Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.54
Peer-review started: November 16, 2015
First decision: December 7, 2015
Revised: December 22, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: March 24, 2016
Processing time: 124 Days and 12.8 Hours
Focal segmental glomerulosclerosis (FSGS) represents one of the most severe glomerular diseases, with frequent progression to end-stage renal disease and a high rate of recurrence in renal allografts (30%-50%). Recurrent FSGS portends a negative outcome, with the hazard ratio of graft failure being two-fold higher then that of other glomerulonephritis. Two patterns of clinical presentations are observed: Early recurrence, which is characterized by massive proteinuria within hours to days after implantation of the renal graft, and late recurrence, which occurs several months or years after the transplantation. Many clinical conditions have been recognized as risk factors for recurrence, including younger age, rapid progression of the disease to end-stage renal disease on native kidneys, and loss of previous renal allografts due to recurrence. However, much less is known about the incidence and risk factors of the so-called “de novo” type of FSGS, for which sufferers are transplanted patients without disease on native kidneys; but, rapid development of allograft failure is frequently observed. Management of both forms is challenging, and none of the approaches proposed to date have been demonstrated as consistently beneficial or effective. In the present review we report an update on the available therapeutic strategies for FSGS in renal transplantation within the context of a critical overview of the current literature.
Core tip: Focal segmental glomerulosclerosis (FSGS) presents as a histological pattern of kidney damage with different, multifactorial, and often undefined pathogenesis. Primary FGSS represents one of the most severe glomerular diseases, with frequent progression to end-stage renal failure and a high rate of recurrence in renal allografts. FSGS recurrence also portends a negative outcome. Despite the proposal of multiple therapeutic approaches, none has emerged as the resolutive option. This review provides an update on the currently available therapeutic strategies for FSGS in renal transplantation, along with a critical overview of the related literature.
- Citation: Messina M, Gallo E, Mella A, Pagani F, Biancone L. Update on the treatment of focal segmental glomerulosclerosis in renal transplantation. World J Transplant 2016; 6(1): 54-68
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/54.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.54
Focal segmental glomerulosclerosis (FSGS) presents as a histological pattern of kidney damage with different, multifactorial, and frequently undefined pathogenesis. FSGS represents one of the most serious glomerular diseases, with frequent progression to end-stage renal disease and a high rate of recurrence in renal allografts. Clinical classification includes the following five forms[1]: Primary or idiopathic FSGS, the etiology of which is largely unknown; secondary or adaptive FSGS, which commonly refers to an adaptive response to glomerular hypertrophy/hyperfiltration and which presents a nonspecific pattern of scarring due to a previous injury; genetic FSGS; drug-induced FSGS; virus-associated FSGS.
In renal transplanted patients, both primary and secondary FSGS are observed. For the primary form, recurrent and de novo types are more severe. Obtaining an accurate estimation of de novo FSGS occurrence, however, is challenging because of the high rate of renal diseases of unknown cause in native kidneys (15.6% and 18.2% in the OPTN-SRTR annual report and ERA-EDTA registry, respectively)[2,3]. FSGS recurrence occurs frequently after transplantation, with reported rates ranging from 30% to 50%[4-6]. The risk of recurrence is substantially higher (up to nearly 100%) in patients who lost their first graft due to a recurrence[7]. Recurrent FSGS portends a negative outcome, with the hazard ratio (HR) of kidney failure being 2.03 compared to other kinds of recurrent glomerulonephritis[8]. Two patterns of clinical presentations are observed: Early recurrence, which is most commonly encountered in pediatric patients and characterized by a massive proteinuria that occurs within hours to days after implantation of the new kidney; late recurrence, which often develops insidiously at several months to years after the transplantation[9].
Many clinical conditions have been recognized as risk factors for recurrence[4,8,10], including younger age (particularly in children who were > 6-year-old at FSGS onset), mesangial proliferation in the native kidneys, rapid progression of the disease to end-stage renal disease (ESRD; < 3 years from onset) for native kidneys, pre-transplant bilateral nephrectomy, non-African race, specific genetic background, heavy proteinuria before transplantation, and, as cited above, loss of previous allografts due to recurrence.
Several lines of evidence have suggested that proteinuria and glomerular histologic alterations can be mediated by the direct activity of a circulating factor. These data were obtained from ex vivo analysis of glomerular changes after incubation with serum from patients with FSGS, as firstly described by Sharma et al[11] in 1999, as well as from analysis of animal models in which kidneys from a specific line of affected mice showed recovery from FSGS after transplantation into normal mice[12]. The most striking data, however, was obtained from a study of a kidney with FSGS recurrence that had been re-grafted from a patient to another and led to total regression of the disease[13]. However, identification of the responsible factor(s) is still a matter of investigation, although some different molecules are considered likely candidates.
In recent years research interest has focused on the soluble form of the urokinase type plasminogen activator receptor (suPAR). suPAR appears to be able to cause podocyte foot effacement in mice[14], and suPAR levels observed in patients with FSGS are higher than those in patients with other glomerulopathies[15]. Nevertheless, the specific involvement of suPAR in glomerulonephritis has not been confirmed by other studies, which showed increased (plasma) suPAR levels in other pathological situations (i.e., bacterial and viral infections, sepsis, and cancer)[16]. Rather, increased suPAR levels were observed primarily in patients with reduced glomerular filtration rate (GFR), suggesting that an elevation of suPAR levels may merely be an indicator of reduced GFR[17]. Finally, the usefulness of suPAR to distinguish between FSGS and non-FSGS glomerulonephritis has been questioned by Bock et al[18], who showed similar (plasma) suPAR levels among FSGS patients, non-FSGS controls, and healthy volunteers.
Other circulating factors, such as cardiothropin-like cytokine 1 (CLC-1), vasodilator-stimulated phosphoprotein and apolipoprotein A-I, have also been proposed as effectors in the glomerular permeability process, but their clinical and pathological roles remain unknown[19]. Recently, detection of a panel of serum antibodies directed towards podocyte antigens was found to be associated with a high percentage of relapses in FSGS (predictive recurrence value of 92%)[20]. The most prominent of these antigens is CD40; the expression of which is up-regulated in podocytes in FSGS, supporting the hypothesis of a potential direct pathogenetic effect of anti-CD40 antibodies.
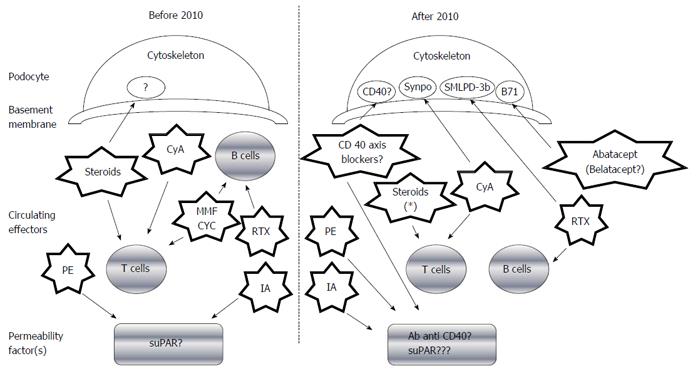
Demonstration of the precise permeability factor(s) remains elusive. Yet, recent findings have confirmed the critical role played by podocytes in FSGS development, and different podocyte antigens/cellular pathways have been associated with the disease course and medical treatment response (Figure 1). For example, it has been postulated that the B71 and sphingomyelin-phosphodiesterase-acid-like-3b (SMLPD-3b) proteins (both of which are expressed on the podocyte membrane) may directly interact with the cytoskeleton-inducing foot process effacement in response to a permeability factor[21,22]; interestingly, this effect could be antagonized by some drugs recently adopted in FSGS treatment [abatacept (Orencia®)/belatacept (Nulojix®)] for B71 and Rituximab® for SMLPD-3b, in particular), as outlined below in the therapeutic section.
Drug-induced or genetic-related alterations of the podocyte metabolic pathways may also lead to a maladaptive response to cell injury, defining a “pro-FSGS” phenotype, as has been observed in some patients with specific donor APOL1 polymorphisms[23] or in animal models with inhibition of the mTOR/Akt axis[24].
Another step forward in defining this disease may be achieved upon increasing our knowledge of the influence of micro (mi)RNAs on podocyte activity. In a mouse model, Gebeshuber et al[25] observed that transgenic expression of miR-193a (a down-regulator of WT1, itself a crucial effector in podocyte homeostasis) rapidly induces FSGS and observed up-regulated expression of miR-193a in isolated glomeruli from individuals with FSGS, as compared to kidney levels in healthy individuals or individuals with other glomerular diseases.
In addition to the probably pivotal role of podocytes in the disease process, it is also likely that T and B cells of the immune system contribute to FSGS development. A Th2 phenotype is commonly observed in patients with idiopathic nephrotic syndrome (NS)[26], and overexpression of IL-13, a characteristic Th2 cytokine, is associated with significant proteinuria in Wistar rats[27]. An indirect confirmation of B cell involvement derives from evidence showing a selective Rituximab®-induced depletion is correlated to disease remission[28]. This association has recently been questioned, however, so the role of B cells in FSGS pathogenesis is still not well defined.
FSGS treatment in renal transplantation, both for recurrent and de novo types, is a significant clinical challenge. Unfortunately, most of the reports consist of few cases or even a single case. Studies of the available strategies are few and have shown unclear and conflicting results for each, possibly due to their retrospective nature, uncontrolled design and limited number of enrolled patients or short follow-up periods. Consequently, while experimental studies have provided major advancements in our knowledge of the pathophysiology of FSGS, the treatment remains largely empirical. Some interesting preliminary data about the use of novel therapies are emerging, but they need further evaluation and validation. Therapeutic indications for de novo idiopathic and non-idiopathic FSGS are even more elusive[29].
Here, we summarize the most frequently reported available strategies for the management of recurrent and de novo FSGS, and suggest the potential benefit of these emerging therapies (summarized in Table 1).
| Treatment schedule | Patients | Outcome | Adjunctive information | |
| Plasma exchange | ||||
| Ponticelli et al[4] | Analysis of PE response in 22 studies | 144 patients (70 < 18 yr, 77 ≥ 18 yr) | Partial/complete remission of proteinuria in 49/70 (70%) children and 49/77 (63%) adults | Analysis also includes Canaud et al[33] 10 patients |
| Gohh et al[31] | Prophylactic course of 8 PE sessions in the peri-operative period in patients at high risk of recurrence | 10 patients (1 < 18 yr, 9 ≥ 18 yr) | 7/10 free of recurrence | |
| Chikamoto et al[32] | Prophylactic course of 4 PE sessions 12 d before transplantation in a high risk patient | 1 patient (< 18 yr) | No recurrence after 8 mo | Patient also received Rituximab® (375 mg/m2; 2 doses), methylprednisolone (1 mg/kg per day), tacrolimus (10 ng/mL) and mycophenolate mofetil (600 mg/m2 per day) 2 wk before transplantation |
| Glucocorticoids | ||||
| Shishido et al[41] | Methylprednisolone pulses (20 mg/kg on three consecutive days in weeks 1, 3 and 5) and increasing CyA target levels | 10 patients (8 < 18 yr, 2 ≥ 18 yr) | Complete remission in 7/10 | |
| CyA | ||||
| Canaud et al[33] | Intravenous CyA (C0 levels between 200-400 ng/mL), followed by oral CyA (C2 levels 1200-1400 ng/mL), high dose oral steroids (1 mg/kg per day for the first 4 wk, then progressively tapered) and a course of PE sessions for 9 mo | 10 patients (≥ 18 yr) | Complete remission of proteinuria in 10/10; proteinuria relapse in 1/10 successfully treated with Rituximab® (2 doses) | |
| Ingulli et al[44] | Progressive up-titration of CyA oral doses | 2 patients (< 18 yr) | Complete remission in 1; partial remission in 1 | |
| Salomon et al[45] | Intravenous CyA (through levels: 250-350 ng/mL) | 16 patients (< 18 yr; 1 re-grafted with a subsequent recurrence) | Complete remission in 14/17 (82%); partial remission in 2/17 (12%) | |
| Raafat et al[46] | Progressive up-titration of CyA oral doses until proteinuria reduction/serum creatinine elevation (CyA doses from 6 to 25 mg/kg per day) | 16 patients (< 18 yr) | Complete remission in 11/16 (69%); partial remission in 2/16 (12%) | |
| CYC/MMF | ||||
| Kershaw et al[53] | CYC (1-2 mg/kg per day, adjusted for white blood cell count) for 8-12 wk | 3 patients (< 18 yr) | Complete remission in 2/3; partial remission in 1/3 | |
| Cheong et al[54] | CYC (2 mg/kg per day) + PE (10 sessions over 2 wk followed by one session per week for 2 mo) | 6 patients (< 18 yr) | Complete remission in 3/6; partial remission in 3/6 | |
| Dall’Amico et al[55] | CYC (2-mo course, 2 mg/kg per day) and PE sessions | 11 patients (< 18 yr) | Complete remission in 9/11 (persistent remission in 7/9) | |
| Gipson et al[57] | 12-mo course of CYC vs MMF + dexamethasone | 138 patients [93/168 (67%) < 18 yr] | CyA arm: complete remission in 14/72 (19%), partial remission in 19/72 (26%) | |
| MMF + dexamethasone arm: complete remission in 6/66 (9%), partial remission in 16/66 (24%) | ||||
| Renin angiotensin system blockers | ||||
| Freiberger et al[62] | Ramipril (10 mg) + candesartan (64 mg) + aliskiren (300 mg) | 1 patient (≥ 18 yr) | Partial remission | Patient was previously treated with Rituximab® (375 mg/m2; 3 doses) and PE without response |
| Galactose | ||||
| Jhaveri et al[64] | High galactose diet + supplemental powder galactose (0.2 g/kg orally 2 times per day) one month later | 1 patient (≥ 18 yr) | Complete remission | No apparent response with previous treatments including Rituximab® (1 g, 2 doses), PE (15 sessions) and IgEv (2 doses) |
| Robson et al[65] | High galactose diet (14 g twice daily in patient 1, 10 g twice daily in patient 2) | 2 patients (≥ 18 yr) | Complete remission in 1; partial remission in 1 | |
| Sgambat et al[66] | High galactose diet (0.2 g/kg per dose twice daily orally) | 7 patients (< 18 yr) with steroid-resistant nephrotic syndrome (2/7 with recurrent FSGS) | Reduction in permeability factor without effect on proteinuria values | |
| Anti-TNF-α agents | ||||
| Leroy et al[69] | Infliximab (3 mg/kg twice monthly) | 1 patient (< 18 yr) | Complete remission | No apparent response with previous treatments including reinforced immunosuppression, CyA (5 mg/kg per day in continuous i.v. perfusion) followed by oral high dose CyA (10 mg/kg per day), methylprednisolone pulses followed by high dose oral prednisone (60 mg/1.73 m2 per day), MMF (600 mg/1.73 m2 per day) switch to cyclophosphamide (100 mg/d, interrupted for hematologic toxicity) and PE (15 sessions within 1 mo) |
| Bitzan et al[70] | Etanercept (twice weekly) | 1 patient (< 18 yr) | Partial remission | |
| Rituximab® | ||||
| Pescovitz et al[28] | Rituximab® (6 doses, 375 mg/m2) | 1 patient (< 18 yr) | Complete remission | |
| Hristea et al[74] | Rituximab® (2 doses, 375 mg/m2) | 1 patient (≥ 18 yr) | Complete remission | Patient also received a short course of oral cyclophosphamide (100 mg/d, days 22-40) and 3 additional PE sessions (days 34, 39, 49) |
| Gossmann et al[75] | Rituximab® (2 doses, 375 mg/m2) | 1 patient (≥ 18 yr) | Complete remission | |
| Fornoni et al[21] | Rituximab® within 24 h after surgery (1 dose, 375 mg/m2) in patients at high risk of recurrence | 41 patients (14 controls vs 27 treated) | Nephrotic proteinuria within 1 mo in 7/27 patients in Rituximab® group vs 9/14 patients in control group (P < 0.005) | Patient mean age: 12.3 ± 5.2 yr (control group), 15.0 ± 5.5 yr (Rituximab® group) |
| Audard et al[76] | Rituximab® induction in patients at high risk of recurrence (first graft lost due to recurrence) | 4 patients (≥ 18 yr) | No evidence of significant proteinuria at the end of follow-up | Single dose of 75 mg/m2 in 2/4 patients, repeated dose of 375 mg/m2 on day 7 in the remaining 2 patients; associated PE sessions (6 and 15, respectively) in 2/4 patients |
| Hickson et al[77] | Rituximab® (375 mg/m2; 2-4 doses) + PE | 4 patients (3 < 18 yr, 1 ≥ 18 yr) | Complete remissions in 4/4 patients | Early Rituximab® treatment in 3/4 (7–63 d post-transplantation), late treatment in 1/4 (982 d post-transplantation during a prolonged PE-dependent remission) |
| Dello Strologo et al[78] | Rituximab® (375 mg/m2; 1-4 doses) + PE | 6 patients (4 < 18 yr; 2 ≥ 18 yr) | Complete remission in 3; partial remission in 2; no response in 1 | 1/7 patients received one dose, 4/7 patients received 2 doses, and 1/7 received 4 doses; 1/7 patients experienced a severe reaction during first infusion and was excluded from the analysis |
| Tsagalis et al[79] | Rituximab® (1 g, 2 doses) + PE | 4 patients (2 < 18 yr; 2 ≥ 18 yr) | Complete remission in 2; partial remission in 2 | |
| Cho et al[80] | Rituximab® (100 mg, 1 dose) | 1 patient (≥ 18 yr) | Complete remission | |
| Yabu et al[87] | Rituximab® + PE | 4 patients (≥ 18 yr) | No response or proteinuria relapse after Rituximab® | Rituximab® schedule: 1 g, 2 doses in 1/4; 375 mg/m2, 4 doses in 1/4; 375 mg/m2, 6 doses in 2/4 |
| Kumar et al[117] | Rituximab® + PE | 8 patients (< 18 yr) | Complete remission in 2/8; partial remission in 4/8; no response in 2/8 | Rituximab® schedule: 375 mg/m2, 4 doses in 4/8; 375 mg/m2, 1 doses in 1/8; 375 mg/m2, 3 doses in 1/8; 375 mg/m2, 8 doses in 1/8; 375 mg/m2, 10 doses in 1/8 |
| Park et al[88] | Rituximab® (375 mg/m2, 1 or 2 doses) before transplantation with or without PE | 9 patients PE ± Rituximab® treated (Rituximab® group) vs 18 patients (control group) | No statistical difference in the prevention of recurrence between PE ± Rituximab® group (2/9, 22%) vs control group (5/18, 28%) | Rituximab® schedule: 375 mg/m2, 1 dose for desensitization in high risk patients; 375 mg/m2, 2 doses in ABO-incompatible transplantation; data not shown for recurrence prevention |
| Kamar et al[89] | Rituximab® (2-4 doses, 375 mg/m2) | 2 patients (≥ 18 yr) | Complete remission in 1 patient; no response in 1 patient | Rituximab® schedule: 75 mg/m2, 2 doses in the first patient (a supplemental dose was repeated after proteinuria relapse in association with PE sessions, achieving a new complete remission); 375 mg/m2, 4 doses in the second patient |
| El-Firjani et al[90] | Rituximab® (6 doses, 375 mg/m2) | 1 patient (≥ 18 yr) | No response | |
| Apeland et al[81] | Rituximab® (3 doses, 375 mg/m2) | 1 patient (≥ 18 yr) | Complete remission | |
| Grenda et al[82] | Rituximab® (4 doses, 375 mg/m2) | 1 patient (< 18 yr) | Complete remission | |
| Sethna et al[83] | Rituximab® (4 doses, 375 mg/m2) + PE | 4 patients (< 18 yr) | Complete remission in 3/4; partial and unsustained response in 1/4 | Proteinuria relapse in 1/3 patients with complete remission response to PE sessions intensification + an adjunctive dose of Rituximab® |
| Prytula et al[91] | Rituximab® (1-5 doses, 375 mg/m2) | 14 patients (< 18 yr) | Complete remission in 6/14; partial remission in 3/14; no response in 5/14 | |
| Stewart et al[92] | Rituximab® (4 doses, 375 mg/m2) | 1 patient (< 18 yr) | Complete remission | |
| Nozu et al[84] | Rituximab® (4 doses, 375 mg/m2) | 1 patient (< 18 yr) | Complete remission | Treatment was adopted after a diagnosis of post-transplant lymphoproliferative disorder |
| Nakayama et al[85] | Rituximab® (1-2 doses, 375 mg/m2) | 2 patients (< 18 yr) | Complete remission in 2 patients | One patient received a single dose; the other patient, after achieving a complete remission with the first dose, experienced a proteinuria relapse and rapidly responded to a second Rituximab® dose |
| Marks and McGraw[93] | Rituximab® (4 doses, 375 mg/m2 in one case; 2 doses 750 mg/m2 in the other one) | 2 patients (< 18 yr) | No response | |
| Bayrakci et al[86] | Rituximab® (4 doses, 375 mg/m2) | 1 patient (< 18 yr) | Complete remission | |
| Rodríguez-Ferrero et al[94] | Rituximab® (4 doses, 375 mg/m2) | 3 patients (≥ 18 yr) | Partial remission in 2/3; no response in 1/3 | |
| CTLA4-Ig (considered as the prevalent treatment) | ||||
| Yu et al[103] | Abatacept | 4 patients (2/4 < 18 yr, 2/4 ≥ 18 yr) with FSGS recurrence; 1 patient (≥ 18 yr) with FSGS on native kidneys | Complete remission in 2/5; partial remission in 3/5 | Patients 1 and 2 received a single dose; patients 3 and 4 received 2 doses; patient 5 (the only one with FSGS on native kidneys) received 3 doses (days 1, 15, 30) and a dose monthly thereafter |
| Alachkar et al[104] | Abatacept (1 dose; 10 mg/kg) in patient 1; belatacept (3 doses 10 mg/kg or continuative treatment) in patients 2-5 | 5 patients (≥ 18 yr) | No response | |
| Garin et al[105] | Abatacept (1 or 2 doses; 10 mg/kg) or belatacept (16 doses 5 mg/kg) | 5 patients (2/5 < 18 yr with minimal change in disease or FSGS on native kidneys; 3/5 with FSGS recurrence (1/3 < 18 yr, 2/3 ≥ 18 yr) | Partial response in minimal change disease patient; no response in primary FSGS patient; partial remission in 1/3 with FSGS recurrence (abatacept treated); no response in 2/3 (abatacept/ belatacept treated respectively) | Patients 1, 2 and 4 received 2 abatacept doses: patient 3 received 1 abatacept dose; patient 5 was treated with belatacept |
| Alkandari et al[106] | Abatacept (3 doses; 10 mg/kg) | 1 patient (< 18 yr) | No response | |
| Grellier et al[107] | Belatacept (days 1, 15, 30 and monthly thereafter, 5 mg/kg) | 5 patients (≥ 18 yr) | Partial response in 2/5; no response in 3/5 (no worsening in proteinuria values pre- and post-belatacept therapy in 1/3) | |
The adoption of plasma exchange (PE) for treatment of FSGS recurrence has been based on the hypothesis of the presence of circulating factor(s) that could be removed in order to treat or prevent the disease. Despite research into this causative factor remaining in a status of “cold case”, PE is still a cornerstone in FSGS recurrence treatment, since the 1985 report of its first positive application by Zimmerman[30]. A systematic review by Ponticelli[4] showed that PE promotes partial or complete remission in 70% of children and 63% of adults with FSGS recurrence. Most of the analyzed studies, however, are limited by their retrospective or uncontrolled design.
Adoption of PE in a pre-emptive protocol to reduce FSGS recurrence has been described by Gohh et al[31] in one of the few prospective studies in the literature. Ten transplanted patients with FSGS and at high risk of recurrence (both children and adults, including 5 transplants from living donors and 5 from deceased donors) were treated with a course of 8 PE sessions in the peri-operative period. Seven of the patients (including all 4 who received first grafts and 3 out of 6 who had prior recurrence) were free of recurrence at the end of follow-up (range of 238-1258 d). The use of pre-emptive PE in a high risk pediatric patient who underwent a second living kidney transplantation (the first kidney was lost due to recurrence) was more recently described by Chikamoto et al[32]. The patient had also received a 2-wk course of Rituximab® (375 mg/m2; 2 doses), methylprednisolone (1 mg/kg per day), tacrolimus (10 ng/mL) and mycophenolate mofetil (MMF) (600 mg/m2 per day) before transplantation; at 12 d before transplantation, 4 PE sessions were performed. No sign of recurrence was found in protocol biopsies at 8 mo after transplantation.
Canaud et al[33] described positive outcome (complete remission at 3 mo after diagnosis) for 10 patients with FSGS recurrence that had been treated with a 9-mo course of intravenous cyclosporine (CyA; C0 levels at 200-400 ng/mL), followed by oral CyA (C2 levels at 1200-1400 ng/mL), high dose oral steroids (1 mg/kg per day for the first 4 wk, then progressively tapered) and a course of PE sessions. The only patient who experienced recurrence of proteinuria after post-transplant year 1, concurrent to PE frequency reduction, had been successfully treated with Rituximab® (2 doses) and PE sessions bimonthly, obtaining a complete proteinuria remission in the 34 ± 6.7 mo of follow-up.
A positive effect is also described for plasma absorption in some papers[34-37], but further studies are needed to define the potential additive benefit in comparison with PE.
KDIGO guidelines suggest for FSGS on native kidneys a 4-wk to 16-wk course of prednisone (1 mg/kg per day, with a maximum of 80 mg and a slow tapering in the 6 mo after remission)[38]. Glucocorticoids may act to stabilize the actin cytoskeleton, thereby preserving glomerular permeselectivity[39] and directly reducing podocyte apoptosis via the PI3K/Akt signal pathway[40]. Efficacy of steroid treatment in recurrent/de novo FSGS has never been evaluated in a randomized trial; on the other hand, considering its pivotal therapeutic role in FSGS on native kidneys, many different regimens have included steroids in post-transplantation FSGS treatment.
Apart from the paper by Canaud et al[33], who described a combined treatment of CyA in association with high dose steroids and PE, Shishido et al[41] also reported a favorable outcome (7/10 complete remission) for pediatric patients with FSGS recurrence in response to a combined treatment with methylprednisolone pulses (20 mg/kg after diagnosis on 3 consecutive days in weeks 1, 3 and 5) and an increase in CyA target levels (area under the curve0–4 4500-5500 ng/h per milliliter for the first month, 4000 ng/h per milliliter for the next 2 mo, and 3000 ng/h per milliliter thereafter).
CyA is commonly applied for the treatment of several immune-mediated diseases and as a second-line therapy for steroid-resistant/dependent FSGS on native kidneys[38]. Conversely, CyA does not appear to prevent post-transplant FSGS recurrence when given as a part of the initial immunosuppressive regimen[42,43]; although, this potential has not been evaluated in more recent studies. Standard oral doses of CyA have not been associated with reduced incidence of recurrent FSGS. Nonetheless, higher intravenous doses have been associated with remission of proteinuria for the first time since reported by Ingulli et al[44] 25 years ago.
Overall, limited evidence has supported the administration of high dose CyA to achieve remission of FSGS recurrence with a persistent effect[45,46]. Salomon et al[45] reported a remission of recurrent proteinuria in 14/17 (82%) of children following administration of intravenous CyA (mean period of 21 d; range of 250-350 ng/mL); after 4 years, 11/17 (64%) patients had achieved sustained remission. In a second series, remission was induced in 13/16 patients (81%), which also included PE sessions for 4 of the cases; CyA doses were from 6 to 25 mg/kg per day[46]. At the latest follow-up (range of 10 mo to 12 years), 11/13 (84%) patients had a functioning allograft. It is noteworthy to mention that in this study, as in the studies by Canaud et al[33] and Chikamoto et al[32], the CyA treatment was combined with PE sessions.
The mechanism by which CyA might decrease proteinuria has been elucidated recently. Briefly, CyA has been shown to act by means of a direct effect on the cytoskeleton via dephosphorylation of synaptopodin, a crucial stabilizer of podocyte actin cytoskeleton, rather than through an immunosuppressive activity such as inhibition of T cells[47,48]. According to these clinical evidence, it was postulated that the anti-proteinuric effect had been observed only with high dose CyA because the hypercholestorelemic state induced by NS limits the CyA active fraction[49].
Currently, the option of CyA therapy in FSGS is more frequently used in combined-therapy regimens. The long-term safety/efficacy ratio of such a therapy, however, remains to be confirmed by study, which is of particular importance in light of the severe toxicities associated with high dose CyA.
Cyclophosphamide (CYC) is an alkalizing agent that inhibits cell DNA duplication, leading to cell death. It is active both on resting and dividing lymphocytes[50]. Anecdotal experiences with CYC therapy (2 mg/kg per day) reported achievement of partial or complete remission in patients with FSGS on native kidneys and also in steroid-dependent patients; however, no benefit was found in steroid-resistant patients[51,52].
In FSGS recurrence, Kershaw et al[53] treated 3 pediatric patients with CYC (1-2 mg/kg per day, adjusted for white blood cell count) for 8-12 wk and obtained two complete remissions and one partial; the patient with the longest follow-up (125 mo) experienced two additional relapses, each of which were treated successfully with pulse intravenous steroids. A more recent report described a series of 6 patients with FSGS recurrence all of whom were treated with a combination of CYC and PE (10 sessions over 2 wk, followed by 1 session per week for 2 mo), with complete remission being achieved in 3 of the patients and partial remission in the other 3[54]. A second case series described 11 pediatric patients with FSGS recurrence who were treated with a 2-mo course of CYC (2 mg/kg per day) and PE sessions, with initial remission being achieved in 9/11 and with 7/9 being free of disease at the last follow-up (32 ± 15 mo)[55].
MMF inhibits the inosine monophosphate dehydrogenase-mediated reduction of T and B lymphocyte proliferation. Gbadegesin et al[56] suggested MMF for treatment of steroid-dependent/resistant FSGS on native kidneys. Subsequently, a randomized controlled trial including 138 patients (both children and adults) with primary FSGS compared CyA and MMF plus dexamethasone, but no difference was observed in complete or partial remission rates after 52 wk of follow-up and both groups showed poor outcome (remission in 46% vs 33%, respectively)[57]. At the present time, as reported by Lau et al[58], no randomized controlled trial has yet to demonstrate the efficacy of MMF in association with other therapies or as a single agent in FSGS treatment on native or transplanted kidneys.
Renin angiotensin system (RAS) blockers have an important role in blood pressure control, but they also have anti-proteinuric and systemic anti-inflammatory effects[59]. RAS inhibition represents an important therapeutic strategy in proteinuric glomerular disease as FSGS, for either recurrent or de novo types.
Despite some reports having suggested RAS blockers as effective therapeutics for this disease[60,61], the association of these drugs with other therapies limits a final judgment on their real effect as a single drug. Freiberger et al[62] reported a favorable outcome after the use of a triple RAS blockage [angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor (blocker) antagonist (ARB), and renin inhibitor] in a transplanted patient with FSGS recurrence; since PE and Rituximab® treatment produced no apparent benefits in the patient previously, a late response to this treatment may not be excluded “a priori”.
It is noteworthy that a close monitoring of serum creatinine and potassium levels is essential in all subjects treated with RAS blockers, especially when all these drugs are prescribed together and even more so when renal function is suboptimal.
The potential effect of galactose on glomerular permeability and proteinuria was firstly hypothesized by Savin et al[63], stating that sucrose binds with high affinity and inactivates the supposed “permeability factor”, thereby facilitating its plasma clearance.
Jhaveri et al[64] described a patient with severe recurrent FSGS (massive proteinuria of 37 g/d at day 2 after transplantation) who had been previously treated with PE, intravenous immunoglobulin and Rituximab®, and achieved complete remission of proteinuria after receipt of a high galactose diet and supplemental oral galactose (0.2 g/kg, two times per day). As for other case series mentioned before, the role played by galactose in disease remission vs the role of previous treatment is indistinguishable. More recently, Robson et al[65] also reported a favorable outcome (1 complete and 1 partial response) in 2 patients with FSGS recurrence treated with high galactose diet. Sgambat et al[66] reported in a recent case series a reduction in permeability factor activity in 7 pediatric patients with steroid-resistant NS (2/7 with recurrent FSGS) treated with high galactose diet (0.2 g/kg, twice daily), without any significant improvement in proteinuria values.
The tumor necrosis factor-alpha (TNF-α) signaling pathway is involved in the development of both NS and FSGS, as evidenced by elevated levels of TNF-α detected in plasma and urine obtained from patients with FSGS[67] and increased glomerular permeability to TNF-α observed in vitro[68].
At the present time, very few cases of FSGS have been treated with anti-TNF-α agents. Leroy et al[69] reported a favorable outcome (complete remission) for a 15-year-old patient with recurrent FSGS that was presumably resistant to other treatments (increased immunosuppressant dose, PE, intravenous immunoglobulin, high dose steroids, CyA, and CYC) after administration of an anti-TNF-α blocker (firstly infliximab, then etanercept). Bitzan et al[70] showed that plasmapheresis effluent or fresh plasma (obtained from a child with recurrent FSGS and from two children with primary FSGS) caused cytoskeleton disturbance on podocyte culture. In detail, the plasma from the patient with FSGS recurrence activated β3 integrin and dispersed focal adhesion complexes, and this effect was reversed by pre-incubation with antibodies against TNF-α or either of the two TNF-α receptors. Following this study’s observation, the patient who was plasma resistant was treated firstly with Etanercept and then with Infliximab, which ultimately led to partial remission of the disease.
Rituximab® is a chimeric monoclonal antibody that recognizes CD20 antigen on B lymphocytes. This agent has several unlabeled applications in the field of kidney transplantation; it has been successfully applied to reduce anti-donor ABO or HLA antibodies[71] and to treat acute humoral rejection of the graft[72], post-transplant lymphoproliferative diseases[73], and also some recurrent/de novo glomerulonephritis.
Rituximab® treatment also has a long history of interest in its potential as a therapeutic option for idiopathic NS before and after transplantation. However, after the initial reports about its favorable use in FSGS recurrence were published in 2006 and 2007[28,74,75], conflicting results were reported by other studies in the literature. Currently, Rituximab® may be adopted as a preventive therapeutic approach to reduce FSGS recurrence rate, or as a treatment of FSGS recurrence.
The use of Rituximab® as a prevention strategy derives from two retrospective studies[21,76]. In the first, Fornoni et al[21] investigated 27 kidney transplanted patients at high risk for FSGS recurrence and showed that use of Rituximab® in the perioperative period (375 mg/m2 within 24 h after the kidney transplantation) was associated with a lower incidence of post-transplant proteinuria and with stabilization of GFR at the 12 mo follow-up. This study also demonstrated for the first time that Rituximab® operates in a B cell-independent manner; sera obtained from FSGS recurrent patients caused a down-regulation of SMLPD-3b, a protein involved in regulation of podocyte cytoskeleton, and this phenomenon was prevented by pre-treatment with Rituximab® through direct binding.
Audard et al[76] observed the absence of a clinical FSGS recurrence (not biopsy proven) in 4 patients who received Rituximab® (375 mg/m2) in their induction protocol for a second kidney transplant (first kidney lost due to a recurrent disease). Nevertheless, the short follow-up (12-54 mo), the difference in Rituximab® schedule (a single administration in 2/4 patients and 2 doses in the other 2 patients), and PE adoption in 2/4 patients partially limit the significance of this uncontrolled study.
To date, Rituximab® has been widely used, alone and in combination protocols, as a treatment for recurrent FSGS in cases of incomplete remission, PE dependence, or as a first-line therapy in specific patient subsets. Despite successful results having been obtained[77-86], other studies have shown a transient or even absent response to Rituximab®[62,87-94] (Table 1).
Abatacept is a biologic agent, specifically the CTLA4-Ig recombinant fusion protein derived from the extracellular portion of CTLA4-Ig and genetically fixated to the high and constant portion of the IgG1 immunoglobulin. Its effect is exerted by interfering with lymphocyte co-stimulation[95,96] upon binding to the APC protein ligands B71 (CD80) or B72 (CD86) and displacing their T cell counterpart or CD28[97]. In some experimental models of organ transplantation, the systemic administration of CTLA4-Ig effectively dampened the immune response, preventing experimental acute and chronic rejection and resulting in prolonged graft survival and tolerance[98-100]. On the basis of these findings, different biological T cell co-stimulation blockers became the subject of clinical trials. A high affinity variant of CTLA4-Ig, named LEA29Y (belatacept, Nulojix®; Bristol-Myers Squibb Pharma, Uxbridge, United Kingdom), has been developed and was awarded approval by the Federal Drug Administration (FDA) in 2011 for prophylactic use for organ rejection in adult kidney recipients[101].
Abatacept was approved by the FDA in 2005 for the treatment of rheumatoid arthritis and active juvenile idiopathic arthritis[102], and quite recently has been proposed as a new treatment strategy for FSGS recurrence. Yu et al[103] reported a positive outcome in 4 patients (2 children) affected by early and Rituximab®-resistant FSGS recurrence and in 1 patient with glucocorticoid-resistant primary FSGS on native kidneys. All these patients received abatacept, at a dose between 250 mg/d and 500 mg/d, the most commonly used dose for rheumatoid arthritis treatment. Before using abatacept, PE sessions were also performed in all 4 patients with FSGS recurrence, while the patient with primary disease on native kidneys received an immunosuppressive treatment composed of prednisone and CyA, with tacrolimus applied as a second line therapy. All patients achieved and maintained a significant proteinuria regression after 10-48 mo of follow-up. The authors suggested that this response was directly correlated with the B71-positive immuno-stained podocytes found in the kidney-biopsy specimens, because B71 may be expressed on the podocyte surface in some proteinuric conditions such as FSGS, thereby altering cytoskeleton organization, a condition that is known to be abrogated by abatacept.
Nevertheless, other studies of patients with FSGS recurrence have shown a slight/absent response after treatment with CTLA4-Ig[104-107], despite the fact that in some of these cases belatacept (able to bind B71 with an higher affinity than abatacept) was adopted.
The use of bone marrow mesenchymal stem cells (BM-MSCs) has been reported to reduce kidney injury in different experimental models of kidney disease[108-111]. Ma et al[111] showed in a well-established murine model of FSGS (adriamycin nephropathy) that human umbilical mesenchymal stem cells (HuMSCs) may improve kidney fibrosis and modulate the inflammatory response. Recently, BM-MSCs have been demonstrated as effective treatment for a wide range of immuno-mediated diseases[112-114].
Belingheri et al[115] reported successful application of their innovative approach with BM-MSCs in a 13-year-old kidney transplanted patient who had developed an immediate biopsy proven FSGS recurrence after renal transplantation and who was non-responsive to PE and Rituximab® (2 doses). The patient had received allogeneic BM-MSCs infusions (6 doses, according to the most commonly adopted protocol for treatment of graft vs host disease) at months 7, 10 and 14 after transplantation and at month 5 after Rituximab® administration. Remission of proteinuria was achieved after three BM-MSCs infusions, and at the last follow-up (22 mo) both renal function and proteinuria values were stable. The treatment appeared as well tolerated, and no adverse events were noted.
In the field of glomerulonephritis, primary FSGS portends one of the most unpredictable and variable outcomes, carrying one of the highest recurrence rates for transplanted kidneys (from 30% to 50% in patients with a history of primary FSGS on native kidneys)[4-6]. FSGS recurrence also remains a “clinical drama”, with almost 50% of allografts lost at 5 years and having a HR of 2.03 compared to other kinds of recurrent glomerulonephritis[8]. Despite the proposal of multiple therapeutic approaches over time, none has yet emerged as the resolutive option, either for the recurrent or de novo types of FSGS; yet, none has been disproven or ruled out and each has several aspects that still need to be studied.
Indeed, PE is still widely applied as FSGS recurrence treatment and as a pre-emptive strategy, despite the absence of controlled trials. Nevertheless, a course of PE treatment is widely used and recommended, titrated according to the clinical/histological response as proposed by Ponticelli[4], even if it remains difficult to determine when to start and when to stop and which schedule of PE sessions is best. Interpretation of the literature data for PE is difficult, partially due to the existence of publication bias, in which positive outcomes of some cases may lead to an overestimation of treatment efficacy. In addition, the reports on PE often describe studies in which the therapy is applied as part of a combination regimen that includes other disease-modifying treatments (i.e., corticosteroids, Rituximab®, CyA), complicating the interpretation of results. Besides, few prospective studies are available and none of them used a control group study design.
On the other hand, application of high dose CyA must be carefully considered on the basis of drug-related toxicities, especially nephrotoxicity. Most of the CyA studies have thus far only included pediatric patients or living-related donors, two populations that are more prone to tolerating high dose CyA. To the contrary, when patients are adult recipients of a kidney from a deceased marginal donor, nephrotoxicity from high dose CyA could be a problematic issue. The previous reported considerations for PE regarding its frequent association with other treatments capable of strengthening its effect are also applicable to CyA (see the study by Canaud et al[33] for an example).
The paucity of data on CYC/MMF adoption for treatment of recurrent FGSS represents another limitation to using the collective literature to draw conclusions about their utility in clinical practice. On the other hand, Rituximab® is one of the most interesting agents proposed to date for treatment of FSGS recurrence; but, again, several limitations lie in the related literature, including the use of a surrogate end-point of disease activity (i.e., clinical/not histological definition of recurrent FSGS in the study by Fornoni et al[21]), short follow-up[76,77], and evidence of absence of positive effects[62,87-94]. Furthermore, the Rituximab® dose is another matter of debate, and the question remains: Should the classic scheme borrowed from hematologic protocols (4 doses of 375 mg/m2 each) or a shorter regimen (titrated to the minimal level necessary to obtain B cell depletion) be adopted? Another first line question involves when the infusion should be performed: As a pre-emptive therapy soon after surgery, in cases at high risk of recurrence, or at the time of recurrence? Although, Rituximab® portends some serious side effects, increasing the risk of opportunistic infections in transplanted patients during the entire time of its blockage of the immune response. Araya et al[116] reported side effects in about 10% of cases (1 case each of neutropenia, severe anaphylactic reaction, BK virus nephropathy, and severe sepsis). Kumar et al[117] observed a significant rate of severe complications (3/8 patients), ranging from Rituximab®-associated lung injury, acute tubular necrosis, and central nervous system malignancy.
The ACEs or ARBs should be considered as adjuvant therapy, especially when other therapies have failed or are not applicable. However, their use may be contraindicated by low GFR and risk of hyperkalemia.
Considering the so-called “anecdotal therapies” (galactose, anti-TNF-α agents), their place in the armamentarium for FSGS treatment in renal transplant is very small in current times, but they could be considered for use in rare conditions as a salvage therapy. Considering the more innovative treatments, BM-MSCs represent a promising treatment[115]. Nevertheless, the results reported in the literature to date need to be evaluated on the basis of the possible influence of previous treatments received by the patients, especially considering a delayed effect of Rituximab® administration, and the natural evolution of the disease, which is often unpredictable.
On the other hand, safety of BM-MSCs remains an open question. On the basis of literature data, auto- and allo-MSCs may interfere with the immune response in a non-defined and unpredictable manner. For example, Reinders et al[118] found auto-MSCs infusion for the treatment of acute rejection to be associated with opportunistic viral infection in 3/6 patients. Allo-MSCs may also induce the production of anti-donor antibodies, as observed in some animal models[119]. Nevertheless, a strong limitation to the adoption of cell therapies is the unknown proneoplastic effect, secondary to a direct (but also indirect) MSCs dedifferentiation[120,121].
A possible way to reduce or abrogate the risk deriving from MSCs infusion is to promote podocyte regeneration. In some experimental models, native parietal epithelial cells (PECs) have been shown to have the potential to migrate to the glomerular tuft after kidney injury, acquiring a phenotype and a morphologic appearance similar to a differentiated podocyte and thereby mitigating the damage[122,123]. On the other hand, PECs have also been associated with glomerular injury and sclerosis[124], so a definitive consideration about their role and potential therapeutic applications is far from being defined.
The therapeutic role of co-stimulatory molecule blockades is emerging for some glomerulonephritis on native kidneys (e.g., lupus nephritis)[125]. Recently, abatacept was associated with interesting results in proteinuria reduction in a small case series of FSGS recurrent patients[103]. Nevertheless, a limitation related to the histological findings reported is intrinsically linked with the efficacy, because all positive results were obtained only in patients with positive B71 staining on renal biopsy and the negative outcomes were reported for patients without this staining pattern on renal specimens[101]. In addition, the absence of response after belatacept use[99,100,102] (abatacept’s “twin drug” with a higher affinity to the B71 receptor) remains an open issue.
In conclusion, no treatment guideline can be proposed at this time to address FSGS in renal transplantation. In our opinion, waiting for improvement in podocyte biology knowledge and taking the perspective that therapeutic protocols should be tailored to the single patient will help to optimize the risk/benefit balance. Protocol biopsy is a useful strategy chosen during the difficult decision-making process involved in cases possibly needing interruption of on-going targeted therapies (maybe with the only exception of RAS blockers). We suggest, as a first line option, the use of Rituximab® at a single dose of 375 mg/m2 (also for induction protocols in patients at high risk of recurrence) with a close monitoring of CD20+ count, that will be applied in combination with steroids and a PE course. The initial schedule could be 5-10 sessions on alternating days, followed by tapering to a 1/wk or less schedule according to the patient’s clinical response. The crucial issue is determining the right time to stop PE after proteinuria disappearance.
Therapy for FSGS in renal transplantation remains an unmet clinical need. Randomized-controlled clinical trials are highly important to resolve this issue and necessary to elucidate the correct approach and the real potentiality of the more recently proposed drugs.
P- Reviewer: Saeki K, Song GB, Wang F S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 575] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 2. | Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14 Suppl 1:11-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant. 2010;25:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, Ouseph R, Peddi VR, Pelz CJ, Roza AM. Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR). Transplantation. 1999;68:635-641. [PubMed] |
| 6. | Hubsch H, Montané B, Abitbol C, Chandar J, Shariatmadar S, Ciancio G, Burke G, Miller J, Strauss J, Zilleruelo G. Recurrent focal glomerulosclerosis in pediatric renal allografts: the Miami experience. Pediatr Nephrol. 2005;20:210-216. [PubMed] |
| 7. | Newstead CG. Recurrent disease in renal transplants. Nephrol Dial Transplant. 2003;18 Suppl 6:vi68-vi74. [PubMed] |
| 8. | Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103-109. [PubMed] |
| 9. | Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Vinai M, Waber P, Seikaly MG. Recurrence of focal segmental glomerulosclerosis in renal allograft: an in-depth review. Pediatr Transplant. 2010;14:314-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Sharma M, Sharma R, McCarthy ET, Savin VJ. “The FSGS factor: “ enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol. 1999;10:552-561. [PubMed] |
| 12. | Le Berre L, Godfrin Y, Günther E, Buzelin F, Perretto S, Smit H, Kerjaschki D, Usal C, Cuturi C, Soulillou JP. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J Clin Invest. 2002;109:491-498. [PubMed] |
| 13. | Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366:1648-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55-63. [PubMed] |
| 15. | Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 648] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 16. | Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 213] [Reference Citation Analysis (0)] |
| 17. | Maas RJ, Wetzels JF, Deegens JK. Serum-soluble urokinase receptor concentration in primary FSGS. Kidney Int. 2012;81:1043-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Bock ME, Price HE, Gallon L, Langman CB. Serum soluble urokinase-type plasminogen activator receptor levels and idiopathic FSGS in children: a single-center report. Clin J Am Soc Nephrol. 2013;8:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, Burke GW, Bruneval P, Naesens M, Jackson A. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6:256ra136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 401] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 22. | Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390-1397. [PubMed] |
| 23. | Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, Hauptfeld V, Bray RA, Gebel HM, Kirk AD. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant. 2015;15:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med. 2013;19:1288-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med. 2013;19:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Sahali D, Pawlak A, Valanciuté A, Grimbert P, Lang P, Remy P, Bensman A, Guellaën G. A novel approach to investigation of the pathogenesis of active minimal-change nephrotic syndrome using subtracted cDNA library screening. J Am Soc Nephrol. 2002;13:1238-1247. [PubMed] |
| 27. | Lai KW, Wei CL, Tan LK, Tan PH, Chiang GS, Lee CG, Jordan SC, Yap HK. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18:1476-1485. [PubMed] |
| 28. | Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med. 2006;354:1961-1963. [PubMed] |
| 29. | Meehan SM, Pascual M, Williams WW, Tolkoff-Rubin N, Delmonico FL, Cosimi AB, Colvin RB. De novo collapsing glomerulopathy in renal allografts. Transplantation. 1998;65:1192-1197. [PubMed] |
| 30. | Zimmerman SW. Plasmapheresis and dipyridamole for recurrent focal glomerular sclerosis. Nephron. 1985;40:241-245. [PubMed] |
| 31. | Gohh RY, Yango AF, Morrissey PE, Monaco AP, Gautam A, Sharma M, McCarthy ET, Savin VJ. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant. 2005;5:2907-2912. [PubMed] |
| 32. | Chikamoto H, Hattori M, Kuroda N, Kajiho Y, Matsumura H, Fujii H, Ishizuka K, Hisano M, Akioka Y, Nozu K. Pretransplantation combined therapy with plasmapheresis and rituximab in a second living-related kidney transplant pediatric recipient with a very high risk for focal segmental glomerulosclerosis recurrence. Pediatr Transplant. 2012;16:E286-E290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Canaud G, Zuber J, Sberro R, Royale V, Anglicheau D, Snanoudj R, Gaha K, Thervet E, Lefrère F, Cavazzana-Calvo M. Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: a pilot study. Am J Transplant. 2009;9:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Belson A, Yorgin PD, Al-Uzri AY, Salvatierra O, Higgins J, Alexander SR. Long-term plasmapheresis and protein A column treatment of recurrent FSGS. Pediatr Nephrol. 2001;16:985-989. [PubMed] |
| 35. | Fencl F, Simková E, Vondrák K, Janda J, Chadimová M, Stejskal J, Seeman T. Recurrence of nephrotic proteinuria in children with focal segmental glomerulosclerosis after renal transplantation treated with plasmapheresis and immunoadsorption: case reports. Transplant Proc. 2007;39:3488-3490. [PubMed] |
| 36. | Fencl F, Vondrák K, Rosík T, Zieg J, Chadimová M, Háček J, Dušek J, Seeman T. Recurrence of nephrotic proteinuria in children with focal segmental glomerulosclerosis - early treatment with plasmapheresis and immunoadsorption should be associated with better prognosis. Minerva Pediatr. 2015;Epub ahead of print. [PubMed] |
| 37. | Lionaki S, Vlachopanos G, Georgalis A, Liapis G, Skalioti C, Zavos G, Boletis JN. Individualized scheme of immunoadsorption for the recurrence of idiopathic focal segmental glomerulosclerosis in the graft: a single center experience. Ren Fail. 2015;37:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139-274. [DOI] [Full Text] |
| 39. | Ransom RF, Lam NG, Hallett MA, Atkinson SJ, Smoyer WE. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 2005;68:2473-2483. [PubMed] |
| 40. | Yu-Shengyou Y. Dexamethasone inhibits podocyte apoptosis by stabilizing the PI3K/Akt signal pathway. Biomed Res Int. 2013;2013:326986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Shishido S, Satou H, Muramatsu M, Hamasaki Y, Ishikura K, Hataya H, Honda M, Asanuma H, Aikawa A. Combination of pulse methylprednisolone infusions with cyclosporine-based immunosuppression is safe and effective to treat recurrent focal segmental glomerulosclerosis after pediatric kidney transplantation. Clin Transplant. 2013;27:E143-E150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Vincenti F, Biava C, Tomlanovitch S, Amend WJ, Garovoy M, Melzer J, Feduska N, Salvatierra O. Inability of cyclosporine to completely prevent the recurrence of focal glomerulosclerosis after kidney transplantation. Transplantation. 1989;47:595-598. [PubMed] |
| 43. | Banfi G, Colturi C, Montagnino G, Ponticelli C. The recurrence of focal segmental glomerulosclerosis in kidney transplant patients treated with cyclosporine. Transplantation. 1990;50:594-596. [PubMed] |
| 44. | Ingulli E, Tejani A, Butt KM, Rajpoot D, Gonzalez R, Pomrantz A, Ettenger R. High-dose cyclosporine therapy in recurrent nephrotic syndrome following renal transplantation. Transplantation. 1990;49:219-221. [PubMed] |
| 45. | Salomon R, Gagnadoux MF, Niaudet P. Intravenous cyclosporine therapy in recurrent nephrotic syndrome after renal transplantation in children. Transplantation. 2003;75:810-814. [PubMed] |
| 46. | Raafat RH, Kalia A, Travis LB, Diven SC. High-dose oral cyclosporin therapy for recurrent focal segmental glomerulosclerosis in children. Am J Kidney Dis. 2004;44:50-56. [PubMed] |
| 47. | Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 764] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 48. | Mathieson PW. Podocyte actin in health, disease and treatment. Nephrol Dial Transplant. 2010;25:1772-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Cravedi P, Kopp JB, Remuzzi G. Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant. 2013;13:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Becker H, Potyka P, Weber C, Renelt M, Federlin K. T-helper cell subsets in patients with inflammatory rheumatic diseases undergoing immunosuppressive therapy. Immun Infekt. 1991;19:26-27. [PubMed] |
| 51. | Tarshish P, Tobin JN, Bernstein J, Edelmann CM. Cyclophosphamide does not benefit patients with focal segmental glomerulosclerosis. A report of the International Study of Kidney Disease in Children. Pediatr Nephrol. 1996;10:590-593. [PubMed] |
| 52. | Schulman SL, Kaiser BA, Polinsky MS, Srinivasan R, Baluarte HJ. Predicting the response to cytotoxic therapy for childhood nephrotic syndrome: superiority of response to corticosteroid therapy over histopathologic patterns. J Pediatr. 1988;113:996-1001. [PubMed] |
| 53. | Kershaw DB, Sedman AB, Kelsch RC, Bunchman TE. Recurrent focal segmental glomerulosclerosis in pediatric renal transplant recipients: successful treatment with oral cyclophosphamide. Clin Transplant. 1994;8:546-549. [PubMed] |
| 54. | Cheong HI, Han HW, Park HW, Ha IS, Han KS, Lee HS, Kim SJ, Choi Y. Early recurrent nephrotic syndrome after renal transplantation in children with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:78-81. [PubMed] |
| 55. | Dall’Amico R, Ghiggeri G, Carraro M, Artero M, Ghio L, Zamorani E, Zennaro C, Basile G, Montini G, Rivabella L. Prediction and treatment of recurrent focal segmental glomerulosclerosis after renal transplantation in children. Am J Kidney Dis. 1999;34:1048-1055. [PubMed] |
| 56. | Gbadegesin R, Lavin P, Foreman J, Winn M. Pathogenesis and therapy of focal segmental glomerulosclerosis: an update. Pediatr Nephrol. 2011;26:1001-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 58. | Lau EW, Ma PH, Wu X, Chung VC, Wong SY. Mycophenolate mofetil for primary focal segmental glomerulosclerosis: systematic review. Ren Fail. 2013;35:914-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system--focusing on the vascular system. Peptides. 2011;32:2141-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 60. | Korbet SM. Angiotensin antagonists and steroids in the treatment of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:219-228. [PubMed] |
| 61. | Kangovi S, Edwards M, Woloszynek S, Mitra N, Feldman H, Kaplan BS, Meyers KE. Renin-angiotensin-aldosterone system inhibitors in pediatric focal segmental glomerulosclerosis. Pediatr Nephrol. 2012;27:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Freiberger V, Amann K, Heemann U, Frank H. Effect of a triple blockade of the renin-angiotensin-system in recurrent focal segmental glomerulosclerosis after kidney transplantation. Transpl Int. 2009;22:1110-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Savin VJ, McCarthy ET, Sharma R, Charba D, Sharma M. Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Transl Res. 2008;151:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Jhaveri KD, Naber TH, Wang X, Molmenti E, Bhaskaran M, Boctor FN, Trachtman H. Treatment of recurrent focal segmental glomerular sclerosis posttransplant with a multimodal approach including high-galactose diet and oral galactose supplementation. Transplantation. 2011;91:e35-e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Robson K, Hill P, Langsford D, Dwyer K, Goodman D, Langham R. Galactose therapy reduces proteinuria in patients with recurrent focal segmental glomerulosclerosis after kidney transplantation. Nephrology (Carlton). 2015;20 Suppl 1:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Sgambat K, Banks M, Moudgil A. Effect of galactose on glomerular permeability and proteinuria in steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2013;28:2131-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Suranyi MG, Guasch A, Hall BM, Myers BD. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. Am J Kidney Dis. 1993;21:251-259. [PubMed] |
| 68. | McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, Savin VJ. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. 1998;9:433-438. [PubMed] |
| 69. | Leroy S, Guigonis V, Bruckner D, Emal-Aglae V, Deschênes G, Bensman A, Ulinski T. Successful anti-TNFalpha treatment in a child with posttransplant recurrent focal segmental glomerulosclerosis. Am J Transplant. 2009;9:858-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Bitzan M, Babayeva S, Vasudevan A, Goodyer P, Torban E. TNFα pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and β3 integrin activation. Pediatr Nephrol. 2012;27:2217-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, Simpkins CE, Singer AL, Stewart ZA. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 72. | Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996-1001. [PubMed] |
| 73. | EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.6.1. Cancer risk after renal transplantation. Post-transplant lymphoproliferative disease (PTLD): prevention and treatment. Nephrol Dial Transplant. 2002;17 Suppl 4:31-33, 35-36. [PubMed] |
| 74. | Hristea D, Hadaya K, Marangon N, Buhler L, Villard J, Morel P, Martin PY. Successful treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation by plasmapheresis and rituximab. Transpl Int. 2007;20:102-105. [PubMed] |
| 75. | Gossmann J, Scheuermann EH, Porubsky S, Kachel HG, Geiger H, Hauser IA. Abrogation of nephrotic proteinuria by rituximab treatment in a renal transplant patient with relapsed focal segmental glomerulosclerosis. Transpl Int. 2007;20:558-562. [PubMed] |
| 76. | Audard V, Kamar N, Sahali D, Cardeau-Desangles I, Homs S, Remy P, Aouizerate J, Matignon M, Rostaing L, Lang P. Rituximab therapy prevents focal and segmental glomerulosclerosis recurrence after a second renal transplantation. Transpl Int. 2012;25:e62-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Hickson LJ, Gera M, Amer H, Iqbal CW, Moore TB, Milliner DS, Cosio FG, Larson TS, Stegall MD, Ishitani MB. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation. 2009;87:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Dello Strologo L, Guzzo I, Laurenzi C, Vivarelli M, Parodi A, Barbano G, Camilla R, Scozzola F, Amore A, Ginevri F. Use of rituximab in focal glomerulosclerosis relapses after renal transplantation. Transplantation. 2009;88:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Tsagalis G, Psimenou E, Nakopoulou L, Laggouranis A. Combination treatment with plasmapheresis and rituximab for recurrent focal segmental glomerulosclerosis after renal transplantation. Artif Organs. 2011;35:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Cho JH, Lee JH, Park GY, Lim JH, Kim JS, Kang YJ, Kwon O, Choi JY, Park SH, Kim YL. Successful treatment of recurrent focal segmental glomerulosclerosis with a low dose rituximab in a kidney transplant recipient. Ren Fail. 2014;36:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Apeland T, Hartmann A. Rituximab therapy in early recurrent focal segmental sclerosis after renal transplantation. Nephrol Dial Transplant. 2008;23:2091-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Grenda R, Jarmużek W, Piątosa B, Rubik J. Long-term effect of rituximab in maintaining remission of recurrent and plasmapheresis-dependent nephrotic syndrome post-renal transplantation - case report. Pediatr Transplant. 2011;15:E121-E125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Sethna C, Benchimol C, Hotchkiss H, Frank R, Infante L, Vento S, Trachtman H. Treatment of recurrent focal segmental glomerulosclerosis in pediatric kidney transplant recipients: effect of rituximab. J Transplant. 2011;2011:389542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Nozu K, Iijima K, Fujisawa M, Nakagawa A, Yoshikawa N, Matsuo M. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol. 2005;20:1660-1663. [PubMed] |
| 85. | Nakayama M, Kamei K, Nozu K, Matsuoka K, Nakagawa A, Sako M, Iijima K. Rituximab for refractory focal segmental glomerulosclerosis. Pediatr Nephrol. 2008;23:481-485. [PubMed] |
| 86. | Bayrakci US, Baskin E, Sakalli H, Karakayali H, Haberal M. Rituximab for post-transplant recurrences of FSGS. Pediatr Transplant. 2009;13:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 87. | Yabu JM, Ho B, Scandling JD, Vincenti F. Rituximab failed to improve nephrotic syndrome in renal transplant patients with recurrent focal segmental glomerulosclerosis. Am J Transplant. 2008;8:222-227. [PubMed] |
| 88. | Park HS, Hong Y, Sun IO, Chung BH, Kim HW, Choi BS, Park CW, Jin DC, Kim YS, Yang CW. Effects of pretransplant plasmapheresis and rituximab on recurrence of focal segmental glomerulosclerosis in adult renal transplant recipients. Korean J Intern Med. 2014;29:482-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Kamar N, Faguer S, Esposito L, Guitard J, Nogier MB, Durand D, Rostaing L. Treatment of focal segmental glomerular sclerosis with rituximab: 2 case reports. Clin Nephrol. 2007;67:250-254. [PubMed] |
| 90. | El-Firjani A, Hoar S, Karpinski J, Bell R, Deschenes MJ, Knoll GA. Post-transplant focal segmental glomerulosclerosis refractory to plasmapheresis and rituximab therapy. Nephrol Dial Transplant. 2008;23:425. [PubMed] |
| 91. | Prytuła A, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, Taylor M, Marks SD, Tuchman S, Camilla R. Rituximab in refractory nephrotic syndrome. Pediatr Nephrol. 2010;25:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 92. | Stewart ZA, Shetty R, Nair R, Reed AI, Brophy PD. Case report: successful treatment of recurrent focal segmental glomerulosclerosis with a novel rituximab regimen. Transplant Proc. 2011;43:3994-3996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Marks SD, McGraw M. Does rituximab treat recurrent focal segmental glomerulosclerosis post-renal transplantation? Pediatr Nephrol. 2007;22:158-160. [PubMed] |
| 94. | Rodríguez-Ferrero M, Ampuero J, Anaya F. Rituximab and chronic plasmapheresis therapy of nephrotic syndrome in renal transplantation patients with recurrent focal segmental glomerulosclerosis. Transplant Proc. 2009;41:2406-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Ashoor IF, Najafian N. Rejection and regulation: a tight balance. Curr Opin Organ Transplant. 2012;17:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Durrbach A, Francois H, Jacquet A, Beaudreuil S, Charpentier B. Co-signals in organ transplantation. Curr Opin Organ Transplant. 2010;15:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Dall’Era M, Davis J. CTLA4Ig: a novel inhibitor of costimulation. Lupus. 2004;13:372-376. [PubMed] |
| 98. | Schaub M, Stadlbauer TH, Chandraker A, Vella JP, Turka LA, Sayegh MH. Comparative strategies to induce long-term graft acceptance in fully allogeneic renal versus cardiac allograft models by CD28-B7 T cell costimulatory blockade: role of thymus and spleen. J Am Soc Nephrol. 1998;9:891-898. [PubMed] |
| 99. | Azuma H, Chandraker A, Nadeau K, Hancock WW, Carpenter CB, Tilney NL, Sayegh MH. Blockade of T-cell costimulation prevents development of experimental chronic renal allograft rejection. Proc Natl Acad Sci USA. 1996;93:12439-12444. [PubMed] |
| 100. | Chandraker A, Russell ME, Glysing-Jensen T, Willett TA, Sayegh MH. T-cell costimulatory blockade in experimental chronic cardiac allograft rejection: effects of cyclosporine and donor antigen. Transplantation. 1997;63:1053-1058. [PubMed] |
| 101. | Vincenti F. Costimulation blockade--what will the future bring? Nephrol Dial Transplant. 2007;22:1293-1296. [PubMed] |
| 102. | Maxwell LJ, Singh JA. Abatacept for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2010;37:234-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 103. | Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 292] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 104. | Alachkar N, Carter-Monroe N, Reiser J. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1263-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 105. | Garin EH, Reiser J, Cara-Fuentes G, Wei C, Matar D, Wang H, Alachkar N, Johnson RJ. Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr Nephrol. 2015;30:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 106. | Alkandari O, Nampoory N, Nair P, Atta A, Zakaria Z, Mossad A, Yagan J, Al-Otaibi T. Recurrent Focal Segmental Glomerulosclerosis and Abatacept: Case Report. Exp Clin Transplant. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Grellier J, Del Bello A, Milongo D, Guilbeau-Frugier C, Rostaing L, Kamar N. Belatacept in recurrent focal segmental glomerulosclerosis after kidney transplantation. Transpl Int. 2015;28:1109-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Choi SJ, Kim JK, Hwang SD. Mesenchymal stem cell therapy for chronic renal failure. Expert Opin Biol Ther. 2010;10:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 109. | Villanueva S, Ewertz E, Carrión F, Tapia A, Vergara C, Céspedes C, Sáez PJ, Luz P, Irarrázabal C, Carreño JE. Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci (Lond). 2011;121:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 110. | Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton). 2011;16:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 111. | Ma H, Wu Y, Xu Y, Sun L, Zhang X. Human umbilical mesenchymal stem cells attenuate the progression of focal segmental glomerulosclerosis. Am J Med Sci. 2013;346:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 113. | Chang JW, Hung SP, Wu HH, Wu WM, Yang AH, Tsai HL, Yang LY, Lee OK. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant. 2011;20:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 114. | Pistoia V, Raffaghello L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev Clin Immunol. 2010;6:211-218. [PubMed] |
| 115. | Belingheri M, Lazzari L, Parazzi V, Groppali E, Biagi E, Gaipa G, Giordano R, Rastaldi MP, Croci D, Biondi A. Allogeneic mesenchymal stem cell infusion for the stabilization of focal segmental glomerulosclerosis. Biologicals. 2013;41:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Araya CE, Dharnidharka VR. The factors that may predict response to rituximab therapy in recurrent focal segmental glomerulosclerosis: a systematic review. J Transplant. 2011;2011:374213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | Kumar J, Shatat IF, Skversky AL, Woroniecki RP, Del Rio M, Perelstein EM, Johnson VL, Mahesh S. Rituximab in post-transplant pediatric recurrent focal segmental glomerulosclerosis. Pediatr Nephrol. 2013;28:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 119. | Seifert M, Stolk M, Polenz D, Volk HD. Detrimental effects of rat mesenchymal stromal cell pre-treatment in a model of acute kidney rejection. Front Immunol. 2012;3:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell. 2012;11:812-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 121. | Mantovani A. MSCs, macrophages, and cancer: a dangerous ménage-à-trois. Cell Stem Cell. 2012;11:730-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Shankland SJ, Smeets B, Pippin JW, Moeller MJ. The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol. 2014;10:158-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 123. | Shankland SJ, Anders HJ, Romagnani P. Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr Opin Nephrol Hypertens. 2013;22:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 124. | Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:1262-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 125. | Askanase AD, Byron M, Keyes-Elstein LL, Cagnoli PC, McCune W, Chatham W, Contreras G, Daikh DI, Dall’ Era M, Wofsy D. Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol. 2014;66:3096-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |