Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.206
Peer-review started: July 4, 2015
First decision: October 16, 2015
Revised: December 3, 2015
Accepted: December 29, 2015
Article in press: January 4, 2016
Published online: March 24, 2016
Processing time: 262 Days and 18.9 Hours
AIM: To investigate the interaction between castanospermine and cyclosporin A (CsA) and to provide an explanation for it.
METHODS: The alkaloid castanospermine was prepared from the seeds of Castanospermum austral consistently achieving purity. Rat heterotopic cardiac transplantation and mixed lymphocyte reactivity were done using genetically inbred strains of PVG (donor) and DA (recipient). For the mixed lymphocyte reaction stimulator cells were irradiated with 3000 rads using a linear accelerator. Cyclosporin A was administered by gavage and venous blood collected 2 h later (C2). The blood levels of CsA (Neoral) were measured by immunoassay which consisted of a homogeneous enzyme assay (EMIT) on Cobas Mira. Statistical analyses of interactions were done by an accelerated failure time model with Weibull distribution for allograft survival and logistic regression for the mixed lymphocyte reactivity.
RESULTS: Castanospermine prolonged transplant survival times as a function of dose even at relatively low doses. Cyclosporin A also prolonged transplant survival times as a function of dose particularly at doses above 2 mg/kg. There were synergistic interactions between castanospermine and CsA in the prolongation of cardiac allograft survival for dose ranges of CsA by castanospermine of (0 to 2) mg/kg by (0 to 200) mg/kg (HR = 0.986; 95%CI: 0.981-0.992; P < 0.001) and (0 to 3) mg/kg by (0 to 100) mg/kg (HR = 0.986; 95%CI: 0.981-0.992; P < 0.001) respectively. The addition of castanospermine did not significantly increase the levels of cyclosporin A on day 3 or day 6 for all doses of CsA. On the contrary, cessation of castanospermine in the presence of CsA at 2 mg/kg significantly increased the CsA level (P = 0.002). Castanospermine inhibited mixed lymphocyte reactivity in a dose dependent manner but without synergistic interaction.
CONCLUSION: There is synergistic interaction between castanospermine and CsA in rat cardiac transplantation. Neither the mixed lymphocyte reaction nor the metabolism of CsA provides an explanation.
Core tip: The authors have established that a biological, castanospermine, interacts with cyclosporin A (CsA) in a synergistic manner when prolonging the survival of cardiac allografts in inbred rats. They suggest that the explanation is not its effect on the mixed lymphocyte reaction nor interference in the metabolism of CsA but rather an inhibition of migration through the basement membrane of the vasculature. They suggest that its effect on heparanase in mononuclear cells and heparan sulphate in the allograft should now be studied. This immunosuppressant holds promise of safe dose reduction of CsA but further assessment of its safety remains.
- Citation: Hibberd AD, Clark DA, Trevillian PR, Mcelduff P. Interaction between castanospermine an immunosuppressant and cyclosporin A in rat cardiac transplantation. World J Transplant 2016; 6(1): 206-214
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/206.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.206
Transplant recipients are at risk from the adverse effects of immunosuppressive agents for the duration of the transplant and beyond. All immunosuppressive agents currently used create adverse effects; this includes cancer[1], infection[2], nephrotoxicity[3] and diabetes mellitus[4]. Hence there is an ongoing need to improve immunosuppressive agents and treatment regimes. One method of managing the adverse effects of cyclosporin A (CsA), a common maintenance immunosuppressive agent, is the addition of a second agent that interacts synergistically with it: This allows reduction in the dose of CsA (thus reducing the risk of adverse effects) while maintaining the overall immunosuppressive effect provided the second agent is well tolerated.
Glycoproteins are essential components of the cell as they are used to construct receptor ligand combinations, membranes and cytokines. Castanospermine disrupts their construction by competitively inhibiting glucosidase 1 and 2. It is a biological found in the Moreton Bay Chestnut Tree. In general construction of glycoproteins takes place in the endoplasmic reticulum and the Golgi apparatus. In the endoplasmic reticulum the oligosaccharide is bound to the polypeptide carried on polysomes[5]. Here it is then refined by removal of glucose by glucosidase 1 and 2, removal of mannose by mannosidase 1 and glycosylation by N acetyl transferase. After moving to the Golgi it is further refined by removal of mannose by mannosidase 2 and glycosylation by N acetyl transferase. Hence the mannose-6-phosphate receptor may be disrupted and the transport of glycoproteins impaired. Overall some glycoproteins become dysfunctional. It is interesting to note that work to date has shown CAST is immunosuppressive and anti-inflammatory: Cardiac allograft rejection[6], thyroid allograft rejection[7], autoimmune encephalomyelitis[8] and chemically induced arthritis[9] are all mitigated.
When developing new immunosuppressive molecules the emphasis has been upon two major targets; the T and B cells. But allograft rejection has other sites that are open to therapeutic intervention including lymphocyte binding to the vascular endothelium and cell migration through the basement membrane of the allograft vasculature. The basement membrane which contains heparan sulphate proteoglycan (HSPG) perlecan[10] protects islet clusters against autoimmune destruction; this protection is broken by heparanase secreted by mononuclear cells which cleaves heparan sulphate from the HSPG[11] thus allowing cell entry. By effecting the membrane expression of adhesion molecules on both lymphocytes and endothelial cells CAST reduces the binding of the two cell types[12]. It may also impair the production of heparanase by MNCs and the degradation of extracellular matrix by endothelial cells[13]. Hence it may conserve the structure of HSPG in the basement membrane of the allograft vasculature and thus protect against rejection. These mechanisms of action are different from those of CsA, to our knowledge, and therefore warrant investigation as a strategy to reduce the adverse effects of CsA. To date an immunosuppressive agent that conserves the function of allograft basement membrane (and also prevents the binding of alloreactive cells to the endothelium) is not in clinical use.
Hence in this study we aimed to determine if there is a synergistic interaction between castanospermine (CAST) and CsA. If so we aimed to provide an explanation for it.
The inbred rat strains PVG (RT1c) (donor) and DA (RT1a) (recipient) were used to study cardiac allograft survival and the mixed lymphocyte reaction (MLR); DA rats were used to study the blood levels of CsA. The rats were housed under standard conditions in the Animal House of the Faculty of Health Sciences, University of Newcastle, Australia.
Heterotopic cardiac transplants were done using a published technique[14]. Cardiac function was assessed daily by abdominal palpation and transplant electrocardiography. The end point of cardiac transplant survival was defined as the last day of palpable heart beating. Care of all rats in this study complied with the Animal Research Act 1985 (NSW, Australia). The protocols were designed to minimise pain and discomfort to the animals. Animals were acclimatised to laboratory conditions (22 °C, 12 h cycle of light and dark, 50% humidity, ad libitum access to food and water) for a minimum of 1 wk prior to experimentation. Intragastric gavage administration was carried out with conscious animals, using curved gavage needles appropriate for animal size (250-300 gm body weight: Gauge 16, 100 mm). All transplanted rats were given post-operative analgesia (Carprofen 4 mg/kg every 12-24 h subcutaneously). They were euthanized by approved carbon dioxide asphyxiation when survival reached 100 d or when the heart stopped beating confirmed by electrocardiography prior to tissue procurement.
This indolizidine alkaloid is extracted from the seeds of Castanospermum australe (the Australian Moreton Bay Chestnut) by a standard technique yielding purity ≥ 99.5%[13]. For the studies on cardiac transplant survival it was administered by Alzet osmotic pumps (Alza Corporation, Palo Alto, United States) at doses of 50, 100, 150, 200 or 300 mg/kg per day by constant subcutaneous infusion (10 μL/h) from day 1 until day 6 when the pump was removed. For the studies of CsA blood levels, CAST was delivered by osmotic pumps at 100 mg/kg per day or 200 mg/kg per day from day 1 until day 6 when the pump was removed. The control was a pump filled with 0.9% saline and removed at day 6. For studies on the MLR, CAST was dissolved in RPMI medium 1640 (Trace Biosciences, Sydney, Australia) supplemented with 10% foetal calf serum (FCS, Trace Biosciences, Sydney, Australia), 2-[4-(2-Hydroxyethyl)]-1-piperazine ethane sulfonic acid buffer 0.02 mol/L (HEPES, Trace Biosciences, Sydney, Australia), sodium bicarbonate 1.5 g/L, penicillin/streptomycin 50 mg/L, 2-mercaptoethanol 5 × 10-5 mol/L and L-glutamine 1 mg/L to a concentration of 65536 μmol/L (micromolar) and then filtered through a 0.22 μm filter (Sartorius, Hannover, Germany). Final concentrations used were quadrupling dilutions of 16384 to 0.0625 μmol/L.
For the transplant survival study CsA (Neoral, Novartis Pharmaceutical, Australia) was diluted in olive oil and administered by gavage at doses of 0.5, 2, 3, 4 mg/kg per day to DA rats. For the study on its blood levels CsA was delivered by gavage at the appropriate dose once daily from day 0 to day 9. Venous blood (0.3 mL) was then collected from the tail veins of DA rats using a 1 mL syringe with a 25 gauge needle two hours after gavage of CsA (C2 level). Samples were then processed at Hunter New England Area Pathology Services (John Hunter Hospital Newcastle, NSW, Australia) using a homogeneous enzyme immunoassay (EMIT 2000, Dade Behring-Syva, Deerfield, Illnois, United States) performed on a Cobas Mira (Roche, Basel, Switzerland). For the MLR CsA was diluted in RPMI medium to 40.96 μmol/L, filtered through a 0.22 μm filter and used in quadrupling dilutions of 10.24 to 0.00015625 μmol/L.
Responder cells were isolated at 4 °C from pooled, all DA available lymph nodes; stimulator cells were isolated at 4 °C from PVG spleens and both were prepared as previously described[6]. Final cell concentrations for use in the MLR were 2 × 106/mL responders and 2 × 106/mL stimulators. The stimulators were irradiated with 3000 rad (radiation absorbed dose) using a linear accelerator (Varian, Palo Alto, California, United States) before use in the MLR.
For the MLR 2 × 105 responder cells were co-cultured with 2 × 105 PVG stimulator cells for 72 h. All assays for given doses of CAST or CsA were done in triplicate. During incubation cells were exposed to final concentrations of CAST in quadrupling dilutions of 16384 to 0.0625 μmol/L or final concentrations of CsA in quadrupling dilutions of 10.24 to 0.00015625 μmol/L or a combination of both drugs. The cultures were pulsed with H3 - thymidine (Amersham, United Kingdom) at 1.0 μCi/well for 18 h and then harvested on to nitrocellulose filters using a Filter Mate Cell Harvester (Packard Instrument Company, Meriden, United States) then counted on a microplate scintillation counter (Packard Instrument Company, Meriden, United States). The mean count per minute (cpm) ± SD was the function used to express the results.
The survival curves for heterotopic cardiac transplants were established for CAST by dose and for CsA by dose separately: Groups received CAST at 50, 100, 150, 200 or 300 mg/kg per day over 7 d; other groups received CsA at 0.5, 2, 3 or 4 mg/kg per day over 7 d. For the interaction studies the groups were: CsA 0.5 mg/kg plus CAST 100 mg/kg, CsA 0.5 mg/kg plus CAST 200 mg/kg, CsA 2 mg/kg plus CAST 50 mg/kg, CsA 2 mg/kg plus CAST 100 mg/kg, CsA 2 mg/kg plus CAST 200 mg/kg, CsA 3 mg/kg plus CAST 50 mg/kg or CsA 3 mg/kg plus CAST 100 mg/kg. The control group consisted of allografts with neither CAST nor CsA. Previous work has established that the osmotic pump with 0.9% saline does not prolong allograft survival[6]. Permanent prolongation was defined as 100 d survival.
The study consisted of 9 groups: CsA 2, 3 or 4 mg/kg each in combination with CAST 0 (saline), 100 or 200 mg/kg. C2 levels (ug/L) were then measured on day 3, 6 (both on pump) and 9 (off pump).
The T cell responses in the MLR relating the proliferation and dose were used to determine the IC50s for CsA and CAST separately. To study the interaction between the two drugs the range of doses selected for CsA or CAST was the IC50 for either drug plus the two dose concentrations that were immediately greater or smaller. A series of MLRs for CsA each with a different CAST dose was then done.
In this study “time to death” was chosen as the outcome measure. The survival time of transplants was truncated at 100 d and therefore the survival times beyond 100 d are unknown. Survival analysis techniques, which model these censored observations, have been used. Specifically, accelerated failure time models that assume survival times follow a Weibull distribution were used[15].
The extent to which dose of CAST can impact on the association between dose of CsA and survival can only be estimated where the marginal effect of either drug does not reach its maximum. Therefore we only examined whether the dose of CAST was an effect modifier of the association between CsA and survival for the dose ranges of CAST by CsA of (0 to 200) mg/kg by (0 to 2) mg/kg and separately (0 to 100) mg/kg by (0 to 3) mg/kg. Hazard ratios (HR) were used to describe the effect of CsA and CAST, and their interaction, on survival. The HR for these data can be interpreted as the relative risk of death at a given follow-up time associated with each one-unit increase in the treatment. With an interaction term in the model, the HR associated with the main effect of one of the treatments is only applicable when the other treatment is held at zero; this is true because the interaction term allows the HR of one treatment to depend on the level of the other treatment. The HR associated with the interaction term is the additional effect of having the two treatments above the individual effects of the two treatments.
The effect of treatment with CsA and CAST on lymphocyte count was explored using linear regression within a linear mixed model framework. The outcome measure in the regression models was the natural logarithm (log) of the lymphocyte count and the main predictors of interest were dose of CsA and CAST. Experimental number was included as the adjusting unit to adjust for any variation that may have occurred in experimental conditions. The likelihood ratio statistic was used to compare the models with and without the interaction term of CsA by CAST. The data indicate that the relationship between CsA and lymphocyte count or between CAST and lymphocyte count is not monotonic with a small increase in the lymphocyte count observed at very low doses. Therefore it was not appropriate to assume that the dose response relationship is linear and so dose of CsA and dose of CAST were included in the model as categorical variables. Therefore no assumption is made about the relationship of dose and the natural log of lymphocyte count.
In this study synergy is defined as a positive interaction between CsA and CAST which means that their combined effects are greater than the sum of their individual effects. The definition of statistical interaction is logically equivalent to the definition of effect-measure modification and is usually described as “departure from additivity of effects on the chosen outcome scale”[16]. This definition implies that the presence or absence of statistical interaction between two factors depends on the scale chosen to measure the effect.
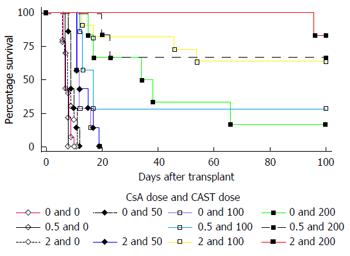
The numbers of transplants that survived to 100 d and the mean transplant survival times are listed in Tables 1 and 2 and Figure 1. Castanospermine clearly prolonged transplant survival times in a dose dependent manner even at relatively low doses. Cyclosporin A also prolonged transplant survival times in a dose dependent manner particularly at doses above 2 mg/kg. The results of statistical analyses of the interactions between the two drugs are listed in Tables 3 and 4. Using accelerated failure time models the effect of dose of CsA on the association between CAST and survival was analysed in the dose ranges of CsA by CAST of (0 to 2) mg/kg by (0 to 200) mg/kg and (0 to 3) mg/kg by (0 to 100) mg/kg. There was a statistically significant interaction between CsA and CAST in both dose ranges (both P < 0.001). In the dose ranges of CsA by CAST of (0 to 2) mg/kg by (0 to 200) mg/kg, the HR associated with CsA was 0.958, with CAST was 0.982 and with the interaction term was 0.986. This means the addition of one mg/kg of CsA together with one mg/kg of CAST reduced the risk of death by 7.2% at each point in the follow-up period, which is captured by the combined HR of 0.928 (0.958 × 0.982 × 0.986).
| Output from the accelerated failure time model with weibull distribution for cyclosporin A doses of 0 to 2 mg/kg per day and castanospermine doses of 0 to 200 mg/kg per day | |||
| Variable | HR | 95%CI | P value |
| Cyclosporin A dose | 0.958 | 0.668-1.374 | 0.817 |
| Castanospermine dose | 0.982 | 0.976-0.988 | < 0.001 |
| Interaction | 0.986 | 0.981-0.992 | < 0.001 |
| Output from the accelerated failure time model with weibull distribution for cyclosporin A doses of 0 to 3 mg/kg per day and castanospermine doses of 0 to 100 mg/kg per day | |||
| Variable | HR | 95%CI | P value |
| Cyclosporin A dose | 0.852 | 0.662-1.0954 | 0.211 |
| Castanospermine dose | 0.978 | 0.968-0.987 | < 0.001 |
| Interaction | 0.986 | 0.981-0.992 | < 0.001 |
This was studied to determine whether the synergistic interaction between CAST and CsA in vivo was simply due to an increased blood level of CsA in the presence of CAST. The results of CsA levels in the presence of CAST are listed in Table 5 and upon cessation of CAST in Table 6. The addition of CAST did not significantly increase the CsA levels on day 3 or day 6 for all CsA doses studied. Furthermore, at day 3 the CsA levels were similar for all doses of CAST but at day 6 the CsA levels tended to decrease with increasing doses of CAST. This difference in the trend of the CsA levels between day 6 and day 3 was statistically significant at each dose of CsA (CsA 2 mg/kg P = 0.02; CsA 3 mg/kg P = 0.04; CsA 4 mg/kg P = 0.001). Cessation of CAST by removal of the pump did not significantly decrease the CsA level: On the contrary, when using CsA at 2 mg/kg cessation of CAST significantly increased the CsA level (P = 0.002).
| Blood level of CsA1 | |||||
| CsA dose2 | d | Castanospermine dose2 | No. | Mean | SD |
| 23,4 | 3 | 0 | 5 | 189.2 | 73.34 |
| 3 | 100 | 5 | 299.8 | 53.53 | |
| 3 | 200 | 5 | 313.0 | 131.56 | |
| 6 | 0 | 5 | 477.0 | 78.97 | |
| 6 | 100 | 5 | 326.6 | 110.48 | |
| 6 | 200 | 5 | 280.2 | 126.69 | |
| 33,5 | 3 | 0 | 5 | 520.6 | 177.18 |
| 3 | 100 | 5 | 450.2 | 218.76 | |
| 3 | 200 | 4 | 506.5 | 271.96 | |
| 6 | 0 | 5 | 1061.80 | 256.22 | |
| 6 | 100 | 5 | 784.80 | 107.83 | |
| 6 | 200 | 4 | 439.75 | 160.51 | |
| 43,6 | 3 | 0 | 5 | 711.80 | 184.61 |
| 3 | 100 | 5 | 601.40 | 121.33 | |
| 3 | 200 | 5 | 1031.60 | 287.18 | |
| 6 | 0 | 5 | 1110.20 | 252.20 | |
| 6 | 100 | 5 | 1152.20 | 127.67 | |
| 6 | 200 | 5 | 556.20 | 192.41 | |
| Blood level of CsA1 | |||||
| CsA dose2 | On pump | Castanospermine dose2 | No. | Mean | SD |
| 23 | Yes | 0 | 10 | 333.10 | 167.84 |
| 100 | 10 | 313.20 | 83.06 | ||
| 200 | 10 | 296.60 | 122.98 | ||
| No | 0 | 5 | 513.20 | 170.76 | |
| 100 | 5 | 560.00 | 254.00 | ||
| 200 | 5 | 355.40 | 105.29 | ||
| 34 | Yes | 0 | 10 | 791.20 | 352.83 |
| 100 | 10 | 617.50 | 239.87 | ||
| 200 | 8 | 473.13 | 209.79 | ||
| No | 0 | 5 | 849.40 | 455.77 | |
| 100 | 5 | 671.20 | 421.57 | ||
| 200 | 4 | 824.50 | 153.44 | ||
| 44 | Yes | 0 | 10 | 911.00 | 295.81 |
| 100 | 10 | 876.80 | 313.14 | ||
| 200 | 10 | 793.90 | 340.42 | ||
| No | 0 | 5 | 968.80 | 429.26 | |
| 100 | 5 | 1188.60 | 453.13 | ||
| 200 | 5 | 589.40 | 290.93 | ||
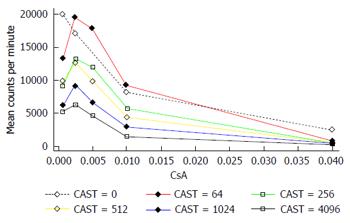
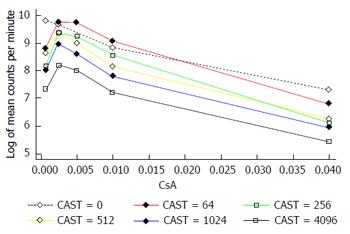
The interaction between CAST and CsA in the MLR is represented in Figures 2 and 3. There is a reduction in the number of lymphocytes with increasing doses of CsA for all dose levels of CAST and the absolute reduction in lymphocytes for a given dose of CsA decreases with decreasing doses of CAST (Figure 2). A more appropriate scale to assess this biological interaction, however, is the natural logarithm (log) of lymphocytes given that proliferation is likely to occur due to a doubling of the current number. The results contained in Figure 3 show there is a reduction in the natural log of the number of lymphocytes with increasing doses of CsA which is similar for all doses of CAST (P < 0.001). This implies that the percentage reduction in the number of lymphocytes with increasing dose of CsA is constant for all doses of CAST. Further, there was no statistically significant interaction between CsA and CAST (P = 0.89).
The major findings in this study are that CAST and CsA interacted synergistically in the prolongation of rat cardiac allograft survival but did not interact synergistically in the MLR despite showing additive dose dependent inhibition with CsA. Further, the blood level of CsA was not increased by the addition of CAST. By contrast it was increased when CAST was ceased while using CsA at 2 mg/kg but not at the other 2 doses of CsA.
In clinical practice the nephrotoxicity of CsA is a major unsolved problem. Cyclosporin A causes interstitial fibrosis, tubular atrophy (IFTA) and arteriolar hyalinosis and therefore can contribute to graft failure[17]. There is controversy, however, about the extent that CsA nephrotoxicity alone causes graft failure; some argue that it is the major cause[17] while others consider it minor causing 0.7% of graft losses[18]. The use of a second agent acting in synergism with CsA provides a method of managing the nephrotoxicity because it allows dose reduction in CsA (and thus toxicity) without compromising graft survival. Reduction in the dose of CsA can be expected to alleviate nephrotoxicity given the inverse relationship between CsA dose and IFTA[19]. Our study shows that because CAST interacts synergistically with CsA and is relatively nontoxic[6] it holds promise of reducing the toxicity of CsA when combined with it. But there are many remaining points of assessment before castanospermine can be considered for the clinic. Other studies have also shown synergistic interactions between CsA and dexamethasone and between CsA and rapamicin which have allowed safe reduction in CsA dose. A second method of managing CsA nephrotoxicity is the use of a specific antagonist: For instance, darusentan alleviates CsA nephrotoxicity in rats by blocking the type A endothelin receptor[20] but to date there is no antagonist in clinical use.
Three explanations for the synergistic interaction between CAST and CsA were examined in our studies. First it is not due to simple inhibition of the hepatic metabolism of CsA because CAST did not increase the CsA level (Table 5). By contrast CAST reduced the blood level of CsA at one of the three doses studied (Table 6). Our hypothesis for these findings is that CAST may impair the mechanism used for the absorption of CsA in the small bowel known to depend upon a glycoprotein transporter. This mechanism may be competitively inhibited at low doses of CsA by CAST but at higher doses of CsA the inhibition is less effective. Second, although CAST inhibits the MLR by inhibiting signal transduction from the IL-2 receptor[21] it did not act synergistically with CsA in the MLR (Figures 2 and 3). It did however reduce the MLR with CsA in an additive dose dependent manner. This finding implies that CAST may act at sites other than the T cell which proliferates in the MLR. Third, our previous immunohistochemistry studies in rats treated with CAST revealed clusters of mononuclear cells (MNCs) about the basement membrane of venules while sparing the interstitial infiltrate in cardiac allografts[6]; these findings are consistent with the observations of Willenborg et al[8] in rats with experimental autoimmune encephalomyelitis treated with CAST.
We therefore propose that CAST may impair the passage of MNCs through this basement membrane of the venules. The evidence for this proposal is the following. The basement membrane contains heparan sulphate proteoglycan perlecan which acts as a barrier to cell entry[10]. It can be broken down by heparanase which is present in MNCs and endothelial cells[11]. Castanospermine has been shown to inhibit heparanase and sulfatase in endothelial cells[13], to inhibit heparanase within intragraft alloreactive cells[22] and to inhibit lysis of extracellular matrix which also contains HSPG[13]. Furthermore, in a murine model of autoimmune insulitis inhibition of heparanase conserved the basement membrane of islet clusters which contained heparan sulphate[11]. Hence an explanation for the synergistic interaction of CAST and CsA may be the reduction in heparanase production from alloreactive cells by CAST thus strengthening the impermeability of the vascular basement membrane. To our knowledge this site is not affected by CsA.
The strengths of our study are that it definitively establishes for the first time that CAST and CsA act synergistically in prolonging rat allograft survival and, second, the explanation cannot be found in its effect on T cell proliferation nor the metabolism of CsA. The weakness of our study is that this work is in inbred rats only and therefore work in higher animal models is required before one can reasonably hope for amelioration of the adverse effects of CsA by dose reduction.
Although we conclude that CAST and CsA interact synergistically in this model further study of its effect on heparanase and heparan sulphate concentrations in organ allograft transplantation is necessary. In vivo and in vitro migration studies are also needed to challenge the proposal that the basement membrane is a key site of action of CAST.
We thank the Directors of Kiriwina Investments Ltd who supplied the grant for the major part of this work. We wish to thank the radiation oncology staff at the Mater Calvary Hospital, Newcastle for access to the linear accelerator used in the MLR studies and Stephen McInally for his expert production of the figures.
The field of organ transplantation which is a major medical advance still has some fundamental problems to solve. One of these is the adverse effects of immunosuppressive drugs that are necessary for the duration of the transplant for the vast majority of recipients. It is an exception for recipients to become tolerant to their transplants implying that their immune responses have accepted the foreign transplants. Now, the major adverse effects of immunosuppressive agents are cancer and infection although nephrotoxicity, diabetes mellitus and osteoporosis are also common. One approach to managing adverse effects is the use of another immunosuppressive agent which acts synergistically with the first agent. Thus reduction in dose of the first agent can be done without inducing rejection. Because dose is reduced its toxicity may also be reduced provided the second agent is relatively non-toxic. In this study the authors have used this strategy when analysing the immunosuppressive ability of castanospermine a biological derived from the Moreton Bay Chestnut tree.
The authors aimed to study the interaction between castanospermine and cyclosporin A (CsA) which is a common maintenance immunosuppressive agent in organ transplantation. The major adverse effect of CsA is nephrotoxicity which is dose dependent. So first the study of the interaction needs to be done in an animal model transplant system.
They study establishes the positive interaction between castanospermine and CsA and therefore justifies studying the mechanism of its immunosuppressive effect. They have found that the synergism is unlikely to be due to inhibition of T-cell proliferation nor interference in the metabolism of CsA. They have other evidence referenced here suggesting that castanospermine may act by inhibiting migration of cells through the basement of the transplant. Impairment of hepararase in T cells seems to be the key.
Although clinical use of castanospermine or a derivative is the long term aim of this work further study of its mechanism and toxicity profile are needed first.
There are several key components of the allograft rejection response. One of these is the T cell that secrets Il-2 a cytokine that causes T cell proliferation. Cyclosporin A interferes with the production of Il-2 and is a strong immunosuppressant. Another is the B cell that presents antigen to the T cell and also enables antibody production from plasmas cells. Rituximab monoclonal antibody inhibits B cell production. Castanospermine acts differently focussing upon migration of cells into the transplant.
The authors have reviewed and answered the peer reviewers’ comments. They liked the idea of developing an immunosuppressive agent that was synergistic with CsA in organ transplantation. They understood that it could have clinical benefit but that other studies in outbred animals about adverse effects and immunosuppressive ability of castanospermine are needed first. They also encouraged further study of the reasons behind synergism and in particular how castanospermine can inhibit cell migration.
P- Reviewer: Holan V, Kin T, Perrault LP, Salvadori M S- Editor: Qiu S L- Editor: A E- Editor: Wang CH
| 1. | Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823-2831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 839] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 2. | Mueller NJ. New immunosuppressive strategies and the risk of infection. Transpl Infect Dis. 2008;10:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Bodziak KA, Hricik DE. New-onset diabetes mellitus after solid organ transplantation. Transpl Int. 2009;22:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Elbein AD. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991;5:3055-3063. [PubMed] |
| 6. | Grochowicz PM, Hibberd AD, Smart YC, Bowen KM, Clark DA, Cowden WB, Willenborg DO. Castanospermine, an oligosaccharide processing inhibitor, reduces membrane expression of adhesion molecules and prolongs heart allograft survival in rats. Transpl Immunol. 1996;4:275-285. [PubMed] |
| 7. | Bartlett MR, Warren HS, Cowden WB, Parish CR. Effects of the anti-inflammatory compounds castanospermine, mannose-6-phosphate and fucoidan on allograft rejection and elicited peritoneal exudates. Immunol Cell Biol. 1994;72:367-374. [PubMed] |
| 8. | Willenborg DO, Parish CR, Cowden WB. Inhibition of experimental allergic encephalomyelitis by the alpha-glucosidase inhibitor castanospermine. J Neurol Sci. 1989;90:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Willenborg DO, Parish CR, Cowden WB. Inhibition of adjuvant arthritis in the rat by phosphosugars and the alpha-glucosidase inhibitor castanospermine. Immunol Cell Biol. 1992;70:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Irving-Rodgers HF, Ziolkowski AF, Parish CR, Sado Y, Ninomiya Y, Simeonovic CJ, Rodgers RJ. Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia. 2008;51:1680-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J Clin Invest. 2012;122:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Hibberd AD, Trevillian PR, Clark DA, McElduff P, Cowden WB. The effects of Castanospermine, an oligosaccharide processing inhibitor, on mononuclear/endothelial cell binding and the expression of cell adhesion molecules. Transpl Immunol. 2012;27:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Bartlett MR, Cowden WB, Parish CR. Differential effects of the anti-inflammatory compounds heparin, mannose-6-phosphate, and castanospermine on degradation of the vascular basement membrane by leukocytes, endothelial cells, and platelets. J Leukoc Biol. 1995;57:207-213. [PubMed] |
| 14. | Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Klein JP, Moeschberger ML. Interference for parametric regression models. Survival Analysis: Techniques for Censored and Truncated Data. Springer-Verlag. 1997;375. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Greenland S, Rothman KJ. Concepts of interaction. Modern Epidemiology 2nd ed. Philadelphia: Lippincott-Raven 1998; 329. |
| 17. | Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1482] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 18. | El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 630] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 19. | Assan R, Timsit J, Feutren G, Bougnères P, Czernichow P, Hannedouche T, Boitard C, Noel LH, Mihatsch MJ, Bach JF. The kidney in cyclosporin A-treated diabetic patients: a long-term clinicopathological study. Clin Nephrol. 1994;41:41-49. [PubMed] |
| 20. | Orth SR, Odoni G, Amann K, Strzelczyk P, Raschack M, Ritz E. The ET(A) receptor blocker LU 135252 prevents chronic transplant nephropathy in the “Fisher to Lewis” model. J Am Soc Nephrol. 1999;10:387-391. [PubMed] |
| 21. | Walter S, Fassbender K, Gulbins E, Liu Y, Rieschel M, Herten M. Glycosylation processing inhibition by castanospermine prevents experimental autoimmune encephalomyelitis by interference with IL-2 receptor signal transduction. J Neuroimmunol. 2002;132:1-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Hibberd AD, Clark DA, Trevillian PR, Cong MA. Castanospermine a novel immunosuppressant inhibits heparanase within intragraft lymphoid cells while conserving heparan sulphate within rat renal transplants. In: Abstracts 33rd Annual Scientific Meeting of the Transplantation Society of Australia and New Zealand. Immunol Cell Biol. 2015;93:A10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |