Published online Jun 24, 2014. doi: 10.5500/wjt.v4.i2.40
Revised: April 9, 2014
Accepted: April 16, 2014
Published online: June 24, 2014
Processing time: 234 Days and 0.3 Hours
Solid organ transplantation is limited by suitable donor organ availability and the geographic limitations that lead to prolonged ischemic times. Ex vivo organ perfusion is an evolving technology that enables assessment of organ function prior to transplantation. As a byproduct, overall out of body organ times are able to be extended. The future implications organ assessment and repair centers utilizing this technology are discussed.
Core tip: Regional organ assessment and repair centers will build upon normo-thermic ex vivo organ perfusion technology, which in turn provides a potential platform to assess, repair and eventually modify donor organs.
- Citation: Whitson BA, Black SM. Organ assessment and repair centers: The future of transplantation is near. World J Transplant 2014; 4(2): 40-42
- URL: https://www.wjgnet.com/2220-3230/full/v4/i2/40.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i2.40
Solid organ transplantation is currently limited by an inadequate number of donor organs and the inability to optimally evaluate and assess those organs prior to transplantation. The lack of donor organs has led to many innovative strategies to increase the number of donor organs available for transplantation. Split organ transplantation (liver), living donor transplantation and the use of marginal or extended criteria donor organs has had a modest effect on organ availability. The organ utilization rate for many donor organ types (i.e., lung) remains low and the benefits of increasing the donor pool are not fully realized. From the initial pioneering work in kidney transplantation by Belzer[1], successful outcomes tended to depend on short ischemic time and good organ quality. Techniques in rapid procurement, implantation and advances in organ preservation were crucial to development of the field of transplantation. Out of the pioneering work of F.O. Belzer, ex vivo kidney perfusion circuits were conceptualized and then utilized with impressive results. Alexis Carrel and Charles Lindberg had telegraphed this possibility with their prescient work in 1935 “The Culture of Organs”[2]. With current advancements in perfusion technology, molecular biology and biomedical engineering the next evolution in organ transplantation will be regional organ assessment and repair centers (ARCs)[3].
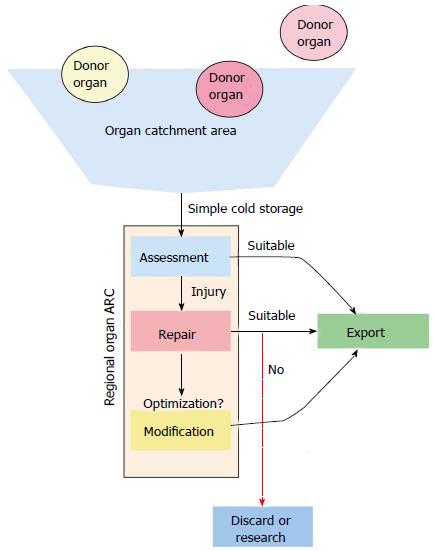
Regional organ ARCs build upon normo-thermic ex vivo organ perfusion (EVOP) technology, which in turn provides a potential platform to assess, repair and eventually modify donor organs. Currently, normo-thermic EVOP allows for assessment of organ function prior to transplantation and is largely focused clinically on lung transplantation. Normo-thermic EVOP allows for potential donor organs to have their function evaluated and the suitability for transplantation assessed[4,5]. Simple cold storage and hypothermic machine preservation, while appreciably lowering the metabolic rate and increasing preservation times do not allow for optimal assessment of an individual organ. Most of the determinations regarding an organ’s function are made pre-procurement and while hypothermia decreases the metabolic rate of the donor organ to 5%-10% of normal, significant anaerobic metabolism continues to take place. Prolonged preservation and marginal donor organ quality can lead to significant delayed graft function or graft non-function in the recipient[3]. At present, there is a multi-institutional clinical trial in the United States to assess the safety and efficacy of ex vivo ung perfusion (EVLP) in clinical transplantation. What’s more, EVLP is used clinically in Canada[4], Australia, and Europe. Ex vivo liver perfusion likewise is in a clinical trial in Europe to assess the feasibility of this approach. A beneficial side effect of EVOP is that organ ischemic times and thus distance between center, donor, and recipient, can be significantly extended.
The potential of ex vivo technology was recently illustrated by the work of Wigfield et al[6] where a marginal lung donor organ was transported from a donor center internationally to the organ repair center at the University of Toronto Lung Transplant Program and then back internationally to the recipient center[6]. This process extended the ischemic time to 15 h and 20 min. In essence, this is the index case for conceptualizing and operationalizing a regional organ ARC approach.
One could envision a time where EVOP would be used as a platform for assessment, repair, and modification of all organs. However, the logistic and financial feasibility of such an approach would likely only allow this technology to be employed when the organs are of marginal quality, of extended donor criteria, or when the best tissue match is in a geographically distinct area.
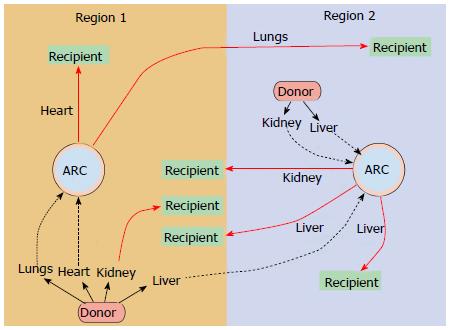
Each specific organ type has a unique function and as such has a unique set of metabolic demands and metrics for assessing viability. With this approach, organs would optimally be assessed and evaluated in a regional ARC (Figure 1). Organs of all types (heart, lung, liver, kidney, intestine and pancreas) that are in geographic proximity to the ARC would be procured and prepared with standard cold static storage. The organs would be transported to the ARC. At the ARC a thorough evaluation of the donor organ takes place, with appropriate repair or regenerative measures employed based on the deficits in function encountered. If the donor organs are deemed to be suitable (or can be made suitable) for transplantation, the organs would then be allocated in a national or international fashion to the most suitable recipient (Figure 2). This more broad, detailed, and individualized matching is only possible with the extended preservation (total ischemic time) that EVOP allows. This ability to match more thoroughly and to repair or resuscitate marginal donor organs would potentially improve graft survival and long-term outcomes.
The impact on organ transplant waitlists will be enormous. Even modest increases in organ availability will markedly increase the total number of transplants performed. For example, in lung transplantation in the United States alone, increasing the overall conversion rate from 17%[7] to 32% (an absolute increase of only 15% more organs transplanted) would practically double the number of transplants performed and eliminates the waiting list. EVLP will have significant impacts by saving the lives of recipients on the waiting list, extending the opportunity for a life-saving and life-extending transplant to patients who currently are not ill enough, and reduce the mortality in transplant recipients.
As the science and technique of organ perfusion and preservation progress, we have the expectation that in the very near future EVOP and regional organ ARCs will serve as the platform for organ repair and modification[8]. Ex vivo perfusion technology has already been used to repair injured livers and lungs prior to transplantation[4-6,8,9]. In livers, bile duct injury can be mitigated[9] and steatotic livers are able to be de-fatted and thus improve overall organ quality and function[9]. In the lung, edema may be removed, infection cleared, and gas exchange improved[4].
By any measure, organ transplantation is one of the success stories of modern medicine. We will look back on EVOP and the development of regional organ ARCs as a similar milestone where the multidisciplinary approach to a complex problem has allowed innovation to propel our science to new frontiers in the treating end-stage organ disease.
P- Reviewers: Dosanjh A, Laubach VE, Nosotti M S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Belzer FO. Organ preservation: A personal perspective. History of Transplantation. Thirty-five Recollections: UCLA Tissue Typing Laboratory 1991; 595-614. |
| 2. | Carrel A, Lindbergh CA. The Culture of Whole Organs. Science. 1935;81:621-623. [RCA] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Black SM, Whitson BA. Regional Organ Assessment and Repair Centers (ARC’s). IJMBS. 2013;5:243-246. |
| 4. | Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, Sato M, Laratta J, Azad S, Madonik M. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 815] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 5. | Knaak JM, Spetzler VN, Selzner N, Selzner M. Normothermic machine perfusion of discarded liver grafts-what is viable? Am J Transplant. 2013;13:2503. [PubMed] |
| 6. | Wigfield CH, Cypel M, Yeung J, Waddell T, Alex C, Johnson C, Keshavjee S, Love RB. Successful emergent lung transplantation after remote ex vivo perfusion optimization and transportation of donor lungs. Am J Transplant. 2012;12:2838-2844. [PubMed] |
| 7. | Punch JD, Hayes DH, LaPorte FB, McBride V, Seely MS. Organ donation and utilization in the United States, 1996-2005. Am J Transplant. 2007;7:1327-1338. [PubMed] |
| 8. | Yeung JC, Wagnetz D, Cypel M, Rubacha M, Koike T, Chun YM, Hu J, Waddell TK, Hwang DM, Liu M. Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig. Mol Ther. 2012;20:1204-1211. [PubMed] |
| 9. | Boehnert MU, Yeung JC, Bazerbachi F, Knaak JM, Selzner N, McGilvray ID, Rotstein OD, Adeyi OA, Kandel SM, Rogalla P. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death. Am J Transplant. 2013;13:1441-1449. [PubMed] |