Published online Apr 24, 2017. doi: 10.5500/wjt.v7.i2.144
Peer-review started: November 24, 2016
First decision: January 16, 2017
Revised: February 6, 2017
Accepted: February 28, 2017
Article in press: March 2, 2017
Published online: April 24, 2017
Processing time: 149 Days and 13.4 Hours
To consolidate the present evidence of effectiveness in renal functioning and graft survival following early introduction of mammalian target of rapamycin (mTOR) inhibitors with or without calcineurin inhibitors (CNIs) in renal transplant recipients.
We analysed the current literature following PROSPERO approval describing the role of immunosuppressive agent, mTOR inhibitors as an alternative to CNI within six months of renal transplant by searching the PubMed, EMBASE, Cochrane, Crossref, and Scopus using MeSH terms.
Six articles of early withdrawal of CNI and introduction of mTOR-inhibitors within six months of renal transplantation were sought. Glomerular filtration rate (GFR) and serum creatinine were significantly better in mTOR inhibitor group with equivalent survival at 12 mo, even though Biopsy Proven Acute rejection was significantly higher in mTOR-inhibitor group.
The evidence reviewed in this meta-analysis suggests that early introduction mTOR-inhibitors substantial CNI minimization. The mTOR inhibitors such as everolimus and sirolimus, due to their complementary mechanism of action and favourable nephrotoxicity profile; better glomerular filtration, lower serum creatinine with equivalent survival. Having said that, due to the higher rejection rate, may influence the use of these regimens to patients with moderate to high immunological risk patients.
Core tip: Early calcineurin inhibitor withdrawal seems to be more pragmatic approach as it bestows better renal functioning in the low immunological risk renal transplant recipients.
- Citation: Kumar J, Reccia I, Kusano T, Julie BM, Sharma A, Halawa A. Systemic meta-analysis assessing the short term applicability of early conversion to mammalian target of rapamycin inhibitors in kidney transplant. World J Transplant 2017; 7(2): 144-151
- URL: https://www.wjgnet.com/2220-3230/full/v7/i2/144.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i2.144
Inventions in medical science enhance life which has been realized in the concept of kidney transplantation and add significant amount of productive years to the patients of chronic kidney disease[1]. The calcineurin inhibitors (CNIs), cyclosporine A (CsA) and tacrolimus (Tac) were instituted in clinical practice in 1980’s. and established themselves as an effective immunosuppressive agent with more than 90% one-year graft survival whilst maintaining a rejection rate of less than 20%[2]. Anyhow, the superlative results of short-term allograft survival have not been maintained for long that could be because of slow, steady decline in renal functioning as, eGFR reduced to below 50% in a span of ten years[3]. Studies have reported chronic allograft nephropathy as the most common cause of late graft loss in 40% kidney transplant patients, whilst the mortality incidence with delayed functioning graft (DFG) was reported in 43% cases. The cardiovascular diseases and malignancies are considered as the most important causes of DFG in transplant patients[4].
The CNI induced nephrotoxicity is considered as an important cause of long-term graft failure in 96.8% of allograft biopsies by virtue of increased production of vasoconstrictors, such as thromboxane and endothelin, together with decreasing the turn-out of vasodilators, such as nitric oxide, prostaglandin E2, and prostacyclin[5,6]. Nankivell et al[7] (2004) outlined that more than 50% of kidney allograft biopsies unveiled attestation of chronic CNI toxicity following ten years transplant as 79.2%-100% exhibit histological alterations as tubular atrophy, nodular arteriolar hyalinosis, tubular vacuolization, luminal narrowing, interstitial fibrosis, focal or global segmental sclerosis and micro-calcifications. Surprisingly, the reward of minimal early acute rejection has not been translated into any long term benefits. In addition, CNIs have been associated with development of various cardiovascular risk factors such as hyperlipidemia, hypertension, and new onset diabetes mellitus after transplantation[8,9].
However, the biggest challenge with immunosuppression therapy is to maintain the balance of immunosuppression need in order to avert any rejection episode whilst keeping the check on the toxicities. The recent introduction of better and more efficient non-nephrotoxic immunosuppressive agents such as the mammalian target of rapamycin (mTOR) inhibitors, sirolimus (SRL) and everolimus (EVR), with mechanism of action similar to that of CNIs, forms the basis of use of these drugs[10,11].
CNIs as Tacrolimus (Tac) and Cyclosporin A (CsA) attach with the intracellular proteins called FKBP and immunophilins to form complex which blocks the effect of calcineurin which normally potentiates the intracellular processes associated with the activation of T-lymphocytes. This causes decreased production of interleukin-2 and inhibit the proliferation of T-cells[12,13].
In the similar manner mTOR inhibitors as SRL and EVR form a complex with FKBP to reduces T-cell activation by blocking growth-factor-mediated cell proliferation in the response to an alloantigen[14-17]. The distinct immunological properties with and limited nephrotoxic potential of mTOR-inhibitors have prevailed clinicians to use them as a surrogate to CNIs in renal transplantation[18-21].
The main aim of this review is to focus on the short term benefit early conversion to mTOR-inhibitors with or without CNI in renal transplant recipients in terms of graft functioning and graft survival.
This meta-analysis was performed following registration in PROSPERO an international database of prospectively registered systematic reviews (CRD42017054458). An extensive search of all the published literature on the role of early conversion to mTOR inhibitors as an alternative to CNI has been made on National Library of Medicine Database (PubMed), EMBASE, Cochrane, Crossref, and Scopus databases on 30th August 2016. The search covered the period 2001 (the year of the first reported early CsA withdrawal with sirolimus in the literature) to September 30th, 2016[22]. The following medical subject headings (MeSH) terms: “Adverse events”, “calcineurin inhibitors”, “cyclosporin”, “everolimus”, “graft rejection”, “graft survival”, “kidney transplantation”, “mTOR inhibitors”, “sirolimus”, “tacrolimus” were searched.
The original English literature articles published between 2001-September 2016 were included. Only studies which systematically and quantitatively assessed the graft functioning and graft survival of more than or equal to 12 mo following early conversion to mTORI with or without CNI in different randomised clinical studies were analysed. All kind of comparative studies, retrospective and prospective were included. We have excluded publications as editorials, reviews and letters (Table 1).
| Study design | Prospective cohort design with a well-defined study population |
| Study group | Post renal transplant |
| Conversion time | Period of 2 wk to 6 mo post-transplant |
| Study size | > 30 patients |
| Length of follow-up | Any |
| Source | Peer-reviewed journals |
| Language | English |
| Outcome measure | Patient safety, exposure-response relationships, adverse events, and graft functioning and long-term survival |
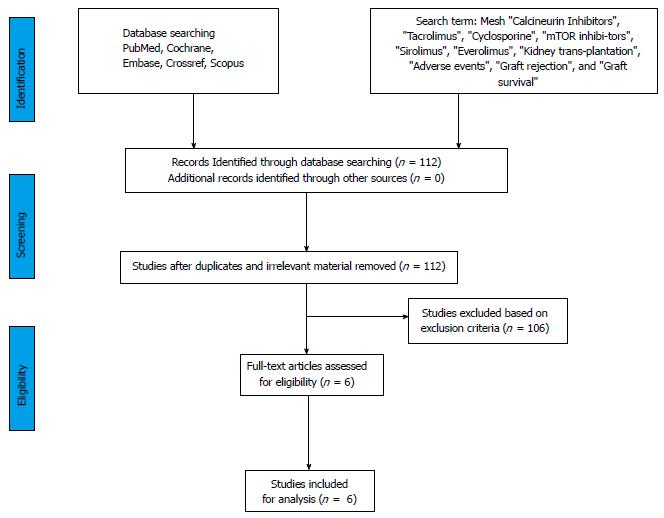
Two separate physician reviewers Kumar J, Reccia I reviewed all the articles. Disagreements were resolved through discussion, whilst in scenarios were consensus could not be achieved were resolved by a third author (Ahmed Halawa). We have analysed all papers with empirical studies using a standardised quality assessment tool and pre-specified inclusion and exclusion criteria. The present meta-analysis was performed using the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and registered in PROSPERO an international database of prospectively registered systematic reviews (Figure 1).
The QUADAS-II (quality assessment of diagnostic accuracy studies-II) based analysis was done to assess the internal validity of pre-specified inclusion and exclusion criteria of the various studies. QUADAS-2 is an evidence-based bias assessment tool to evaluate the quality of diagnostic accuracy studies in a systematic review.
A total of six peer-reviewed multi-institutional studies were included in the present meta-analysis. We reviewed each study comprehensively, and data were extracted for the outcomes such as patient safety, exposure-response relationships, adverse events, and various shortcomings or weaknesses to improve the graft functioning and long-term survival (Table 2).
| Ref. | Study design | Time of conversion | Group 1 | Group 2 |
| Everolimus | ||||
| Budde et al[23], 2011 (ZEUS Study) | Multicentre, Prospective, Randomized Study (n = 300), 12 mo | 4.5th month | EVR (C0, 6-10 ng/mL) Induction: Basiliximab (n = 155) | CsA (C0, 120-180 ng/mL till 4.5-6 mo then decreased to 100-150 ng/mL) Induction: Basiliximab (n = 145) |
| Mjörnstedt et al[24], 2012 (CENTRAL trial) | Multicentre, Prospective, Randomized Study, (n = 269), 12 mo | 7th week | EVR (C0, 6-10 ng/mL) + MMF (1.4 g/d till 2 wk then decreased to 1.08 g/d) + S (n = 92) | Low CsA (C0, 75-200 ng/mL till 2 wk then decreased to 50-150 ng/mL) + MMF (1.4 g/d) + S (n = 90) |
| Sirolimus | ||||
| Lebranchu et al[25], 2009 (CONCEPT Study) | Multicentre Prospective, Randomized Study, (n = 193), 12 mo | 3rd month | SRL (C0, 8-15 ng/mL till 39 wk then decreased to 5-10 ng/mL) + MMF + S (Induction: Daclizumab) (n = 95) | CsA (C0, 500-800 ng/mL) + MMF + S (Induction: Daclizumab) (n = 97) |
| Guba et al[26], 2010 (SMART Trial) | Multicentre Prospective, Randomized Study, (n = 140), 12 mo | 10-24th day | SRL (C0, 8-12 ng/mL then decreased to 5-10 ng/mL) + MMF (1.5 g/d) + S (Induction: ATG) (n = 69) | CsA (C0, 150-200 ng/mL then decreased to 100-150 ng/mL) + MMF (2 g/d) + S (Induction: ATG) (n = 71) |
| Weir et al[27], 2010 (Spare the Nephron Trial) | Multicentre, Prospective, Randomized Study, (n = 299), 12 mo | Within 115 d | MMF + SRL (n = 148) | MMF + CNI (n = 151) |
| Heilman et al[28], 2011 | Multicentre Prospective, Randomized Study, (n = 122), 12 mo | 1 mo | SRL (C0, 9.8 ± 3.6 ng/mL) + MMF + S (Induction: Basiliximab) (n = 62) | TAC (C0, 6.9 ± 4.6 ng/mL) + MMF + S (Induction: Basiliximab) (n = 60) |
Review Manager (RevMan) Version 5.3 was used to analyse continuous and dichotomous trial data when at least two trials reported. Odds ratios (OR) for dichotomous outcomes, mean difference (MD) for continuous outcomes including a 95%CI, heterogeneity between the trials was measured using the statistic with > 30% considered as significant. The random effects model was used in cases of significant heterogeneity by visualizing the forest plot of involved trials.
The initial search yielded a total of 112 manuscripts. After careful evaluation, 98 articles were excluded on basis period of introduction was not within six months of transplantation. Eventually, a total of six articles matched the previously described inclusion criteria, i.e., ZEUS trial (2011), CENTRAL trial (2012), CONCEPT trial (2009), SMART trial (2010), Spare the Nephron trial (2010)[23-27] and Heilman et al[28] (2011) (Table 2). The comprehensive data of all these studies summarizing the renal functioning, Biopsy Proven Acute rejection (BPAR), survival and adverse events were included in Table 3, below we have further analyzed these studies in the time frame of 12 mo following transplantation.
| Ref. | Renal function (Gp1 vs Gp 2) | BPAR (Gp1 vs Gp 2) | Adverse event (Gp1 vs Gp 2) | Remarks |
| Everolimus | ||||
| Budde et al[23], 2011, (ZEUS Study) | 12 mo Sr. Cr: 141.7 ± 44 μmol/L vs 137.0 ± 43 μmol/L (P = NS) eGFR: 71.8 ± 18 mL/min vs 61.2 ± 16 mL/min (P = 0.000) | 9.7% vs 3.4% (P = 0.03) | SAE/Infection: 61% vs 59% (P = NS) UTI: 57.0% vs 53% (P = NS) Diarrhoea: 36% vs 27% (P = NS) HPL: 14% vs 10% (P = NS) | Graft survival: 100% vs 100% (P = NS) Patient survival 100% vs 99% (P = NS) |
| Mjornstedt et al[24], 2012 (CENTRAL trial) | 12 mo Sr. Cr: 122.0 ± 35 μmol/L vs 132.0 ± 45 μmol/L (P = NS) eGFR: 68.1 ± 21.5 mL/min vs 69.4 ± 22.9 mL/min (P = NS) | 27.5% vs 11.0% (P = 0.004) | SAE/Infection: 53.9% vs 38.0% (P = 0.025) CMV infection: 8.8% vs 13.0% (P = NS) Edema: 29.4% vs 21.0% (P = NS) Anaemia: 16.7% vs 6.0% (P = 0.02) HPL: 12.7% vs 9.0% (P = NS) Proteinuria: 4.9% vs 0% (P = 0.06) Acne: 12.7% vs 2.0% (P = 0.006) Mouth Ulceration: 12.7% vs 2.0 % (P = 0.001) | Graft survival: 100% vs 100% (P = NS) Patient survival 98% vs 98% (P = NS) |
| Sirolimus | ||||
| Lebranchu et al[25], 2009 (CONCEPT Study) | 12 mo: Sr. Cr: 117.4 μmol/L vs 132.3 μmol/L (P < 0.001) eGFR: 68.9 mL/min vs 64.4 mL/min (P = 0.017) | 16.8% vs 8.2% (P = NS) | Peripheral Edema: 28.1% vs 22.6% (P = NS) SAE/infection: 60% vs 44% (P = 0.025) Diarrhoea: 30.2% vs 9.2% (P < 0.001) Dyslipidemia: 5.20% vs 4.12% (P = NS) Proteinuria: 9.3% vs 3.09 % (P = NS) NODAT: 3.1% vs 2.06% (P = NS) Apthous Stomatitis: 45.8% vs 5.15% (P < 0.001) | Graft Survival: 99% (P = NS) Patient Survival 97% (P = NS) |
| Guba et al[26], 2010, (SMART Trial) | 12 mo: Sr Cr: 111.5 ± 45 mg/dL vs 142.6 ± 74 mg/dL (P = 0.004) eGFR: 64.5 ± 25.2 mL/min vs 53.4 ± 18.0 mL/min (P = 0.001) | 17.4% vs 15.5% (P = NS) | Wound Healing Disorder: 10.1% vs 11.3%, (P = NS) Infection: 52.2% vs 60.6% (P = NS) CMV: 7.3% vs 28.2% (P < 0.001) HPL: 20.3% vs 7.0% (P = 0.02) Diarrhoea: 13.0% vs 9.9% (P = NS) Lymphocele: 27.5% vs 23.9% (P = NS) | Graft Survival: 99% vs 97% (P = NS) Patient Survival 99% vs 99% (P = NS) |
| Weir et al[27], 2010 (Spare the Nephron Trial) | 12 mo Sr. Cr: 126.2 ± 82.8 μmol/L vs 145.0 ± 96.5 μmol/L (P = NS) eGFR: 74.6 ± 17.9 mL/min vs 71.5 ± 21.2 mL/min (P = 0.06) | 7.4% vs 6.0% (P = NS) | Infection: 16.2% vs 18.3% (P = NS) HPL: 24.3% vs 10.5% (P = 0.000) CMV: 4.7% vs 9.2% (P = NS) Polyoma virus: 2% vs 4% (P = NS) Diarrhoea: 29.7% vs 9.8% (P = 0.001) Malignancy: 4.7% vs 6.5% (P = NS) | Graft Survival: 98% vs 97.4% (P = NS) Patient Survival 100% vs 98% (P = NS) |
| Heilman et al[28], 2011 | 12 mo Sr. Cr: 96.1 ± 28 μmol/L vs 106.1 ± 61 μmol/L (P = NS) eGFR: 63.0 ± 19.1 mL/min vs 59.8 ± 18.9 mL/min (P = NS) | 13% vs 5% (P = NS) | CMV: 13% vs 13% (P = NS) Polyoma virus: 2% vs 4% (P = NS) | NA |
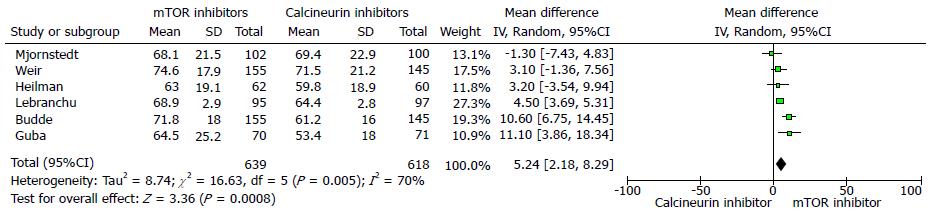
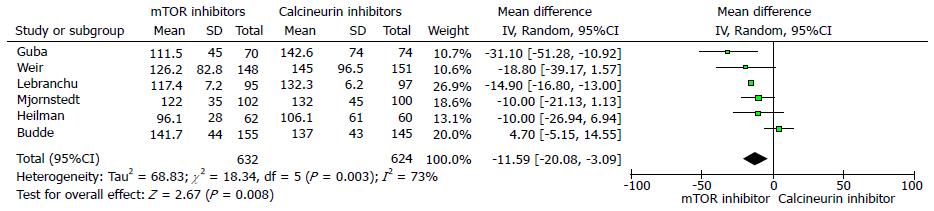
The 12 mo estimated renal function (eGFR) was significantly better in the mTOR inhibitor group compared to CNI group (six trials, 1257 patients, mean difference 5.24 mL/min per 1.73 m2, 95%CI: 2.18 to 8.29, P = 0.00, I2 = 70%) (Figure 2). Similarly, the measured serum creatinine was significantly lower in the mTOR inhibitors groups at 12 mo (six trials, 1256 patients, mean difference = -11.59 μmol/L, 95%CI: -20.08 to -3.09, P < 0.00, I2 = 73%) (Figure 3).
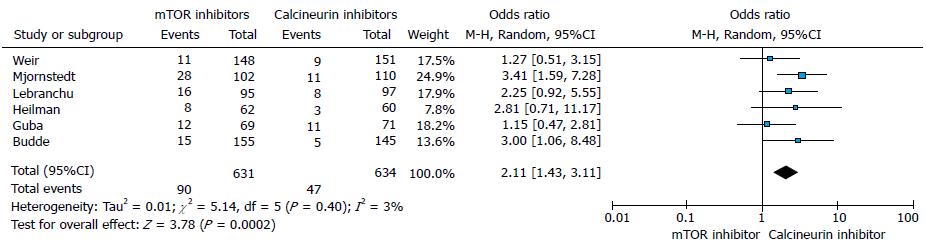
The incidence of BPAR was significantly higher in mTORs groups compared to CNIs groups (six trials, 1265 patients, OR = 2.11, 95%CI: 1.43 to 3.11, P = 0.00, I2 = 3%) at 12 mo (Figure 4).
At 12 mo, the rates of graft survival were comparable for mTOR inhibitor group and the CNI groups (Table 3). There was no significant difference in the incidence of serious adverse events/infection between the mTOR inhibitors and CNI groups in majority of studies.
The initiation of mTOR-inhibitors in early post-transplant period is one of the arduous decision taken by clinicians as it should be done following the period of the heightened immunological risk is over, but no evidence of CNI related toxicity evolved[29,30]. Various CNI free or reduced dosing regimens have been tried to minimize nephrotoxic adverse effect. The peril of increased risk of rejection with the denovo use of CNI free protocols, has been pared down with the early introduction mTOR inhibitors. Howbeit, data regarding optimal transmutation time to mTOR inhibitor based immunosuppression is not clear. Though, the present literatures support the notion of early conversion to mTOR inhibitors within the six months of transplant whereas the reward of conversion after month 6 is not that encouraging. The major hindrance in the expected outcome following late conversion might be because the CNI related nephrotoxicity has already settled in[23,25].
In the present rationale, mTOR inhibitors should be introduced within a period of 2 wk to 6 mo, i.e., following the period of increased risk for rejection and wound infection has been over.
In a ZEUS study, which was multicenter randomised trial done by Budde et al[23] (2011) considered early conversion from CsA to everolimus at 4.5 mo after renal transplantation. Two hundred and sixty-nine patients were randomised into two groups the first group received everolimus with MMF, while another group was maintained on gradually tapered lower dose of CsA with MMF. The group has reported a statistically significant improvement in renal functioning, i.e., eGFR for the everolimus group (71.8 ± 18 mL/min vs 61.2 ± 16 mL/min; P = 0.000), at 12 mo while, BPAR was a higher in the everolimus group (13.9% vs 7.5%, P = 0.09). Nevertheless, they heralded no difference in terms of graft and patient survival[23].
In a CENTRAL trial by Mjörnstedt et al[24] (2012) they studied the effect of early conversion from CsA to everolimus in the seventh week of the post-transplant. About two hundred and two patients who were randomised to receive intervention group everolimus (C0, 3-8 ng/mL) and were compared with CsA (C0, 75-200 ng/mL for two weeks then reduced, further maintained at 50-150 ng/mL) with oral steroids and MMF. They didn’t report significant improvement in GFR in everolimus group (68.1 ± 21.5 mL/min vs 69.4 ± 22.9 mL/min, P = NS) at 12 mo, although serum creatinine was lower in mTOR inhibitor group (122.0 ± 35 μmol/L vs 132.0 ± 45 μmol/L, P = NS).
Though the reported incidence of BPAR was significantly higher in EVR group than in CsA group (27.5% vs 11.0%, P = 0.004), the survival outcomes were similar at 12 mo. The reported side effects as proteinuria, anaemia, hyperlipidemia, acne and mouth ulceration were significantly more frequent in the everolimus group[24].
In the CONCEPT study 2009 by Lebranchu et al[25], instituted Sirolimus by replacing CsA in the third month of the post-transplantation. Their literature listed significantly better eGFR (68.9 mL/min vs 64.4 mL/min) and significantly lower serum creatinine (117.4 μmol/L vs 132.3 μmol/L, P < 0.001) in the sirolimus group at 12 mo. The detailed BPAR was similar for entire period of observation. The side effects such as diarrhoea, SAE, aphthous stomatitis, proteinuria and new onset diabetes mellitus were either significantly higher or higher in the sirolimus group[25].
Guba et al[26] (2010) carried out a multicenter randomised SMART trial, to explore the effects of very early conversion to sirolimus from CsA only 10 to 24 d after the renal transplantation. They randomised one hundred and forty-one patients were into two groups to confer sirolimus with MMF and steroid, on the other hand the second group was maintained on gradually tapered lower dose of CsA with MMF and steroid. They reported statistically significant improvement in renal functioning, eGFR (64.5 ± 25.2 mL/min vs 53.4 ± 18 mL/min; P = 0.001) with significantly reduced serum creatinine (111.5 ± 45 μmol/L vs 142.6 ± 74 μmol/L, P = 0.004) for the sirolimus group at 12 mo. The detailed incidence of BPAR (17.4% vs 15.5%, P = NS) was similar in both groups, likewise, the graft and patient survival were quite similar. In addition, the recipients in the sirolimus group reported a significantly higher number of adverse effects such as acne, hyperlipidemia and lower number CMV viremia withal the incidence of BPAR was similar in both groups (20.2% vs 19.7%, P = NS)[26].
In Spare the Nephron Trial, Weir et al[27] (2010) randomized 299 kidney transplant recipients into two groups following 115 d of the transplant. The first group received sirolimus with MMF while the second group was maintained on CNI and MMF. They reported significant improvement in renal function in terms of higher eGFR (74.6 ± 17.9 mL/min vs 71.5 ± 21.2 mL/min; P = 0.06) and lower serum creatinine (126.2 ± 82.8 μmol/L vs 145.0 ± 96.5 μmol/L, P = NS) in the sirolimus group. They delineated the likewise patient and graft survival in both groups. However, patients in the sirolimus group reported a significantly higher number of adverse effects as hyperlipidemia and diarrhoea[27].
In the 2011 study by Heilman et al[28], sirolimus introduced in the first month of the renal transplant. They have given the account of significant improvement in eGFR (63.0 ± 19.1 mL/min vs 59.8 ± 18.9 mL/min; P = NS) and set out lower serum creatinine in the sirolimus group at 12 mo while the reported BPAR was likewise in both groups[28].
Publication bias is an important point to consider in a meta-analysis because all the researches which take place are not published. Studies with a significant result are more likely to be published. Studies with a significant result are more likely to be placed in a higher impact journal compared to the studies with null results. Moreover, well controlled and properly carried out studies are less likely to achieve significance.
In general, early CNI withdrawal in the wake of mTOR inhibitor based regimen institution seems a more empirical and constructive approach towards immunosuppressive management of renal transplant recipients. Natheless, taking into account of the high rejection rate contemplated in these studies, it will be a judicious decision of not to proffer this therapy to patients with moderate to high immunological risk though additional studies with long duration of follow-up are demanded to confirm present conjecture[29-33].
Despite the fact that the data on the Tac minimization strategies are limited, the present evidence suggest that treatment with mTOR-inhibitors allows early and substantial CNI minimization and provides better renal functioning at the end of first year of transplantation. Thus, it is not judicious to extend these regimens to patients with moderate to high immunological risk. However, further trials directed towards different ethnicity and geography are needed to determine further evidence.
The aim of this review is to assess the one-year effectiveness of the early introduction of mammalian target of rapamycin (mTOR) inhibitors with or without calcineurin inhibitors (CNIs) within six months of renal transplantation.
The current literature was reviewed to assess the role of immunosuppressive agent, mTOR inhibitors as an alternative to CNI within six months of renal transplant in terms of better renal functioning and survival by assessing glomerular filtration rate (GFR), serum creatinine, Biopsy Proven Acute Rejection (BPAR) and survival.
The major advantages were observed regarding better renal functioning, GFR and serum creatinine were better in mTOR inhibitor group at 12 mo. BPAR was significantly higher in the mTOR-inhibitor group though survival was comparable.
In general, early CNI withdrawal seems to be a more empirical and constructive approach as it provides better renal functioning in the low immunological risk transplant recipients.
This study is a systemic review and meta-analysis of the effect on renal function and graft survival following early conversion of CNI to mTOR inhibitors with or without CNI after kidney transplantation. The authors initially selected 112 manuscripts, and of them, only 6 papers were useful for meta-analysis. They conclude that introduction of mTOR-inhibitors allows early and substantial CNI minimization.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Novosel MK, Okumura K, Salvadori M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Salvadori M, Bertoni E. Is it time to give up with calcineurin inhibitors in kidney transplantation? World J Transplant. 2013;3:7-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Knops N, Levtchenko E, van den Heuvel B, Kuypers D. From gut to kidney: transporting and metabolizing calcineurin-inhibitors in solid organ transplantation. Int J Pharm. 2013;452:14-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Diekmann F, Andrés A, Oppenheimer F. mTOR inhibitor-associated proteinuria in kidney transplant recipients. Transplant Rev (Orlando). 2012;26:27-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Krieger NR, Becker BN, Heisey DM, Voss BJ, D’Alessandro AM, Becker YT, Odorico JS, Kalayoglu M, Pirsch JD, Sollinger HW. Chronic allograft nephropathy uniformly affects recipients of cadaveric, nonidentical living-related, and living-unrelated grafts. Transplantation. 2003;75:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Cornell LD, Colvin RB. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2005;14:229-234. [PubMed] |
| 6. | Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RD. Delta analysis of posttransplantation tubulointerstitial damage. Transplantation. 2004;78:434-441. [PubMed] |
| 8. | Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, Babineau D, Kurian S, Salomon D, Novick AC. Kidney transplantation with sirolimus and mycophenolate mofetil-based immunosuppression: 5-year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation. 2007;83:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Flechner SM. Sirolimus in kidney transplantation indications and practical guidelines: de novo sirolimus-based therapy without calcineurin inhibitors. Transplantation. 2009;87:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Augustine JJ, Knauss TC, Schulak JA, Bodziak KA, Siegel C, Hricik DE. Comparative effects of sirolimus and mycophenolate mofetil on erythropoiesis in kidney transplant patients. Am J Transplant. 2004;4:2001-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hernández D, Martínez D, Gutiérrez E, López V, Gutiérrez C, García P, Cobelo C, Cabello M, Burgos D, Sola E. Clinical evidence on the use of anti-mTOR drugs in renal transplantation. Nefrologia. 2011;31:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Gullestad L, Iversen M, Mortensen SA, Eiskjaer H, Riise GC, Mared L, Bjørtuft O, Ekmehag B, Jansson K, Simonsen S. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation. 2010;89:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Serre JE, Michonneau D, Bachy E, Noël LH, Dubois V, Suberbielle C, Kreis H, Legendre C, Mamzer-Bruneel MF, Morelon E. Maintaining calcineurin inhibition after the diagnosis of post-transplant lymphoproliferative disorder improves renal graft survival. Kidney Int. 2014;85:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Zaza G, Tomei P, Ria P, Granata S, Boschiero L, Lupo A. Systemic and nonrenal adverse effects occurring in renal transplant patients treated with mTOR inhibitors. Clin Dev Immunol. 2013;2013:403280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 347] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 16. | Nashan B. Induction therapy and mTOR inhibition: minimizing calcineurin inhibitor exposure in de novo renal transplant patients. Clin Transplant. 2013;27 Suppl 25:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Gonzalez-Vilchez F, Vazquez de Prada JA, Paniagua MJ, Gomez-Bueno M, Arizon JM, Almenar L, Roig E, Delgado J, Lambert JL, Perez-Villa F. Use of mTOR inhibitors in chronic heart transplant recipients with renal failure: calcineurin-inhibitors conversion or minimization? Int J Cardiol. 2014;171:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Filler G. Calcineurin inhibitors in pediatric renal transplant recipients. Paediatr Drugs. 2007;9:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Rostaing L, Kamar N. mTOR inhibitor/proliferation signal inhibitors: entering or leaving the field? J Nephrol. 2010;23:133-142. [PubMed] |
| 20. | Ganschow R, Pape L, Sturm E, Bauer J, Melter M, Gerner P, Höcker B, Ahlenstiel T, Kemper M, Brinkert F. Growing experience with mTOR inhibitors in pediatric solid organ transplantation. Pediatr Transplant. 2013;17:694-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Peddi VR, Wiseman A, Chavin K, Slakey D. Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev (Orlando). 2013;27:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Pescovitz MD, Govani M. Sirolimus and mycophenolate mofetil for calcineurin-free immunosuppression in renal transplant recipients. Am J Kidney Dis. 2001;38:S16-S21. [PubMed] |
| 23. | Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet. 2011;377:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Mjörnstedt L, Sørensen SS, von Zur Mühlen B, Jespersen B, Hansen JM, Bistrup C, Andersson H, Gustafsson B, Undset LH, Fagertun H. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant. 2012;12:2744-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, Moulin B, Frouget T, Le Meur Y, Glotz D. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant. 2009;9:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J, Andrassy J, Reinke P, Pressmar K, Hakenberg O. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation. 2010;90:175-183. [PubMed] |
| 27. | Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, Kalil RN, Pearson TC. Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial. Kidney Int. 2011;79:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Heilman RL, Younan K, Wadei HM, Mai ML, Reddy KS, Chakkera HA, Gonwa TA. Results of a prospective randomized trial of sirolimus conversion in kidney transplant recipients on early corticosteroid withdrawal. Transplantation. 2011;92:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Silva HT, Felipe CR, Garcia VD, Neto ED, Filho MA, Contieri FL, de Carvalho DD, Pestana JO. Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant. 2013;13:3155-3163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Groetzner J, Kaczmarek I, Schulz U, Stegemann E, Kaiser K, Wittwer T, Schirmer J, Voss M, Strauch J, Wahlers T. Mycophenolate and sirolimus as calcineurin inhibitor-free immunosuppression improves renal function better than calcineurin inhibitor-reduction in late cardiac transplant recipients with chronic renal failure. Transplantation. 2009;87:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Höcker B, Tönshoff B. Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option? Paediatr Drugs. 2011;13:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Kaczmarek I, Zaruba MM, Beiras-Fernandez A, Reimann R, Nickel T, Grinninger C, Sadoni S, Hagl C, Meiser B. Tacrolimus with mycophenolate mofetil or sirolimus compared with calcineurin inhibitor-free immunosuppression (sirolimus/mycophenolate mofetil) after heart transplantation: 5-year results. J Heart Lung Transplant. 2013;32:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Gatault P, Lebranchu Y. Conversion to mTOR-inhibitor-based immunosuppression: which patients and when? Transplant Res. 2013;2:S3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |