Published online Dec 24, 2013. doi: 10.5500/wjt.v3.i4.127
Revised: June 4, 2013
Accepted: July 18, 2013
Published online: December 24, 2013
Processing time: 291 Days and 16.3 Hours
AIM: To investigate patient and graft outcomes in isolated small bowel transplant (SBTx) recipients and immunosuppressant induction agent impact on outcomes.
METHODS: A retrospective review of the perioperative data of patients who underwent SBTx transplant during an 8-year period was conducted. The intraoperative data were: patient demographics, etiology of short gut syndrome, hemodynamic parameters, coagulation profiles, intraoperative fluid and blood products transfused, and development of post-reperfusion. The postoperative data were: hospital/intensive care unit stays, duration of mechanical ventilation, postoperative incidence of acute kidney injury, and 1-year patient and graft outcomes. The effects of the three immunosuppressant induction agents (Zenapax, Thymoglobulin, Campath) on patient and graft outcomes were reviewed.
RESULTS: During the 8-year period there were 77 patients; 1-year patient and graft survival were 95% and 86% respectively. Sixteen patients received Zenapax, 22 received Thymoglobulin, and 39 received Campath without effects on patient or graft survival (P = 0.90, P =
0.14, respectively). The use of different immune induction agents did not affect the incidence of rejection and infection during the first 90 postoperative days (P = 0.072, P = 0.29, respectively). The Zenapax group received more intraoperative fluid and blood products and were coagulopathic at the end of surgery. Zenapax and Thymoglobulin significantly increased serum creatinine at 48 h (P = 0.023) and 1 wk (P = 0.001) post-transplant, but none developed renal failure or required dialysis at the end of the first year.
CONCLUSION: One-year patient and graft survival were 95% and 86%, respectively. The use of different immunosuppressant induction agents may affect the intraoperative course and short-term postoperative morbidities, but not 1-year patient and graft outcomes.
Core tip: Small bowel transplant (SBTx) is the treatment of choice for patients with intestinal failure. However, patient and graft survival can be affected by multiple factors, such as the choice of immunosuppressant and immune induction agent. Studying the effects of these agents may help care providers customize the immunosuppressant protocol to the individual patient. In this study, we reviewed in great detail how different immune induction agents can impact the intraoperative and postoperative course, as well as the short term outcome of these patients. Such information can be of great value to physicians who treat SBTx recipients.
- Citation: Hilmi IA, Planinsic RM, Nicolau-Raducu R, Damian D, Al-Khafaji A, Sakai T, Abu-Elmagd K. Isolated small bowel transplantation outcomes and the impact of immunosuppressants: Experience of a single transplant center. World J Transplant 2013; 3(4): 127-133
- URL: https://www.wjgnet.com/2220-3230/full/v3/i4/127.htm
- DOI: https://dx.doi.org/10.5500/wjt.v3.i4.127
Isolated small bowel transplant (SBTx) is a quickly-growing curative procedure for patients with short gut syndrome (SGS)[1]. Improvements in surgical techniques, immunosuppressant drugs, and anesthetic management[2] have resulted in great improvement of patient outcome. In this retrospective single-center study, we reviewed the medical records of 77 consecutive patients who underwent first time isolated SBTx. Patient and graft outcome, along with the effects of different immunosuppressant induction agents, are reported.
After Institutional Review Board approval, the medical records of 77 consecutive adult patients who underwent first time isolated SBTx during an 8-year period (April 2000-June 2007) were reviewed. Preoperative data included patient demographics, etiology of SGS, and renal function status. Intraoperative hemodynamic data were recorded at six time points during the surgery: I (baseline before the surgical incision), I+60 (60 min after the surgical incision), II (completion of enterectomy/dissection), III+5 (5 min post-reperfusion), III+60 (60 min post-reperfusion), and IV (at completion of surgery). These data were: heart rate, mean arterial blood pressure (MABP), central venous pressure, pulmonary artery wedge pressure, mean pulmonary artery pressure, cardiac output (CO), systemic and pulmonary vascular resistance (SVR, PVR), end-diastolic volume, right ventricular ejection fraction, and mixed venous oxygen saturation. At each of these six time points, a thromboelastogram (TEG) was performed and the results were recorded and analyzed.
In order to investigate the severity of the post reperfusion syndrome (PRS) in SBTx, patients were divided into two groups according to PRS severity: patients with mild PRS and patients with significant PRS[3]. PRS was defined as mild when the decrease in blood pressure and/or HR was < 30% baseline, was short-lived (≤ 5 min), and responded to the administration of small doses of vasopressors [calcium chloride (1 g IV) and/or epinephrine (≤ 100 μg) IV] without requiring continuous infusion of these vasopressors during the remaining transplantation procedure. PRS was defined as significant when severe hemodynamic instability occurred, such as persistent hypotension (> 30% baseline), asystole or hemodynamically significant arrhythmias requiring intraoperative infusion of vasopressors, and fibrinolysis that required treatment with antifibrinolytic agents.
Patients received one of three immunosuppressant induction agents: Zenapax, Campath, or Thymoglobulin; the effects of these agents on patient and graft outcomes were reviewed. As part of the immunosuppressant regimen, all patients received 1 g methylprednisolone before reperfusion of the graft and postoperative tacrolimus.
Postoperative data collected were: hospital/intensive care unit (ICU) stay, days requiring postoperative mechanical ventilation, postoperative incidence of acute kidney injury (AKI), incidence of infection and rejection within the first 90 postoperative days, and 1-year patient and graft outcomes. To document the presence of post-transplant infection, we utilized the International Sepsis Forum Consensus Conference definition of infection in the ICU[4]. AKI was defined by modified RIFLE (risk, injury, failure, loss, end-stage renal disease) criteria as recommended by the Kidney Disease Improving Global Outcomes (KDIGO) AKI guideline, but without urine output data. According to the KDIGO, AKI was defined as a 50% increase in SCr from the baseline (preoperative value as in our study) or a 0.3 mg/dL increase within 48 h[5-7].
The statistical analysis was performed using SPSS Statistics 17.0 software (SSPS Inc., Chicago, IL). Descriptive statistics were reported as mean ± SD. For categorical data, the χ2 test or Fisher exact test were used and for normally distributed continuous variable data, the paired t-test or ANOVA were used. For analysis of the continuous variables that were not normally distributed, the Kruskal Wallis test was used and the median and range were reported. For patient and graft 1-year survival, Kaplan-Meier survival analysis was used.
Seventy-seven patients received an isolated SBTx during the 8-year study period. Sixteen patients received Zenapax, 22 received Thymoglobulin, and 39 received Campath. Patient demographics were: age (range 28-66 years old, mean of 40), more females than males (26/51) with P = 0.02. Etiologies of SGS were: volvulus in 11 patients, vascular in 24, inflammatory in 20, trauma and adhesion in 10, radiation in four, and miscellaneous in eight. Intraoperative data showed that patients who received Zenapax had significantly longer surgical times (P = 0.0001), longer cold ischemia times (P = 0.05), and required more crystalloid (P = 0.002), more colloid (P = 0.00001), and packed red cells (P = 0.0008) (Table 1). The intraoperative coagulation profile (Table 2) as monitored by TEG showed no significant differences between the groups until the completion of surgery. At that point, the Zenapax group had a longer R-time (P = 0.011) and K-time (P = 0.0001), indicating less coagulability. Significant changes were found in almost all hemodynamic parameters during the reperfusion phase when compared to the baseline readings (Table 3). These changes were reflected in a drop in the SVR (P = 0.0001), increase in CO (P = 0.0001), and decrease in MABP (P = 0.0001); however, these changes in hemodynamic parameters were considered as mild PRS according to our definition. There was a trend toward more hemodynamic instability in the Zenapax group, but it did not reach statistical significance (P < 0.065).
| Zenapax (n = 16) | Thymoglobulin (n = 22) | Campath (n = 39) | P value | |
| Surgical time (h) | 14.16 ± 2.59 | 12.01 ± 1.73 | 10.59 ± 1.59 | < 0.000 |
| Cold ischemia time (min) | 514.0 ± 86.73 | 480.18 ± 111.60 | 441.0 ± 100.33 | 0.05 |
| Warm ischemia time (min) | 31.18 ± 5.19 | 30.95 ± 4.94 | 32.18 ± 4.62 | 0.636 |
| Crystalloids (mL) | 7214.67 ± 2286.39 | 5622.73 ± 1707.95 | 4705.26 ± 1791.26 | 0.0002 |
| Colloids (mL) | 6383.33 ± 2277.34 | 4428.64 ± 1611.94 | 3721.05 ± 1516.51 | < 0.0001 |
| Packed red cell (units) | 6.47 ± 3.44 | 3.45 ± 1.95 | 3.66 ± 2.39 | 0.0008 |
| Fresh frozen plasma (units) | 0.73 ± 1.71 | 0.36 ± 1.05 | 1 ± 1.61 | 0.208 |
| Cryoprecipitate (units) | 0 | 0.27 ± 1.27 | 1.58 ± 3.32 | 0.377 |
| Platelets (units) | 1.20 ± 3.36 | 4.36 ± 8.52 | 3.95 ± 6.58 | 0.541 |
| Zenapax(n = 16) | Thymoglobulin(n = 22) | Campath(n = 39) | P value | |
| Stage-I (baseline) | ||||
| R I | 9.26 ± 3.6 | 9.09 ± 3.88 | 7.36 ± 4.35 | 0.195 |
| K I | 2.72 ± 0.91 | 2.73 ± 0.97 | 2.21 ± 2.96 | 0.630 |
| ANG I | 71.64 ± 5.46 | 65.9 ± 14.1 | 66.1 ± 12.25 | 0.291 |
| MA I | 65.11 ± 11.1 | 59.53 ± 14.56 | 66.04 ± 13.72 | 0.223 |
| Stage-II (organ on the field) | ||||
| R II | 9.12 ± 5.23 | 7.36 ± 3.17 | 7.15 ± 3.54 | 0.327 |
| K II | 4.26 ± 1.53 | 3.99 ± 2.1 | 3.10 ± 2.12 | 0.176 |
| ANG II | 64.25 ± 8.26 | 60.82 ± 11.66 | 55 ± 15.72 | 0.109 |
| MA II | 53.58 ± 10.42 | 53.71 ± 9.7 | 54.44 ± 15.16 | 0.974 |
| Stage-III (reperfusion) | ||||
| R III | 9.3 ± 2.7 | 7.44 ± 2.53 | 7.74 ± 3.4 | 0.356 |
| K III | 6.13 ± 2.56 | 4.77 ± 2.1 | 3.9 ± 2.54 | 0.088 |
| ANG III | 56.13 ± 9.23 | 58.6 ± 7.36 | 49.2 ± 15.57 | 0.072 |
| MA III | 47.56 ± 12.5 | 50.7 ± 10.67 | 48.67 ± 16.1 | 0.855 |
| Closing the abdominal incision (final) | ||||
| R end | 9.26 ± 2.58 | 8.77 ± 3.39 | 6.38 ± 2.31 | 0.011a |
| K end | 6.21 ± 2.83 | 5.13 ± 2.1 | 2.79 ± 1.62 | < 0.0001a |
| ANGe nd | 56.75 ± 12.8 | 57.47 ± 7.6 | 58.1 ± 11.13 | 0.949 |
| MA end | 50.56 ± 8.51 | 51.32 ± 11.76 | 55.46 ± 9.23 | 0.339 |
| Baseline | Reperfusion | P value | |
| Cardiac output (L/min) | 7.32 ± 1.88 | 9.1 ± 2.62 | < 0.0001 |
| Ejection fraction (%) | 38.14 ± 7.78 | 39.75 ± 7.76 | 0.183 |
| End diastolic volume (mL) | 216.24 ± 55.82 | 242.34 ± 64.54 | < 0.0001 |
| Mixed venous oxygen saturation | 83.80 ± 3.99 | 85.17 ± 4.37 | 0.017 |
| Heart rate (beat/min) | 89.81 ± 13.49 | 101.41 ± 13.71 | < 0.0001 |
| Mean arterial blood pressure | 77.71 ± 9.47 | 71.29 ± 11.64 | < 0.0001 |
| Systemic vascular resistance | 793.76 ± 244.67 | 581.92 ± 196.8 | < 0.0001 |
| Mean pulmonary artery pressure | 17.52 ± 3.82 | 20.17 ± 4.8 | < 0.0001 |
| Pulmonary vascular resistance | 58.07 ± 26.32 | 52.13 ± 24.76 | 0.078 |
| Pulmonary capillary wedge pressure | 12.42 ± 3.44 | 14.82 ± 3.8 | < 0.0001 |
| Central venous pressure | 9.53 ± 3.63 | 11.42 ± 3.46 | < 0.0001 |
Changes in postoperative SCr and the effects of the different immune induction agents showed a significant increase in SCr in patients who received Zenapax (P = 0.023) and Thymoglobulin (P = 0.001). Overall, 15 patients developed AKI (19.5%) during the first 72 h post-transplant, which increased to 31.2% (24 patients) after completion of the first postoperative week, but none progressed to renal failure or required dialysis at any time during the first post-transplant year. Classical outcome criteria showed a mean ICU stay of 5 d (range 4-62 d), mean hospital stay of 26 d (range 7-89 d), and mean time on ventilator of 2 d (range 1-95 d). Infection within the first 90 postoperative days was reported in 24 patients with no prevalence among immune induction agent used (P = 0.29).
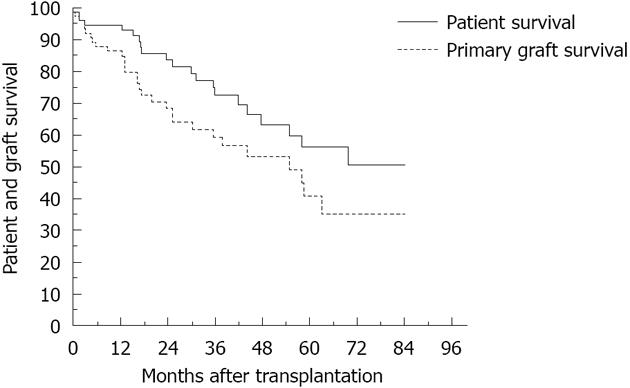
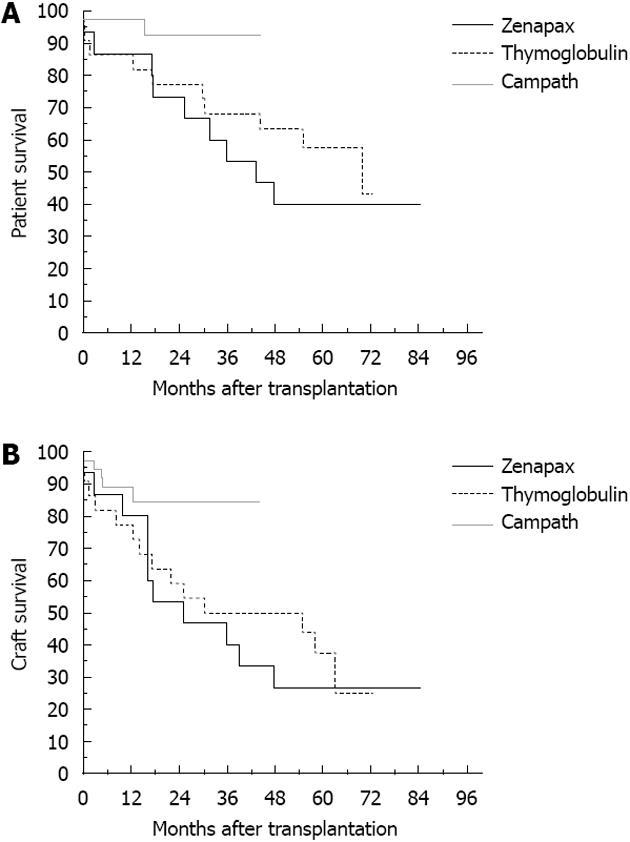
Forty-three rejection episodes occurred during the first 90 d post-transplant; 21 were considered severe episodes. The use of different immunosuppressant induction agents had no impact on the incidence of rejection within the first 90 d post-transplant. The 1-year patient survival was 95% and the 1-year graft survival was 86% (Figure 1), with no impact of immunosuppressant induction agent type on graft and patient survival (Figure 2, P = 0.09, P = 0.14, respectively).
Patients with SGS presenting for isolated SBTx are known to have multiple problems related to the absence of intestinal functions and/or chronic dependency on total parenteral nutrition (TPN). One of the challenging issues in caring for this group of patients is vanishing vein syndrome due to the use of TPN and utilization of most if not of all of the patient’s central venous access[8,9].
Other challenges are attributed to the fact that intestinal grafts are very susceptible to rejection due to high concentration of lymphoid tissue, which may contribute to high incidence of graft versus host disease (GVHD)[10-12]. A high level of immunosuppression is required to prevent rejection, which can lead to serious and life threatening sepsis[13]. Selective bowel decontamination and low-dose radiation were implemented in the donor to ameliorate or prevent the occurrence of GVHD reaction. However, in our group of SBTx recipients, we did not see GVHD or lymphoproliferative diseases within the first post-transplant year. The Epstein-Barr virus (EBV) has been linked to the development of lymphoproliferative disorders in SBTx recipients[14]. The absence of post-transplant lymphoproliferative disorders in our series is due to the fact that we reviewed 1 year post-transplant outcomes; also, all the donors in our series were screened for EBV and all recipients were put on EBV prophylaxis regardless of their preoperative viral status. Historically, GVHD affects about 5% of SBTx recipients, which is much higher than in other solid organ transplant recipients[11]. The absence of GVHD in our series may be related to the use of immunosuppressant induction agents. The diagnosis of GVHD was suspected on the basis of clinical symptoms, according to the Consensus Conference on Acute GVHD grading, with its usual attacks on the skin, liver, and gastrointestinal tract[15].
When compared to liver transplant recipients, SBTx recipients have a theoretical advantage in that the graft can be removed in cases of graft-related problems without serious impact on the recipient’s well-being. Our results showed improved patient 1-year survival (95%) when compared to the series from England and Wales[16], which was reported to be 57% for cases performed during the late nineties to early 2000s. Our results are better, as they reflect the experience of the surgical team and/or improvement in the immunosuppression protocol and patient selection. The England and Wales study reported a 1-year graft survival of at best, 40%; in our series it was 85%. In our study, the SBTx patients and grafts showed much improvement, even when compared to the first report from our center in 1997[17], which documented patient and graft survival as 73% and 60%, respectively. In most SBTxs that were performed during the nineties, the main immunosuppressants used were tacrolimus and steroids. Tacrolimus is still the main immunosuppressant agent used for SBTx recipients, but new immunoinduction agents have been added while steroids and/or OKT3 have been reserved to treat rejection episodes.
The use of three different immunoinduction agents for this group of patients reflects three periods in the 8-year timeline in which the immunosuppressant protocol at our institution was modified. The overall improvement in SBTx outcome after the introduction of the immunoinduction agent Zenapax was documented in children in an early report by the University of Miami[18]. In our report, the use of different induction agents did not affect short-term patient or graft outcomes. However, in the University of Miami report, patient and graft outcomes were better for isolated SBTx than for transplant of combined liver-small bowel or multivisceral grafts. Although this contradicted our report that was published in 2001[1], the difference in the results may be due to the difference in the recipients’ age (pediatric vs adult). The indications for SBTx at our center are not different from what are used in other transplant centers due to the fact that morbidity and mortality for patients with SGS on TPN are low, especially with advances in technology and pharmacological applications of TPN[19,20]. SBTx is reserved for patients with SGS who develop TPN-related complications, such as loss of venous access, repeated line infection, dehydration, and hepatobilliary complications (cholestasis, fatty liver)[8,19].
Rejection remains the most serious challenge in post-transplant patients. The fact that no single laboratory test can predict or detect rejection makes the diagnosis of rejection even more difficult and an almost impossible task. The diagnosis of rejection depends on adapting a high suspicious index in interpretation of clinical presentation and performing an early endoscopic examination with biopsy. The SBTx service at our center has adopted a standardized protocol for detection and follow up of rejection in SBTx recipients, which includes regular endoscopic examination and biopsy. Treatment of rejection is usually accomplished with OKT3 and supplemental dose of tacrolimus with or without high dose of steroids[21-23].
Intestinal transplantation can be a quite lengthy procedure due to the fact that SBTx recipients typically undergo multiple abdominal surgeries that make the dissection phase very prolonged and complicated. Although the SBTx procedure is longer than the orthotopic liver transplant (OLT) procedure[3], it is associated with less blood loss than OLT (as compared to results from our center); this may be related to the fact that SBTx recipients are hypercoagulable and less likely to lose excessive blood[24].
The presentation of PRS in SBTx recipients was determined and compared to the incidence of PRS in liver transplant recipients, which is a well-documented phenomenon. In this study, criteria established by Hilmi et al[3]
to define PRS and its severity were used. In the Hilmi study, PRS was found to occur in all liver transplant recipients, with 50% developing mild PRS and the other 50% developing more severe PRS, while in this study, most SBTx recipients suffered mild PRS and almost none suffered severe PRS. Although PRS was common in SBTx recipients, it was not as dramatic as in liver transplant recipients. Residual hypotension, which usually follows initial hypotension in PRS, typically dissipates during the first hour after reperfusion in SBTx.
The duration of ICU and hospital stay can be prolonged; in our series the mean was 3 and 26 d respectively, which is comparable to what is published and known about this procedure. The occurrence of complications, especially infection and rejection, and AKI can further prolong hospital and ICU stay[25]. Tacrolimus-based maintenance therapy is used in most if not all transplant centers world-wide, while steroid and OKT3 are reserved for treatment of rejection episodes. During the last 10 years, application of immune induction agents in SBTx recipients increased such medications include anti-lymphocyte globulin (Thymoglobulin), anti-interleukin receptor globulin (Zenapax), and the latest therapy, alemtuzumab (Campath). The introduction of these agents in clinical practice has significantly reduced the incidence of early rejection and almost eliminated early graft loss as we demonstrated in this study. However, the uses of the immune induction agents are not without toxicity and unwanted side effects. Recently Campath was reported to cause serious complications that prompted care providers to re-consider the use of Thymoglobulin, especially in renal transplant recipients[26-28].
Although short-term patient and graft outcomes have greatly improved, 5-year survival remains to be improved. In our series, 5-year survival was 40% for grafts and 60% for patients. While still better than the survival rates reported for recipients in the 2009 Transplant Registry report, there is still a long way to go to improve overall survival.
In summary, the overall short-term outcome for SBTx recipients has greatly improved since our first report. Changes in immunosuppressant protocol with introduction of induction agents and refinement of surgical techniques may play a role in this improvement. However, this improvement has yet to be reflected in long-term patient and graft outcomes and scientists and clinicians have many challenges to overcome.
Intestinal transplantation was made possible by the advancement in immunosuppressant medications (tacrolimus) and it soon became the treatment of choice for patients with irreversible intestinal failure. However, long-term patient and graft health and survival are challenged by the high incidence of rejection and sepsis. As a result, new methods of controlling the immune response in small bowel transplant (SBTx) recipients continue to emerge, such as the use of irradiated grafts to control the intestinal lymphatic tissues and the application of immune induction agent to control the immune system. None of these methods are without risks or side-effects; some of these complications are documented in this study.
Although SBTx is a well-established procedure for patients with short gut syndrome, it is only in its infancy; researchers and clinicians are still looking for answers and solutions to the problems associated with this procedure. Reporting the outcomes from a well-known transplant center and documentation of the impact of the immune induction agents on the perioperative course constitutes valuable information for care providers.
The use of different immune modulation agents may impact short-term patient and graft outcomes, but not long-term outcomes. Zenapax proved to be the most notorious agent in causing more unwanted side effects and can significantly impact the intraoperative and hospital course. Campath became the most commonly used agent due to lower incidence of complications when compared to Zenapex. Recently, the use of Thymoglobulin has been rising after reports of serious complications related to Campath, especially in renal transplant recipients. Tailoring an immunosuppressant regimen that is appropriate for a particular patient will be the optimal way to control and modulate the immune response without added risk of sepsis.
This study showed that SBTx recipients have better short-term outcomes with a theoretical advantage in that the graft can be removed in cases of graft-related problems without serious impact on the recipient’s well-being. The results showed an improved patient 1-year survival (95%) when compared to series from other centers, which reflects the experience of the surgical team and/or improvement in the immunosuppression protocol and patient selection.
This study defined post reperfusion syndrome according to the standard that is accepted by the anesthesiology team at our institution, which is not widely used outside our practice. The authors defined acute kidney injury (AKI) using the definition of the modified RIFLE (risk, injury, failure, loss, end-stage renal disease) criteria, as recommended by the Kidney Disease Improving Global Outcomes AKI guideline but without urine output data. This definition uses a 50% increase in SCr from the baseline (pre-operative value) or a 0.3 mg/dL increase within 48 h.
The nature of a retrospective study may lead to important limitations on the identification and analysis of different confounding factors. However, the data we used in this study were carefully collected, maintained, and tabulated for each SBTx recipient as a part of our institutional policy, which gives credibility to the authors’ study findings.
P- Reviewer: Hardinger KL S- Editor: Zhai HH L- Editor: A E- Editor: Zheng XM
| 1. | Abu-Elmagd K, Reyes J, Bond G, Mazariegos G, Wu T, Murase N, Sindhi R, Martin D, Colangelo J, Zak M. Clinical intestinal transplantation: a decade of experience at a single center. Ann Surg. 2001;234:404-416; discussion 416-417. [PubMed] |
| 2. | Planinsic RM. Anesthetic management for small bowel transplantation. Anesthesiol Clin North America. 2004;22:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Hilmi I, Horton CN, Planinsic RM, Sakai T, Nicolau-Raducu R, Damian D, Gligor S, Marcos A. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538-1548. [PubMed] |
| 5. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4984] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 6. | Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 7. | Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. 2012;2:1-138. |
| 8. | Matsusaki T, Sakai T, Boucek CD, Abu-Elmagd K, Martin LM, Amesur N, Thaete FL, Hilmi IA, Planinsic RM, Aggarwal S. Central venous thrombosis and perioperative vascular access in adult intestinal transplantation. Br J Anaesth. 2012;108:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Seisdedos Elcuaz R, Conde García MC, Castellanos Monedero JJ, García-Manzanares Vázquez-de Agredos A, Valenzuela Gámez JC, Fraga Fuentes MD. [Central venous catheters-related infections in patients with parenteral nutrition]. Nutr Hosp. 2012;27:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Shaffer BC, Modric M, Stetler-Stevenson M, Arthur DC, Steinberg SM, Liewehr DJ, Fowler DH, Gale RP, Bishop MR, Pavletic SZ. Rapid complete donor lymphoid chimerism and graft-versus-leukemia effect are important in early control of chronic lymphocytic leukemia. Exp Hematol. 2013;41:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Wu G, Selvaggi G, Nishida S, Moon J, Island E, Ruiz P, Tzakis AG. Graft-versus-host disease after intestinal and multivisceral transplantation. Transplantation. 2011;91:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Mazariegos GV, Abu-Elmagd K, Jaffe R, Bond G, Sindhi R, Martin L, Macedo C, Peters J, Girnita A, Reyes J. Graft versus host disease in intestinal transplantation. Am J Transplant. 2004;4:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Akhter K, Timpone J, Matsumoto C, Fishbein T, Kaufman S, Kumar P. Six-month incidence of bloodstream infections in intestinal transplant patients. Transpl Infect Dis. 2012;14:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Reyes J, Green M, Bueno J, Jabbour N, Nalesnik M, Yunis E, Kocoshis S, Kauffman M, Todo S, Starzl TE. Epstein Barr virus associated posttransplant lymphoproliferative disease after intestinal transplantation. Transplant Proc. 1996;28:2768-2769. [PubMed] |
| 15. | Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825-828. [PubMed] |
| 16. | Middleton SJ, Pollard S, Friend PJ, Watson C, Calne RY, Davies M, Cameron EA, Gimson AE, Bradley JA, Shaffer J. Adult small intestinal transplantation in England and Wales. Br J Surg. 2003;90:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Todo S, Reyes J, Furukawa H, Abu-Elmagd K, Lee RG, Tzakis A, Rao AS, Starzl TE. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270-280; discussion 280-282. [PubMed] |
| 18. | Kato T, Tzakis AG, Selvaggi G, Gaynor JJ, David AI, Bussotti A, Moon JI, Ueno T, DeFaria W, Santiago S. Intestinal and multivisceral transplantation in children. Ann Surg. 2006;243:756-764; discussion 764-766. [PubMed] |
| 19. | O’Keefe SJ. Candidacy for intestinal transplantation. Am J Gastroenterol. 2006;101:1644-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Pironi L, Joly F, Forbes A, Colomb V, Lyszkowska M, Baxter J, Gabe S, Hébuterne X, Gambarara M, Gottrand F. Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut. 2011;60:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Trevizol AP, David AI, Dias ER, Mantovani D, Pécora R, D’Albuquerque LA. Intestinal and multivisceral transplantation immunosuppression protocols--literature review. Transplant Proc. 2012;44:2445-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | O’Keefe SJ, El Hajj II, Wu T, Martin D, Mohammed K, Abu-Elmagd K. Endoscopic evaluation of small intestine transplant grafts. Transplantation. 2012;94:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Remotti H, Subramanian S, Martinez M, Kato T, Magid MS. Small-bowel allograft biopsies in the management of small-intestinal and multivisceral transplant recipients: histopathologic review and clinical correlations. Arch Pathol Lab Med. 2012;136:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Giraldo M, Martin D, Colangelo J, Bueno J, Reyes J, Fung JJ, Starzl TE, Abu-Elmagd K. Intestinal transplantation for patients with short gut syndrome and hypercoagulable states. Transplant Proc. 2000;32:1223-1224. [PubMed] |
| 25. | Suzuki M, Mujtaba MA, Sharfuddin AA, Yaqub MS, Mishler DP, Faiz S, Vianna RM, Mangus RS, Tector JA, Taber TE. Risk factors for native kidney dysfunction in patients with abdominal multivisceral/small bowel transplantation. Clin Transplant. 2012;26:E351-E358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Adams PS, Shapiro R, Hilmi IA. Postoperative cardiac tamponade after kidney transplantation: a possible consequence of alemtuzumab-induced cytokine release syndrome. Transplantation. 2013;95:e18-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Castelli R, Gritti G, Cannavò A, Moreo G, Conti G, Reda G, Cortelezzi A. Successful management with intravenous immunoglobulins in alemtuzumab-induced acute inflammatory demyelinating neuropathy: clinical report of three patients. Immunopharmacol Immunotoxicol. 2012;34:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Muzaffar M, Taj A, Ratnam S. Aggressive posttransplant lymphoproliferative disease in a renal transplant patient treated with alemtuzumab. Am J Ther. 2010;17:e230-e233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |