Published online Sep 24, 2016. doi: 10.5500/wjt.v6.i3.599
Peer-review started: March 17, 2016
First decision: April 18, 2016
Revised: July 4, 2016
Accepted: July 29, 2016
Article in press: August 1, 2016
Published online: September 24, 2016
Processing time: 192 Days and 4.6 Hours
To characterize major determinants of 20-year survival after liver transplantation (LT).
This longitudinal single-institution study includes 313 consecutive patients who received a LT between 1988 and 1992. Pretransplant clinical characteristics and laboratory values were assessed and compared between 20-year survivors and non-survivors. Particular attention was paid to the Model for End-Stage Liver Disease (labMELD)-score and the Eurotransplant Donor Risk Index (ET-DRI) to unravel their impact on 20-year survival after LT.
Twenty-year survivors were significantly younger (44 vs 50 years, P = 0.001), more likely to be female (49% vs 36%, P = 0.03) and less likely to be obese at the time of LT (19% vs 32%, P = 0.011). Mean labMELD-score (P = 0.156), rate of high-urgency LT (P = 0.210), cold-ischemia time (P = 0.994), rate of retransplantation (P = 0.12) and average donor age (28 vs 33 years, P = 0.099) were not statistically different. The mean estimated glomerular filtration rate was higher among survivors (P = 0.007). ET-DRI > 1.4 (P = 0.020) and donor age ≥ 30 years (P < 0.022) had significant influence on 20-year survival. The overall survival was not significantly impacted by labMELD-score categories (P = 0.263).
LT offers excellent long-term results in case of optimal donor and recipient conditions. However, mainly due to the current organ shortage, these ideal circumstances are rarely given; thus algorithms for donor-recipient matching need to be refined, in order to enable a maximum benefit for the recipients of high quality as well as marginal organs.
Core tip: We compare characteristics of 20-year survivors and non-survivors after liver transplantation. The lab model for end-stage liver disease-score seems not to be an adequate tool for predicting long-term (20 years) outcome. The Eurotransplant Donor Risk Index (ET-DRI) has a significant impact on long-term survival. While close to 60% of patients that received a donor organ with an ET-DRI < 1.2 survived for 20 years and longer, only less than 40% of the patients with an ET-DRI > 1.4 survived the same number of years. Only about 20% survivors had overweight before transplantation, compared to about 33% non-survivors. The mean estimated glomerular filtration rate was higher among survivors.
- Citation: Buescher N, Seehofer D, Helbig M, Andreou A, Bahra M, Pascher A, Pratschke J, Schoening W. Evaluating twenty-years of follow-up after orthotopic liver transplantation, best practice for donor-recipient matching: What can we learn from the past era? World J Transplant 2016; 6(3): 599-607
- URL: https://www.wjgnet.com/2220-3230/full/v6/i3/599.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i3.599
Over the last three decades, liver transplantation (LT) has become the standard therapeutic treatment for patients with terminal liver failure[1-4]. Short- and long-term results have improved, resulting in dramatic prolongation of recipients’ life expectancy[5]. Surgical techniques, pharmaceutical regimens, and intensive care management were continuously refined[6,7]. Equally as important, LT centers have gained invaluable experience regarding the long-term management of LT patients[3,4,8]. Many obstacles resulting in patient and graft loss have been identified, and means to overcome them have been developed. This has led to a broad increase in the number of potential LT recipients[9].
However, with growing waiting lists and an increasing number of LT-centers, the LT community is now facing the issue of fair organ allocation. The limited amount of donor organs led to the implementation of different liver allocation policies[10,11] and a more liberal acceptance of extended criteria donor (ECD) organs[12,13]. The implementation of Model for end-stage liver disease (MELD) allocation in 2006 within the Eurotransplant area has reduced waiting list mortality to about 10%[14], but has also increased the one-year mortality in many European centers, e.g., at our center from 8.2% to about 17.4%[15]. Donor-recipient-matching has become crucial to achieving reasonable one year mortality[16] and acceptable waiting list mortality, especially when allocating marginal organs to progressively sicker recipients.
With this study, we aim to evaluate the influence of pretransplant labMELD and Eurotransplant Donor Risk Index (ET-DRI) on the long-term survival of a cohort of LT-recipients. Furthermore, we compared the pretransplant characteristics of recipients who survived ≥ 20 years after their LT to those who died within the 20-year observation period.
A longitudinal single-institution study was performed to characterize 20-year LT survivors. Institutional Review Board approval was obtained for this study.
The cohort has been described previously[17]. Indications for primary transplants are presented in Table 1. Patients were divided into groups with regards to their underlying disease: Cholestatic/autoimmune comprises all patients with primary (n = 19) or secondary (n = 3) sclerosing cholangitis, primary (n = 29) or secondary (n = 1) biliary cirrhosis and autoimmune hepatitis (n = 12). The group hepatobiliary malignancy includes all cases of hepatocellular carcinomas (HCC, n = 27), cholangiocarcinomas (n = 5) as well as Klatskin tumors (n = 4), while virus-related cirrhosis includes all patients with hepatitis B (n = 47), hepatitis C (n = 32), hepatitis B and C (n = 3) and hepatitis B and D (n = 10) virus cirrhosis. Overall, virus-related cirrhosis (29.4%), cholestatic/autoimmune liver disease (20.4%), alcoholic cirrhosis (16.0%), hepatobiliary malignancy (11.5%), cryptogenic cirrhosis (9.3%) and acute liver failure (7.3%) were the most common indications for primary LT. Of the twenty-seven HCC patients, seven did not fall under the later defined Milan criteria.
| All patients n = 313 (100%) | 20-yr survivors n = 157 (50%) | 20-yr non- survivors n = 141 (45%) | Ratio1 | Lost n = 15 (5%) | |
| Virus-related cirrhosis | 92 (29.4%) | 46 (29.30%) | 39 (27.70%) | 1.18 | 7 |
| Hepatitis B | 47 (15.0%) | 26 (16.6%) | 19 (13.5%) | ||
| Hepatitis C | 32 (10.2%) | 13 (8.3%) | 17 (12.1%) | ||
| Hepatitis B and D | 10 (3.2%) | 5 (3.2%) | 2 (1.4%) | ||
| Hepatitis B and C | 3 (1.0%) | 2 (1.3%) | 1 (0.7%) | ||
| Cholestatic/ autoimmune | 64 (20.4%) | 38 (24.2%) | 20 (14.2%) | 1.90 | 6 |
| Alcoholic cirrhosis | 50 (16.0%) | 23 (14.6%) | 27 (19.1%) | 0.85 | |
| Hepatobiliary malignancy | 36 (11.5%) | 7 (4.5%) | 28 (19.9%) | 0.25 | 1 |
| HCC | 27 (8.6%) | 6 (3.8%) | 20 (14.2%) | ||
| CCC | 5 (1.6%) | 0 (0.0%) | 5 (3.5%) | ||
| Klatskin tumor | 4 (1.3%) | 1 (0.6%) | 3 (2.1%) | ||
| Cryptogenic cirrhosis | 29 (9.3%) | 15 (9.6%) | 13 (9.2%) | 1.15 | 1 |
| Acute liver failure | 23 (7.3%) | 16 (10.2%) | 7 (5.0%) | 2.29 | |
| Others | 19 (6.1%) | 13 (8.3%) | 6 (4.3%) | 2.20 |
Characteristics of donors and recipients are depicted in Table 2. In summary, the cohort consists of 313 consecutive patients who received a primary LT at the Charité, Campus Virchow-Klinikum, between 1988 and 1992. During the twenty-year follow-up those patients received a total of 365 livers including 54 retransplantations (46 first retransplantations). There were 178 male and 135 female recipients. At the date of primary LT, median patient age was 47 (14-66) years including two patients who were minors at the age of 14 and 16, while median donor age was 30 (9-64) years. Mean labMELD-Score was 18.6 ± 7.6 and mean ET-DRI was 1.35 ± 0.2.
| All patients n = 313 | 20-yr-survivors n = 157 | 20-yr-non-survivors n = 141 | P | |
| Recipients | ||||
| Age (yr) | 47 (14-66) | 44 (14-66) | 50 (25-65) | 0.001 |
| Age < 18, n (%) | 2 (0.6) | 2 (1.3) | 0 (0) | 0.06 |
| Age > 55, n (%) | 57 (18) | 19 (12) | 36 (26) | 0.03 |
| Gender, n (%) female | 135 (43) | 77 (49) | 51 (36) | 0.03 |
| labMELD-score | 18.6 (± 7.6) | 19.4 (± 8.3) | 18.1 (± 7.0) | 0.156 |
| Urgent LT, n (%) | 23 (7) | 15 (10) | 8 (6) | 0.21 |
| BMI (kg/m2) | 23.0 ± 3.3 | 22.7 ± 3.0 | 23.5 ± 3.7 | 0.037 |
| HBMI, n (%) | 78 (25%) | 30 (19%) | 45 (32%) | 0.011 |
| HLIP, n (%) | 45 (14%) | 20 (15%) | 23 (19%) | 0.376 |
| Donors | ||||
| Donor age (yr) | 30 (9-64) | 28 (14-64) | 33 (9-60) | 0.099 |
| ET-DRI | 1.35 (± 0.2) | 1.32 (± 0.2) | 1.37 (± 0.2) | 0.121 |
| Transplant | ||||
| Cold ischemia time, h | 10.6 (± 4) | 10.6 (± 4) | 10.7 (± 4) | 0.994 |
| Retransplantation, n (%) | 46 (15) | 18 (11) | 25 (18) | 0.120 |
| Liver function | ||||
| tBili | 8.1 ± 11.9 | 9.0 ± 12.6 | 7.7 ± 11.6 | 0.363 |
| AST | 115 ± 460 | 124 ± 486 | 111 ± 454 | 0.820 |
| ALT | 102 ± 233 | 102 ± 177 | 108 ± 286 | 0.849 |
| INR | 1.76 ± 0.8 | 1.82 ± 0.8 | 1.7 ± 0.8 | 0.226 |
| Clinical characteristics | ||||
| Systolic BP (mmHg) | 120 ± 20 | 119 ± 20 | 122 ± 21 | 0.340 |
| Diastolic BP (mmHg) | 71 ± 11 | 71 ± 12 | 72 ± 11 | 0.353 |
| Laboratory parameters | ||||
| Glucose (mg/dL) | 120 ± 58 | 116 ± 46 | 126 ± 70 | 0.174 |
| Cholesterol (mg/dL) | 134 ± 72 | 129 ± 55 | 138 ± 86 | 0.311 |
| Triglycerides (mg/dL) | 95 ± 67 | 91 ± 56 | 100 ± 80 | 0.326 |
| Creatinine (mg/dL) | 1.0 ± 0.8 | 1.06 ± 1.0 | 0.95 ± 0.4 | 0.247 |
| eGFR (mL/min per 1.73 m2) | 98 ± 59 | 106 ± 70 | 88 ± 39 | 0.007 |
Patients were observed until their death, loss to follow-up, or graft loss. Data were censored at time of patients’ death, loss to follow-up, graft loss or at 20 years after transplantation, respectively. A graft survival analysis was performed in which labMELD-scores, pretransplant laboratory values (median 0 d before LT, range 0-84 d), clinical characteristics and ET-DRI were evaluated for the primary LT as well as for the primary graft, in order to compare characteristics of 20 year-survivors and non-survivors.
LabMELD-scores were retrospectively calculated using the pretransplant serum bilirubin level, serum creatinine level, and INR according to Kamath et al[18].
Given Quick values were converted into INR with the help of the corresponding batch numbers. Serum bilirubin, INR, or serum creatinine values of less than 1.0 were set to 1.0 to preclude negative scores. Serum creatinine level was capped at 4.0. MELD-scores were capped at 40. We were able to retrieve MELD-scores for 308 patients. For the compilation of Kaplan-Meier curves, recipients were grouped into three different categories: MELD ≤ 15 (n = 126), MELD = 16-25 (n = 134) and MELD > 25 (n = 48).
The ET-DRI was assessed using the required donor and transplant factors according to Braat et al[19].
We were able to calculate the corresponding ET-DRI for 179 patients (57%). For the remaining donors the latest GGT level was unknown, which is an essential factor for ET-DRI calculation. Ninety-four of these recipients were 20-year survivors, 85 were non-survivors. For Kaplan-Meier estimates, the grafts were divided into three groups: ET-DRI < 1.21 (n = 54), 1.21-1.40 (n = 61) and > 1.4 (n = 64).
Laboratory parameters were obtained after a fasting period of at least 12 h and included serum levels of total cholesterol, triglycerides, creatinine, Quick-value, total bilirubin (tBili), aspartate aminotransferase, alanine aminotransferase and glucose.
Overweight (HBMI) was defined as body-mass-index (BMI = weight/height2) above 25. Blood cholesterol levels of more than 200 mg/dL, triglyceride levels above 175 mg/dL, or statin treatment were considered “hyperlipidemia” (HLIP). The MDRD-formula was used to estimate glomerular filtration rate (eGFR). An eGFR < 60 mL/min per 1.73 m2 was considered moderately impaired renal function (MIRF), while rates < 30 mL/min per 1.73 m2 were defined as severely impaired renal function (SIRF)[20].
Categorical variables were compared by the χ2 test and summarized as percentages and frequencies. Continuous variables were compared using unpaired t test and summarized as median and range, or mean ± SD. A P value of less than 0.05 was interpreted as statistically significant. Kaplan-Meier estimates were used to calculate survival curves. Differences in survival curves were compared using log-rank statistics. All calculations were done using the SPSS software package (version 22.0 for Windows, SPSS Inc., Chicago, IL).
After a median follow-up of 233 mo (0-260), 157 patients were alive (141 with complete sets of data, 16 with incomplete sets of data) and 141 had died (27 patients within 6 mo after LT) while 15 patients were lost to follow-up 99 to 243 mo after LT.
Table 1 depicts the distribution of primary indication for LT among survivors and non-survivors. The most common indications among survivors were virus-related cirrhosis (29.3%), cholestatic/autoimmune liver disease (24.2%), and alcoholic cirrhosis (14.6%), while among non-survivors virus-related cirrhosis (27.7%), hepatobiliary malignancy (19.9%) and alcoholic cirrhosis were the most frequent. The ratio of survivors/non-survivors was lowest for hepatobiliary malignancies (0.25) and highest for cholestatic/autoimmune liver disease (1.90) and acute liver failure (2.29).
As shown in Table 2, median age of 20-year-survivors and non-survivors was 44 (14-66) and 50 (25-65) years, respectively (P = 0.001). Both minors (primary indication PSC and ALF) were alive after twenty years of follow-up. The group of non-survivors includes significantly more LT recipients over the age of 55 (26% compared to 12% of the survivors, P = 0.03) while the group of survivors has a significantly larger amount of female recipients (49% compared to 36% of the non-survivors, P = 0.03). Mean BMI for survivors and non-survivors was 22.7 ± 3.0 and 23.5 ± 3.7 kg/m2, respectively (P = 0.037). There were no significant differences for survivors and non-survivors regarding pretransplant labMELD-score (19.4 ± 8.3 and 18.1 ± 7.0, P = 0.156), rate of high-urgent LT (10% and 6%, P = 0.210), cold-ischemia time (10.6 ± 4 and 10.7 ± 4 h, P = 0.994) and rate of retransplantation (11% and 18%, P = 0.12).
Among survivors, median donor age was 28 years (14-64) compared to a median donor age of 33 years (9-60) among non-survivors (P = 0.099). Mean ET-DRI for survivors and non-survivors was 1.32 ± 0.2 and 1.37 ± 0.2, respectively (P = 0.121).
The overall actuarial patient survival rates at 1, 10 and 20 years were 88.4%, 72.7% and 52.5%, respectively. The overall graft survival rates were 83.7%, 64.7% and 46.6% after 1, 10 and 20 years, respectively.
None of the liver function tests that were compared showed a statistically significant difference between survivors and non-survivors (Table 2). Prior to LT, mean total bilirubin was 9.0 ± 12.6 mg/dL for survivors and 7.7 ± 11.6 mg/dL for non-survivors (P = 0.363). Mean aspartate aminotransferase was 124 ± 486 U/L for survivors and 111 ± 454 U/L for non-survivors (P = 0.820). Mean pretransplant alanine aminotransferase was 102 ± 177 U/L for survivors and 108 ± 286 U/L for non-survivors (P = 0.849).
Systolic BP and diastolic BP were not significantly different between survivors and non-survivors. 20-year survivors’ mean blood glucose was 116 ± 46 mg/dL compared to 126 ± 70 mg/dL among non-survivors (P = 0.174). Cholesterol (129 ± 55 and 138 ± 86, P = 0.311) and triglycerides (91 ± 56 and 100 ± 80, P = 0.326) values did not differ significantly between survivors and non-survivors. Regarding the renal function, mean eGFR of 106 ± 70 mL/min per 1.73 m2 in survivors was significantly higher than mean eGFR of 88 ± 39 mL/min per 1.73 m2 in non-survivors (P = 0.007). Detailed data are presented in Table 2, where the percentages relate to the amount of patients with complete data in the specific category.
Nineteen percent of the twenty-year survivors had HBMI before transplantation, while 32% of the non-survivors had HBMI (P = 0.016). Comparing survivors and non-survivors, prevalence of HLIP (15% and 19%, P = 0.407), MIRF (20% and 21%, P = 0.886) and SIRF (5% and 3%, P = 0.547) did not show a significant difference.
To further analyze the impact of renal function, patients were split up into separate groups, based on their eGFR before transplantation (Table 3). Eighty percent of the survivors and 79% of the non-survivors had an eGFR > 60 (P = 0.860), pointing to normal renal function. The groups that comprise eGFR values of 60 to 69 and 70 to 79 contain significantly more non-survivors than survivors (20.0% and 15.7% compared to 6.5% and 6.5%, P = 0.001 and P = 0.011, respectively), while 30.3% of the survivors had an eGFR > 120 compared to 20.0% of the non-survivors (P = 0.042).
| 20-yr survivors n = 155 | 20-yr non-survivors n = 140 | P | |
| eGFR > 60 | 126 (80%) | 112 (79%) | 0.860 |
| MIRF | 31 (20%) | 29 (21%) | 0.879 |
| SIRF | 7 (4.5%) | 4 (2.9%) | 0.453 |
| eGFR 30-39 | 10 (6.5%) | 5 (3.6%) | 0.261 |
| eGFR 40-49 | 8 (5.2%) | 7 (5.0%) | 0.950 |
| eGFR 50-59 | 8 (5.2%) | 13 (9.3%) | 0.169 |
| eGFR 60-69 | 10 (6.5%) | 28 (20%) | 0.001 |
| eGFR 70-79 | 10 (6.5%) | 22 (15.7%) | 0.011 |
| eGFR 80-89 | 22 (14.2%) | 13 (9.3%) | 0.193 |
| eGFR 90-99 | 16 (10.3%) | 14 (10.0%) | 0.927 |
| eGFR 100-109 | 15 (9.7%) | 9 (6.4%) | 0.308 |
| eGFR 110-119 | 10 (6.5%) | 7 (5.0%) | 0.593 |
| eGFR > 120 | 47 (30.3%) | 28 (20.0%) | 0.042 |
A subgroup analysis was performed to assess the underlying diseases among those patients who later developed MIRF and SIRF. The most common indications for primary LT among patients with MIRF at 20 years after LT (n = 85) were virus-related cirrhosis (n = 32), CD/AIH (n = 18) and alcoholic liver disease (n = 15). Among patients who later developed SIRF (n = 10), the most common primary indications were CD/AIH (n = 4), virus-related cirrhosis (n = 3) and polycystic liver disease (n = 2).
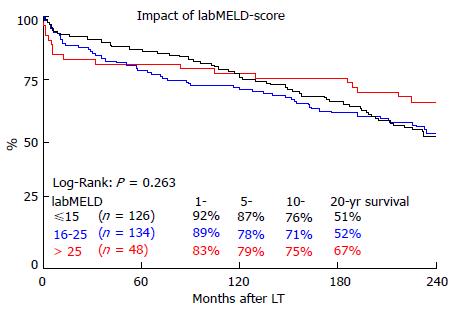
As shown in Figure 1, the overall survival at 1, 5, 10 and 20 years for the three different groups of labMELD-Scores, was 92.1%, 86.5%, 76.2% and 51.3% for group 1 (labMELD ≤ 15), 88.8%, 77.6%, 70.9% and 51.9% for group 2 (labMELD = 16-25) and 83.3%, 79.2%, 75.0% and 66.7% for group 3 (labMELD > 25). The 20-year survival did not differ significantly (P = 0.263). This was also true for 0.5- (P = 0.226), 1- (P = 0.293), 5- (P = 0.293), 10- (P = 0.522) and 15-year (P = 0.241) survival. Survival of recipients with labMELD > 25 was not significantly worse compared to all others at 6 mo after LT, (P = 0.095), also not at 1-year (P = 0.158), 5-year (P = 0.704) and 10-year (P = 0.726). At 15-year (P = 0.143) and 20-year (P = 0.107), recipients with MELD > 25 showed better overall survival, but this difference was not statistically significant.
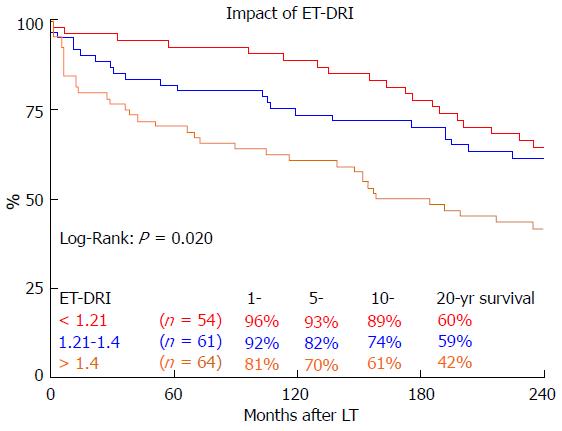
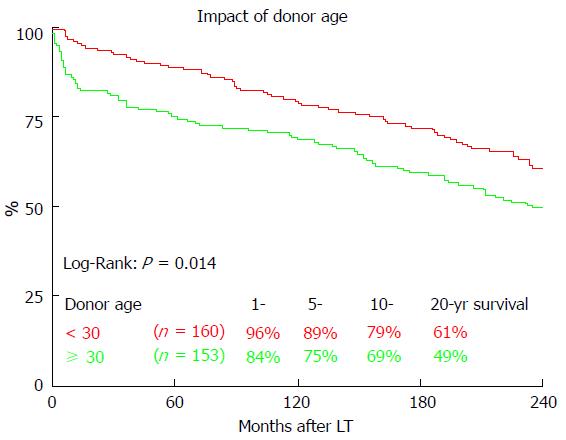
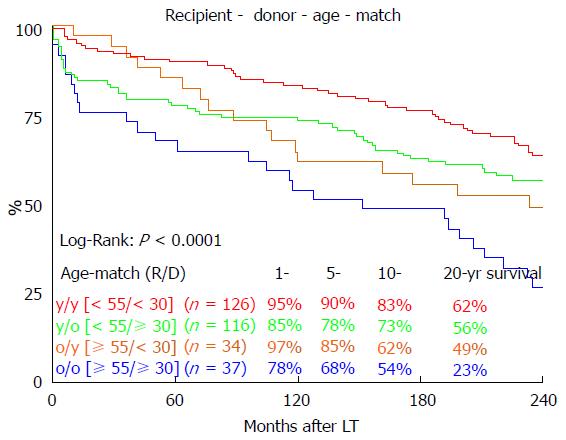
Long-term survival was significantly influenced by ET-DRI (P = 0.020, Figure 2). Comparing only two groups, ET-DRI ≤ 1.4 and >1.4, the survival outcome showed a significant difference as well (P = 0.011) (data not shown). Looking at the donor age separately (< vs≥ 30 years), we also found a significant impact on long-term survival as shown in Figure 3 (P < 0.014). A more detailed analysis of donor and recipient age based on a recipient age of < and ≥ 55 years and a donor age of < vs≥ 30 years revealed a highly significant impact on long term outcome in the comparison of these four categories (P < 0.0001, Figure 4).
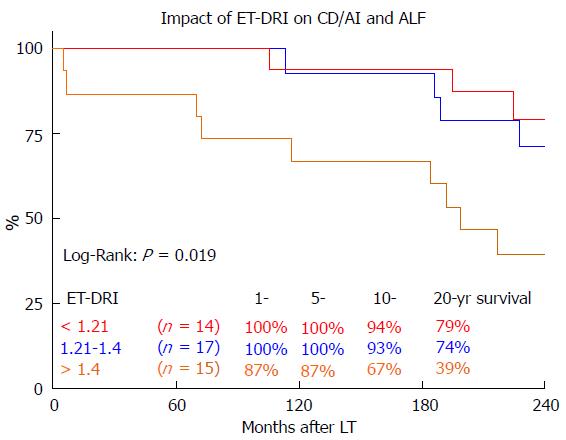
In a sub-analysis of patients with the best long-term survival[17] (CD/AIH and ALF) the effect of donor quality (ET-DRI) was even more pronounced: Transplanting an ET-DRI < 1.21 organ resulted in an 20 year survival of 79% compared to 39% for an ETDRI > 1.4 organ (Figure 5).
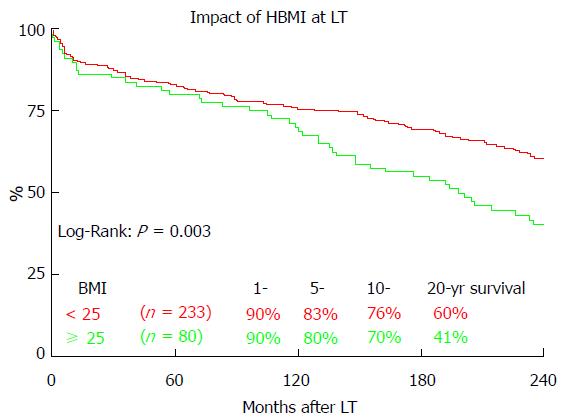
Figure 6 shows the impact of the BMI on the long-term outcome after LT. Patients without pretransplant HBMI (< 25) showed significantly better overall 20-year survival (60.4% vs 40.6%, P = 0.003). HBMI did not significantly impact 1 year (90.0% vs 90.6%, P = 0.703), 5 year (80.0% vs 82.8%, P = 0.471) or 10 year (70.0% vs 75.5%, P = 0.191) survival.
Presence of MIRF and SIRF before transplantation did not significantly influence the overall 20-year survival (P = 0.936 and 0.387, respectively) (data not shown).
As we have previously published[17], the most common causes of death overall were recurrence of primary disease (21.3%), infection (20.6%) and de-novo malignancy (19.9%). While recurrent disease was most common in the first decade after LT, followed by infection and de novo malignancy, de novo malignancy was the most common cause of death during the second decade after LT, followed by infection and cardiovascular events. Recurrence of primary disease was especially common in patients with hepatobiliary malignancy and virus-related cirrhosis. Among the de-novo malignancies, squamous-cell carcinomas were most common. Pneumonia and sepsis were the most common infections.
Recently, our center published the first European single-institution 20-year survival data and the most promising long-term outcomes worldwide to this point[17]. More than half of our cohort survived for two decades after LT. With the present study, we aimed to compare the characteristics of 20-year survivors and 20-year non-survivors in order to characterize those patients who achieved outstanding long-term survival.
Not surprisingly, on average 20-year survivors were significantly younger and predominantly female. Previous studies have also found that survival for female recipients is slightly higher compared to male recipients. The prevalence of cardiovascular risk factors, as well as cardiovascular events, is higher in male long-term survivors, which may explain this finding[17,21].
The Kaplan-Meier analyses of the long-term survival in this cohort show that the greatest disparity in outcome based on ET-DRI categories (Figure 2) seems to occur within the first year after LT; after this there is little divergence in the Kaplan-Meier curves according to donor risk. Thus, after the short-term post-transplant period has passed, the underlying disease and further recipient characteristics seem to play a more important role than the initial graft quality. Long-term outcome studies, such as this one, are valuable in identifying such recipient characteristics. One example is the fact that in our cohort, presence of HBMI does not become a significant prognostic factor until 10 years after LT.
As far as the distribution of primary indications for LT goes, we found that hepatobiliary malignancies had a particularly low survival rate[17]. In this cohort, the ratio of survivors/non-survivors for patients with hepatobiliary malignancy was 0.25; several patients in this group presented at an advanced stage. Due to the high prevalence of recurrent disease among patients with HCC far beyond the Milan criteria[22] and advanced cholangiocellular carcinomas[23], they are no longer eligible for LT. The European Liver Transplant Registry states 20-year patient survival rates of 27% for primary liver tumors, which make up for 14% of the total indications for LT[24]. On the other hand, patients with autoimmune and cholestatic liver disease (ratio 1.9) as well as patients with acute liver failure (ratio 2.29), made up a significant part of the 20-year survivors, which is in line with the findings of the European Liver Transplant Registry, which lists 20-year patient survival rates of 44% for cholestatic disease, 55% for autoimmune liver disease and 47% for acute hepatic failure, which make up for a total of 21% of all indications[24].
Unexpectedly, the labMELD-score did not significantly influence 20-year survival in our cohort. Our study supports the findings of previous studies[25] showing that the labMELD score is particularly relevant during the first couple years after LT. LabMELD categories showed a strong trend regarding the differences in 1-year survival, even if not statistically significant. After ten years, these differences evened out. Most surprisingly, after 20-years, recipients with labMELD > 25 showed the best overall survival. Even though the labMELD-score is able to predict waiting list mortality, it does not seem to be an adequate tool for predicting long-term outcome and thus survival benefit[26]. With a mean labMELD-score of 18.6, the patients in our cohort can be considered relatively healthy compared to German patients receiving transplants in the current era, with an average matchMELD of 34[14]. Also, the mean ET-DRI of 1.35 suggests excellent donor organ quality. In summary excellent overall conditions for transplantation, which are hardly realized under the current LT conditions. This makes it difficult to interpret the impact of our data on the era of MELD-allocation with ECD organs. The MELD-score has contributed to reduce the waiting list mortality[27] and decrease the waiting time for LT[28]. However, there are several weaknesses: Interlaboratory variability of creatinine, bilirubin and INR causes a lack of objectivity[29,30]. Secondly, the score does not adequately represent the necessity for LT for many indications, making it necessary to assign priority-based extra-points, which have seen a rather arbitrary up- and down-regulation[31,32]. Most importantly, the MELD score neglects all donor characteristics in the allocation process whatsoever. Therefore, organ allocation according to a MELD-based policy is not true donor-recipient matching at all. Our findings suggest that, depending on the quality of a given donor organ, the underlying disease, the recipients’ age and many other factors, a similar MELD value may result in very different long-term outcomes.
Another unexpected finding was the lack of significant impact of an impaired renal function prior to transplantation on long-term survival. The significant difference in mean eGFR between survivors and non-survivors (106 ± 70 mL/min per 1.73 m2vs 88 ± 39 mL/min per 1.73 m2, respectively, P = 0.007) is most likely due to the large amount of survivors with eGFR > 120 mL/min per 1.73 m2 (30% vs 20%) and the fact that the MDRD-formula does not adequately represent the renal function for patients without impairment[33]. In our previous publication mentioned above, we showed that a moderately or severely impaired renal function at 6 mo after LT was an independent risk factor for long-term survival in this cohort[17]. However, in this study, neither patients with pretransplant MIRF nor those with SIRF showed significantly lower overall survival. This is contrary to what other authors have described[34-36]. What was striking was the high number of non-survivors that had an eGFR that was just above 60, making these patients barely off the limit for an impaired renal function. Possibly, a number of non-survivors were pushed into renal impairment just after their LT. Ojo et al[36] found that the 5-year incidence of SIRF after LT was 18.1%, resulting in a 4.55-fold increased risk of death and Sanchez et al[35] described that the lower the initial GFR after LT, the earlier renal failure develops within the next 5 years, emphasizing the importance of a well-controlled post-transplant renal function.
Only about one in five survivors had HBMI before transplantation, compared to every third non-survivor (P = 0.011). Obese patients with terminal liver failure are not only at increased risk for perioperative morbidity and mortality[37], but also for experiencing cardiovascular events[38], which make up for a major proportion of deaths after LT[3,17,39].
We found a significant impact of ET-DRI on long-term survival. While close to 60% of patients that received a donor organ with an ET-DRI < 1.2 survived for two decades and longer, only less than 40% of the patients with an ET-DRI > 1.4 survived for twenty years. In recent years, more than 60% of all LT donor organs in Germany have an ET-DRI of > 1.5[14], a number that is likely to increase even more with decreasing rates of organ donation. The impact of donor age by itself, which is one of the factors of the ET-DRI, on long-term survival was also significant. Regarding the recipient-donor age match it seems that “older” livers may be suitable for younger recipients, but the benefit of younger organs for elderly recipients evens out 10 years after transplant.
Schaubel et al[40] described that regardless of the organ quality, higher labMELD recipients have a significant survival benefit from LT, whereas lower labMELD candidates who receive higher ET-DRI organs demonstrate higher mortality and no significant survival benefit. According to that particular study, 2000 life-years could be saved per year if benefit-based allocation was implemented.
Our data suggest that the ideal LT recipient is a young woman with acute liver failure or CD/AIH, who has a BMI < 25, a normal kidney function and no dyslipidemia. Such a patient would benefit the most from a donor organ < 30 years old with an ET-DRI of < 1.2. Since this combination of characteristics may hardly be found in recent years, it is even more important to match a specific donor organ to an adequate recipient, based on benefit-based allocation.
With major improvements in outcomes after liver transplantation and growing experience regarding transplant management, both the indications for liver transplantation (LT) and donor criteria have been expanded over the years. Shortage of donor organs has led to changes in liver allocation policies and the use of marginal organs.
Very long-term outcome data (20 years) after LT are scarce. In the presented cohort the best 20-year survival published ever so far was described. This retrospective analysis focuses on donor and recipient characteristics of survivors and non-survivors to elucidate factors that may be predictive of long-term survival.
Several factors influencing long-term survival after liver transplantation could be identified. It seems that “older” livers may be suitable for younger recipients, but the benefit of younger organs for elderly recipients evens out 10 years after transplant. The labMELD score seems not to be an adequate tool in prediction of long term survival. HBMI becomes predictive only ten years after transplant. A high number of non-survivors had an estimated glomerular-filtration-rate that was just above 60, making these recipients barely off the limit for an impaired renal function. Possibly, a number of non-survivors were pushed into renal impairment just after their LT. Immunosuppressive regimens should take this into account and may be adapted accordingly.
This study gives valuable insights in donor-recipient matching, when trying to achieve excellent long-term outcome, especially when allocating marginal organs to progressively sicker recipients.
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BP: Blood pressure; COD: Cause of death; DCD: Donation after cardiac death; ECD: Extended-criteria donor; ET-DRI: Eurotransplant Donor Risk Index; eGFR: Estimated glomerular-filtration-rate; HBMI: Overweight; HLIP: Hyperlipidemia; HCC: Hepatocellular carcinoma; INR: International normalized ratio; LT: Liver transplantation; MELD: Model for end-stage liver disease; MIRF: Moderately impaired renal function; SIRF: Severely impaired renal function; tBili: Total bilirubin.
This retrospective study concerning characteristics of more than 20 years survivors after LT is very interesting and useful.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mizuno S, Morioka D, Pandey CK, Qin JM S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] |
| 2. | Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, Abu-Elmagd K, Marsh W, Madariaga J, Mazariegos G. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 410] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, Saab S, Han S, Durazo F, Weaver M. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905-916; discussion 916-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 303] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, Holt C, Harlander-Locke M, Hong JC, Rana AR. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013;258:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Åberg F, Isoniemi H, Höckerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100:14-21. [PubMed] |
| 6. | Busuttil RW, Lake JR. Role of tacrolimus in the evolution of liver transplantation. Transplantation. 2004;77:S44-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Burroughs AK, Sabin CA, Rolles K, Delvart V, Karam V, Buckels J, O’Grady JG, Castaing D, Klempnauer J, Jamieson N. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Pfitzmann R, Nüssler NC, Hippler-Benscheidt M, Neuhaus R, Neuhaus P. Long-term results after liver transplantation. Transpl Int. 2008;21:234-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Oosterlee AR. Eurotransplant, Annual Report, Leiden, 2011. Available from: http://www.eurotransplant.org/cms/mediaobject.php?file=ar_2011.pdf. |
| 10. | Ioannou GN, Perkins JD, Carithers RL. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, Geier A, Clavien PA. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745-753; discussion 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Dutkowski P, Schlegel A, Slankamenac K, Oberkofler CE, Adam R, Burroughs AK, Schadde E, Müllhaupt B, Clavien PA. The use of fatty liver grafts in modern allocation systems: risk assessment by the balance of risk (BAR) score. Ann Surg. 2012;256:861-868; discussion 868-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Schlitt HJ, Loss M, Scherer MN, Becker T, Jauch KW, Nashan B, Schmidt H, Settmacher U, Rogiers X, Neuhaus P. Current developments in liver transplantation in Germany: MELD-based organ allocation and incentives for transplant centres. Z Gastroenterol. 2011;49:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Seehofer D, Schöning W, Neuhaus P. [Deceased donor liver transplantation]. Chirurg. 2013;84:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Bahra M, Neuhaus P. Liver transplantation in the high MELD era: a fair chance for everyone? Langenbecks Arch Surg. 2011;396:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Schoening WN, Buescher N, Rademacher S, Andreou A, Kuehn S, Neuhaus R, Guckelberger O, Puhl G, Seehofer D, Neuhaus P. Twenty-year longitudinal follow-up after orthotopic liver transplantation: a single-center experience of 313 consecutive cases. Am J Transplant. 2013;13:2384-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 19. | Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, Porte RJ, Rogiers X, Ringers J. The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 20. | Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3156] [Cited by in RCA: 3214] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 21. | Guckelberger O, Byram A, Klupp J, Neumann UP, Glanemann M, Stockmann M, Neuhaus R, Neuhaus P. Coronary event rates in liver transplant recipients reflect the increased prevalence of cardiovascular risk-factors. Transpl Int. 2005;18:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Molmenti EP, Klintmalm GB. Liver transplantation in association with hepatocellular carcinoma: an update of the International Tumor Registry. Liver Transpl. 2002;8:736-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Pascher A, Jonas S, Neuhaus P. Intrahepatic cholangiocarcinoma: indication for transplantation. J Hepatobiliary Pancreat Surg. 2003;10:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 644] [Article Influence: 49.5] [Reference Citation Analysis (2)] |
| 25. | Onaca NN, Levy MF, Sanchez EQ, Chinnakotla S, Fasola CG, Thomas MJ, Weinstein JS, Murray NG, Goldstein RM, Klintmalm GB. A correlation between the pretransplantation MELD score and mortality in the first two years after liver transplantation. Liver Transpl. 2003;9:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Desai NM, Mange KC, Crawford MD, Abt PL, Frank AM, Markmann JW, Velidedeoglu E, Chapman WC, Markmann JF. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Brown RS, Lake JR. The survival impact of liver transplantation in the MELD era, and the future for organ allocation and distribution. Am J Transplant. 2005;5:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Freeman RB. Model for end-stage liver disease (MELD) for liver allocation: a 5-year score card. Hepatology. 2008;47:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Francoz C, Prié D, Abdelrazek W, Moreau R, Mandot A, Belghiti J, Valla D, Durand F. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the model for end-stage liver disease score. Liver Transpl. 2010;16:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Cholongitas E, Marelli L, Kerry A, Senzolo M, Goodier DW, Nair D, Thomas M, Patch D, Burroughs AK. Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl. 2007;13:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Argo CK, Stukenborg GJ, Schmitt TM, Kumer SC, Berg CL, Northup PG. Regional variability in symptom-based MELD exceptions: a response to organ shortage? Am J Transplant. 2011;11:2353-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Washburn K. Model for End Stage Liver Disease and hepatocellular carcinoma: a moving target. Transplant Rev (Orlando). 2010;24:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 774] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 34. | Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 399] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Sanchez EQ, Melton LB, Chinnakotla S, Randall HB, McKenna GJ, Ruiz R, Onaca N, Levy MF, Goldstein RM, Klintmalm GB. Predicting renal failure after liver transplantation from measured glomerular filtration rate: review of up to 15 years of follow-up. Transplantation. 2010;89:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 37. | Flancbaum L, Choban PS. Surgical implications of obesity. Annu Rev Med. 1998;49:215-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 39. | Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, Venick RS, Feist S, Goldstein L, Saab S. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |