Published online Jul 25, 2022. doi: 10.5501/wjv.v11.i4.170
Peer-review started: December 24, 2021
First decision: March 7, 2022
Revised: March 25, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: July 25, 2022
Processing time: 210 Days and 0.5 Hours
Vaccination for coronavirus disease 2019 (COVID-19) is a critical strategy in controlling the current pandemic of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). After widespread COVID-19 vaccine imple-mentation, isolated case reports about myocarditis as a potential adverse reaction started coming. As of November 12, 2021, Centers for Disease Control and Prevention (CDC) has reported 1793 cases of myocarditis or pericarditis among young people with age 12-29 years, most cases have been reported in the male adolescent age group after the second dose of mRNA COVID-19 vaccines. It is very important to monitor the safety standards and adverse reactions of vaccines to effectively implement the vaccination policies. The CDC and the United States Food and Drug Administration actively monitor vaccine-associated adverse reactions a well-known platform such as Vaccine Adverse Event Reporting System. CDC continues to recommend COVID-19 vaccines and booster doses for eligible individuals (age limit according to the type of vaccine) after careful consideration from risk-benefit assessment and favorable outcomes from vaccination. Mechanisms behind COVID-19 vaccine-induced myocarditis are not clear yet but several possibilities such as molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens, immune response to mRNA, and activation of host immunological system, trigger of the pre-existing dysregulated immunological system have been documented in the literature. Overall, data suggests a good prognosis, especially in young patients. In this review article, we cover currently available data on COVID-19 vaccine-related myocarditis incidence, concerns, possible mechanisms of myocarditis, current treatment, and outcome trends, risk vs benefit assessment of COVID-19 vaccination in this current pandemic.
Core Tip: Coronavirus disease 2019 (COVID-19) vaccination campaign is progressing successfully, and more than 400 million vaccine doses have been administered in the United States. We support the COVID-19 vaccination drive given positive data on preventing significant morbidities from COVID-19 disease in fully vaccinated people and relatively rare occurrences of serious side effects. Many questions remain open such as: whether patients with a history of vaccine-associated myocarditis should receive the subsequent vaccines or booster doses, the long-term effect of vaccine-associated myocarditis, how to identify the high-risk individuals for such adverse reactions to selectively save vulnerable populations, etc. There is still substantial research to be done in this direction to answer unsolved questions.
- Citation: Dhaduk K, Khosla J, Hussain M, Mangaroliya V, Chauhan S, Ashish K, Gupta R, Pal S. COVID-19 vaccination and myocarditis: A review of current literature. World J Virol 2022; 11(4): 170-175
- URL: https://www.wjgnet.com/2220-3249/full/v11/i4/170.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i4.170
Coronavirus disease 2019 (COVID-19), a disease that originated from a viral infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was declared a global pandemic on March 11, 2020, by the World Health Organization. Since the declaration of the pandemic, tremendous efforts have been made towards the development of safe and effective COVID-19 mitigation, specifically through the administering of vaccines. Three of the COVID-19 vaccines were approved by the United States Food and Drug Administration (FDA) for emergency use: the first approval was for Pfizer-BioNTech COVID-19 Vaccine on August 23, 2020[1], the second approval was for Moderna COVID-19 vaccine on December 18, 2020[2] and the third approval was for Janssen COVID-19 vaccine on February 27, 2021[3].
Viral infections are known to cause acute myocarditis by a direct effect on cardiac myocytes causing myonecrosis[4]. Additionally, other mechanisms have also been described including autoimmune response and vasculitis leading to injury. Post-immunization myocarditis as a rare adverse reaction after vaccination has been reported historically after the administering of the smallpox, anthrax, Haemophilus type B, influenza type B, BCG, typhoid fever, influenza and hepatitis B vaccines[5]. The Centers for Disease Control and Prevention (CDC) and the FDA monitor vaccine-associated adverse reactions through the use of a system known as the Vaccine Adverse Event Reporting System (VAERS)[6]. The CDC and FDA use extensive data and statistical methods to generate recommendations relative to vaccine safety and continue to recommend COVID-19 vaccination among everyone ages 5 and older[7]. VAERS is also contingent upon reporting bias, including underreporting (mild adverse events) and overreporting (especially with intense media attention and public awareness)[6].
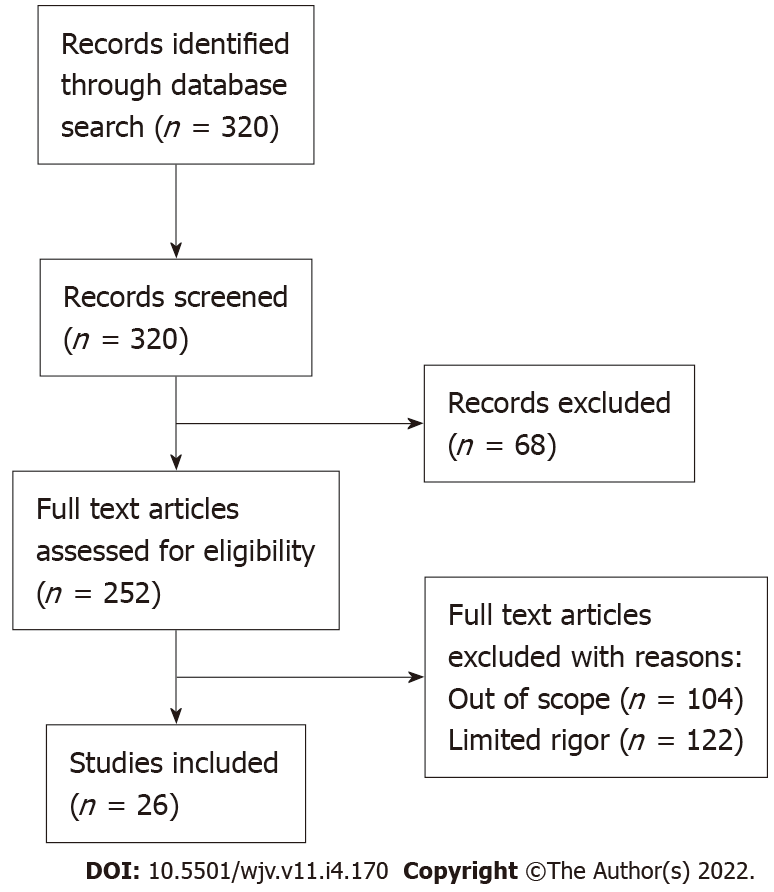
The data and literature continue to accumulate each day regarding COVID-19 vaccine-related adverse events. Myocarditis, in general, has been reported as a rare adverse reaction after the 2nd dose of the COVID-19 vaccine, especially in young males. Within the contents of this review article, we intend to expound upon COVID-19 vaccine-related myocarditis incidence, available data and statistics, possible mechanisms of vaccine-related myocarditis, current treatment trends, outcomes of such events, and risk vs benefit assessments of COVID-19 vaccination in the current pandemic. PRISMA flow diagram for article review is shown in Figure 1.
The CDC reported that more than 448 million doses of the COVID-19 vaccine had been administered in the United States as of November 19, 2021. Furthermore, upwards of 195 million people have been fully vaccinated, and 33.5 million people have received the COVID-19 booster vaccine[8]. The CDC has reported 1793 cases of myocarditis or pericarditis among young individuals (age 12-29 years) vaccinated for COVID-19, predominantly in adolescent males after the second dose of mRNA COVID-19 vaccines[9].
The estimated incidence rate appears to be very low; a comprehensive cohort study from Israel reported 2.13 cases per 100000 vaccinated persons with an incidence rate per 100000 persons for disease severity as 1.62 for mild myocarditis, 0.47 for intermediate myocarditis, and 0.04 for fulminant myocarditis[10]. Another study observed an incidence of 0.8 cases per 1 million doses of first dose COVID-19 vaccine and 5.8 cases per 1 million doses of second dose in 10-d observation window[11]. There is substantial heterogeneity in the incidence rate of vaccine-associated myocarditis, in respect to age and sex of the population as well. Numerous case series and case reports have reported findings consistent with post-COVID-19 immunization myocarditis. Such association was not found in clinical trials for these vaccines. One of the explanations could be a rarity of such adverse reactions, which gained clinical attention after the beginning of the global scale vaccine administration program.
Data from the available literature suggest patients experienced the symptoms within 12 h to 5 d and primarily after the second dose of COVID-19 vaccine administration; the majority were young male individuals and had good clinical outcomes[12-14]. A study from Israel reported 54 cases of postimmunization myocarditis within approximately 3 to 5 d after the second dose of the vaccine; only one case was fulminant and required extracorporeal membrane oxygenation, 83% of patients did not have co-existing medical conditions, 69% of patients developed myocarditis after the second dose of COVID-19 vaccine[10].
The key to identifying such cases is a high index of suspicion in young patients presenting with chest pain or cardiac symptoms within a few days of COVID-19 immunization. A review of the literature suggests patients with acute myocarditis typically present with chest pain (100% cases); myalgia, fatigue, fever (33%-86% cases); palpitations, dyspnoea, fatigue[15]. Blood work shows elevated troponin, C-reactive protein, brain natriuretic peptide; common electrocardiography (EKG) findings are ST-segment elevation, diffuse ST-T changes, ventricular and supraventricular tachycardias, but no EKG changes have also been reported. Echocardiogram findings varied from being normal to reduced systolic heart function, but a review article has reported primarily normal cardiac function in patients ages 18 years and younger, and more systolic dysfunction in patients ages 30 years and older. Cardiac magnetic resonance imaging was only available in a selective number of patients; late gadolinium enhancement in anterolateral and inferolateral cardiac walls was consistently noticed, few patients had myocardial edema on T2 mapping. Cardiac biopsy is a confirmatory test for acute myocarditis, it was noted very infrequently in available literature[10,15,16].
The Pfizer-BioNTech and Moderna COVID-19 vaccines are mRNA vaccines, which contain nucleoside modified mRNA encoding the SARS-COV-2 viral spike protein. Once administered, mRNA particles induce viral spike protein synthesis in the host cells which then stimulates adaptive immune response to produce IgG antibodies to this spike proteins. Such vaccine induced IgG antibodies help neutralising the virus by preventing the attachment of SARS-COV-2 virus to host cell receptors via spike protein[15].
The generation of heart reactive autoantibodies against multiple antigens can have a functional effect on cardiac cells[17]. Autoantibodies develop more frequently in first degree relatives of patients with cardiomyopathy, raising possibility of genetically susceptible subgroup of patients. The role of molecular mimicry between the spike protein in the SARS-COV-2 virus and self-antigens has been extensively studied. A study has demonstrated possible cross-reactions between viral spike protein and many tissue proteins including alfa-myosin[18] which may potentially play a role in molecular mimicry mechanism affecting cardiomyocytes. Nucleoside modification plays a critical role in effective and safe mRNA vaccine development as it selectively activates the innate immune system to appropriate target cells and regulates the immune response which is essential in vaccine development[19]. The innate immune system can recognize genetic materials of pathogen that usually lack RNA modifications[19]. A potential immune mediated adverse event such as triggering of the pre-existing dysregulated immune pathways in susceptible individuals leading to exaggerated immune response could be a potential mechanism of myocarditis after mRNA vaccination, such hypothesis have already been generated for COVID-19 viral infection[20]. The overresponse and overproduction of the innate immune system with adaptive mechanisms leads to pathological response. The overexpression of interferon-gamma that drives innate and viral responses to the vaccine booster leads to cardiac events which involve MAPK and JAK-STAT pathways[21]. A report demonstrated no significant elevation of IgM and IgG antibody levels in patients with myocarditis compared to patients without myocarditis after mRNA vaccination[22], provided an evidence against a hyperimmune response as a potential mechanism in general population. Young male predominance for myocarditis cases have been reported in the literature but there is no clear understanding of it. One possibility may be that gender differences in the stimulation of neutrophils and release of cytokines such as TNF-α and IFN-γ with predisposition in males and higher hormonal stimulation at puberty may explain propensity of males to get higher rate of vaccine associated myocarditis, but this needs to be validated[23]. Significant research is still required to better understand the molecular and genetic aspects of such potential mechanisms and vaccine complications.
Fortunately, most cases of myocarditis after COVID-19 vaccination had favorable outcomes. Those patients responded well to medical therapy and recovered from their symptoms within a short period. All patients presenting with chest pain within a week after receiving the COVID-19 vaccination should undergo complete evaluation for broad differentials of diagnoses. Initial evaluation includes EKG, troponin levels, and inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein. Further workups including echocardiography, cardiac catheterization, and cardiac magnetic resonance imaging should be considered to establish the diagnosis of myocarditis/pericarditis. Besides cardiology, infectious diseases and rheumatology should also be consulted to rule out other causes of myocarditis/pericarditis[15]. All these cases should be reported to VAERS. Further management of these patients is based on their clinical presentation, hemodynamic and rhythmic stability, and disease progression. Patients with acute chest pain, up-trending troponins, EKG changes, or signs of hemodynamic/rhythm instability need to be hospitalized and closely monitored. Many patients responded well with supportive care, nonsteroidal anti-inflammatory drugs, colchicine, and steroids. In patients with left ventricular systolic failure, it may be reasonable to consider additional treatments such as beta-blockers, aspirin, and angiotensin-converting enzyme inhibitors. The average duration of hospital stay is found to be less than a week in the cases reported so far[15,24]. Recently, a case report was published mentioning fulminant myocarditis after COVID-19 vaccination, which unfortunately proved to be fatal for one patient in particular[25]. While such an outcome is certainly alarming, the CDC has not confirmed any death that could be directly attributed to myocarditis related to COVID-19 vaccination.
Despite reports of myocarditis, COVID-19 vaccination data is reassuring in terms of safety standards. The CDC reports a significantly higher rate of hospitalization for unvaccinated adults compared to vaccinated adults; risk is 10 times higher for unvaccinated adolescents ages 12 to 17 years, 9 times higher for unvaccinated adults more than 18 years of age, 14 times higher for unvaccinated adults 18 to 49 years of age, 13 times higher for unvaccinated adults 50 to 64 years of age, and around 6 times higher in unvaccinated adults older than 65 years of age[26]. In terms of mortality risk, CDC data from August 2021 reported unvaccinated individuals had a risk of death from COVID-19 related complications around 11 times higher compared to vaccinated individuals[27]. Infection rate and mortality rate were lower in vaccinated individuals irrespective of vaccine brand (Pfizer, Moderna, or Janssen). As of November 19, 2021, more than 448 million doses of the COVID-19 vaccine have been administered in the United States[8] and during this time, VAERS has received 9810 reports of death (0.0022%) related to the COVID-19 vaccine[9]. Reports of adverse events including mortality reports do not necessarily mean a vaccine-related complication. Total deaths from COVID-19 disease as of November 20, 2021 are more than 770461 in United States alone[28]. Considering the risks and benefits of COVID-19 vaccines, it is evident that vaccines have a positive impact on COVID-19 related morbidity and mortality. Therefore, the CDC continues to recommend COVID-19 immunisation to all eligible individuals and continues to monitor upcoming data very closely.
In conclusion, the COVID-19 vaccination campaign is progressing successfully, and more than 400 million vaccine doses have been administered in the United States. The CDC and other organizations are actively monitoring the safety standards and adverse reactions related to COVID-19 vaccination. After a thorough evaluation of risk vs benefit, the CDC continues to recommend COVID-19 vaccination in everyone ages 5 or older[7]. We support the COVID-19 vaccination drive given positive data on preventing significant morbidities from COVID-19 disease in fully vaccinated people and relatively rare occurrences of serious side effects. Many questions remain open such as: whether patients with a history of vaccine-associated myocarditis should receive the subsequent vaccines or booster doses, the long-term effect of vaccine-associated myocarditis, how to identify the high-risk individuals for such adverse reactions to selectively save vulnerable populations, etc. There is still substantial research to be done in this direction to answer unsolved questions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Freij BJ, United States; Odhar HA, Iraq A-Editor: Liu X, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. [cited 19 November 2021]. In: Food and Drug Administration. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine. |
| 2. | Moderna COVID-19 Vaccine. [cited 19 November 2021]. In: Food and Drug Administration. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine. |
| 3. | Janssen COVID-19 Vaccine. [cited 19 November 2021]. In: Food and Drug Administration. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine. |
| 4. | Leonard EG. Viral myocarditis. Pediatr Infect Dis J. 2004;23:665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Su JR, McNeil MM, Welsh KJ, Marquez PL, Ng C, Yan M, Cano MV. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 406] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 7. | Clinical Considerations: Myocarditis after mRNA COVID-19 Vaccines. [cited 14 November 2021]. In: Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. |
| 8. | CDC COVID-19 Response Team, Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 201] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 9. | Selected Adverse Events Reported after COVID-19 Vaccination. [cited 19 November 2021]. In: Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html.. |
| 10. | Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, Kornowski R. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021;385:2132-2139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 444] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 11. | Simone A, Herald J, Chen A, Gulati N, Shen AY, Lewin B, Lee MS. Acute Myocarditis Following COVID-19 mRNA Vaccination in Adults Aged 18 Years or Older. JAMA Intern Med. 2021;181:1668-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 12. | Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021;6:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 398] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 13. | Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessy R, Caron R, Fuss C, Corbin KJE, Emuren L, Faherty E, Hall EK, Di Pentima C, Oster ME, Paintsil E, Siddiqui S, Timchak DM, Guzman-Cottrill JA. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 2021;148:e2021052478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 267] [Article Influence: 66.8] [Reference Citation Analysis (2)] |
| 14. | Schauer J, Buddhe S, Colyer J, Sagiv E, Law Y, Mallenahalli Chikkabyrappa S, Portman MA. Myopericarditis After the Pfizer Messenger Ribonucleic Acid Coronavirus Disease Vaccine in Adolescents. J Pediatr. 2021;238:317-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 621] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 16. | Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: What Do We Know So Far? Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 19. | Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1722] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 20. | Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 21. | Hajjo R, Sabbah DA, Bardaweel SK, Tropsha A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Muthukumar A, Narasimhan M, Li QZ, Mahimainathan L, Hitto I, Fuda F, Batra K, Jiang X, Zhu C, Schoggins J, Cutrell JB, Croft CL, Khera A, Drazner MH, Grodin JL, Greenberg BM, Mammen PPA, Morrison SJ, de Lemos JA. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 mRNA Vaccine. Circulation. 2021;144:487-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 23. | Aomatsu M, Kato T, Kasahara E, Kitagawa S. Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem Biophys Res Commun. 2013;441:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, Damluji AA, de Lemos JA, Desai SS, Emaminia A, Flanagan MC, Khera A, Maghsoudi A, Mekonnen G, Muthukumar A, Saeed IM, Sherwood MW, Sinha SS, O'Connor CM, deFilippi CR. Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation. 2021;144:502-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 25. | Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA Vaccination. N Engl J Med. 2021;385:1332-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 26. | Rates of laboratory-confirmed COVID-19 hospitalizations by vaccination status. [cited 19 November 2021]. In: Centers for Disease Control and Prevention. Available from: https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalizations-vaccination. |
| 27. | Rates of COVID-19 Cases and Deaths by Vaccination Status. [cited 21 November 2021]. In: Centers for Disease Control and Prevention. Available from: https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status. |
| 28. | United States COVID-19 Cases, Deaths, and Laboratory Testing (NAATs) by State, Territory, and Jurisdiction. [cited 21 November 2021]. In: Centers for Disease Control and Prevention. Available from: https://covid.cdc.gov/covid-data-tracker/#cases_totalcases. |