests that COVID-19 has both immediate and long-term metabolic consequences associated with inflammation[4,7,17,22,23]. Immunometabolism, which is the direct link between metabolic diseases and inflammation, has recently emerged as a key study subject. Correlation analyses reveal strong links between metabolites and proinflammatory cytokines and chemokines[22,23]. In this sense, studies have found that arginine, tryptophan, and purine metabolism have a regulatory interaction with inflammation. Therefore, targeting metabolism to modulate the release of proinflammatory cytokines could be a viable method for treating cytokine storms in COVID-19 patients. This review article discusses the spectrum of metabolic dysfunctions in COVID-19 patients, their pathophysiological aspects, and clinical implications in patients with underlying metabolic comorbidities and liver disorders.
In patients with severe COVID-19, considerable changes in hepatic metabolic and biosynthetic pathways have been discovered[6-8,24-28]. Lipids, glycoproteins, amines, aromatic compounds, amino acids, steroids, and flavone metabolism were all found to be altered (Table 1). A study on liver autopsy samples of COVID-19 patients has demonstrated a significant downregulation of transcripts implicated in the metabolic pathways. The most downregulated genes were acyl-CoA dehydrogenases 11 (involved in mitochondrial β-oxidation and metabolism of long-chain fatty acyl-CoAs), CIDEB (a liver-specific regulator of lipids metabolism), glycine N-methyltransferase (contributes to liver steatosis and fibrosis), and glycerol-3-phosphate acyltransferase (implicated in triglyceride biosynthesis)[6,24-26]. The suppression of lipid and amino acid metabolism has resulted in the accumulation of amino acids and steroids in the sera of COVID-19 patients[7]. More than 100 Lipids were discovered to be downregulated in COVID-19 patient sera, including sphingolipids, glycerophospholipid, and fatty acids, most likely due to liver damage. Many steroid hormones, such as progesterone, androgens, and estrogens, have been found to accumulate, which can enhance immune cell activation. Increased levels of 21-hydroxypregnenolone could imply that corticosterone is protective against COVID-19[7].
| Metabolite alteration | Implications/association |
| Increased branched chain amino-acids | Insulin resistance, reactive oxygen species production, and pro-inflammatory responses |
| Decreased tryptophan; Increased kynurenine | Increased kynurenine tryptophan ratio indicates inflammatory response |
| Increased glutamic acid; Decreased glutamine | Lower glutamine level is associated with insulin resistance and an increased risk of diabetes |
| Decrease arginine; Increased ornithine | Attempt to suppress virus-specific CD8+ T cell. Delayed interferon response or metabolic syndrome tend to increase arginine/ornithine ratio, causing tissues damage |
| Increased spermidine and spermine | Help structural assembling and genome replication |
| Increased serum triglycerides and VLDL; Decreased total cholesterol, HDL and LDL; Upregulation of fatty acid synthesis | Viral replication, inflammation, atherogenic risk, hepatic steatosis |
| Increased ketone bodies and 2-hydroxybutyric acid | Altered energy metabolism and oxidative stress |
| Decreased glycerophospholipid; Increased lysophospholipids | Indicates inflammation and tissue damage |
| increased levels of pyruvate, pyruvate kinase and lactate dehydrogenase | Indicates enhanced glucose metabolism. Increased glycolysis promotes replication of SARS-CoV-2 and cytokine storm |
| Increased methionine sulfoxide levels; Decreased glutathione levels | Indicative of increased oxidative stress |
In this context, many such metabolic changes aid SARS-CoV-2 replication and have been linked to the severity of COVID-19 cases. Glycolysis and glutaminolysis were found to be required for virus replication[8,27]. In this regard, glutaminolysis is a process that converts glutamine to tricarboxylic acid (TCA) cycle intermediates and is required for protein, lipid, and nucleic acid production. Inhibiting glutaminolysis has been shown to impede viral replication and production. Furthermore, patients with severe COVID-19 disease had higher glucose and mannose levels in their blood[8], and mannose was found to be a reliable biomarker for the severity of COVID-19 disease. Chen YM found that the TCA cycle and glycolytic pathways were significantly dysregulated in COVID-19 patients[28]. Significant suppression of cytochrome P450 enzymes has been observed in COVID-19 patients, suggesting a compromised hepatic detoxification capacity[6]. In a metabolomic study, Shen et al[7] have detected elevated levels of glucuronate, which is a bilirubin degradation product, and bile acid derivatives in severe COVID-19 patients, also indicating a decline in the liver’s detoxification function. It is noteworthy that the suppressed hepatic metabolic pathways in COVID-19 patients are consistent with mitochondrial dysfunction[6]. In this regard, Scozzi et al[29] have reported that circulating levels of mitochondrial DNA (MT-DNA), inflammatory nucleic acids released by injured tissues, were highly elevated in patients who eventually died or required ICU admission. Thus, MT-DNA in blood could be a potential early prognostic marker for poor outcomes in COVID-19 cases. Moreover, mitochondrial dysfunction also appears to play a role in COVID-19-induced porphyrin accumulation occurring due to interference with the heme biosynthetic pathway[30]. Heme synthesis is dependent on the sequential action of eight enzymes, which are mainly expressed in the liver and erythroid cells.
Amino acids (AAs), which are mostly synthesized in the liver, are essential for metabolism, immunological function, and redox balance[31]. The potential effects of glutamine, arginine, methionine, and cysteine on immunological function have been well documented[32]. Branched-chain amino acids (BCAAs) play an important role in metabolism and inflammation. In this sense, BCAAs stimulate the synthesis of glycogen and proteins such as albumin via activating the mammalian target of rapamycin complex 1 (mTORC1) signaling[33]. Serine and glycine are important components of the one-carbon cycle, which aids redox balance and several biosynthetic activities[34].
BCAA levels in the blood increase in severe COVID-19 patients[35]. Through the transcription factor NF-kB, elevated levels of BCAA increase reactive oxygen species generation and proinflammatory responses in endothelial cells[33]. Additionally, BCAAs also cause insulin resistance (IR) via activating mTORC1[36]. Furthermore, increased BCAA levels in the blood are linked to a higher risk of metabolic diseases, including DM. On the other hand, a decrease in the BCAA/aromatic amino acids ratio, also known as Fischer's ratio, has been linked to hepatic impairment in COVID-19 patients[37]. A meta-bolomics study has linked the severity of COVID-19 to a reduction in serotonin and increased plasma levels of aspartate, glutamate, phenylalanine, and succinic acid[38]. The rise in such amino acids and succinic acid could be related to a dysregulation of central carbon metabolism in the liver as well as metabolic and oxidative stress.
On another note, changes in tryptophan metabolism along the kynurenine pathway have been reported in COVID-19 patients[39]. This pathway is activated by proinflammatory cytokines such as interleukin-6 (IL-6) in response to diverse situations. Indeed, the kynurenine and tryptophan ratio (KTR) is frequently used to assess inflammation and immunological responses in a variety of disorders. In COVID-19 patients, an increased KTR was also indicative of the disease severity and progression[40]. Other alterations indicative of hepatic dysfunction in COVID-19 patients include elevated levels of taurine and ethanolamine[37]. Increased taurine levels in the blood have been identified as indicators of liver failure. Furthermore, glutamate and glutamine are important in energy metabolism. In this regard, glutamic acid levels are higher in COVID-19 patients; however, glutamine levels are much lower, and this is linked to IR and an increased risk of DM[41]. Low glutamine levels in COVID-19 patients may be due to an abnormal cysteine catabolism secondary to increased hepatic glutathione biosynthesis, induced by proinflammatory cytokines[42]. On another note, the hepatic urea cycle is dysregulated in severe COVID-19 patients[40]. The urea cycle, which converts ammonia to urea, is the principal metabolic pathway implicated in detoxification processes, with a fumarate shunt connecting the urea cycle and the TCA cycle[43]. In moderate and severe COVID-19 patients, an increased level of ornithine, the main metabolite of the urea cycle, is observed. This, along with increased levels of aspartate and glutamate, which are also linked to the cycle, suggests that SARS-CoV-2 disturbs the hepatic urea cycle. Moreover, ornithine and glutamate demonstrate a positive correlation with lactic acid in severe COVID-19[38].
In viral infections, modification of liver metabolism and the urea cycle may be an endogenous immunoregulatory mechanism to minimize tissue damage[44]. The reprogramming of liver metabolism that occurs after a viral infection is correlated with type I interferon (IFN-I) responses. In this sense, the IFN-I response modifies the urea cycle, resulting in lower arginine and higher ornithine concentrations in the blood, thus suppressing virus-specific CD8+ T-cell responses and reducing the liver damage[45]. However, in COVID-19 patients, the IFN-I response is frequently delayed, and this may weaken the protective response. Metabolic syndrome, which is common in COVID-19 patients, causes decreased arginine availability and an elevated arginine/ornithine ratio, which may further worsen tissue damage[46]. Furthermore, the synthesis of polyamines may be increased if ornithine metabolism is dysregulated. Metabolomics analysis has reported increased levels of spermidine and spermine in the serum of COVID-19 patients[47]. As polyamines are involved in various viral activities, including viral assembly and genome replication, blocking polyamine synthesis could be a useful antiviral strategy.
Lipids play an important role throughout the viral life cycle, and viruses exploit host lipid metabolism to facilitate their replication. Several studies have looked at lipidomic profiling in COVID-19 patients. Even though these studies are heterogeneous, several consistent findings have been reported[48]. COVID-19 patients exhibited downregulation of several serum lipids, including sphingolipids, glycerophospholipids, and fatty acids[49]. The liver damage caused by SARS-CoV-2 infection has been linked to dyslipidemia and oxidative stress[50]. In this regard, the levels of blood triglycerides and very-low-density lipoprotein are significantly elevated, whereas the levels of high-density lipoprotein and low-density lipoprotein are much lower[37,50,51]. Notably, COVID-19-related dyslipidemia occurs primarily in patients with high severity and not in those who recover from a mild uneventful infection. Bruzzone et al[50] used nuclear magnetic resonance spectroscopy to determine the lipidomic serum profile of 389 COVID-19 patients, revealing a pathogenic redistribution of lipoprotein particle size and composition with atherosclerosis risk. In the same study, the metabolomics analysis revealed unusually high levels of ketone bodies, which are produced in the liver from free fatty acids, and 2-hydroxybutyric acid, which is a marker of oxidative stress and a consequence of glutathione synthesis in the liver. Ketosis in COVID-19 patients has been associated with a longer hospitalization and increased mortality rates[52]. Furthermore, a shift to fatty acid oxidation is a common metabolic response observed during many severe illnesses, and COVID-19 is no exception[49,53]. In this regard, a reduction in sphingosine-1-phosphate (S1P), which is a sphingosine molecule that regulates a variety of biological processes such as inflammation and apoptosis, is observed in COVID-19 patients[7,54]. In a study, serum level of S1P was found to be inversely associated with COVID-19 severity[55]. Additionally, glycerophospholipid levels are also reduced, whereas the levels of the corresponding lysophospholipids are increased, indicating increased phospholipase A2 activation[7,54,56]. Elevated levels of phospholipase A2 may be an early marker of severe COVID-19.
On another note, SARS-CoV-2 infection is also associated with dysregulated glucose metabolism. Regardless of previous diabetes status, hyperglycemia frequently develops in COVID-19 patients, and many develop new-onset DM and diabetes ketoacidosis[9-11,57]. Furthermore, the abnormal glucose metabolism has been reported to persist even after recovery from COVID-19. In a hospitalized sample of 551 COVID-19 patients, 46% were hyperglycemic, and glycemic abnormalities were detected for at least 2 mo following COVID-19 recovery[58]. In several observational studies, more severe hyperglycemia has been linked to a worse prognosis in COVID-19 patients[2,8,9,10,59]. IR and/or decreased insulin production are the primary causes of abnormal glucose metabolism in COVID-19 patients, and proinflammatory cytokines play an essential role in this process. The increased glucose metabolism due to sustained hyperglycemia may further enhance entry of SARS-CoV-2, with exacerbated immune response[60]. In this sense, elevated glucose levels and glycolysis lead to an increase in SARS-CoV-2 replication[27,61]. Furthermore, COVID-19 causes glycemic control to deteriorate in patients with pre-existing DM, and new-onset hyperglycemia is an independent predictor of mortality in such patients[11,62,63]. As glycemic control deteriorates, the severity of illness and the risk of mortality increases.
The occurrence of metabolic dysfunction in COVID-19 has been well documented; however, the molecular mechanisms behind these dysfunctions are sparsely known. Infection with SARS-CoV-2 can affect several metabolic organs such as the liver, pancreas, adipose tissue, and muscles, either directly or indirectly. Proinflammatory cytokines, oxidative stress, and IR all appear to contribute to metabolic dysregulation in COVID-19, and the association between metabolism and inflammation is well-known and still being investigated. The by-products of glycolysis increase cytokine maturation and, as a result, T-cell proliferation[64]. One such glycolytic metabolite necessary for IL-1β synthesis is 3-phosphoglycerate. Moreover, alterations in the levels of fatty acids and tryptophan metabolites have been associated with inflammatory markers in COVID-19 patients[40]. A study found high-affinity interactions between the viral spike protein and toll-like receptors (TLRs), particularly TLR4[65]. TLR4 activation is known to cause inflammation and cellular metabolic alterations[66]. Additionally, hyperglycemia has been linked to delayed IFN response and cytokine storm in COVID-19 patients[67,68].
The interaction between the spike protein and ACE2 allows SARS-CoV-2 to enter host cells. Virus entry is facilitated through the priming of spike proteins by specific proteases such as transmembrane serine protease 2 (TMPRSS2) and furin protease. At first, SARS-CoV-2 targets epithelial cells in the lungs; however, viral RNA has been found in a variety of organs, including the liver, suggesting that other organs could be targeted as well. ACE2 is expressed by many cells, and its expression is further upregulated in a variety of conditions, including inflammatory and liver diseases[12,69]. As a result, increased ACE2 expression could be a risk factor as well as an effect of SARS-CoV-2 infection. In particular, the delta and omicron variants of SARS-CoV-2 have an even higher affinity for ACE2 than other variants[70]. While ACE2 expression is low in healthy livers, cirrhotic livers exhibit higher levels of ACE2 expression[12]. As a result, patients with liver cirrhosis may be more susceptible to SARS-CoV-2 infection.
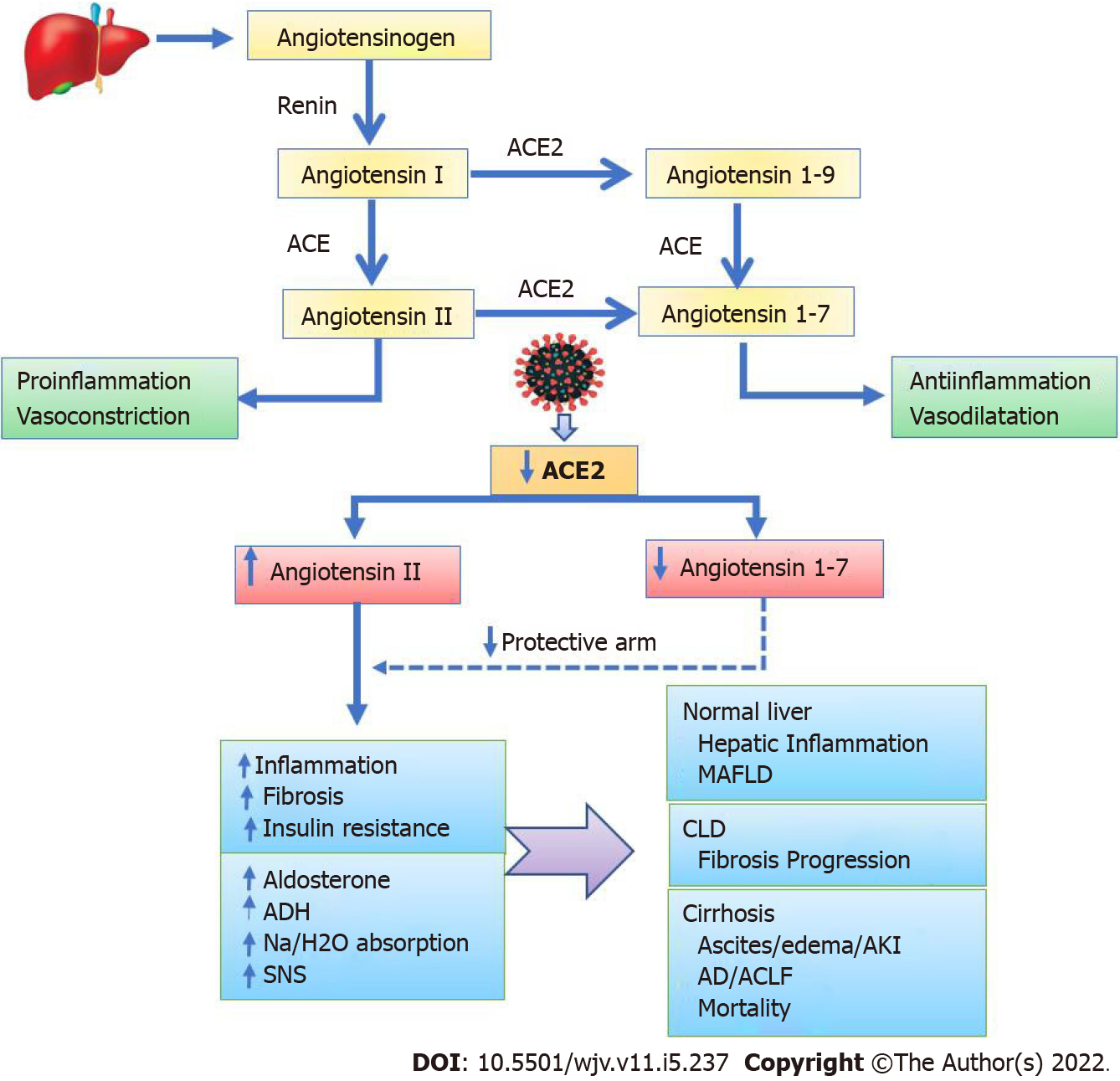
SARS-CoV-2 infection significantly influences the RAAS since ACE2 is a key part of it[71]. The discovery of functional local RAAS in several organs, including the liver, has changed our knowledge of the RAAS[14]. Alternative RAAS pathways mediated by ACE2 in the local RAAS result in the opposite effects of classic RAAS (Figure 1). ACE2 is a major regulator in the alternative RAAS pathways, regulating the production of angiotensin 1–7 (Ang 1-7) from angiotensin II (Ang II). Additionally, ACE2 converts angiotensin I to angiotensin 1–9 (Ang 1-9), which can be further converted to Ang 1–7 by the angiotensin-converting enzyme. Importantly, the protective arm of the RAAS is made up of ACE2, Ang 1–7, and its Mas receptor, and this results in anti-inflammatory and antifibrotic responses (Figure 1). However, when ACE2 is downregulated, Ang II gets upregulated, and upon binding to the Ang II receptors, it causes proinflammatory, profibrotic, vasoconstrictive, and antidiuretic responses that can lead to end-organ damage[71]. The plasma level Ang II rises in COVID-19 patients and is linearly associated with viral load[72]. Ultimately, SARS-CoV-2 infection causes inflammatory reactions due to the downregulation of ACE2[71-73]. Generally, ACE2 coupled to virions is internalized, reducing its availability on the cellular surface. Moreover, some unknown mechanism induces the gene expression of disintegrins and metalloproteinase domain-17 (ADAM-17)[74]. ADAM-17 is a membrane sheddase protease that releases ACE2, IL-4, and IFN from cell membranes. Finally, free IFN-γ and IL-4 suppress membrane-bound ACE2, further shifting the RAAS to a higher Ang II and lower Ang1-7 tone[71,74].
The one-carbon pathway is a metabolic network that include the methionine and folate cycles and is involved in many biological functions such as synthesis of amino acids, polyamines, nucleic acids, adenosine triphosphate, phospholipids and glutathione[75]. In particular, the metabolic pathways of methionine, folate, and choline have been implicated in the pathogenesis of hepatic steatosis[76]. One-carbon metabolism appears to have a crucial role in COVID-19[78-83]. In this regard, SARS-CoV-2 uses folate and one-carbon metabolism to gain a competitive advantage in replication[77]. It modifies host folate metabolism at the post-transcriptional level to enhance de novo purine synthesis. Several observational studies on COVID-19 patients have linked one-carbon metabolism to the disease severity, although mechanistic insights are still being developed[78]. The results of various studies on the link between one-carbon metabolism and COVID-19 have been varied and conflicting, except for a few metabolites such as glutathione, choline, and methionine sulfoxide, which were consistently altered by COVID-19[78]. These discrepancies could be related to the confounding effects of non-matched study subjects, variances in disease severity, and the time points at which samples were collected in different studies.
Furthermore, metabolomic studies have ascertained that S-adenosylmethionine (SAM), the universal methyl donor, is significantly increased in severe and fatal cases of COVID-19[79,80]. As the generation of SAM requires vitamin B12-dependent methionine synthase, many symptoms of long COVID-19 are similar to those of vitamin B12 deficiency, a condition in which methylation is disturbed[81]. Multiple independent metabolic studies have reported higher serum levels of methionine sulfoxide in COVID-19 patients, implying increased oxidative stress[82,83]. Moreover, glutathione, the most important an-tioxidant, is consistently depleted in COVID-19 patients and is often associated with increased lipid peroxidation markers[84]. In children with mild COVID-19, higher levels of methylmalonic acid (MMA), which is a catabolic product of certain amino acids, have been found[83]. A vitamin B12-dependent enzyme further metabolizes MMA to succinic acid, which is a TCA cycle substrate. The antiviral and anti-inflammatory properties of MMA are thought to protect children from severe infection. Polyamines, including as spermidine and spermine, have been found to have a role in the replication and attachment of SARS-CoV-2, with serum levels of these compounds being greater in COVID-19 patients[47]. Overall, it appears that the virus exploits one-carbon metabolism pathways for its replicative advantages, producing metabolic disturbance in the host cells.
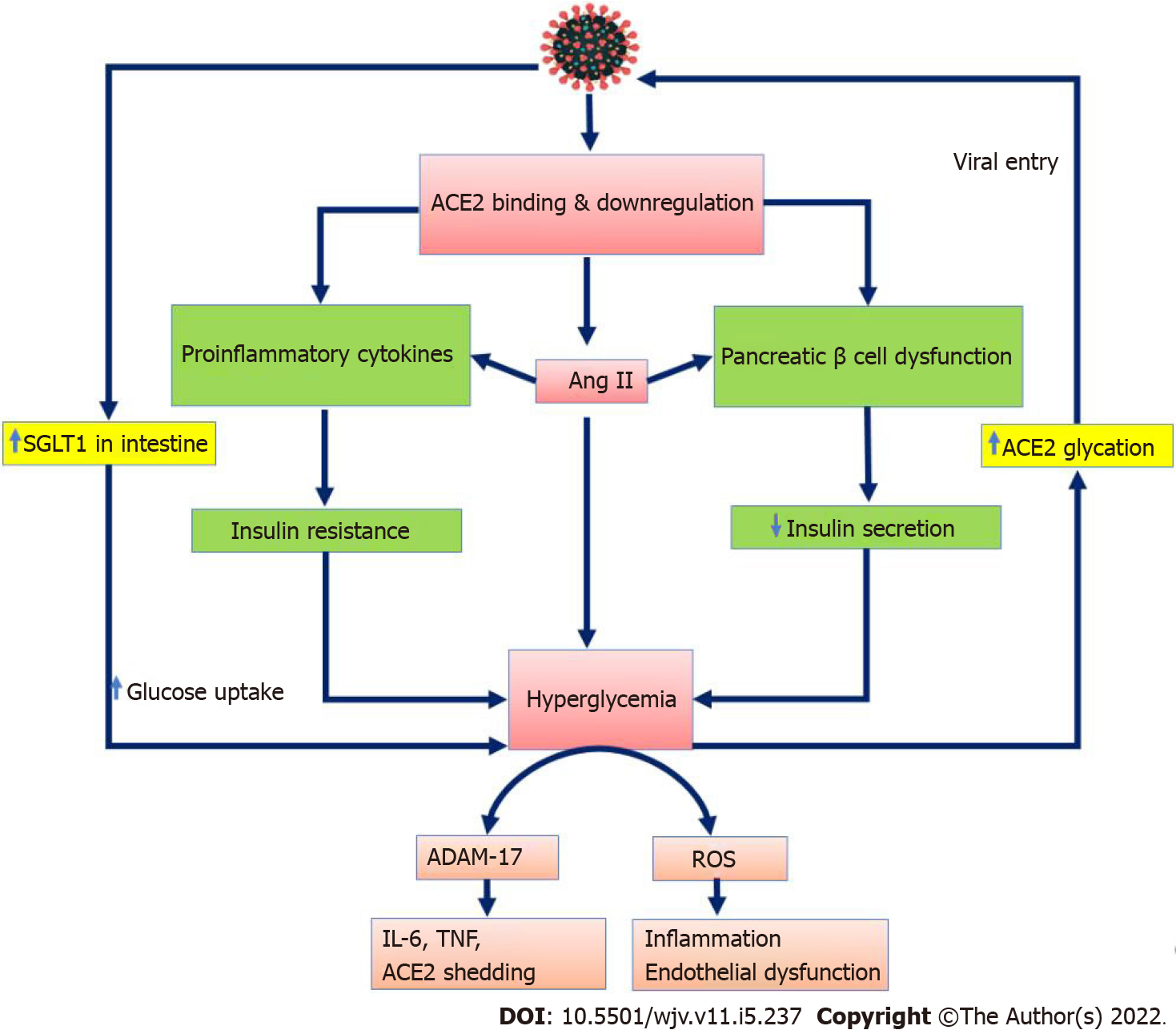
The pathophysiological basis of hyperglycemia in COVID-19 patients appears to be the development of IR and pancreatic β-cell dysfunction (Figure 2). Peripheral IR is caused by SARS-CoV-2-induced hyperinflammation and cytokine storm. Metaflammation, defined as a rise in TNF, IL-6, and IL-1 Levels in patients with metabolic syndrome, can further increase IR[85]. Furthermore, pancreatic damage with subsequent impairment of insulin secretion is evident in COVID-19 patients[10,86]. However, SARS-CoV-2 does not appear to infect β-cells. directly, as ACE2 and TMPRSS2 have only been detected in pancreatic microvasculature and ducts, not in β-cells[87]. Pancreatic injury caused by SARS-CoV-2 increases the release of pancreatic lipase, resulting in lipolysis and the release of unsaturated fatty acids, thus causing mitochondrial damage and inflammation[86,88]. When ACE2 is downregulated in the intestinal epithelium, SGLT1 is upregulated, resulting in hyperglycemia[89]. The unopposed action of Ang II leads to oxidative stress that triggers β-cell damage and further impairment of insulin secretion. Hyperglycemia per se can cause β-cell dysfunction by upregulating the Ang II receptor on β-cells and causing glucolipotoxicity[11]. Furthermore, persistent hyperglycemia may exacerbate COVID-19 by glycating ACE2, which facilitates the entry of SARS-CoV-2[10]. Recently, a circulating protein GP73, which is a glucogenic hormone that enhances hepatic gluconeogenesis, has been found in COVID-19 patients, and it appears to modulate SARS-CoV-2-induced glucose metabolic alteration[90].
Multiple studies have found that metabolic comorbidities are more common in COVID-19 patients and are associated with poorer outcomes. However, the pathophysiologic mechanisms that underpin this adverse metabolic interaction are still poorly understood. The proinflammatory environment in patients with metabolic disorders may aggravate immune dysregulation, inflammation, microvascular dysfunction, and thrombosis, which may intensify the essential interaction between virus and host components. Additionally, patients with metabolic illnesses are more likely to respond to infection in a proinflammatory rather than protective manner, which could contribute to increased cytokines in COVID-19 infection. Obesity[91,92], DM[2,59], hypertension[93], dyslipidemia[94,95], and metabolic-associated fatty liver (MAFLD)[5,96] have all been shown to be associated with a more severe disease course and increased mortality in COVID-19 (Table 2). A pooled data analysis of 20 studies determined that obese individuals had a 46% (Odds ratio [OR]: 1.46) higher chance of testing positive for COVID-19 than non-obese people[97]. Moreover, a history of prior bariatric surgery is associated with a reduced severity in COVID-19 patients[98]. In severe COVID-19 patients, the prevalence of DM (OR: 3.5) and hypertension (OR: 2.6) is higher than that in non-severe patients[99]. In a meta-analysis of 33 studies, including 16003 COVID-19 patients, the pooled odds ratio of mortality or severity in presence of DM was 2.16 (95%; CI: 1.74-2.68; P < 0.01)[59]. Poor outcome of COVID-19 associated with DM or hyper-glycemia may be attributed to higher glucose levels that provide huge substrates for increased glycolysis thus producing energy and substrates for SARS-CoV-2 replication. On the other hand, improved glycemic control is associated with better outcomes in COVID-19 patients with DM[62]. Lactic acidosis has been documented frequently in severe COVID-19 patients with DM treated with metformin[100]. A meta-analysis of 7 studies (n = 6922) showed that dyslipidemia is associated with severe COVID-19 infections [RR 1.39][95].
| Ref. | Metabolic condition | COVID-19 (N); Studies/Patients | Main results |
| Ho et al[91] | Obesity | 61/270241 | Obesity was associated with more severe disease (OR 3.13, 95%CI: 1.41-6.92) and mortality (OR 1.36, 95%CI: 1.09-1.69) |
| Yang et al[92] | Obesity | 50/18 260 378 | Obesity was associated with a higher risk of SARS-CoV2 infection (OR: 1.39, 95%CI: 1.25-1.54), increased disease severity (OR: 3.74, 95%CI: 1.18-11.87) and mortality (OR: 1.65, 95%CI: 1.21-2.25) |
| Huang et al[2] | DM | 30/6452 | DM was associated with composite poor outcome (RR 2.38 [1.88, 3.03], P < 0.001) |
| Kumar et al[59] | DM | 33/16003 | The combined corrected pooled OR of mortality or severity was 2.16 (95%CI: 1.74-2.68; P < 0.01) |
| Atmosudigdo et al[94] | Dyslipidemia | 09/3663 | Dyslipidemia was associated with poor outcome (RR 1.39 [1.02, 1.88], more so in patients with older age, male, and hypertension |
| Hariyanto et al[95] | Dyslipidemia | 07/6922 | Dyslipidemia was associated with severe disease (RR 1.39 (95%CI: 1.03-1.87) |
| Du et al[93] | Hypertension | 24/99918 | Patients with hypertension had a 1.82-fold higher risk for critical COVID-19 (OR: 1.82; 95%CI: 1.19-2.77; P = 0.005) and a 2.17-fold higher risk for COVID-19 mortality (OR: 2.17; 95% CI: 1.67-2.82; P < 0.001) |
| Zuin et al[4] | Metabolic syndrome | 06/209.569 | Pre-existing metabolic syndrome was associated with higher risk of mortality (OR: 2.30, 95%CI: 1.52-3.45). Meta-regression showed a direct correlation with hypertension, DM and hyperlipidaemia |
| Tao et al[5] | MAFLD | 07/2141 | MAFLD increased the risk of severe COVID-19 (OR: 1.80, 95%Cl: 1.53-2.13) |
| Pan et al[96] | MAFLD | 06/1293 | MAFLD increased the risk of disease severity, with a pooled OR of 2.93 (95%CI: 1.87, 4.60) |
SARS-CoV-2 produces steatosis and lobular and portal inflammation in the liver[101]. Microthrombi have been found in the hepatic sinusoids in fatal cases due to coagulopathy and endothelial dysfunction. Despite the preponderance of ACE2 on the biliary epithelium, significant cholestasis is rare. In a histological study of patients who died of complications of COVID-19, macrovesicular steatosis was the most common finding as it was observed in 75% of patients, and PCR for viral ribonucleic acid in liver tissue was positive in 55% of patients tested[102]. Such a high frequency of hepatic steatosis suggests a role of some metabolic derangements associated with COVID infection, which in turn lead to fatty liver disease. In this regard, COVID-19 patients with NAFLD have a higher risk of developing liver injury[103], a higher risk of disease progression (44.7% vs 6.6%), more likelihood of impaired liver function, and a longer viral shedding time compared to those without NAFLD[19]. It is noteworthy that NAFLD is now known as MAFLD, which refers to the hepatic manifestation of metabolic health. In two meta-analyses, MAFLD was found to increase the risk of severe COVID-19 (OR: 1.8 and 2.9, respectively)[4,5]. After adjusting for confounders, the pooled OR for severe COVID-19 in NAFLD was 2.358, demonstrating that NAFLD alone, without confounding factors, may contribute to worse COVID-19 outcomes; however, the exact explanation is still unknown[104]. Therefore, further research on the impact of NAFLD in COVID-19 patients is needed.
In patients with liver cirrhosis, the RAAS plays a key role in the development of portal hypertension and ascites[14,105,106]. The hyperdynamic circulation seen in portal hypertension is caused by overexpression of ACE2 and enhanced Ang1-7 production in the mesenteric arterioles[105,106]. As per the combined SECURE-liver and COVID-Hep registries, 38% of patients with cirrhosis and COVID-19 had worsening ascites, acute kidney injury (AKI), or encephalopathy[107]. In cirrhosis, RAAS activation occurs as a compensatory response to the systemic and splanchnic arterial vasodilation, resulting in renal water and sodium retention, which contributes to the development of the complications of cirrhosis such as ascites and AKI[14,108,109]. COVID-19 can increase the risk of these complications by interacting with the RAAS. On another note, hyperammonemia has been reported in COVID-19 patients, and it could be linked to hepatic dysfunction and urea cycle interference[110]. Ammonia is a neurotoxin that affects astrocytes and plays a role in the development of cerebral edema and hepatic encephalopathy. By causing IR and pancreatic dysfunction, COVID-19 can increase the risk of hepatogenous diabetes in patients with liver cirrhosis, and it can aggravate pre-existing gut dysbiosis in cirrhosis. On the one hand, gut dysbiosis can result in the translocation of endotoxins and bacteria leading to inflammation; on the other hand, it reduces the anti-inflammatory effects by reducing the production of commensal bacterial metabolites such as butyrate, bile acid derivatives, and indole[111]. Overall, the proinflammatory environment with metabolic alterations in COVID-19 patients with liver cirrhosis leads to the development of acute decompensation, acute-on-chronic liver failure, and increased mortality[109,112].
During the early convalescence of COVID-19 patients, distinct profiles of metabolites and cytokines have been observed. One study reported a reduction in saturated fat palmitic acid while unsaturated fatty acids such as docosapentaenoic acid and docosahexaenoic acid were elevated[29]. These changes correspond to the prevention of hepatocyte apoptosis and facilitation of liver repair. Furthermore, a rise in tryptophan levels was observed, and this could aid in the reversal of liver injury by preserving protein synthesis activity[113]. On another note, the glycemic abnormalities persist for at least 2 mo following recovery from COVID-19[58]. A long-term follow-up study on patients who had recovered from the original SARS-CoV-1 infection found a significant prevalence of hyperlipidemia (68%) and glycemic abnormalities (60%)[114]. Given the structural similarity of the SARS-CoV-2 virus to the original SARS-CoV-1 virus, comparable outcomes can be expected; however, this remains to be seen. The metabolic abnormality appears to persist more in patients with metabolic comorbidities. In this sense, a study with 1-year follow-up following discharge reported significant abnormalities in metabolic indicators such as blood lipids, uric acid, and liver function in obese COVID-19 patients compared to non-obese ones[18]. Another study demonstrated incomplete metabolic phenorversion in post-COVID patients. Even though most metabolic markers showed a high level of normalization, plasma taurine, and lower glutamine/glutamate ratios indicated little normalization in the majority of patients, indicating probable liver and muscle injury[17]. Further research is needed to determine the long-term clinical implications of these findings. In a study published recently, MAFLD was highly prevalent after hospital discharge, indicating potential long-term metabolic health implications. The prevalence of MAFLD was 55.3% at follow-up, while it was 37.3% on admission[115]. As metabolic alterations such as dysglycemia, hyperlipidemia, and inflammation are important in the progression of MAFLD, a high prevalence of MAFLD-induced advanced CLD may be expected in the years to come.
Human SARS-CoV-2 infection triggers a complex viral-host interaction that results in metabolic reprogramming, altered immunological response, and a variety of clinical consequences. As the liver is the metabolic hub of the body, it is targeted in this process. In metabolomics and lipidomic studies on COVID-19 patients, a variety of alterations in amino acids, lipids, carbohydrates, and energy metabolism have been identified. Although the impact of each metabolic change remains to be determined, pathophysiological alterations in the RAAS, insulin sensitivity, pancreatic functions, biosynthesis pathways, and ammonia metabolism can be used to make various extrapolations in the clinical setting. Furthermore, evidence suggests a direct link between metabolic changes and inflammatory responses in the body. Patients with underlying low-grade chronic inflammation, such as metabolic syndrome or CLD, may be particularly affected by COVID-19-induced metabolic changes. Therefore, obesity, DM, hypertension, dyslipidemia, and MAFLD in COVID-19 patients have all been associated with a more severe disease course and higher mortality. Moreover, preliminary data suggest that metabolic changes in COVID-19 can also have long-term health implications. Improved metabolic parameters such as blood glucose, blood pressure, and body weight may help control the systemic inflammatory response and reduce the severity of COVID-19 disease. Furthermore, metabolic changes may reflect the molecular profile of SARS-CoV-2-infected individuals, opening up new avenues for targeted therapeutic interventions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corrales FJ, Spain; Salim J, Indonesia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. 2021;93:257-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 2. | Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 3. | Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 4. | Zuin M, Rigatelli G, Bilato C, Cervellati C, Zuliani G, Roncon L. Prognostic Role of Metabolic Syndrome in COVID-19 Patients: A Systematic Review Meta-Analysis. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Tao Z, Li Y, Cheng B, Zhou T, Gao Y. Risk of Severe COVID-19 Increased by Metabolic Dysfunction-associated Fatty Liver Disease: A Meta-analysis. J Clin Gastroenterol. 2021;55:830-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Hammoudeh SM, Hammoudeh AM, Bhamidimarri PM, Mahboub B, Halwani R, Hamid Q, Rahmani M, Hamoudi R. Insight into molecular mechanisms underlying hepatic dysfunction in severe COVID-19 patients using systems biology. World J Gastroenterol. 2021;27:2850-2870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, Quan S, Zhang F, Sun R, Qian L, Ge W, Liu W, Liang S, Chen H, Zhang Y, Li J, Xu J, He Z, Chen B, Wang J, Yan H, Zheng Y, Wang D, Zhu J, Kong Z, Kang Z, Liang X, Ding X, Ruan G, Xiang N, Cai X, Gao H, Li L, Li S, Xiao Q, Lu T, Zhu Y, Liu H, Guo T. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182:59-72.e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 1091] [Article Influence: 218.2] [Reference Citation Analysis (0)] |
| 8. | Krishnan S, Nordqvist H, Ambikan AT, Gupta S, Sperk M, Svensson-Akusjärvi S, Mikaeloff F, Benfeitas R, Saccon E, Ponnan SM, Rodriguez JE, Nikouyan N, Odeh A, Ahlén G, Asghar M, Sällberg M, Vesterbacka J, Nowak P, Végvári Á, Sönnerborg A, Treutiger CJ, Neogi U. Metabolic Perturbation Associated With COVID-19 Disease Severity and SARS-CoV-2 Replication. Mol Cell Proteomics. 2021;20:100159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Albulescu R, Dima SO, Florea IR, Lixandru D, Serban AM, Aspritoiu VM, Tanase C, Popescu I, Ferber S. COVID-19 and diabetes mellitus: Unraveling the hypotheses that worsen the prognosis (Review). Exp Ther Med. 2020;20:194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Singh AK, Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract. 2020;167:108382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 12. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Brojakowska A, Narula J, Shimony R, Bander J. Clinical Implications of SARS-CoV-2 Interaction With Renin Angiotensin System: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:3085-3095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 14. | Di Pascoli M, La Mura V. Renin-angiotensin-aldosterone system in cirrhosis: There's room to try! Dig Liver Dis. 2019;51:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6518] [Article Influence: 1303.6] [Reference Citation Analysis (0)] |
| 16. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18878] [Article Influence: 3775.6] [Reference Citation Analysis (7)] |
| 17. | Holmes E, Wist J, Masuda R, Lodge S, Nitschke P, Kimhofer T, Loo RL, Begum S, Boughton B, Yang R, Morillon AC, Chin ST, Hall D, Ryan M, Bong SH, Gay M, Edgar DW, Lindon JC, Richards T, Yeap BB, Pettersson S, Spraul M, Schaefer H, Lawler NG, Gray N, Whiley L, Nicholson JK. Incomplete Systemic Recovery and Metabolic Phenoreversion in Post-Acute-Phase Nonhospitalized COVID-19 Patients: Implications for Assessment of Post-Acute COVID-19 Syndrome. J Proteome Res. 2021;20:3315-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Shang L, Wang L, Zhou F, Li J, Liu Y, Yang S. Long-term effects of obesity on COVID-19 patients discharged from hospital. Immun Inflamm Dis. 2021;9:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 20. | Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol. 2021;13:522-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Saviano A, Wrensch F, Ghany MG, Baumert TF. Liver Disease and Coronavirus Disease 2019: From Pathogenesis to Clinical Care. Hepatology. 2021;74:1088-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Batabyal R, Freishtat N, Hill E, Rehman M, Freishtat R, Koutroulis I. Metabolic dysfunction and immunometabolism in COVID-19 pathophysiology and therapeutics. Int J Obes (Lond). 2021;45:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Sathish T, Tapp RJ, Cooper ME, Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2021;47:101204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Li JZ, Ye J, Xue B, Qi J, Zhang J, Zhou Z, Li Q, Wen Z, Li P. Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes. 2007;56:2523-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | He M, Pei Z, Mohsen AW, Watkins P, Murdoch G, Van Veldhoven PP, Ensenauer R, Vockley J. Identification and characterization of new long chain acyl-CoA dehydrogenases. Mol Genet Metab. 2011;102:418-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Varela-Rey M, Martínez-López N, Fernández-Ramos D, Embade N, Calvisi DF, Woodhoo A, Rodríguez J, Fraga MF, Julve J, Rodríguez-Millán E, Frades I, Torres L, Luka Z, Wagner C, Esteller M, Lu SC, Martínez-Chantar ML, Mato JM. Fatty liver and fibrosis in glycine N-methyltransferase knockout mice is prevented by nicotinamide. Hepatology. 2010;52:105-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo Dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32:498-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 28. | Chen YM, Zheng Y, Yu Y, Wang Y, Huang Q, Qian F, Sun L, Song ZG, Chen Z, Feng J, An Y, Yang J, Su Z, Sun S, Dai F, Chen Q, Lu Q, Li P, Ling Y, Yang Z, Tang H, Shi L, Jin L, Holmes EC, Ding C, Zhu TY, Zhang YZ. Blood molecular markers associated with COVID-19 immunopathology and multi-organ damage. EMBO J. 2020;39:e105896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 29. | Scozzi D, Cano M, Ma L, Zhou D, Zhu JH, O'Halloran JA, Goss C, Rauseo AM, Liu Z, Sahu SK, Peritore V, Rocco M, Ricci A, Amodeo R, Aimati L, Ibrahim M, Hachem R, Kreisel D, Mudd PA, Kulkarni HS, Gelman AE. Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 30. | San Juan I, Bruzzone C, Bizkarguenaga M, Bernardo-Seisdedos G, Laín A, Gil-Redondo R, Diercks T, Gil-Martínez J, Urquiza P, Arana E, Seco M, García de Vicuña A, Embade N, Mato JM, Millet O. Abnormal concentration of porphyrins in serum from COVID-19 patients. Br J Haematol. 2020;190:e265-e267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Miyajima M. Amino acids: key sources for immunometabolites and immunotransmitters. Int Immunol. 2020;32:435-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 32. | Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 987] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 33. | Zhenyukh O, Civantos E, Ruiz-Ortega M, Sánchez MS, Vázquez C, Peiró C, Egido J, Mas S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med. 2017;104:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 34. | Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1211] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 35. | Wu J, Zhao M, Li C, Zhang Y, Wang DW. The SARS-CoV-2 induced targeted amino acid profiling in patients at hospitalized and convalescent stage. Biosci Rep. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 1028] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 37. | Kimhofer T, Lodge S, Whiley L, Gray N, Loo RL, Lawler NG, Nitschke P, Bong SH, Morrison DL, Begum S, Richards T, Yeap BB, Smith C, Smith KGC, Holmes E, Nicholson JK. Integrative Modeling of Quantitative Plasma Lipoprotein, Metabolic, and Amino Acid Data Reveals a Multiorgan Pathological Signature of SARS-CoV-2 Infection. J Proteome Res. 2020;19:4442-4454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 38. | Caterino M, Costanzo M, Fedele R, Cevenini A, Gelzo M, Di Minno A, Andolfo I, Capasso M, Russo R, Annunziata A, Calabrese C, Fiorentino G, D'Abbraccio M, Dell'Isola C, Fusco FM, Parrella R, Fabbrocini G, Gentile I, Castaldo G, Ruoppolo M. The Serum Metabolome of Moderate and Severe COVID-19 Patients Reflects Possible Liver Alterations Involving Carbon and Nitrogen Metabolism. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Essa MM, Hamdan H, Chidambaram SB, Al-Balushi B, Guillemin GJ, Ojcius DM, Qoronfleh MW. Possible role of tryptophan and melatonin in COVID-19. Int J Tryptophan Res. 2020;13:1178646920951832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, Hudson KE, Zimring JC, Hansen KC, Hod EA, Spitalnik SL, D'Alessandro A. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 414] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 41. | Liu X, Zheng Y, Guasch-Ferré M, Ruiz-Canela M, Toledo E, Clish C, Liang L, Razquin C, Corella D, Estruch R, Fito M, Gómez-Gracia E, Arós F, Ros E, Lapetra J, Fiol M, Serra-Majem L, Papandreou C, Martínez-González MA, Hu FB, Salas-Salvadó J. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: Case-cohort study within the PREDIMED trial. Nutr Metab Cardiovasc Dis. 2019;29:1040-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Bilinsky LM, Reed MC, Nijhout HF. The role of skeletal muscle in liver glutathione metabolism during acetaminophen overdose. J Theor Biol. 2015;376:118-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Shambaugh GE 3rd. Urea biosynthesis I. The urea cycle and relationships to the citric acid cycle. Am J Clin Nutr. 1977;30:2083-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Lercher A, Bhattacharya A, Popa AM, Caldera M, Schlapansky MF, Baazim H, Agerer B, Gürtl B, Kosack L, Májek P, Brunner JS, Vitko D, Pinter T, Genger JW, Orlova A, Pikor N, Reil D, Ozsvár-Kozma M, Kalinke U, Ludewig B, Moriggl R, Bennett KL, Menche J, Cheng PN, Schabbauer G, Trauner M, Klavins K, Bergthaler A. Type I Interferon Signaling Disrupts the Hepatic Urea Cycle and Alters Systemic Metabolism to Suppress T Cell Function. Immunity. 2019;51:1074-1087.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 45. | Nishio A, Rehermann B. Virus-Induced Interferon Regulates the Urea Cycle. Immunity. 2019;51:975-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Moon J, Kim OY, Jo G, Shin MJ. Alterations in Circulating Amino Acid Metabolite Ratio Associated with Arginase Activity Are Potential Indicators of Metabolic Syndrome: The Korean Genome and Epidemiology Study. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Firpo MR, Mastrodomenico V, Hawkins GM, Prot M, Levillayer L, Gallagher T, Simon-Loriere E, Mounce BC. Targeting Polyamines Inhibits Coronavirus Infection by Reducing Cellular Attachment and Entry. ACS Infect Dis. 2021;7:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Theken KN, Tang SY, Sengupta S, FitzGerald GA. The roles of lipids in SARS-CoV-2 viral replication and the host immune response. J Lipid Res. 2021;62:100129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 49. | Casari I, Manfredi M, Metharom P, Falasca M. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog Lipid Res. 2021;82:101092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 50. | Bruzzone C, Bizkarguenaga M, Gil-Redondo R, Diercks T, Arana E, García de Vicuña A, Seco M, Bosch A, Palazón A, San Juan I, Laín A, Gil-Martínez J, Bernardo-Seisdedos G, Fernández-Ramos D, Lopitz-Otsoa F, Embade N, Lu S, Mato JM, Millet O. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience. 2020;23:101645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 51. | Meoni G, Ghini V, Maggi L, Vignoli A, Mazzoni A, Salvati L, Capone M, Vanni A, Tenori L, Fontanari P, Lavorini F, Peris A, Bartoloni A, Liotta F, Cosmi L, Luchinat C, Annunziato F, Turano P. Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog. 2021;17:e1009243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 52. | Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935-1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 53. | Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, Zhang Q, Lu T, Yue L, Chen S, Li X, Sun Y, Li L, Xu L, Li Y, Yang M, Xue Z, Liang S, Ding X, Yuan C, Peng L, Liu W, Yi X, Lyu M, Xiao G, Xu X, Ge W, He J, Fan J, Wu J, Luo M, Chang X, Pan H, Cai X, Zhou J, Yu J, Gao H, Xie M, Wang S, Ruan G, Chen H, Su H, Mei H, Luo D, Zhao D, Xu F, Zhu Y, Xia J, Hu Y, Guo T. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775-791.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 314] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 54. | Hao Y, Zhang Z, Feng G, Chen M, Wan Q, Lin J, Wu L, Nie W, Chen S. Distinct lipid metabolic dysregulation in asymptomatic COVID-19. iScience. 2021;24:102974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Marfia G, Navone S, Guarnaccia L, Campanella R, Mondoni M, Locatelli M, Barassi A, Fontana L, Palumbo F, Garzia E, Ciniglio Appiani G, Chiumello D, Miozzo M, Centanni S, Riboni L. Decreased serum level of sphingosine-1-phosphate: a novel predictor of clinical severity in COVID-19. EMBO Mol Med. 2021;13:e13424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 56. | Barberis E, Timo S, Amede E, Vanella VV, Puricelli C, Cappellano G, Raineri D, Cittone MG, Rizzi E, Pedrinelli AR, Vassia V, Casciaro FG, Priora S, Nerici I, Galbiati A, Hayden E, Falasca M, Vaschetto R, Sainaghi PP, Dianzani U, Rolla R, Chiocchetti A, Baldanzi G, Marengo E, Manfredi M. Large-Scale Plasma Analysis Revealed New Mechanisms and Molecules Associated with the Host Response to SARS-CoV-2. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 57. | Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, Dobesh DP, Brufsky A, Connor RI. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. 2021;93:409-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 58. | Montefusco L, Ben Nasr M, D'Addio F, Loretelli C, Rossi A, Pastore I, Daniele G, Abdelsalam A, Maestroni A, Dell'Acqua M, Ippolito E, Assi E, Usuelli V, Seelam AJ, Fiorina RM, Chebat E, Morpurgo P, Lunati ME, Bolla AM, Finzi G, Abdi R, Bonventre JV, Rusconi S, Riva A, Corradi D, Santus P, Nebuloni M, Folli F, Zuccotti GV, Galli M, Fiorina P. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;3:774-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 59. | Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? Diabetes Metab Syndr. 2020;14:535-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 460] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 60. | Ardestani A, Azizi Z. Targeting glucose metabolism for treatment of COVID-19. Signal Transduct Target Ther. 2021;6:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 61. | Wu L, Girgis CM, Cheung NW. COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol (Oxf). 2020;93:390-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 62. | Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068-1077.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1099] [Article Influence: 219.8] [Reference Citation Analysis (0)] |
| 63. | Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ, Young B, Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 665] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 64. | Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 65. | Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol. 2020;92:2105-2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 360] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 66. | Andrade Silva M, da Silva ARPA, do Amaral MA, Fragas MG, Câmara NOS. Metabolic Alterations in SARS-CoV-2 Infection and Its Implication in Kidney Dysfunction. Front Physiol. 2021;12:624698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 67. | Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol. 2020;20:397-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 68. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 69. | Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 497] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 70. | Candido KL, Eich CR, de Fariña LO, Kadowaki MK, da Conceição Silva JL, Maller A, Simão RCG. Spike protein of SARS-CoV-2 variants: a brief review and practical implications. Braz J Microbiol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, Tkachuk VA, Markov AG, Lehnert H, de Angelis MH, Rietzsch H, Rodionov RN, Khunti K, Hopkins D, Birkenfeld AL, Boehm B, Holt RIG, Skyler JS, DeVries JH, Renard E, Eckel RH, Alberti KGMM, Geloneze B, Chan JC, Mbanya JC, Onyegbutulem HC, Ramachandran A, Basit A, Hassanein M, Bewick G, Spinas GA, Beuschlein F, Landgraf R, Rubino F, Mingrone G, Bornstein SR. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 72. | Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Xu Y, Cao Z, Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 703] [Article Influence: 140.6] [Reference Citation Analysis (1)] |
| 73. | Iwasaki M, Saito J, Zhao H, Sakamoto A, Hirota K, Ma D. Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation. 2021;44:13-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 74. | Healy EF, Lilic M. A model for COVID-19-induced dysregulation of ACE2 shedding by ADAM17. Biochem Biophys Res Commun. 2021;573:158-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu Rev Anim Biosci. 2019;7:263-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 76. | Radziejewska A, Muzsik A, Milagro FI, Martínez JA, Chmurzynska A. One-Carbon Metabolism and Nonalcoholic Fatty Liver Disease: The Crosstalk between Nutrients, Microbiota, and Genetics. Lifestyle Genom. 2020;13:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Zhang Y, Guo R, Kim SH, Shah H, Zhang S, Liang JH, Fang Y, Gentili M, Leary CNO, Elledge SJ, Hung DT, Mootha VK, Gewurz BE. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat Commun. 2021;12:1676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 78. | Perła-Kaján J, Jakubowski H. COVID-19 and One-Carbon Metabolism. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Danlos FX, Grajeda-Iglesias C, Durand S, Sauvat A, Roumier M, Cantin D, Colomba E, Rohmer J, Pommeret F, Baciarello G, Willekens C, Vasse M, Griscelli F, Fahrner JE, Goubet AG, Dubuisson A, Derosa L, Nirmalathasan N, Bredel D, Mouraud S, Pradon C, Stoclin A, Rozenberg F, Duchemin J, Jourdi G, Ellouze S, Levavasseur F, Albigès L, Soria JC, Barlesi F, Solary E, André F, Pène F, Ackerman F, Mouthon L, Zitvogel L, Marabelle A, Michot JM, Fontenay M, Kroemer G. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 2021;12:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 80. | Roberts I, Wright Muelas M, Taylor JM, Davison AS, Xu Y, Grixti JM, Gotts N, Sorokin A, Goodacre R, Kell DB. Untargeted metabolomics of COVID-19 patient serum reveals potential prognostic markers of both severity and outcome. Metabolomics. 2021;18:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 81. | McCaddon A, Regland B. COVID-19: A methyl-group assault? Med Hypotheses. 2021;149:110543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Su Y, Chen D, Yuan D, Lausted C, Choi J, Dai CL, Voillet V, Duvvuri VR, Scherler K, Troisch P, Baloni P, Qin G, Smith B, Kornilov SA, Rostomily C, Xu A, Li J, Dong S, Rothchild A, Zhou J, Murray K, Edmark R, Hong S, Heath JE, Earls J, Zhang R, Xie J, Li S, Roper R, Jones L, Zhou Y, Rowen L, Liu R, Mackay S, O'Mahony DS, Dale CR, Wallick JA, Algren HA, Zager MA; ISB-Swedish COVID19 Biobanking Unit, Wei W, Price ND, Huang S, Subramanian N, Wang K, Magis AT, Hadlock JJ, Hood L, Aderem A, Bluestone JA, Lanier LL, Greenberg PD, Gottardo R, Davis MM, Goldman JD, Heath JR. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell. 2020;183:1479-1495.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 439] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 83. | Wang C, Li X, Ning W, Gong S, Yang F, Fang C, Gong Y, Wu D, Huang M, Gou Y, Fu S, Ren Y, Yang R, Qiu Y, Xue Y, Xu Y, Zhou X. Multi-omic profiling of plasma reveals molecular alterations in children with COVID-19. Theranostics. 2021;11:8008-8026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 84. | Kumar P, Osahon O, Vides DB, Hanania N, Minard CG, Sekhar RV. Severe Glutathione Deficiency, Oxidative Stress and Oxidant Damage in Adults Hospitalized with COVID-19: Implications for GlyNAC (Glycine and N-Acetylcysteine) Supplementation. Antioxidants (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 85. | Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 996] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 86. | Goyal H, Kopel J, Ristić B, Perisetti A, Anastasiou J, Chandan S, Tharian B, Inamdar S. The pancreas and COVID-19: a clinical conundrum. Am J Transl Res. 2021;13:11004-11013. [PubMed] |
| 87. | Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, Saunders DC, Bottino R, Aramandla R, Jenkins R, Stein R, Kaestner KH, Vahedi G; HPAP Consortium, Brissova M, Powers AC. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in β Cells. Cell Metab. 2020;32:1028-1040.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 88. | Taneera J, El-Huneidi W, Hamad M, Mohammed AK, Elaraby E, Hachim MY. Expression Profile of SARS-CoV-2 Host Receptors in Human Pancreatic Islets Revealed Upregulation of ACE2 in Diabetic Donors. Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 89. | Koufakis T, Metallidis S, Zebekakis P, Kotsa K. Intestinal SGLT1 as a therapeutic target in COVID-19-related diabetes: A "two-edged sword" hypothesis. Br J Clin Pharmacol. 2021;87:3643-3646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Wan L, Gao Q, Deng Y, Ke Y, Ma E, Yang H, Lin H, Li H, Yang Y, Gong J, Li J, Xu Y, Liu J, Zhang X, Huang L, Feng J, Zhang Y, Huang H, Wang H, Wang C, Chen Q, Huang X, Ye Q, Li D, Yan Q, Liu M, Wei M, Mo Y, Tang K, Lin C, Zheng F, Xu L, Cheng G, Wang P, Yang X, Wu F, Sun Z, Qin C, Wei C, Zhong H. GP73 is a glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia. Nat Metab. 2022;4:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 91. | Ho JSY, Fernando DI, Chan MY, Sia CH. Obesity in COVID-19: A Systematic Review and Meta-analysis. Ann Acad Med Singap. 2020;49:996-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 92. | Yang J, Ma Z, Lei Y. A meta-analysis of the association between obesity and COVID-19. Epidemiol Infect. 2020;149:e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 93. | Du Y, Zhou N, Zha W, Lv Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: A meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31:745-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 94. | Atmosudigdo IS, Pranata R, Lim MA, Henrina J, Yonas E, Vania R, Radi B. Dyslipidemia Increases the Risk of Severe COVID-19: A Systematic Review, Meta-analysis, and Meta-regression. J Clin Exp Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14:1463-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 96. | Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: A meta-analysis. Dig Liver Dis. 2021;53:153-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 97. | Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, Alsukait RF, Alluhidan M, Alazemi N, Shekar M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 814] [Cited by in RCA: 744] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 98. | Aminian A, Fathalizadeh A, Tu C, Butsch WS, Pantalone KM, Griebeler ML, Kashyap SR, Rosenthal RJ, Burguera B, Nissen SE. Association of prior metabolic and bariatric surgery with severity of coronavirus disease 2019 (COVID-19) in patients with obesity. Surg Obes Relat Dis. 2021;17:208-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 99. | Yanai H. Adiposity is the Crucial Enhancer of COVID-19. Cardiol Res. 2020;11:353-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Lui DTW, Lee CH, Tan KCB. One year into the clash of pandemics of diabetes and COVID-19: Lessons learnt and future perspectives. J Diabetes Investig. 2022;13:19-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 740] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 102. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 103. | Huang R, Zhu L, Wang J, Xue L, Liu L, Yan X, Huang S, Li Y, Zhang B, Xu T, Li C, Ji F, Ming F, Zhao Y, Cheng J, Wang Y, Zhao H, Hong S, Chen K, Zhao XA, Zou L, Sang D, Shao H, Guan X, Chen X, Chen Y, Wei J, Zhu C, Wu C. Clinical features of COVID-19 patients with non-alcoholic fatty liver disease. Hepatol Commun. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 104. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020;2:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 105. | Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N, Walther T, Sauerbruch T, Burrell LM, Angus PW, Trebicka J. Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. 2013;145:874-884.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Casey S, Schierwagen R, Mak KY, Klein S, Uschner F, Jansen C, Praktiknjo M, Meyer C, Thomas D, Herath C, Jones R, Trebicka J, Angus P. Activation of the Alternate Renin-Angiotensin System Correlates with the Clinical Status in Human Cirrhosis and Corrects Post Liver Transplantation. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Kushner T, Cafardi J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin Liver Dis (Hoboken). 2020;15:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 108. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 109. | Singh V, Kumar R, Nain CK, Singh B, Sharma AK. Terlipressin versus albumin in paracentesis-induced circulatory dysfunction in cirrhosis: a randomized study. J Gastroenterol Hepatol. 2006;21:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Bobermin LD, Quincozes-Santos A. COVID-19 and hyperammonemia: Potential interplay between liver and brain dysfunctions. Brain Behav Immun Health. 2021;14:100257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | Chen J, Vitetta L. The gut-liver axis in chronic liver disease associated with severe COVID-19. Eur J Gastroenterol Hepatol. 2021;33:e1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 112. | Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 113. | Koike S, Kabuyama Y, Obeng KA, Sugahara K, Sato Y, Yoshizawa F. An Increase in Liver Polyamine Concentration Contributes to the Tryptophan-Induced Acute Stimulation of Rat Hepatic Protein Synthesis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, Xu L, Li X, Liu H, Yin P, Li K, Zhao J, Li Y, Wang X, Zhang Q, Xu G, Chen H. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci Rep. 2017;7:9110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 282] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 115. | Milic J, Barbieri S, Gozzi L, Brigo A, Beghé B, Verduri A, Bacca E, Iadisernia V, Cuomo G, Dolci G, Yaacoub D, Aprile E, Belli M, Venuta M, Meschiari M, Sebastiani G, Clini E, Mussini C, Lonardo A, Guaraldi G, Raggi P. Metabolic-Associated Fatty Liver Disease Is Highly Prevalent in the Postacute COVID Syndrome. Open Forum Infect Dis. 2022;9:ofac003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |