Published online Sep 24, 2015. doi: 10.5500/wjt.v5.i3.110
Peer-review started: April 21, 2015
First decision: May 13, 2015
Revised: July 16, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: September 24, 2015
Processing time: 156 Days and 21.3 Hours
AIM: To evaluate the short and long-term effects of the complex cell therapy of 202 cases of spinal cord injury (SCI).
METHODS: The main arm included 202 cases of SCI and the control arm included 20 SCI cases. For the therapy the hematopoietic stem cells (HSCs) and progenitor cells (PCs) were mobilized to peripheral blood by 8 subcutaneous injections of granulocyte colony-stimulating factor (G-CSF) for 4 d and are harvested at day 5. The cells were administered to the main arm intrathecally every 3 mo for a long term (3-5 years) according to the internal research protocol international medical institute of tissue engineering. Magnetic resonance imaging of the site of injury and urodynamic tests were performed every 6 mo. Motor evoked potentials (MEP), somatosensory evoked potentials (SSEP) were evaluated every 3 mo. The patients were evaluated with american spianl injury association (ASIA) index, functional independence measure index, the Medical Research Council Scale, the International Standards for Neurological Classification of Spinal Cord Injury (ISCSCI-92) and specifically developed scales. The function of bladder was evaluated by a specifically developed clinical scale. The long-term clinical outcomes were assessed for the SCI patients who received no less than 20 intrathecal transplantations of HSCs and hematopoietic precursors (HPs).
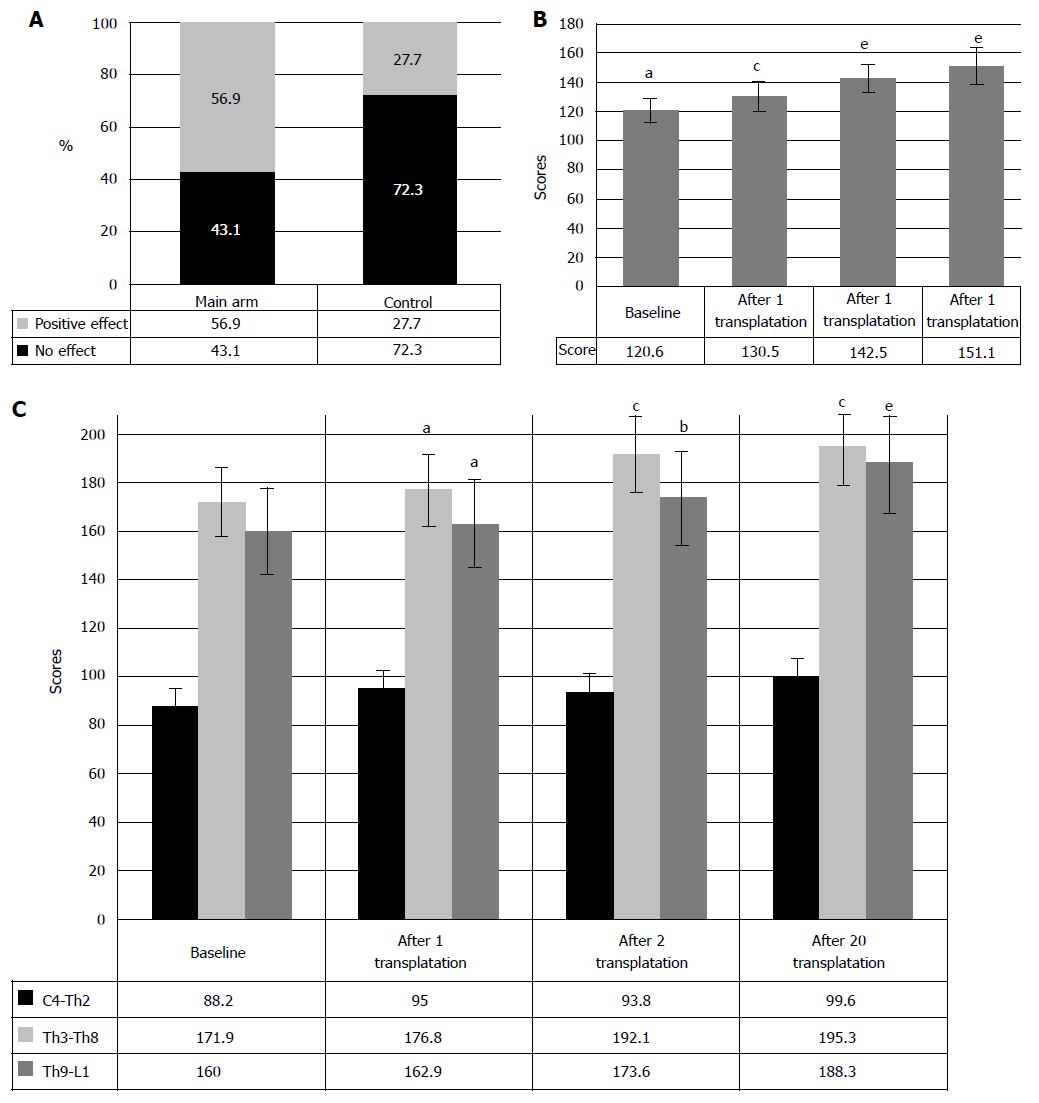
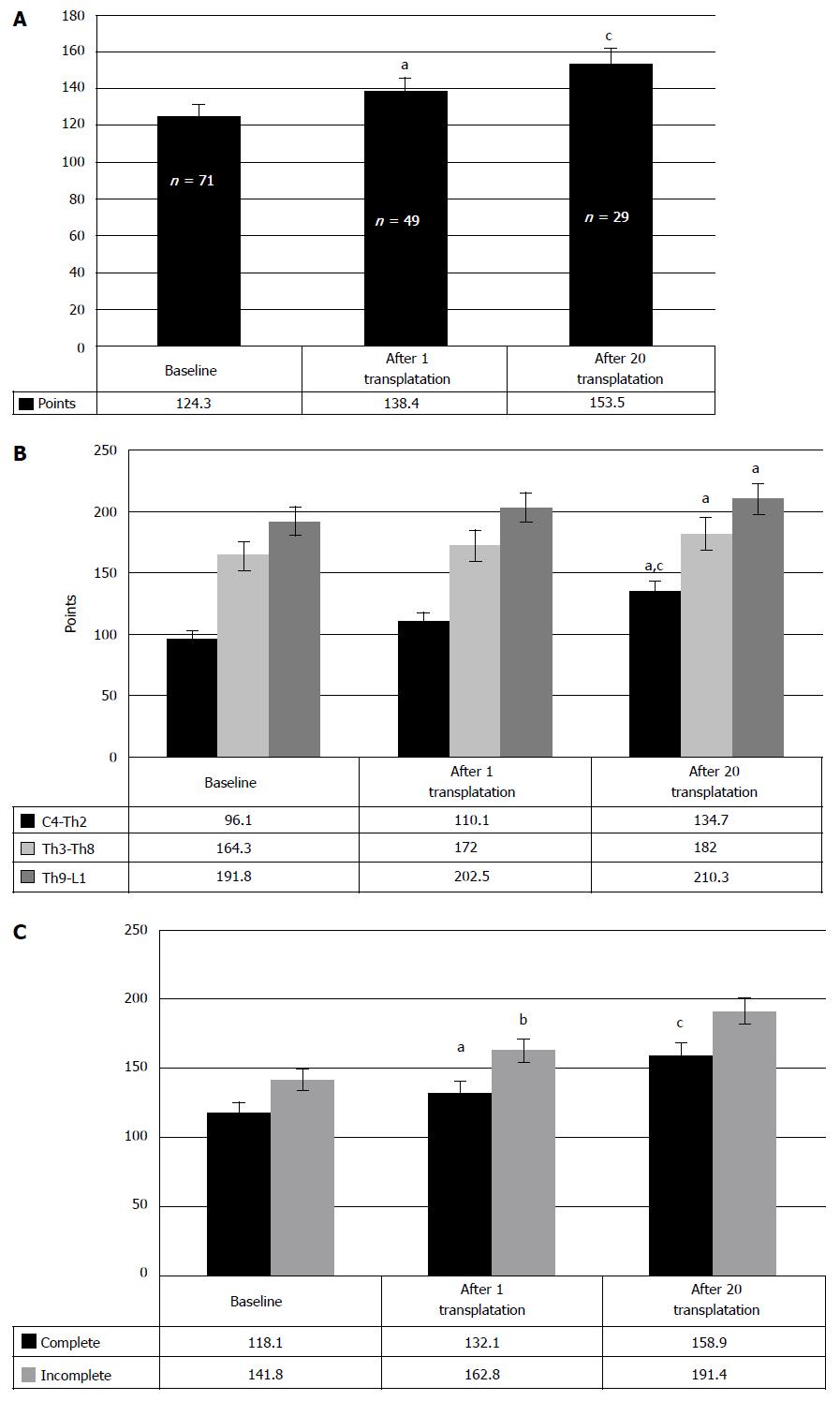
RESULTS: The restoration of neurologic deficit after HSCs and HPs transplantations was proved stable and evident in 57.4% of the cases. In 42.6% cases no neurologic improvement has been observed. In 50% of the cases the motor restoration began after the first transplantation, which is confirmed in average by 9.9 points improvement in neurologic impairment as compared to the baseline (P < 0.05). Repair of the urinary system was observed in 47.7% of the cases. The sensitivity improved from baseline 124.3 points to 138.4 after the first and to 153.5 points after the second transplantations of HSCs and HPs (P < 0.05, between the stages of research). The evaluation with ASIA index demonstrated regress of neurologic symptoms in 23 cases. Motor progress was also assessed with the ISCISCI-92 motor and sensory scores, and the data coincided with those received with the specifically developed scale. The number of the patients with the signs of locomotive repair was 56.9%. No life threatening complications or adverse effects have been observed.
CONCLUSION: The method is safe, effective and considerably improves the life quality of SCI patients. The therapy is approved for clinical use as the treatment of choice.
Core tip: The work summarizes the 12 year experience of stem cell therapy for chronic spinal cord injury. The unique preparation of autologous hematopoietic stem cells and hematopoietic precursors was multiply administered to 202 patients. The article analyzes short and long-term benefits, short and long-term complications and the instruments that were used for their evaluation.
- Citation: Bryukhovetskiy AS, Bryukhovetskiy IS. Effectiveness of repeated transplantations of hematopoietic stem cells in spinal cord injury. World J Transplant 2015; 5(3): 110-128
- URL: https://www.wjgnet.com/2220-3230/full/v5/i3/110.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i3.110
A global incidence rate of traumatic spinal cord injury (SCI) is estimated as 23 cases per million[1]. Regional incidence rates vary from 15 (Australia) to 40 (United States) cases per 1 million of population[1]. The average age at injury increased from 28.7 years in the 1970s to 42.6 years since 2010[2], still, the incidence of traumatic SCI peaks in young people[1,3].
Although spinal fractures constitute only 0.44% of all injury types, the percentage of spinal traumas has dramatically increased (over 200-fold) for the past 7 decades. The analysis predicted 800 of new SCI per 10 million of population.
For the past two decades the therapeutic advances hold a lot of promise for the patients with SCI, but none of the available therapies led to restoration of the morphological structure of spinal cord and its functions. Various therapeutic programs improve outcomes and life quality of the injured only in a few cases, but still they remain unable to repair severe neurologic deficit and restore lost functions. Surgical approaches to repair SCI are aimed at orthopedic restoration of vertebral canal anatomy, and their results remain controversial. To date, an SCI is a final verdict that entails impossibility to return to the previous way of life, to restore previous working capacity and reproductive functions, resulting in tremendous social and economic losses. The total direct costs of SCI in the United States alone are estimated at about 7.7 billion United States dollar[4].
Inefficiency of the available SCI therapies was used to be explained by the absence of regeneration potential of adult neurons, and the opportunity to restore damaged neural cells has only recently been proved[5]. By now, the first steps to develop new neurorestorational therapy of SCI have been made[6,7], although no universally acknowledged methods to restore the spinal cord after the injury are observed. Novel cell techniques and tissue engineering methods can provide the solution; so, according to the Stem Cell Summit (2009) data, 34 million of patients received transplantations of stem cells of various origin, and 1 million of them were SCI patients[8]. However, outcomes and long-term consequences of such transplantations remain as yet unknown.
The available experience is minimally documented and rather obscure, due to insufficient theoretical and experimental evidence of cell technologies, as well as underdeveloped methods of their application, when the fate of transplanted cells, their further differentiation and transformation are unclear. The crucial question of cancer development, triggered by the transplantation of stem cells, also remains unanswered. The myths and fears of possible negative consequences of stem cell therapy significantly interfere with the research and progress in the area.
We have transplanted cells for SCI for 25 years both in research and in clinical practice and have accumulated substantial experience of victories and defeats administrating allogeneic and xenogeneic fetal neural and mesenchymal cells, isolated from animal and human embryos of 10-24 gestation weeks, as well as embryonic stem neural cells, obtained from human blastocyst. This experience is summed up in our book[9], and to date, we have refused from the clinical application of allogeneic and xenogeneic cell material for SCI. We believe the future of the SCI therapy to belong to the suspensions, prepared from autologous stem and progenitor cells (PCs), as under the SCI condition the organism specifies and individually tailors the cells for the treatment of their own SCI, along with the advantage of null immunologic and transplantation side effects and absence of undesirable paramedical ethic, legal and religious aspects[10]. The only option to use the allogeneic stem cells for SCI is haploidentical stem cells or those of close relatives, and only after the human leukocyte antigen typing.
In the present article we would like to determine the basic parameters for the beginning of the cell therapy for SCI and the criteria to terminate it in clinical practice.
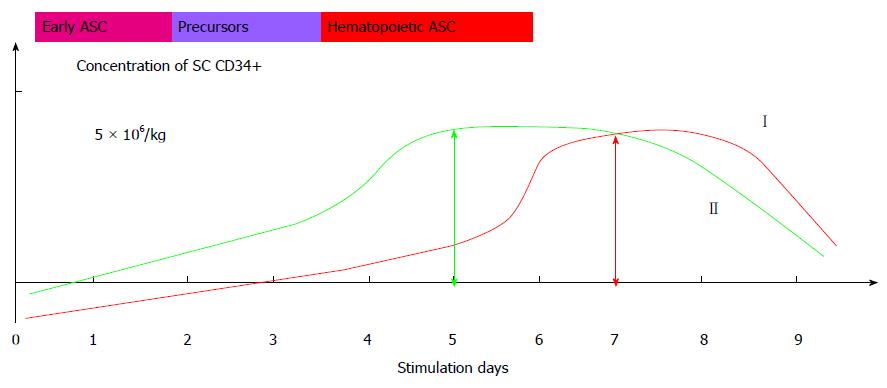
The 12 year trial was performed under the branch program of the Russian Academy of Medical Sciences New Cell Techniques to Medicine, with the approval and under the supervision of the Scientific Board and Ethics Committee of the Russian State Medical University (Moscow, Russia). The trial was launched 2002 and was not registered in the international database for their absence. It is an open parallel controlled trial (phase I/II) that followed IMITE protocol (Switzerland). The trial included 202 SCI patients (1008 case histories) that made trial group 1, see Table 1. According to the protocol, we evaluated the control group that included 20 SCI patients matched by age, sex and level of injury, see Table 2. The enrolled patients signed the Informed Consent. Trial participants met the following eligibility criteria: SCI occurred at least 12 mo prior to the inclusion into the trial; age between 15 and 60; adequate end organ function; adequate bone marrow function, negative pregnancy test; written, voluntary, informed consent. Exclusion criteria were acute infections, severe hematologic disorders; contraindications for MRI, pregnancy or breast feeding, grade III/IV cardiac problems as defined by the New York Heart Association Criteria; severe and/or uncontrolled medical diseases; known diagnosis of human immunodeficiency virus (HIV) infection; previous radiotherapy to ≥ 25% of the bone marrow; major surgery within 6 wk prior to study entry; known malignant tumours. All patients received conventional pharmaceutical treatment and intensive rehabilitation: exercise therapy, physiotherapy and massage. The suspension of HSCs and hematopoietic precursors (HPs) was intrathecally administered to the patients of the main arm every three months for 3-5 years. To produce HSCs and HPs suspension the stem cells (SC) and PCs are mobilized to peripheral blood by 8 subcutaneous injections of granulocyte-colony stimulating factor (G-CSF) every 10-12 h for 4 d. First three days the G-CSF dose is 2.5 μg per kilogram of body weight, the last day the dose is doubled. The stem cells and precursors are harvested at day 5 in blood cell separator (COBE-spectra, Gambro BCT, United States), using a disposable system for separation and standard solutions. The separation lasts 3-4 h, depending on the speed of the procedure, weight of the patient and blood test results. The red blood cells are removed from the obtained material in a conventional way, and the received leukoconcentrate is examined. On average, the volume of the material varies from 300 to 400 mL. The material is evaluated according to total number of nuclear cells (NCs) in the sediment and according to CD34+ cells per a kilogram of the patient’s weight. The NCs in the sediment are determined by counting in Gorjaev’s chamber. The percentage of CD34+ is determined by flow cytometry method by FACScan (Becton Dickinson, United States). Previously we have provided a detailed analysis of the preparation[10]. The standardized and certified HSCs and HPs were uniformly dispensed in 20 tubes and cryopreserved by adding dimethyl sulfoxide in 5% final concentration, frozen down at a rate of 1 °C/min up to a temperature point of -80 °C or -120 °C in a programmed freezer and further stored in liquid nitrogen or liquid nitrogen vapor. The cell material is characterized in Figure 1 and Table 3. Before administration the cells are thawed in +37 °C water bath and washed by double centrifugation with 0.9% NaCl. According to CD34+ count, an average dose of the cells is 5.8 × 106 in a tube. The main trial group received intrathecal administrations (no less than 20) of the HSCs and HPs suspension. The autologous HSCs and HPs were harvested once in 101 patients (50%), twice in 68 patients (33.7%), and three times in 33 patients (16.3%). Totally, during the whole period of observation, the patients received 1790 intrathecal transplantations of autologous HSCs and HPs. The control group patients received analogous treatment, excluding intrathecal administration of HSCs and HPs.
| No. of patients | 202 (1008 case histories) |
| Age | From 19 to 51 yr |
| Gender | Males - 156, females - 46 |
| Years post injury | Less than 1 yr - 11 |
| From 1 to 5 yr - 144 | |
| Over 5 yr - 47 | |
| Level of spinal cord injury | Cervical level - 93 |
| Thoracic level - 98 | |
| Lumbar level - 11 | |
| Type of injury | Complete - 43 |
| Incomplete - 159 | |
| No. of transplantations | No less than 20 HSCs and HPs transplantations |
| Average number of transplanted cells | 5.8 × 106 |
| No. of patients | 20 (62 case histories) |
| Age | From 18 to 44 yr |
| Gender | Males - 13, females - 7 |
| Years post injury | < 1 yr - 6 |
| From 1 to 5 yr - 10 | |
| Over 5 yr - 4 | |
| Level of spinal cord injury | Cervical level - 14 |
| Thoracic level - 4 | |
| Lumbar level - 2 | |
| Type | Complete - 12 |
| Incomplete - 8 |
| Technique | G-CSF dose | Period of administration (d) | Stimulation regimen | Cell markers | Cryopreservant |
| Administration of HSCs in blood | 10-20 μg/kg | 6-7 | 1 in 24 h | CD34+, CD45+ | 10%-20% DMSO |
| HLA DR+, CD38+ | |||||
| Gp130± | |||||
| Administration of HSC and HPs in CSF | 5 μg/kg; double dose at day 4 | 5 | 2 in 24 h | CD34+, CD45- | 5%-10% DMSO + |
| HLA DR±, CD38± | polyglucin | ||||
| Gp130+ |
The patients were clinically and paraclinically evaluated according to the protocol. Evaluation of neurologic condition included tests for locomotion and sensation, bladder and bowel functions, level of injury and its completeness/incompleteness. Safety evaluation was based on the frequency of adverse events, particularly adverse events leading to discontinuation of treatment and on the number of abnormal laboratory values.
Neurological response was assessed every 3 mo, by an examination performed by a neurologist and recorded according to ASIA scale and functional independence measure (FIM) scale. Changes from baseline in neurological status grades and body weight were summarised at defined intervals and produced in the tables of summary statistics.
MRI scan of the CNS and urodynamic tests were performed every 6 mo. Motor evoked potentials (MEP), Somatosensory evoked potentials (SSEP) examinations were performed every 3 mo. Urodynamic tests were performed every 6 mo. To evaluate motor activity we used specifically developed scale of clinical restoration of motor function[9,10] that estimated muscle force in the extremities, range of active movement and movement pace, to calculate the total score of motor activity. Additionally, motor restoration was evaluated with the Medical Research Council Scale that estimates (from 0 to 5 points, depending on the degree of manifestation) the range of active and passive movements, as well as the strength of a body and extremities. Sensitive disorders were evaluated with specifically developed scale of sensation restoration[10] that included 2-point testing of pain, temperature and deep sensation on dermatome on each side, and evaluation of the feeling of “heaviness” in resting muscles and after training in the lower and upper extremities, abdomen and back. Completeness/incompleteness of SCI was assessed according to neurologic symptoms: lower paraplegia, conduction anesthesia and urine retention. Minimal movements or hypoesthesia below the level of injury were evaluated as an incomplete injury (no injury equals 0, an incomplete functional injury of spinal cord equals 1, a complete functional injury of spinal cord is 2).
The function of bladder was evaluated by specifically developed clinical scale to estimate the restoration of bladder function that included 3-point assessment of urination feeling and 5-point assessment of urine retention[10]. The total score, denoting absence of neurologic bladder disorders, equals 8 points. All patients passed complex urodynamic tests. Besides, the effectiveness of the intrathecal transplantation of HSCs and HPs in chronic SCI was evaluated with ASIA index, FIM index and the International Standards for Neurological Classification of Spinal Cord Injury (ISCSCI-92).
The main criteria of effectiveness were improvement of neurologic symptoms (motor, sensitive and bladder and bowel function). The expectation period for the improvement to manifest was individual in every case, depending on the scope of injury, years post injury and functional impairment. The results of the therapy manifested from 1-3 d to 24-36 mo post transplantation and were evaluated by the clinical indexes of ASIA and FIM. Patients were considered in response if at least one of the following criteria were met: (1) An unequivocal improvement of SSEP, MEP; (2) An unequivocal sign of tissue regeneration at MRI; (3) An unequivocal improvement of UT; and (4) Changes from baseline in neurological status grades (ASIA, FIM).
The statistical review of the study was performed by the biomedical statistician of the School of Biomedicine, Far Eastern Federal University. The material was statistically processed with SPSS 13 software. Statistical significance of the data was evaluated with Student’s coefficient, and analysis of variance analysis of variance and χ2 method. The data were considered statistically significant at P < 0.05.
Clinical efficacy was evaluated after three years of therapy by standard neurologic examination and registration of the results in specifically developed forms. The analysis of the registered data demonstrated efficacy of the intrathecal transplantation of HSCs and HPs in 57.4% of the patients, concerning motor and sensitive restoration, as well as repair of bowel and bladder functions (Figure 2). As it can be seen from Figure 2, we observed no neurologic improvement in 42.6% cases, which can be explained by underdeveloped inclusion/exclusion criteria. To date, it is clear that the method demands rigorous screening of the patients for this therapy that will further entail the development of clearer indications and contraindications for the intrathecal transplantation of HSCs and HPs. The size of lesion, its location, type and anatomic continuity of bone structures were of prior importance in this therapy. The analysis of ineffective cases of HSCs and HPs transplantation showed that in major part of the cases (25.2%) the size of spinal cord (SC) lesion exceeded 50% of the spinal cord cross-wise and one segment long-wise, according to MRI. Other reason for the inefficacy of the intrathecal transplantation of HSCs and HPs seems to be the unnoticed moderate or slight disorder of CSF circulation, associated with CSF hypertension, instability of the spinal segment in the injury site and/or scars and cicatrices of the spinal cord that hinder the circulation of CSF. Refusal of the patients from rehabilitative therapy (40.6% of cases) has also significantly contributed to the inefficacy of the therapy. The patients considered administered transplantations sufficient for the recovery and neglected the rehabilitation. In 10.6% cases, the patients negated positive results of the therapy, although the medical exercise instructors and attending doctors observed neurologic progress. Only video records that were taken in the beginning of the treatment and in the course of it, served a decisive argument to confirm functional repair. The therapy that took from 5 to 8 years showed that these patients demonstrated good clinical results of SC functions’ repair. However, this trial included only the patients who received no less than 20 transplantations of HSCs and HPs. In other cases (8.2%) the reason of inefficacy remained unclear, prompting necessity of further research. Moreover, we did not find correlation between the number of transplanted HSCs and HPs and transplantation efficacy [P = 0.1 (P > 0.1)], which was also confirmed by the absence of difference between the number of the transplanted cells to the patients with no effect and those with positive effect, resulting from HSCs and HPs transplantation (5.3 ± 0.9 × 106, as compared to 106.4 ± 0.9 × 106, P > 0.1, respectively). The hypothesis that the process of repair after intrathecal administration of HSCs and PCS depends on the amount of the cells (5.3 ± 0.9 × 106 as compared to 106.4 ± 0.9 × 106) was not confirmed at a 90% significance level.
Evaluation of motor function repair: The efficacy of the intrathecal transplantation of HSCs and HPs was evaluated with the help of the assessment of neurologic condition that included 5-point test of muscle strength, active movements and pace of movements of the extremities on both sides. Total score for no neurologic disorder is 300 points. As seen from Figure 3A, 56.9% of the cases demonstrated improvement of neurologic symptoms, accompanied by muscle strength and muscle tone build-up, visual contractions of some groups of muscles, frequently unilateral, and further development of movements in lightweight positions. Largely, the active movements appeared 12-18 mo later during exercises on press machines. Accordingly, in 50% of the patients the motor restoration began after the first HSCs and HPs transplantation, which is confirmed in average by 9.9 points improvement in neurologic impairment as compared to the baseline (P < 0.05) (Figure 3B). Repeated HSCs and HPs transplantations further enhanced neurologic improvement, that made 142.5 ± 9.7 points (P < 0.05, as compared to baseline and first HSCs and HPs transplantation results). Usually, intensive exercise led to strengthening of extremities’ muscles, increase of range and pace of the movements, stabilization of the knee joints, ability to stand independently in the knee supporting position and development of the elements of walking with assisting devices (walkers). It should be noted that 91.2% reported no restoration of motor functions for several years, and development of the first controllable movements was extremely important for the patients and served an incentive for further training. However, the improvement of the muscle strength was often admitted by the patient no earlier than in 6-12 mo and became objective reality by the end of the second or even third year. By the sixth year, the patients are deeply convinced in the effectiveness and practicability of the therapy.
As our research demonstrated, the intrathecal transplantations of HSCs and HPs led to gradual recovery of the lost movements in chronic SCI patients, only being accompanied by specific rehabilitation. Still, rehabilitation without HSCs and HPs transplantation before enrollment into the program produced only limited effect.
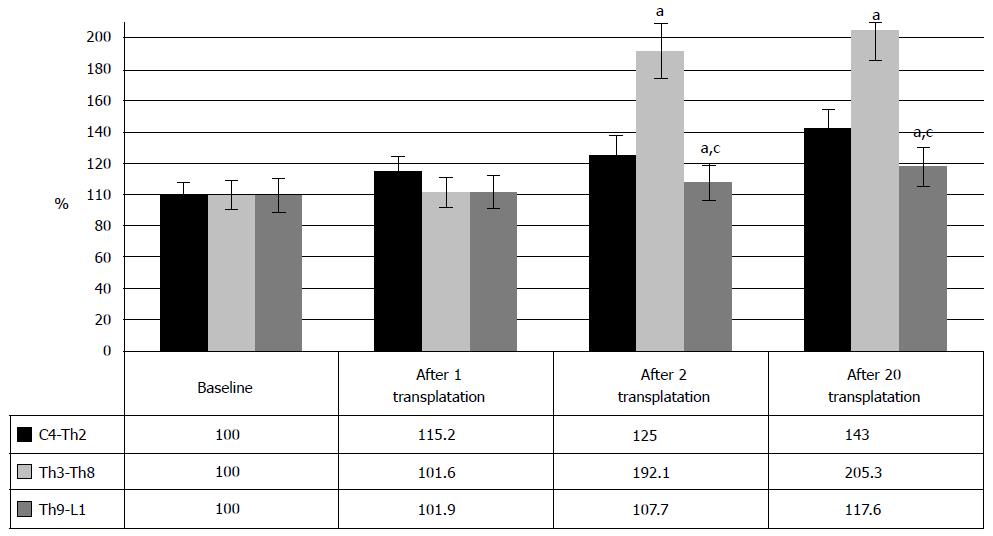
The motor improvement was mostly observed at Th3-Th8 level of injury, specifically in 81.3% of the cases (Figure 3C). Meanwhile, cervical and lumbar SCI cases showed lesser benefit from the therapy, and functional restoration was less illustrative (Figure 4). However, the level Th3-Th8 cases demonstrated considerable repair.
Due to baseline diversity, the comparison of the clinical data between the levels of injury was done in per cent and showed maximal improvement of Th3-Th8 SCI cases after the second and consequent HSCs and HPs transplantations. After the first HSCs and HPs transplantation neurologic improvement was observed only in the cases of cervical injury, which can be explained by the fact that the first feeling of the slight changes in motor functionality (mostly of upper limbs) was much brighter in this category of the patients. By 5-8-th transplantations the quadriplegics were able to turn in their beds independently, the strength in upper extremities and back increased, and they did not require fixation to a wheelchair with the belts or any other devices. However, three years after the first transplantation, the most positive results were observed in lumbosacral cases and, strangely enough, in cervical SCI. At least, the improvement of life quality was more obvious in quadriplegics, both for the patient and for their relatives.
Accordingly, these data report more vigorous repair of motor functions at Th3-Th8 level of SCI after HSCs and HPs transplantations. Although, the represented data show limited opportunity for the restoration at the level of cervical and lumbar enlargement, we observed the benefits of cell transplantations at these levels. Follow-up of the SCI patients after the HSCs and HPs transplantations demonstrated neurologic progress in 61.1%, and it was associated with strengthening of the muscles, development and/or increase of motor activity, regress of sensitive disorders, and improvement of bowel and bladder functions. The most notable clinical effect was achieved in locomotion. In most cases, the changes in motor functions were minimal after the first HSCs and HPs transplantation and manifested in lightweight positions. Further intensive rehabilitation led to strengthening of extremities muscles, increase of pace and range of movements during exercise tests. After the second HSCs and HPs transplantation 33 patients were able to stabilize knee joints, to stand in knee supporting position independently and developed some elements of walking with assisting devices (walkers). It should be noted that 96% of the patients demonstrated no signs of neurologic restoration for several years before HSCs and HPs therapy. One of the patients from the United States restored independent automatic walk in a month of the therapy that included 4 administrations of the HSCs and HPs, and left the hospital on their own feet, although their previous treatment in the United States lasted 5 years. The similar recovery was observed in the patient from Bosnia and Herzegovina, when two administrations were enough to restore the walking function after 6 years of ineffective therapies in various clinics of the world.
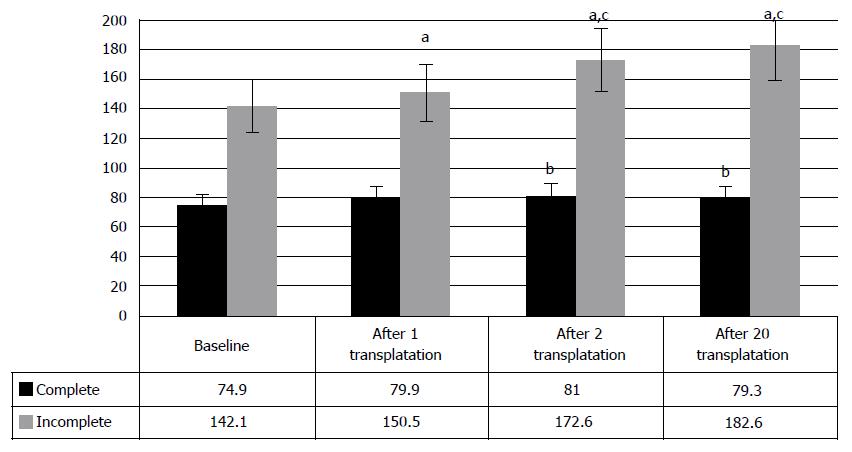
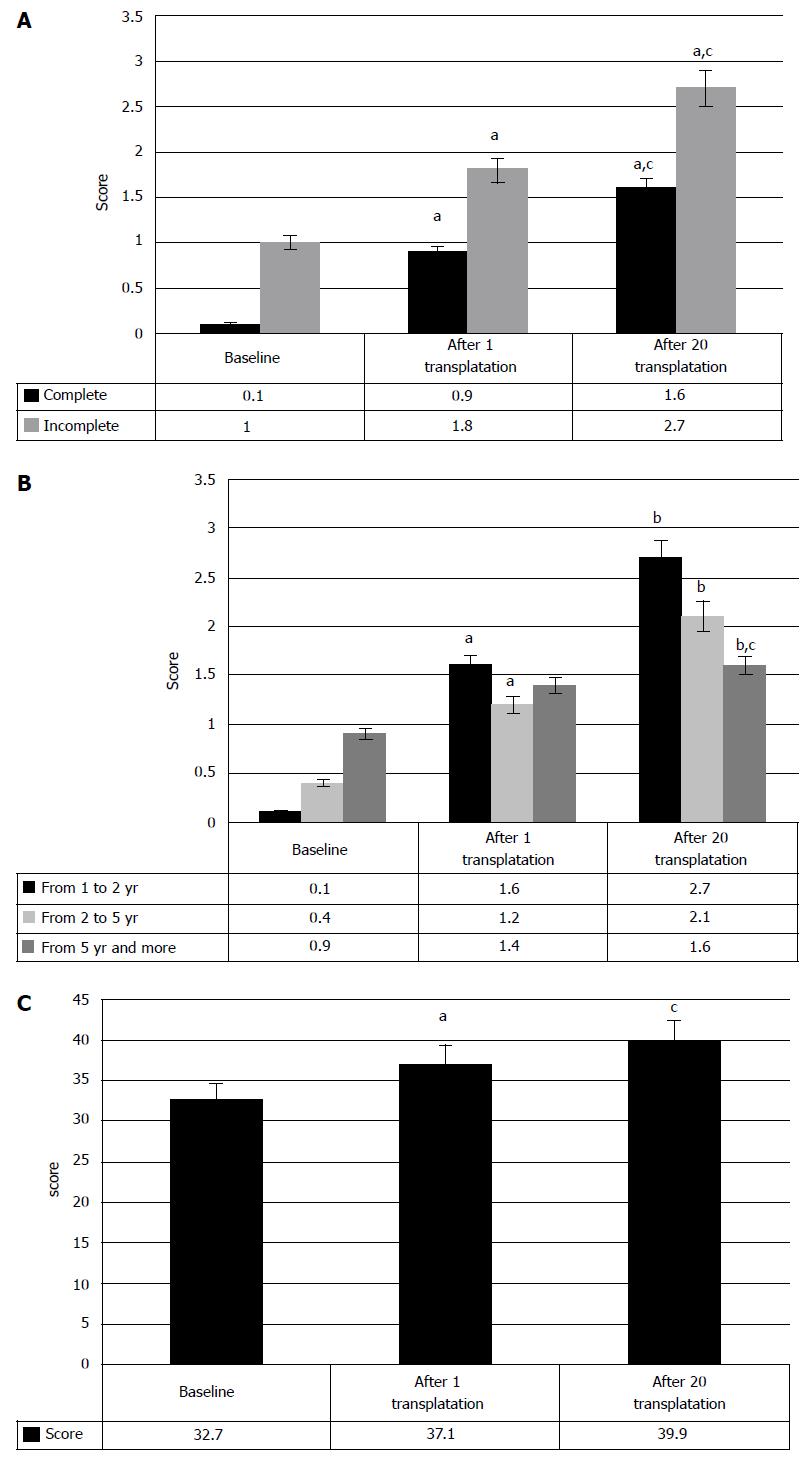
As expected, comparison of the results, depending on type of injury, showed better progress in the cases of incomplete SCI. Sixty percent of incomplete injury cases demonstrated improved locomotion, as compared to 46.7% of complete SCI cases (Figure 5). The patterns, identified at early period of the therapy, were fully confirmed 1-3 years post therapy beginning. They are supported by the changes of clinical condition in incomplete SCI cases, manifested in the increase of motor points from baseline 142.1 ± 5.7 to 150.5 ± 5.7 after the first transplantation, and 172.6 ± 8.1 after the second transplantation (P < 0.05) (Figure 5). In complete SCI cases neurologic improvements were minimal and made only 5 points after the first HSCs and HPs transplantation (P < 0.05). The tendency to improve to 81 ± 7.9 points was observed after the second transplantation (P < 0.1), which can be explained by the insignificant number of cases (n = 11); Due to different baseline scores of incomplete SCI and complete SCI cases, the comparison between the stages of therapy was done in percent and did not demonstrated significant difference in results after the first, or after the second, and even after the twentieth HSCs and HPs transplantations.
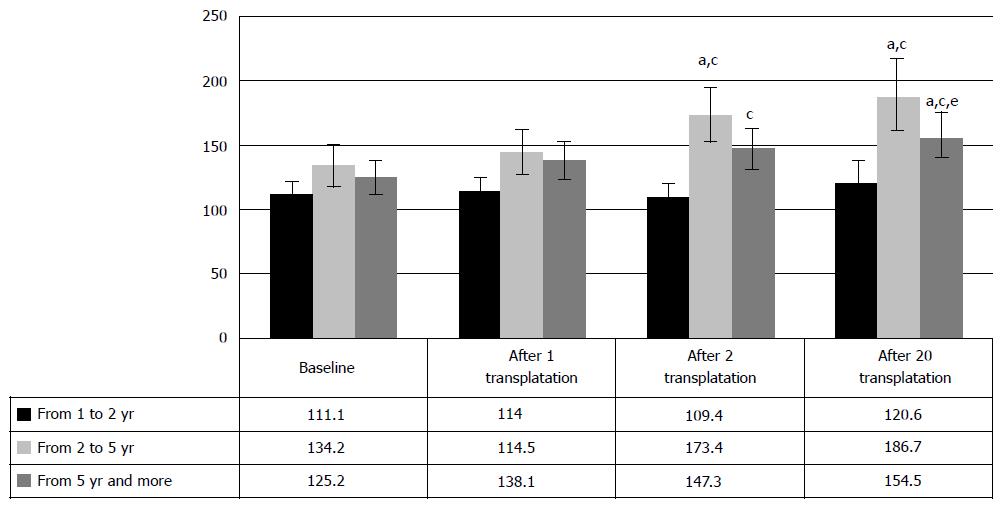
The increase of motor activity increase (Figure 6) after HSCs and HPs transplantation was observed only in the cases of 2-5 years post SCI; it was manifested in the motor activity increase from baseline 134.5 ± 7.3 points to 144.5 ± 8.6 points after the first transplantation and to 173.4 ± 10.7 after the second P < 0.05 between baseline and transplantations, respectively). Neither cases of 1-2 years post SCI, nor the cases over 5 years post injury showed statistically significant changes of clinical symptoms. These results seem to be conditioned by the inability of HSCs and HPs to realize their regeneration potential, due to residual inflammation and apoptosis in the patients with the period post SCI, varying from 1 to 2 years and due to degenerative changes in spinal cord in over 5 years old SCI cases. Still, regress of motor neurologic symptoms was observed in some of the patients with such SCI, so that in one of the cases the motor functions were considerably repaired 29 years post injury.
The Medical Research Council Scale was used to confirm the obtained results of motor progress after the HSCs and HPs transplantation in chronic SCI patients. The scale seems to be one of the most convenient and clear measurements of the strength of separate muscles, and originally was meant to detect locomotion deficit in the injuries of peripheral nerves. Total score for the absence of neurologic impairment makes 100 points.
As seen in Figure 7, the HSCs and HPs transplantation, accompanied by intensive rehabilitation, resulted in the increase of the muscle strength at all stages of research (P < 0.05). The second HSCs and HPs transplantation did not lead to muscle strength increase in damaged extremities. These data can be explained by insensitivity of the measurement tool to paresis improvements, the so called ceiling effect, that agrees with the data of Belova[11]. It is also confirmed by the analysis of muscle strength, the patients being distributed according to the level and type of injury (Figure 8). Strengthening of the muscles was observed in the cases of more severe injuries: at the level of cervical intumescence and with complete SCI.
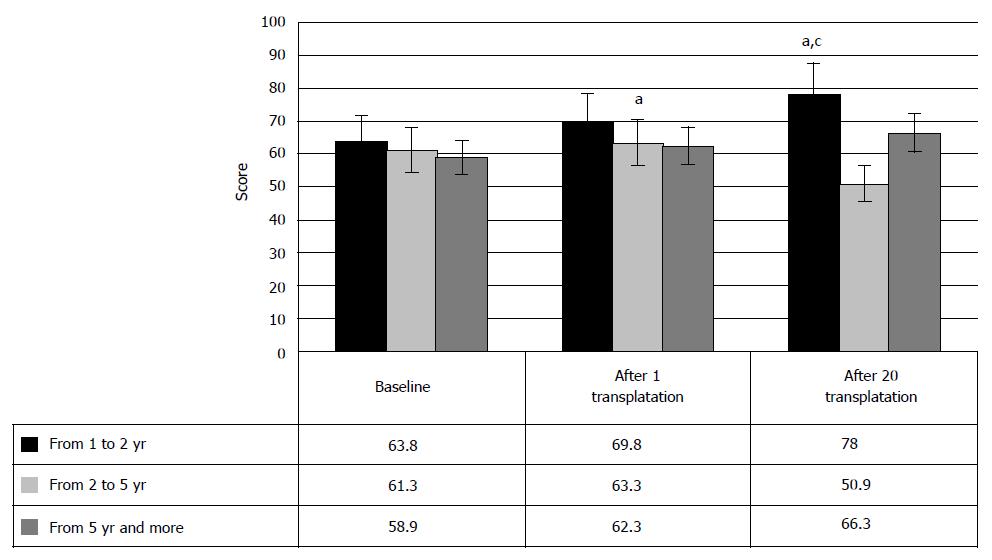
On the other hand, recovery of the muscle strength after HSCs and HPs transplantation repeated the pattern of the progress of motor functions, depending on the years post injury. This manifested in the slight score increase in the cases of 2-5 years post SCI after the first HSCs and HPs transplantation (from baseline 63.8 ± 4.6 points to 78 ± 7.1 points after HSCs and HPs transplantation, P < 0.05, respectively). However, after the second HSCs and HPs transplantation, muscle strength increase was registered only in the patients with 1-2 years old injury. The cases of over 5 years old SCI demonstrated no statistically valid increase of muscle strength, herewith, confirming the hypothesis of hindered motor restoration, due to degenerative changes in spinal cord in these cases. Hence, the changes in muscle strength, measured by Medical Research Council Scale, demonstrated improvement of locomotion after HSCs and HPs transplantation despite low sensitivity of the tool and consequent low increase of the score (Figure 9).
Sensation repair after the intrathecal transplantation of HSCs and HPs was evaluated in 71 patients by the assessment of neurologic condition that included 2-point tests of pain, temperature, deep sensation on dermatomes on both sides, as well as the assessment of the feeling of muscle “heaviness” in rest and after exercise in upper and lower extremities, abdomen and back. Total score, denoting absence of neurologic motor disorders, made 312 points.
As different from the locomotion, the repair of sensation was registered in a much fewer number of chronic SCI cases (Figure 10), the reason as yet remaining unclear. At the same time, the analysis of the obtained clinical data showed (Figure 11A) that the cell therapy led to the increase of sensitivity from baseline 124.3 points to 138.4 after the first and to 153.5 points after the second transplantations of HSCs and HPs (P < 0.05, between the stages of research).
Clinically, the repair manifested in the expansion of sensation areas, accompanied by gradual involvement of new dermatomes. Major part of the patients observed the elements of deep sensation after the first transplantation and characterized them as the “heaviness” of muscles in rest and after physical training. Further, it was noted that development of the feeling of the position of lower extremities in space preceded stabilization of knee joints and development of the first elements of walking.
Expansion of the areas of surface sensation did not depend on the level of injury, i.e., the sensation could manifest with separate dermatomes of lower and/or upper extremities, anterior chest or abdomen walls. In most of the cases the dermatomes did not restore in full, but only partially the sensation seldom restored unilaterally. Having received 5-7 HSCs and HPs transplantations, some of the patients restored sensation in all or almost all dermatomes of extremities and body. Hence, after the transplantation of HSCs and HPs, the sensation restores in chronic SCI cases, but in fewer cases than locomotion.
Case distribution, depending on the level or type of injury, demonstrated restoration of sensation in the most severe cases (complete SCI of cervical intumescence) (Figures 11B and C). These results are likely to be conditioned by low sensitivity of the measurement scale, i.e., “ceiling effect”. However, gradation of the sensation disorders was copied from widely applied measurement scales, including ISCSCI-92, and, hence, demonstrated the inefficiency of applied evaluation methods that demand upgrade.
No clinical changes were observed in the distribution of the cases, depending on the years post injury. This can be explained by lesser damage of posterolateral parts of spinal cord that agrees with the multiple data of pathomorphological tests. However, additional tests are necessary to confirm this hypothesis. Obtained clinical data of sensation repair were objectified with SSEP[12].
Efficacy of the rapier of bladder functions was evaluated in 72 patients with the assessment of neurologic condition that included 3-point assessment of the feeling of urination and 5-point assessment of urine retention. Total score that denotes absence of neurologic signs of urinary disorder is 8 points.
Repair of the urinary system was observed in 47.7% of the cases after the intrathecal transplantation of HSCs and HPs. Clinically, the restoration of urinary system manifested in creeping sensation in the body or unpleasant feelings in the lower abdomen that preceded involuntary urination, but complete syndrome of vegetative hyperreflexia was absent (changes in blood pressure and heart rate, arrhythmia, sweating, fever above the injury level). Many patients observed the feeling of weak “swelling” above pubic symphysis that allowed beginning of bladder training with closing urethral or cystostomic catheter. Further restoration of the capacity to retain urine for at least 1-3 min led to intermittent catheterization, or refusal from the cystostomy. In some cases, 3-5 intrathecal transplantations of HSCs and HPs resulted in full refusal from intermittent catheterizations and further complete repair of urinary function.
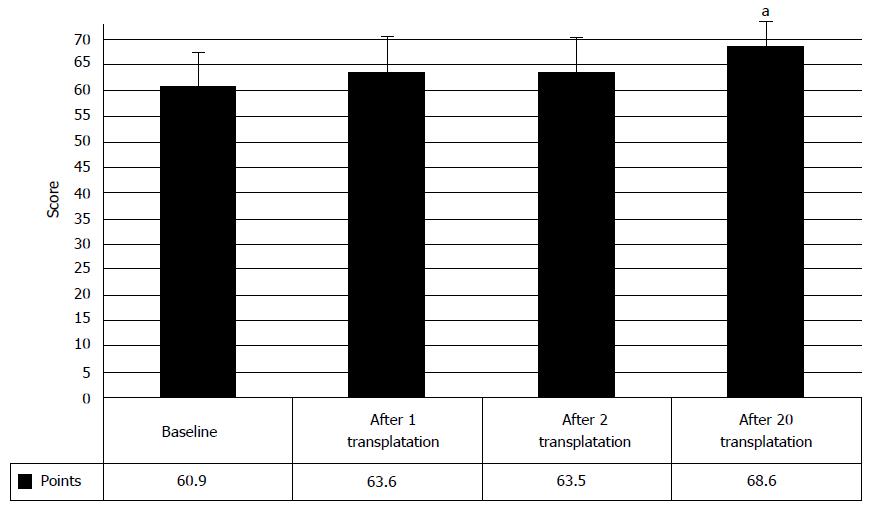
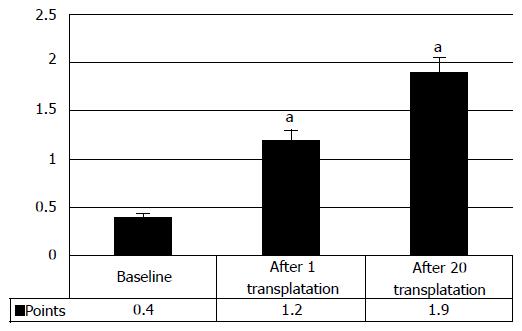
Analysis of the clinical data showed that in 33.8% cases the manifestations of urinary restoration began after the first transplantation of HSCs and HPs, showing clinical improvement from baseline 0.4 ± 0.2 points to 1.2 ± 0.2 points after the first transplantation of HSCs and HPs (P < 0.05) (Figure 12). Consequent transplantations improved the urinary function further, thus increasing the score to 1.9 ± 0.4 points.
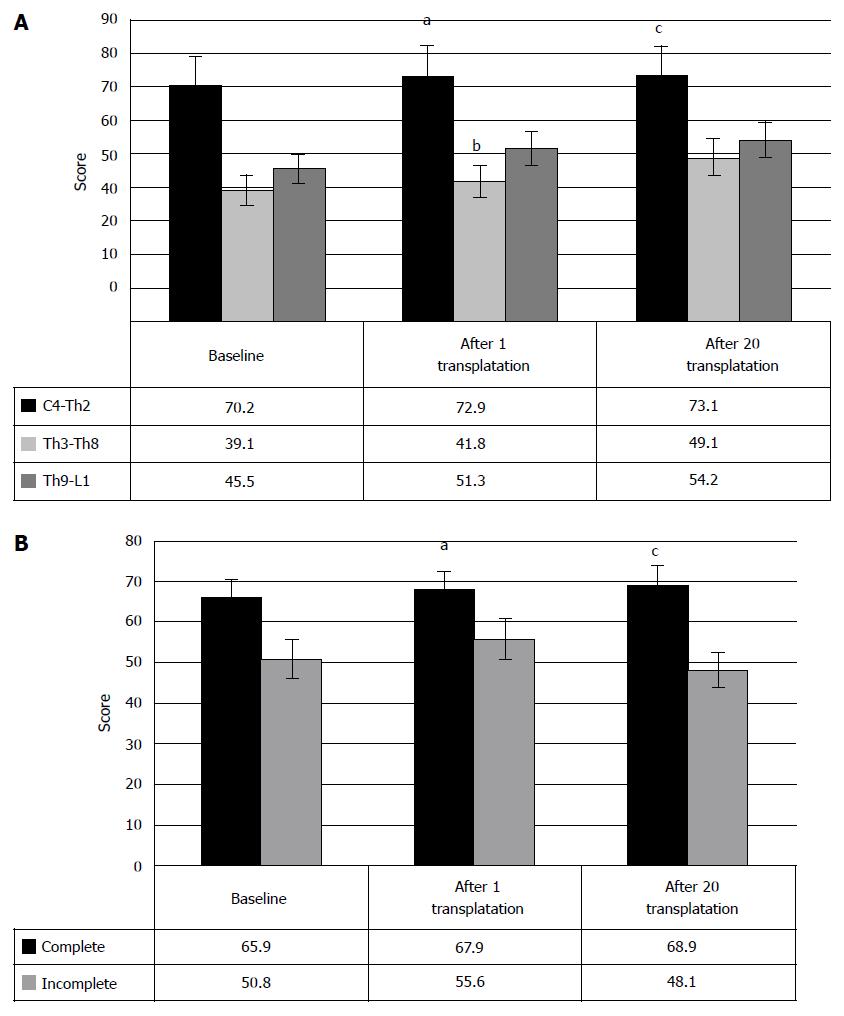
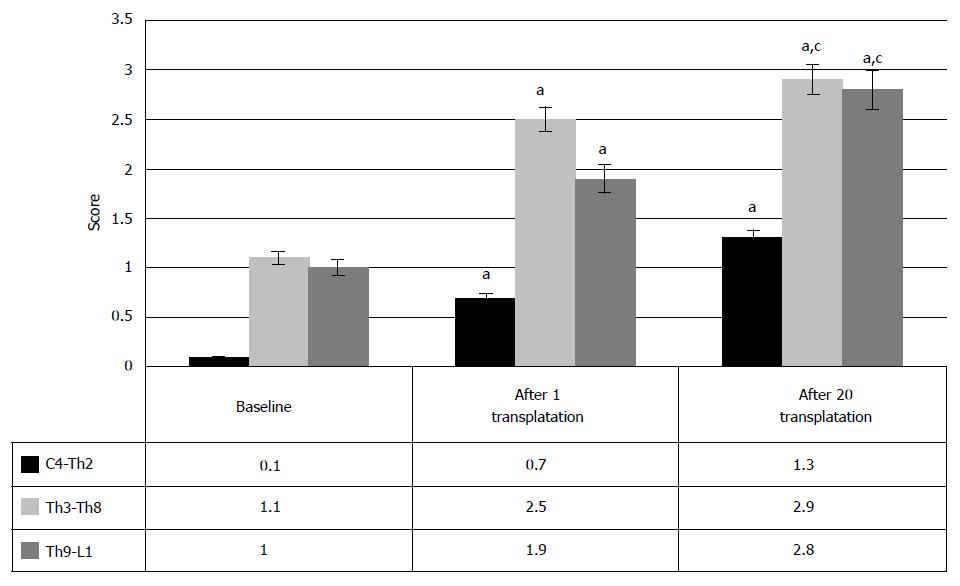
Hence, the transplantation of HSCs and HPs can lead to gradual restoration of urinary function in chronic SCI cases. Analysis of the data, depending on the level of injury (Figure 13), showed that largely, the improvement in the urinary system after HSCs and HPs transplantation was noted at Th3-Th8 level of SCI and at the level of lumbar enlargement (70%). It manifested in the increasing urinary restoration (Figure 13) from baseline 1.1 ± 0.8 points to 2.5 ± 0.8 points after the first transplantation and to 2.9 ± 0.9 points after the repeated HSCs and HPs transplantations (P < 0.05 between the therapy stages) in Th3-Th8 SCI cases. In SCI at the level of lumbar enlargement the urinary function changed from baseline 1 point to 1.9 and 2.8 after the first and the second transplantations, respectively (P < 0.05 between the therapy stages).
Despite fewer number of the SCI patients at the level of cervical intumescence, who showed the urinary system repair (36.8%), the restoration from baseline 0.1 points to 0.7 points and to 1.3 points was clinically registered after the first HSCs and HPs transplantation after the second HSCs and HPs transplantation, respectively (P < 0.05 between the therapy stages).
Thus, the urinary system after the intrathecal transplantation of the HSCs and HPs restores irrespective of the level of the SCI. However, the urinary system restores more efficiently in the cases of SCI at the level of Th3-Th8 and lumbar enlargement.
The repair of the urinary system after HSCs and HPs transplantation did not depend on the type of SCI, as shown in Figure 14A. However, in the cases of the incomplete SCI the urinary disorder at a baseline was less significant, as well as after the first transplantation. After the second transplantation, no statistically significant changes in the clinical evaluation of urinary system have been observed.
Restoration of the urinary system did not depend on period post injury, either. As seen in Figure 14B, some restoration of urinary function was observed irrespectively from years post injury. There is a clear tendency for further improvement of urinary function after 2 or 3 years of HSCs and HPs therapy, as compared to baseline.
Consequently, the intrathecal transplantation of HSCs and HPs in chronic SCI patients is an efficient method to repair urinary function. The lower levels of SCI are more prone to restore urinary function, which can be explained by closer location of urination centers in sacral spinal segments to lesion sites and, possibly, by larger concentration of HSCs and HPSs in the sites of injury. Herewith, neither the type of injury, nor years post injury, do not influence restoration of urinary function.
The functional repair of spinal cord was analyzed for 72 chronic SCI cases; it was measured with ASIA, ISCISCI-92 and FIM indexes at all stages of HSCs and HPSs transplantation. The evaluation with ASIA index demonstrated regress of neurologic symptoms only in 23 cases. Two patients with complete SCI (ASIA A) showed restoration of the locomotion below neurologic level of injury with muscle force of no less than 3 points (ASIA C) after HSCs and HPs transplantation, and over 3 points (1 patient, ASIA D). The ASIA B patient after HSCs and HPs transplantation showed neurologic restoration to ASIA C.
Nevertheless, the above shown analysis of clinical regress of neurologic symptoms demonstrates inefficiency of the ASIA impairment scale that was used to evaluate restoration of the spinal cord functions. On the one hand, it is associated with the specific features of restoration of spinal cord functions, and on the other, with low sensitivity of the index that gives only general estimation of regress of the neurologic damage. According to Belova[11] the ASIA index is applicable only for screening of the spinal functions during acute period of SCI. To evaluate neurologic progress in SCI, the more detailed characterization of locomotion, sensation and urination is required in every individual case.
As shown above, the sensation recovered after the manifestations of the restoration of motor functions analysis, especially in S4-S5 segments. The sensation restored mosaically, frequently after the development of passive or active movements, and involved the segments only partially. Absence of sensation in S4-S5 segments conditioned ASIA A level of impairment, even if motor functions of certain muscles below injury level were preserved to a certain extent. In this respect, 10 patients observed restoration of muscle force in most of the muscles below the level of injury that enabled their walking with assisting devices after 4-8 transplantations, while currently, two patients are able to cover short distances independently. However, only one of these patients demonstrated restoration of sensation in S4-S5 segments.
Hence, the ASIA impairment scale is effective to assess the degree of disability, but is ineffective, when used to assess the restoration of spinal cord functions in chronic SCI after HSCs and HPs transplantation.
Motor progress was also assessed with the ISCISCI-92 motor and sensory scores, and the data coincided with those received in evaluation of motor functions by the specifically developed scale. The number of the patients with the signs of locomotive repair was 56.9%. Moreover, the motor activity rates increased from baseline 32.7 points to 37.1 after the first transplantation and to 39.9 after repeated transplantation of HSCs and HPs (P < 0.05, at each stage of transplantation) (Figure 14C).
In spite of clinical restoration of sensation in 38.6% of the patients, the ISCISCI-92 scores did not confirm these data. This is conditioned by the absence of evaluation of deep sensation in ISCISCI-92, and, as noted before, by the “ceiling effect”, when the neurologic status changes within the partial restoration of sensation.
Hence, the assessment of motor restoration with the ISCISCI-92 scores demonstrated effectiveness of the HSCs and HPs transplantation in chronic SCI patients. The ISCISCI-92 score confirms the data of our specifically developed scale to assess the clinical motor restoration of spinal cord, thus, demonstrating its applicability in practice. The advantage of our evaluation scale of clinical motor restoration over the ISCISCI-92 lies in the multi-factor analysis of the motor activity, based on standard neurologic examination. Absence of changes in sensation as measured by ISCISCI-92 scores that, however, are accompanied by the clinical signs of restoration, demands development of new tools to measure changes both in surface sensation (touch and pain) and deep sensation. Despite partial solution of this issue in the specifically developed scale of clinical motor restoration, the “ceiling effect” was not overcome in partial restoration of this function.
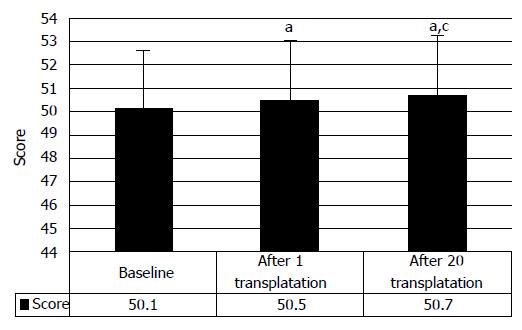
We would like to focus on the restoration of the functional independence after HSCs and HPs transplantation that was evaluated in 64 patients with the functional independence measurement (FIM) scale. The signs of the restoration of life activity was observed in 36.2% patients and were minimal (from baseline 50.1 points to 50.5 points after the first transplantation, and to 50.7 points after repeated HSCs and HPs transplantation; P < 0.05 at all stages of therapy, respectively) (Figure 15). It is associated with moderate restoration of the spinal functions after the first HSCs and HPs transplantations that manifested mostly in locomotion. However, as shown above, further transplantations resulted in more profound clinical progress. Besides, the FIM scale, when applied to chronic SCI cases has significant disadvantage: in the cases of considerable disorders of nerve impulse conductance, the FIM displayed very low sensitivity, due to absence of detailed functional evaluation. Accordingly, the analysis of the obtained data showed very slight improvement of the FIM scores, demonstrating improvement of the functional independence conditioned by the motor function of spinal cord.
During 12 years of follow up we observed no life threatening complications resulting from the HSCs and HPs transplantation. The complications of HSCs and HPs administration were evaluated at three stages: stage 1 after the first transplantation; stage 2 after one year of the therapy that included 5.3 ± 0.5 administrations; stage 3 two years of regular administrations (10.1 ± 1.1). The complications were summed up in Table 4. We observed one case of cancer (femoral carcinoma) of 202 followed up cases. However, according to the conclusion of the experts of the Russian Cancer Research Centre, it was not associated with HSCs and HPs transplantation. Surprisingly, in the control group of 20 cases we registered one case of spontaneous brain cancer development (pituitary adenoma) too, for which the patient was operated on.
| Symptoms | Stages of research | Control group patients | ||
| 1 stage | 2 stage | 3 stage | ||
| Increased spasticity | 46% | 49.9% | 54.5% | 0 |
| Fever | 15% | 19% | 18.8% | 0 |
| Post-puncture headache | 11% | 14.9% | 12.2% | 14.9% |
| High blood pressure | 10% | 8.1% | 14.5% | 0 |
| Coordination disturbance | 2.3% | 1.3% | 0 | 0 |
| Dizziness | 2% | 3.4% | 0 | 4.2% |
| Sleepiness | 2.1% | 1.7% | 0 | 0 |
| Emotional lability | 1.6% | 1.7% | 0 | 0 |
| Disordered consciousness | 1.2% | 0.8% | 0.8% | 0 |
| Meningism | 3.7% | 2.95% | 0.8% | 0 |
| Low blood pressure | 1.68% | 2.95% | 5.9% | 0 |
| General % of the patients with complications | 63.5% | 72.9% | 75% | 19.1% |
The therapy of SCI with autologous HSCs and HPs demonstrated high efficiency (to 95.1%) of stem (CD34+) peripheral cells mobilization in SCI patients. It is well known that under the conditions of undamaged hematopoiesis, the hematopoietic stem cells (HSCs) circulate in peripheral blood of a human. But their concentration is extremely low (less than 0.01%) that makes their detailed study and transplantation almost impossible. High concentration of HSCs results from the damage of hematopoiesis (usually as a result of chemotherapy), or administration of colony-stimulating factors (CSF). In the clinical practice, the granulocyte CSF (G-CSF) and granulocyte macrophage CSF (GM-CSF) are the most widely used. These factors increase the concentration of HSCs 100-1000 times, thus allowing the harvest of the cells and their use for transplantation. It should be noted that mobilization of the stem cells and precursor cells into peripheral blood vessels in the patients with traumatic disease of spinal cord was efficient in all cases - both absolute number and the percentage of CD34+ in leukoconcentrate received after 1 session of leukapheresis meet the transplantation standard of the number of mononuclears (> 2 × 106/kg).
As seen from the description, we applied the suspe nsion of autologous HSCs and HPs, and not a standard suspension of autologous HSCs (CD34+). We consider this cell suspension to reflect systemic specific response of bone marrow of each patient to the injury of the central nervous system, and the cell composition received in specific stimulation conditions and cryopreservation is unique and obligate. In case of stimulation of an SCI patient with G-CSF in the dose of 10 or 20 mg/kg for 5-6 d as it is recommended in the manuals of hematooncology, we harvest mature differentiated hematopoietic cells, able to restore hematopoiesis, and not injured nervous system. We applied the standard sparing scheme of simulation, which is ubiquitously used in pediatric oncology. This empirically selected mode of stimulation allowed us for new property of cell suspension that conditions its clinical effectiveness.
As seen from Table 3, we refused from cryopreservation with 10% DMSO with polyglycine, although the combination is considered ideal to protect HSCs. We applied lower concentration for cryopreservation, and, namely 5% DMSO and polyglycine that demonstrated its high efficiency and safety for intrathecal transplantation.
The stem cells and committed precursor cells form a so-called pool of HSCs. The expression of CD34 molecule on the surface of a membrane is common to all cells of the pool, and this property enabled use of flow cytometry methods to detect the precursor cells and to provide their quick count in any hematopoietic material. Last decades the peripheral blood was the main source of stem and precursor cells. Thus, for example, transplantation of separated fraction of mononuclear cells of peripheral blood with hematopoiesis stimulation permits considerable reduction of critical cytopenia in patients after high-dose chemotherapy. The phenomenon is conditioned by stem and precursor cells entering peripheral blood under colony stimulating factors influence. Special attention should be given to the composition of subpopulation of CD34+ cells, that is, the number of the cells of different compartments of HSCs and HPs pool.
Subpopulation composition of CD34+ cells was assessed by flow cytometry with triple-labeling method. Our analysis of efficiency of the suspension in SCI patients demonstrated that best motor restoration in SCI cases was observed only when the membrane of an autologous stem cell expressed gp130 protein. Gp130 is a transducing molecule of IL-6 cytokines and a receptor of cell functional condition. Basic pleiotropic action of these receptors is to contribute to cell differentiation, gene expression, stimulation or inhibition of cell growth and control of cell apoptosis. At day 4.5 and 5 of stimulation with G-CSF the abrupt decrease of gp130 expression was observed, which reduced activity of the cell preparation and therapy effect. The HSCs and HPs harvested according to standard protocol at day 6 of stimulation did not lead to any clinical effect. We received the pool of formed mononuclears with highly differentiated and well-diagnosed genuine hematopoietic and mesenchymal stem cells, therapeutic effect of which is disputable in our case. The cell suspension we use for therapy does not contain conventional HSCs, although they are assessed in CD34+ gate, when evaluated in flow cytometer, the suspension contains heterogeneous mixture of mobilized low-differentiated precursors, promoting regeneration of nervous system. In this technique the dose has no relevance and can considerably vary. The standard cell composition is of the key importance, reflecting the level and concentration of the output of non-differentiated PC at the proposed sparing G-CSF stimulation modes in the patients with post-traumatic neurologic deficit. The proposed individual preparation contains the mixture of highly efficient mobilized stem precursor cells of bone marrow, including hematopoietic-like cells. To date, we are unable to accurately identify what exactly type of cells of this pool make the treatment effective, but this seems unimportant for the patients and the clinical practice. We know that using the proposed method of harvest, we receive a standard cell preparation that gives a steady, reproducible and progressing clinical effect.
This preparation has no prototype, as well as the presence or absence of HSCs (CD34+) is not pivotal. Novelty of this preparation is determined by the presence of the mixture of non-differentiated cell precursors, restoring neurogenesis and regeneration in the damaged brain/spinal cord. The researches in cell medicine mention the facts of using HSCs to treat multiple sclerosis and amyotrophic lateral sclerosis. None of the authors used cryopreservation, they applied a single bolus injection of stem cell preparation. Our experience clearly demonstrated that only multiple and long-term (for 5-8 years) administration of the preparation will provide the maximal benefit of the existing regenerative potential of the cells and the opportunity to restore the damaged brain/spinal cord functions. It is the sparing 4 d long mode of G-CSF administration in the patients with neurologic deficit that provides for the harvest of all necessary nuclear cell precursors.
According to our evaluation of long-term outcomes of SCI cell therapy, the transplantation of autologous HSCs and HPs is an efficient method to repair lost functions in SCI patients, and it is not directly dependant on the dose and number of autologous HSCs and HPs transplantation. The patients with the lesion exceeding 50% of spinal cord cross-wise and 1 segment long-wise, and possibly those with moderate CSF circulation disorders should be excluded from therapy. Presumably, such patients require reconstructive surgical intervention with meningoradiculomyelolysis, spine stabilization and, possibly, tissue engineering of spinal cord. The HSCs and HPs transplantation only will hardly result in the restoration of spinal functions in these cases.
The rehabilitation is a requisite component in the therapy of chronic SCI patients.
Research of the SCI therapy demonstrated that to restore the functions, the conductance along various nervous pathways (pyramidal, extrapyramidal, spinothalamic, etc.) must be restored, and new synaptic links between injured segments of spinal cord must be established. Under these circumstances, the grey matter of spinal cord need not be replaced due to availability of cross innervation of dermatomes and myotomes in humans. Mere surgery and/or rehabilitation do not lead to the expected outcomes, as they do not eliminate the main cause of the disorder and do not restore injured neural structures of spinal cord. Application of the systems of adult stem and PCs confirmed the opportunity to restore spinal cord. The regulatory action of the mobilized progenitors, and not their regenerative potential, seem to be the main mechanism of functional restoration in SCI, activating synaptogenesis in adult brain, increasing plasticity of injured neural tissue of SC and developing functional neurophysiologic bypass. The intensity of HSCs and HPs regulatory potential depends on the size of SCI and directly proportional to intact neural structures of spinal cord.
The analysis of treatment efficiency depending on the level of injury deserves special attention. The reason for better clinical restoration at thoracic level seems to lie in morphological feature of the spinal cord structure and cervical and lumbar intumescence, where great number of neurons is located (second motor neurons, interneurons, etc.). The SCI at the level of intumescences leads to larger damage of spinal cell components and more intense pathologic processes; hence, the restoration in such cases of SCI is more difficult. The axons of motor neurons are located mostly at the thoracic level, the bodies of them are found in motor cortex of brain, and hence, less number of bodies of neural cells is involved into the injury. Restoration of the motor functions is associated with the increase of regeneration potential of the spinal cord, mainly at the level of cortical influence of HSCs and HPs on the intact bodies of motor neurons. The mechanism of HSCs and HPs effect does not seem to be associated with their differentiation into neurons and glial cells of SC. Most likely, the regulatory influence of HSCs and HPs at the site of SC injury leads to gene expression and secretion of neurotrophic factors, entailing growth and regeneration of axons in the site of injury and restoration of nerve impulse conductance along the intact but functionally inactive axons. As a result, the available ensembles of neurons are differentiated due to the development of new synaptic contacts below and above the injury site. The phenomenon is only observed when the stem cells are transplanted into the injured spinal cord, and it fully agrees with the data offered by Snyder[13]. The development of new synaptic links below and above injury level can serve as an explanation of the clinical results of motor restoration that we have observed.
The analysis of the obtained data indirectly confirms the hypothesis of HSCs and HPs influence on axonal growth in the site of injury or development of conductance along functionally inactive, but anatomically intact fibers, as it is the patients with incomplete injury, who demonstrate maximal restoration of motor functions. Consequently, incomplete SCI is prognostically more favorable for restoration of motor functions of spinal cord. However, to obtain representative results the clinical data have to be compared depending on the level of SCI. In the cases of complete SCI we observed intensively restoring functions, too.
The issue of termination of HSCs and HPs therapy of SCI remains important for us. Many patients, who have completed 3 year and 6 year courses, insist on continuation of the therapy. Their arguments are quite simple: “My own cells cannot hurt me and I see steadily increasing positive effect from them, so it is harmless to continue the therapy”. To date, 15 patients received HSCs and HPs transplantation for 8 years on a regular basis and no negative effects have been observed either at the level of clinical picture, or at the level of thorough paraclinical examination.
Summing up, we can conclude that the method is safe, effective and considerably improves the life quality of SCI patients. Administration of the autologous cell systems of hematopoiesis precursors led to real restoration of various movements and improved life quality in major part of our patients. About 15 patients are able to walk independently or with supporting devices, over half of them restored sensation of different types and the function of the bowel and bladder. The therapy was approved for clinical use as the treatment of choice. In terms of the long-term clinical outcomes, we can discuss complexity of the processes, observed in the central nervous system after SCI and under HSCs and HPs therapy, which are often hard to explain from clinical point of view. Being limited by the size of journal article, we are unable to demonstrate the whole range of long-term neurophysiologic and urodynamic paraclinical results, and their correlations with the mentioned clinical data, but we would be happy to offer them in our other works.
Contemporary healthcare have greatly improved the survival rates in spinal cord injuries (SCI) cases as well as their life expectancy, leading to the overall growth of the national economic burden. However, current healthcare advances have not led to any breakthrough in restoration of the functions of spinal cord after the injury, and ever since the Edwin Smith Papyrus the SCI has been classified as the ailment not to be treated. To date SCI is a verdict that entails impossibility to return to previous way of life, to restore previous working capacity and reproductive functions, resulting in tremendous social and economic losses. Inefficiency of the available SCI therapies used to be explained by the absence of the regeneration potential in adults, and the restoration of the damaged neural cells has been demonstrated only recently.
By now, the first steps to develop new restorative therapy of SCI have been made, and the cell transplantation is the most obvious choice, although no universally acknowledged methods to restore spinal cord after the injury are observed. The methods of transplantation and the types of cells significantly vary; the evidence gathered is mostly limited by a one or two years follow up. Being involved into stem cell transplantation for SCI for about 25 years in research and in clinical practice we have accumulated substantial experience of achievements and failures in stem cell therapy. In the current work, the authors describe the cell therapy that proved the safest and the most effective both in the short-term period and in long-term follow-up.
The method implies multiple long-term transplantations of the preparation of hematopoietic stem cells and hematopoietic precursors that was harvested from peripheral blood after sparing mode of administration of granulocyte colony-stimulating factor. The composition of the applied preparation is characterized. The cells are administered intrathecally in a subarachnoid space every three months and the transplantation is followed by vigorous specialized rehabilitation. The effects are evaluated by conventional indexes and tests, including somatosensory evoked potentials tests and urodynamic tests, as well as by specifically developed scales. The effects of 20 consecutive transplantations for each case are measured.
The method is safe, effective and is applicable to chronic SCI cases when no further restoration of the functions is observed. It considerably improves the life quality of the SCI patients. The method received official approval in the Russian Federation in 2005 and in 2006 and is recommended as the therapy of choice.
Intrathecal transplantation means the infusion of the cells in the subarachnoid space in the course of lumbar puncture.
This is an important manuscript describing the clinical outcome of cellular therapy for spinal cord injury.
P- Reviewer: Liu L, Tanabe S S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 543] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 2. | National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2014;37:243-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Noonan VK, Fingas M, Farry A, Baxter D, Singh A, Fehlings MG, Dvorak MF. Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology. 2012;38:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord. 1997;35:809-813. [PubMed] |
| 5. | Raisman G. Sniffing out new approaches to spinal cord repair. Nat Med. 2000;6:382-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Huang H, Chen L, Sanberg P. Cell Therapy From Bench to Bedside Translation in CNS Neurorestoratology Era. Cell Med. 2010;1:15-46. [PubMed] |
| 7. | Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G, Maia CA, Capucho C, Hasse-Ferreira A, Peduzzi JD. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. 2010;24:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Zorin VL, Cherkasov VR, Zorina AI, Deev RV. The Characteristics of World Market Cell Technologies. Kletochnaya Transplantologiya i tkanevaya engineeriya. 2010;3:96-115 (in Russian). |
| 9. | Bryukhovetskiy AS. Transplantatsiya nervnikh kletok i tkanevaya engineeriya mozga pri nervnikh bolezniakh [Transplantation of nerve cells and tissue engineering of brain in nerve diseases]. Moscow, ZAO Neurovita, Neurovita, 2003: 1-400 (in Russian). . |
| 10. | Bryukhovetskiy AS. Travma spinnogo mozga: kletochniye tekhnologii v lechenii i reabilitatsii [Spinal cord injury: Cellular technologies in the treatment and rehabilitation]. Moscow, Prakticheskaya meditsina, 2010: 1-341 (in Russian). . |
| 11. | Belova AN. Neiroreabilitatsiya: Rukovodstvo dlya vrachei [Neurorehabilitation: A Manual for Physicians]. Moscow: Antiodor 2000; 1-736 (in Russian). |
| 12. | Frolov AA, Bryukhovetskiy AS. Effects of hematopoietic autologous stem cell transplantation to the chronically injured human spinal cord evaluated by motor and somatosensory evoked potentials methods. Cell Transplant. 2012;21 Suppl 1:S49-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Snyder E. Neural stem cells: Developmental insights may suggest therapeutic options. Proceedings of the 7th International Congress of the Cell Transplant Society; 2004, November 17-20; Boston, MA, USA, 2004: 53. . |