Copyright
©The Author(s) 2016.
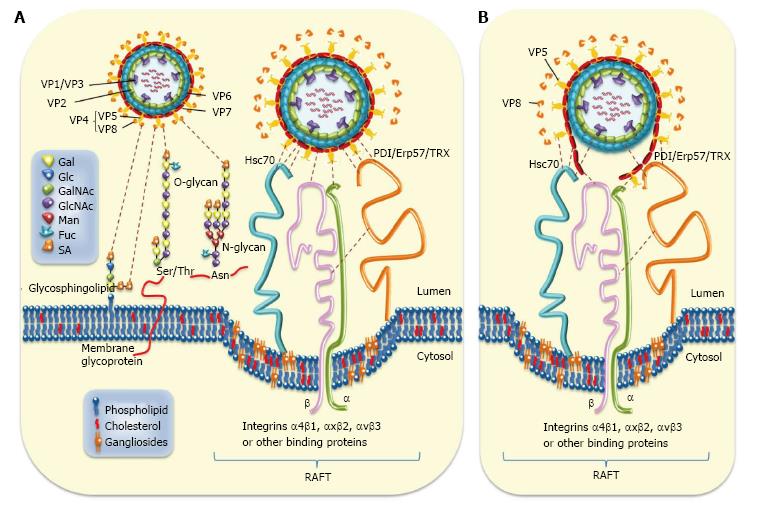
Figure 1 Rotavirus-cell surface interactions during entry.
A: The rotavirus particle-associated proteins (VP1/2/3/4/5/6/7) that enclose the viral genome are represented. Cell surface molecules including sialic acid (SA), Hsc70, PDI, Erp57, thioredoxin (TRX), and integrins α4β1, αxβ2, and αvβ3 are also represented. Infection is initiated by the VP8*-mediated binding (attachment) of virion to terminal or non-terminal (neuraminidase -resistant) SAs located on cell surface glycolipids including gangliosides or to SAs located on cell surface glycoproteins. The N- and O-substituted derivatives of neuraminic acid (SAs) are indicated. Neuraminidase-resistant rotavirus strains can bind directly to integrin α2β1 through VP5* DGE sequence. SA-dependent strains bind first through VP8* to SA before interacting with integrin α2β1 through VP5*. A putative caveolae containing raft-associated cell surface receptors is depicted. The sequence of virion-cell interactions taking place after binding to α2β1 has not yet been established. However, several interactions involving rotavirus structural protein (VP5*, VP7 and VP6) and raft-associated cell surface receptors (Hsc70, PDI and integrins α4β1, αxβ2 and αvβ3) have been documented. Interactions between rotavirus structural proteins and cell surface molecules are illustrated; B: Disruption of rotavirus proteins (VP5*, VP7 and VP6) caused by cell surface-associated chaperone (Hsc70, PDI) and oxido-reductase activities (PDI, integrin αvβ3) is depicted. Hsc70: Heat shock cognate protein 70; PDI: Protein disulfide isomerase.
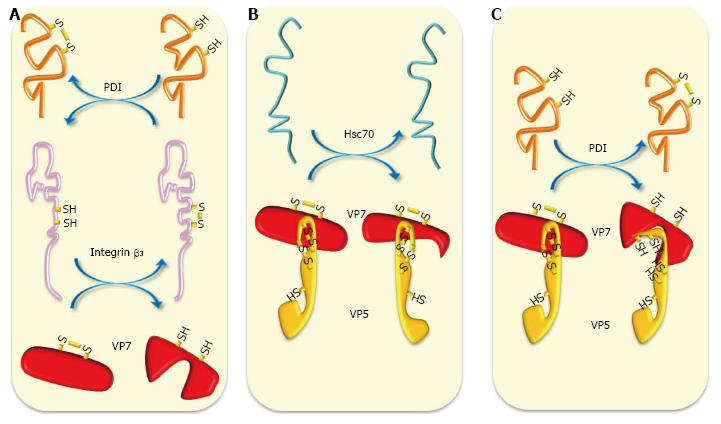
Figure 2 Schematic representation of chaperone and oxidoreduction activities during rotavirus entry.
A: Cell surface reducing PDI has been shown to form complexes with integrins α2β1 and αvβ3 to generate free thiols in these integrins. Reducing integrin β3 can reduce thiol-disulfide bonds present in VP7; B: Chaperone activity of Hsc70 can induce conformational changes in VP5* and VP7 priming them for further interactions; C: Cell surface reducing PDI can reduce thiol-disulfide-containing VP5* and VP7 generating in them conformational changes needed for further interactions and entry. Hsc70: Heat shock cognate protein 70; PDI: Protein disulfide isomerase.
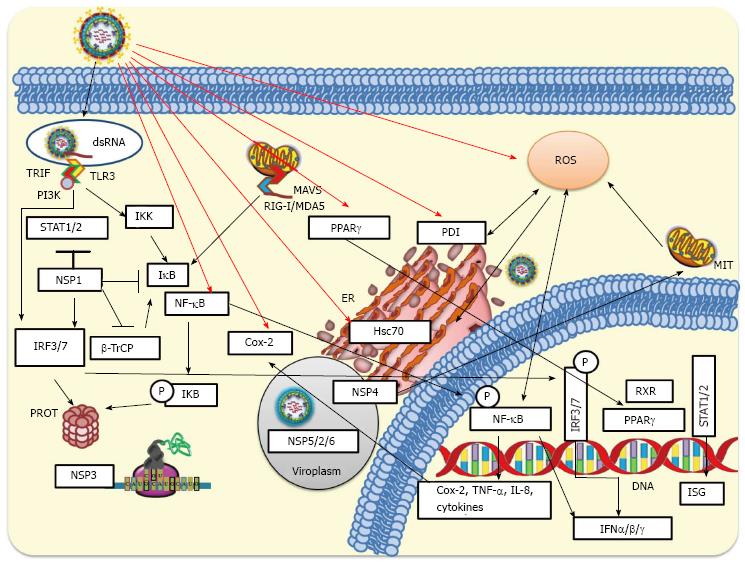
Figure 3 Cellular innate response to rotavirus infection.
During rotavirus internalization viral nucleic acid may be exposed and recognized by either Toll-like receptors (TLR3) or intracellular RIG-I-like receptors (RLRs). Activated RLRs can bind and activate mitochondrial antiviral-signaling protein (MAVS), which recruits a signaling complex needed to activate cytoplasmic transcription factors including interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB). On the other hand, activation of endosomal TLR3 facilitates the adaptor TRIF recruitment, which allows the recruitment of signaling molecules such as IKKs that phosphorylate IRF3 or NF-κB. Phosphorylated IRF3 is dimerized and then translocated to the nucleus. Signaling pathways induced by rotavirus infection produce phosphorylation of IκB (inhibitor of NF-κB) and its subsequent ubiquitination and proteasomal degradation mediated by SCFβ-TrCP E3 ligase. This signaling pathway leads to NF-κB translocation to the nucleus where, jointly with IRF3 and IRF7, binds to the interferon (IFN)-β promoter for transcription of IFN-β mRNA. Rotavirus can early counteract signaling pathways of innate response by NSP1-mediated degradation of IRF3 and IRF7. NSP1 encoded by some rotavirus strains can target SCFβ-TrCP for proteasomal degradation, whereas NSP1 from other strains has been implicated in the direct inhibition of the IFN-mediated STAT1 activation. NSP3 can interfere with the translation of cellular-encoded proteins including those induced by the IFN signaling. The viroplasm, which includes some viral non-structural proteins (NSP2/5/6), can protect viral RNAs from being recognized by some pattern-recognition receptors (RIG-I, MDA-5, among others) involved in antiviral response. MIT, ER and PROT are indicated. IKK: IκB kinase; MIT: Mitochondria; ER: Endoplasmic reticulum; PROT: Proteasome; TNF: Tumor necrosis factor.
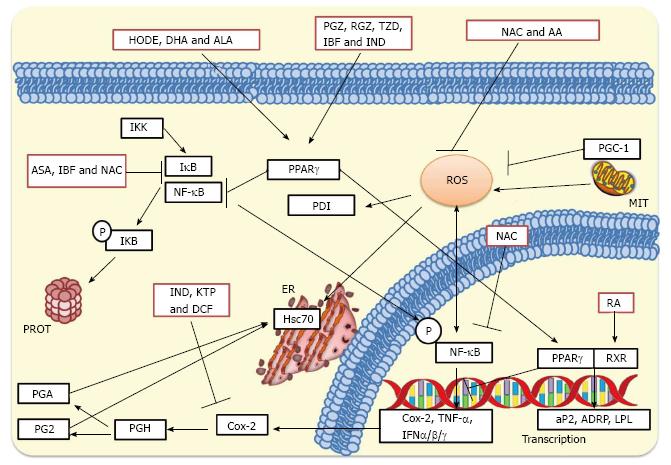
Figure 4 Inhibition of rotavirus infection by treatment with antioxidants, nonsteroidal antiinflammatory drugs and peroxisome proliferator-activated receptor gamma agonists.
NAC and AA can inhibit the production of ROS, whereas NAC can also affect IκB preventing the cytoplasmic activation of NF-κB. NAC can further inhibit nuclear phosphorylated NF-κB preventing the transcription of pro-inflammatory genes. NSAIDs such as KTP, IND and DCF inhibit Cox-2 leading to a significant inhibition of prostaglandin accumulation. On the other hand, ASA and IBF inhibit activation of NF-κB suppressing the transcription of IFN-α, IFN-β and IFN-γ, cytokines and interleukins. These NSAID treatments significantly inhibit rotavirus infections in cultured cells and mice. PPARγ agonists such as 13(S)-hydroxyoctadecadienoic acid (HODE), ALA and DHA, and thiazolidinediones such as PGZ, RGZ, and 2, 4-thiazolidinedione (TZD) activate PPARγ leading to inhibition of cytoplasmic NF-κB. PPARγ can heterodimerize with the RA-activated RXR for promoting transcription of anti-inflammatory genes. This complex can also cause inhibition of phosphorylated NF-κB which in turn leads to decreased transcription of pro-inflammatory genes. MIT, ER, and PROT are indicated. NAC: N-acetylcysteine; NSAIDs: Nonsteroidal antiinflammatory drugs; PPARγ: Peroxisome proliferator-activated receptor gamma; AA: Ascorbic acid; ROS: Reactive oxygen species; NF-κB: Nuclear factor-κB; KTP: Ketoprofen; IND: Indomethacin; DCF: Diclofenac; Cox-2: Cyclooxygenase-2; ASA: Acetylsalicylic acid; IBF: Ibuprofen; IFN-α: Interferon-α; ALA: Alpha-linolenic acid; DHA: Docosahexaenoic acid; PGZ: Pioglitazone; RGZ: Rosiglitazone; RA: Retinoic acid; RXR: Retinoid X receptor; MIT: Mitochondria; ER: Endoplasmic reticulum; PROT: Proteasome.
- Citation: Guerrero CA, Acosta O. Inflammatory and oxidative stress in rotavirus infection. World J Virol 2016; 5(2): 38-62
- URL: https://www.wjgnet.com/2220-3249/full/v5/i2/38.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i2.38