Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.104308
Revised: March 2, 2025
Accepted: March 10, 2025
Published online: September 18, 2025
Processing time: 121 Days and 22.6 Hours
The Luminex platform, where beads are coated with single human leukocyte antigens (HLA), detects HLA antibodies with higher sensitivity and specificity compared to complement-dependent cytotoxicity (CDC) assay and flow cross-match (FCXM). The clinical significance of donor-specific antibodies (DSAs) detected by this method is still under investigation.
To report the impact of low-level pretransplant DSAs detected by the Luminex platform on the rates of acute rejection (AR), allograft function, and long-term graft survival.
This retrospective study was conducted at the Immunology Department of Sindh Institute of Urology and Transplantation, Karachi, Pakistan between January 2013 and December 2022. During this period 2714 patients were transplanted. Out of these patients 78 (2.9%) patients had low-level DSAs detected by the Luminex flow beads method and were negative by CDC and FCXM with their donors. All recipients received ABO-compatible live-related kidney transplants. All patients had a minimum follow-up of 1 year. Graft rejection rates, graft function, and patient and graft survival were analyzed. The es
The mean age of all recipients was 29.57 ± 10.11 years and 34.53 ± 9.09 years for the donors. In 48 (61.5%) patients, the cause of end-stage kidney disease was unknown. DSA against HLA class I was detected in 36 (46.1%) patients, class II in 35 (44.8%) patients, and both class I and II in 7 (8.9%) patients. AR episodes were encountered in 8 (10.3%) cases. Seven (87.5%) had T cell mediated rejection (type IA) and one acute antibody-mediated rejection. Antibody status was re-evaluated at the time of biopsy-proven ARs. Five (62.5%) patients lost their DSAs, while three (37.5%) had persistent DSAs. The mean estimated glomerular filtration rate at 1 year was 80.56 ± 27.48 mL/min/1.73 m2 and at the last follow-up 73.41 ± 28.80 mL/min/1.73 m2. The 1-year and 10-year patient and graft survival rates were 99% and 79% and 95% and 75%, respectively. During the follow-up period, 10 (12.8%) patients died, 8 patients had a functioning graft, and 2 patients had failed grafts. Eight patients died due to cardiopulmonary arrest, and two died due to sepsis with failed grafts.
Patients with pretransplant low-level DSAs on Luminex without CDC and FCXM reactivity had good allograft outcomes at 1 year and 10 years as long as they are induced with biological agents and given potent maintenance immunosuppressants.
Core Tip: Pretransplant low-level donor-specific antibodies (DSAs) detected by the Luminex platform are associated with favorable graft outcomes after 1 year and 10 years in kidney transplant recipients. This study highlighted the appropriate immunosuppressive induction and maintenance protocols. These patients experienced low rates of acute rejection and sustained satisfactory long-term graft function and survival. The majority of patients who experienced acute rejection showed a resolution of DSAs, underscoring the dynamic nature of antibodies. These findings support the clinical relevance of Luminex-detected low-level DSAs and reinforce the efficacy of contemporary immunosuppressive strategies in optimizing graft survival in this subset of transplant recipients.
- Citation: Abbas K, Mubarak M, Musharraf W, Hafeez AR, Aziz T, Zafar MN. Impact of low-level pretransplant donor-specific antibodies detected by the Luminex platform on acute rejection and long-term graft survival. World J Transplant 2025; 15(3): 104308
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/104308.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.104308
The presence of pretransplant donor-specific antibodies (DSAs) represents a significant barrier to kidney transplantation for highly sensitized patients as they are less likely to undergo transplantation compared with their non-sensitized counterparts[1,2]. When DSAs are detected pretransplantation, there may be an increased immunological risk of proceeding with transplantation with that particular donor, impacting transplant decision-making[3,4].
Luminex single antigen bead (SAB) assays are widely used to detect pretransplant DSAs to stratify patients according to immunological risk of rejection and complications[5,6]. The presence of pretransplant DSAs augments the immunological risk and requires modification of the immunosuppression protocol compared to negative pretransplant DSAs[3,7]. However, the level at which DSAs become clinically significant is still unsettled[8,9]. Previous studies have described the association between pretransplant DSAs and an increased risk of antibody-mediated rejection (ABMR)[10,11]. Pretransplant DSAs can cause accelerated acute rejection (AR), acute ABMR, and graft loss[12,13]. On the other hand, we and others did not observe an increased risk of rejection in DSA-positive patients[9,10,14]. These results may be related to induction by biological agents and the use of potent maintenance immunosuppressants[12-16].
The levels of DSAs by SAB are not quantitative and are based on the mean fluorescence index (MFI). Different laboratories use different MFI cutoffs, ranging from 1000 to 10000[10-14]. This is because there is no universally agreed-upon MFI threshold, leading to variations in cutoff values. These differences arise due to variations in the sensitivity of the assay platforms, reagent sensitivities, and the lack of universally standardized protocols. These variations can potentially influence clinical decision-making by affecting the interpretation of DSA levels, which in turn impact im
Several other potential confounding factors can influence the interpretation of DSA levels. Adjustments in immunosuppressive medications can alter DSA levels. Intravenous immunoglobulin can mask or reduce DSA levels. Moreover, DSA levels can vary over time due to immune activation or suppression. High antibody concentrations may lead to false negative results. Viral infections such as cytomegalovirus (CMV) and polyomavirus can induce transient DSA formation. In addition, the use of different vendor kits for the same sample for DSA follow-up can potentially confound the interpretation of results. Hence, MFI values should be interpreted in context with clinical and historical factors and not in isolation.
Against the above backdrop, this study aimed to evaluate the impact of low-level DSAs by Luminex when com
This retrospective observational study was conducted at the Department of Immunology, Sindh Institute of Urology and Transplantation (SIUT), Karachi, Pakistan from January 2013 to December 2022. A total of 2714 patients underwent kidney transplantation during this period. Out of these, 78 (2.9%) patients had DSAs detected by the Luminex method and were negative by CDC and FCXM cross-matches with their donors. All recipients received live-related and ABO-compatible kidney transplants. All patients had a minimum follow-up of 1 year. Graft rejection rates, graft function, and patient and graft survivals were recorded in all study subjects. The estimated glomerular filtration rate was calculated by the full CKD-EPI formula[17]. Graft survival was censored for death.
All kidney transplant recipients and donors were typed for human leukocyte antigens (HLA) class I (HLA, A, B and C loci) and class II (DR and DQ loci) by sequence-specific primers by Collaborative Transplant Study Heidelberg, Germany by PCR as in our previous study[14]. CDC-XM was performed in all recipients for T and B cells at room temperature, 4 °C, and 37 °C and by antihuman globulin-CDC for T cells. The lymphocytes were separated from the whole blood using a lymphocyte separation medium (MP biomedical, LLC, France) by density gradient centrifugation. Lymphocytes were then separated into B and T cells using Dyna electromagnetic beads for HLA class II (Invitrogen, Lithuania). IgM antibodies were excluded by testing for CDC-XM in the presence of dithiothreitol. CDC auto-XMs were also performed using recipient sera incubated with recipients’ cells to detect autoantibodies in selected cases. CDC-XMs were interpreted as positive when the observed cell death exceeded ≥ 10% above the background, and auto-XM was negative.
FCXM was performed using polyclonal rabbit anti-human IgG/FITC and IgM/FITC (Dako, Glostrup, Denmark) on the Beckman Coulter Analyzer (BD) for both T and B cells. In brief, donor peripheral blood lymphocytes were obtained from whole blood using the same method as for CDC-XM. We did not treat the donor cells with pronase. FCXM was in
The Luminex 200 system (One lambda, Canoga Park, CA, United States) with Luminex screening and SAB assays was used for the detection of HLA class I and class II antibodies in the sera of recipients in that order in all cases. Sera were not treated with EDTA, dithiothreitol, or heat in any of the recipients. The tests were repeated multiple times before transplantation, and the trend was noted. Both positive and negative control beads were used to minimize the chances of false negative and false positive results, respectively. In particular, high background results of negative control beads were carefully examined and run with AdosrboutTM beads. We also ran LABScreenTM negative control serum with every run. False positive reactivity was also excluded by examining FCXM mean channel shift as a function of DSA MFI rather than using FCXM output as positive/negative results. A positive result was defined as a baseline normalized MFI > 1000. The mismatched antigens between donors and recipients were compared to the antibody profile for each patient sample to determine the donor-specific reactivity.
The 78 patients with low-level preformed DSAs were induced with anti-thymocyte globulin (ATG, Fresenius) at a dose of 3-5 mg/kg for 7 days. The maintenance immunosuppression comprised tacrolimus (Tac) (0.15 mg/kg), prednisolone (0.5 mg/kg), and mycophenolate mofetil (10-15 mg/kg). The target Tac trough levels were kept around 8-10 ng/mL during the early post-transplant period. The dose was reduced to 0.1 mg/kg at 3 months if there was no episode of biopsy-proven rejection.
All graft dysfunction events were thoroughly investigated using serum drug levels, color Doppler ultrasonography, and kidney allograft biopsies. All documented rejection events were biopsy-proven. The indicated allograft biopsies were conducted when serum creatinine increased by ≥ 20% above the baseline. The diagnostic criteria for acute T cell-mediated rejection (TCMR) and ABMR were used as per the updated Banff 2019 classification[18]. At the time of graft dysfunction, all patients were tested for CMV by real-time PCR (Abbott RealTime CMV kit) and polyomavirus infections (Artus kit). Drug levels were measured on an immunoanalyzer (Architect i1000 SR, Abbott, IL, United States). The patients were also tested for DSA by Luminex when graft biopsies revealed features of rejection.
The treatment of acute TCMR consisted of methylprednisolone (MP) boluses (500 mg/day) for 3 days and 250 mg/day for the next 2 days. MP-resistant ARs were treated with ATG at a dose of 3-5 mg/kg for 10-14 days. The treatment for ABMR consisted of 500 mg MP for 5 days, ATG for 10-14 days, plasmapheresis for 5-7 sessions, two doses of rituximab (375 mg /m2), and one cycle of bortezomib in variable combinations in individual patients. Desensitization strategies were not employed in any patient before the transplant. All transplant recipients were given prophylaxis against Pneumocystis jiroveci in the form of sulfamethoxazole and trimethoprim. In addition, patients who received ATG induction were given valganciclovir for 3 months for prophylaxis against CMV infection.
The study was approved by the Institutional Ethical Review Committee of SIUT, Karachi, Pakistan (No: SIUT-ERC-2022/A-417). The study was conducted in accordance with the tenets of the Declaration of Helsinki.
The data analyses were performed by SPSS version 22.0 (IBM Corp., Armonk, NY, United States). The continuous variables were summarized as mean ± SD or median ± interquartile range (IQR), depending on the normality of the distribution of data. The categorical variables were presented as frequencies and percentages. Kaplan-Meier survival curves were used to calculate the graft survival. A P value < 0.05 was deemed as statistically significant.
The demographic and clinical parameters of 78 patients are given in Table 1. The mean age of recipients was 29.57 ± 10.11 years and was 34.53 ± 9.09 years for donors. In 48 (61.5%) patients, the cause of end-stage renal disease was unknown. The median follow-up of the whole cohort was 4 (3-5) years. Pretransplant DSA positivity against class I and class II HLA and the corresponding MFI values are given in Table 2. DSA against HLA class I was detected in 36 (46.1%) patients, class II in 35 (44.8%) patients, and both class I and II in 7 (8.9%) patients. ARs and MFI values in relation to antibody classes and the MFI values are also given in Table 2. AR episodes were encountered in 8 (10.3%) of the cases. Seven (87.5%) had TCMR (type IA), and one patient had acute ABMR.
| Parameter | n = 78 |
| Age of recipients (years) | 29.57 ± 10.11 |
| Recipient gender | |
| Males | 49 (62.8) |
| Females | 29 (37.2) |
| Age of donors (years) | 34.53 ± 9.09 |
| Donor gender | |
| Males | 46 (59.0) |
| Females | 32 (51.0) |
| Donor relation | |
| Siblings | 46 (58.9) |
| Parents | 14 (17.9) |
| Spouses | 17 (21.8) |
| Offspring | 1 (1.3) |
| HLA mismatches | |
| 1-2 | 21 (27.0) |
| 3-4 | 37 (47.4) |
| 5-6 | 20 (25.6) |
| Duration of hemodialysis (months) | 14.01 ± 15.48 |
| End-stage kidney disease cause | |
| Unknown | 48 (61.5) |
| Chronic glomerulonephritis | 20 (25.6) |
| Stone disease | 5 (6.4) |
| Bladder outlet obstruction and neurogenic bladder | 3 (3.8) |
| Parameter | Mean DSA MFI |
| DSA Class I, n = 36 (46.2%) | 3286.58 ± 4530.50; Range: 933-9994 |
| DSA Class II, n = 35 (44.8%) | 3207.47 ± 4219.00; Range: 1041-9383 |
| DSA Class I and II, n = 7 (8.9%) | Class I = 3245.00 ± 2117.00; Range: 5515-6518 |
| Class II = 2101.00 ± 959.00; Range: 2336- 3610 | |
| In rejection (n = 8) | |
| Class I, n = 2 (25.0%) | Median = 4994 (IQR = 3922-4994) |
| Class II, n = 6 (75.0%) | Median = 2718 (IQR = 1221-5173) |
| In no rejection (n = 63) | |
| Class I, n = 34 (48.5%) | Median = 2481 (IQR = 1732-4136) |
| Class II, n = 29 (41.4%) | Median = 3088 (IQR = 1479- 5249) |
Antibody status was re-evaluated at the time of biopsy-proven ARs. Five (62.5%) patients lost their DSA, while three (37.5%) had persistent DSAs. Among these 3 patients, 2 patients had preformed DSAs against HLA class I with a mean MFI of 4994, while 1 patient had DSA against HLA class II with an MFI of 2718.
Among the 8 patients who developed rejection, 6 patients had preformed DSAs against class II antigen with a median MFI of 2718 (IQR: 1221-5173), and 2 patients had DSAs against HLA class I with a median MFI of 4994 (IQR: 3922-4994). In the group with no rejection, 34 (48.5%) patients had DSA against HLA class I with a median MFI of 2481 (IQR: 1732-4136), and 29 (41.4%) patients had DSA against HLA class II with a median MFI of 3088 (IQR: 1479-5249). In the study period, 27 (34.6%) patients developed CMV infection, and 26 (33.3%) had urinary tract infections.
The mean estimated glomerular filtration rate at 1 year was 80.56 ± 27.48 mL/min/1.73 m2 and at the last follow-up was 73.41 ± 28.80 mL/min/1.73 m2. No cases of polyomavirus nephropathy was detected during the study period.
Antibody class and MFI values in the AR and no rejection groups are given in Table 2. Overall, ARs were encountered in 8 (10.0%) patients. The mean MFI was 4994 in class I and 2718 in class II. In the group with no rejection the mean MFI was 2481 in class I and 3088 in class II.
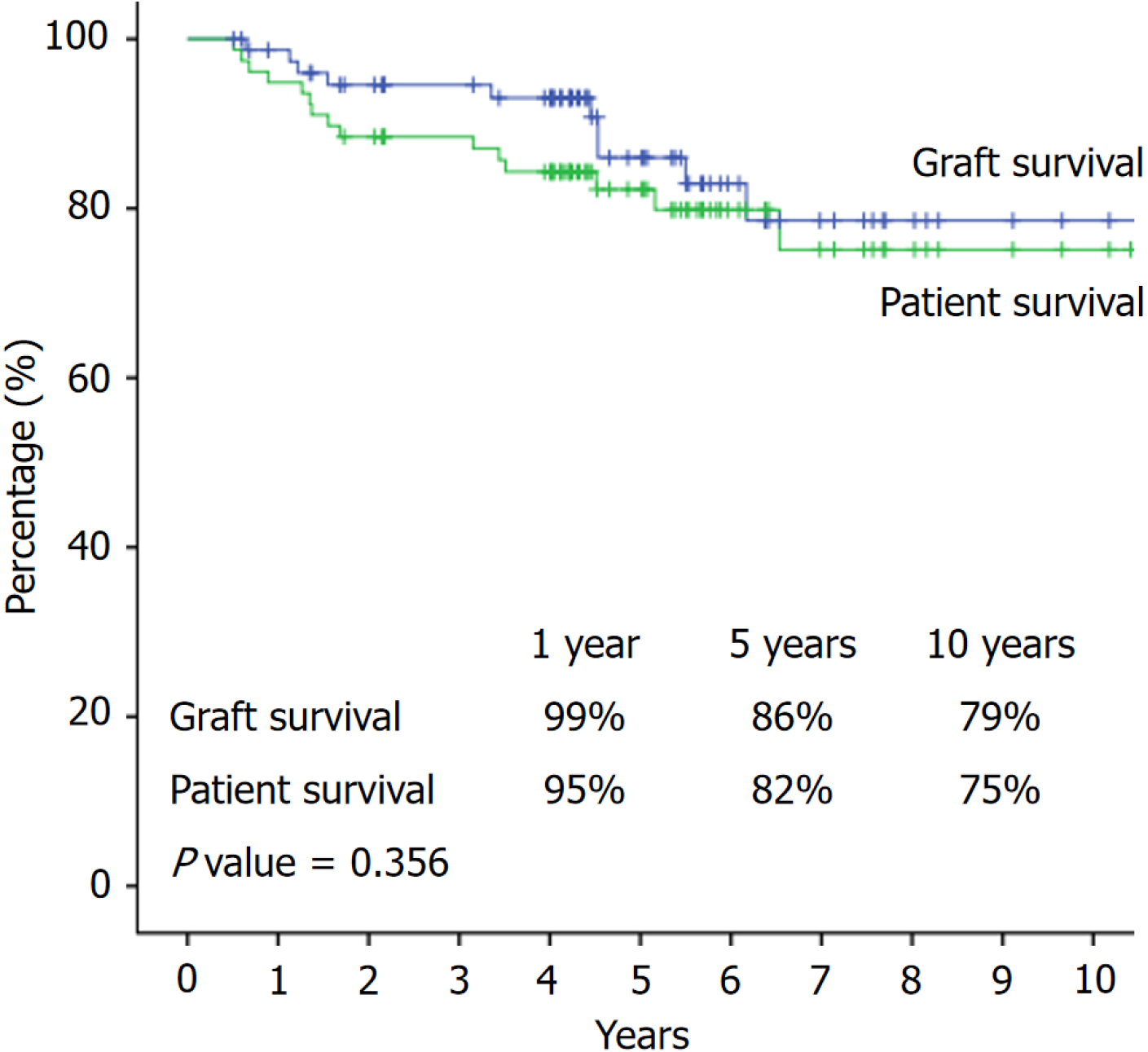
Overall, death-censored 1-year and 10-year patient and graft survival are given in Figure 1. The 1-year and 10-year patient and graft survival were 99% and 79% and 95% and 75%, respectively. In brief, during the follow-up period, 10 (12.8%) patients died of which 8 patients had a functioning graft, and 2 patients had failed grafts. Eight patients died due to cardiopulmonary arrest and two due to sepsis with failed grafts.
This study described the outcome of 78 patients with low-level DSAs detected by the Luminex platform when CDC and FCXM results were negative. In the follow-up period, 8 (10.3%) patients developed ARs, 7 patients developed TCMR, and 1 patient developed ABMR. The 1-year and 10-year graft survival was 99% and 79%, and patient survival was 95% and 75%, respectively. In the follow-up period, 10 patients died, 8 with functional graft and 2 with graft failure. Eight patients died of cardiopulmonary arrest and two due to sepsis.
Our results show that low-level pretransplant DSAs detected by the Luminex had no adverse impact on AR and graft outcomes. In fact, AR rates were markedly lower in these patients as compared to our historical rates[14]. Moreover, the majority of these were TCMR and not ABMR. This may be because of the use of induction with ATG and the use of more potent immunosuppression regimens in these patients. Several other recent studies have yielded similar results[16,19]. Our results are concordant with the results of Aubert et al[20] who indicated that preformed low-level DSAs are largely clinically insignificant. Parajuli et al[21] also demonstrated that low-level preformed DSAs without concomitant inflammation and rejection do not pose a risk for graft failure. A study by Adebiyi et al[15] showed that preformed DSAs on Luminex with a negative FCXM pose little immunological risk in the medium term and do not warrant desensitization treatment. They also concluded that these antibodies do not represent a barrier to transplantation. Gupta et al[16] showed that DSAs detected by Luminex with negative CDC and FXM had no association with ABMR or inferior outcomes at 1 year. It is notable that the study by Gupta et al[16] was conducted two decades ago when ATG induction was not widely used because a CyA-based regimen comprised the mainstay of maintenance immunosuppression.
On the contrary, our findings are in disagreement with some other previous studies showing that whatever the cause, preformed DSAs are associated with an increased risk of early ABMR and inferior graft outcomes. Betjes et al[22] found that low-level preformed DSAs are predisposing factors for early ABMR and graft failure. Notably in their study among patients with preformed DSAs, only 24% received induction therapy as compared to all patients in the present study. In maintenance immunosuppression, Tac was used in 60% of patients as compared to 100% in our study. Tian et al[13] also showed that solid-phase antibodies are not strong enough to elicit a positive crossmatch but may influence long-term graft outcomes. They did not use induction therapy or desensitization treatment strategies[13]. Lee et al[11] retro
Similarly, Thammanichanond et al[23] found a higher incidence of ABMR in patients with pre-existing DSAs on Luminex and negative CDC crossmatch. However, they did not find a significant impact of DSA on graft survival. In the latter study, induction therapy was used in 58% of patients, and Tac-based maintenance suppression was given in only 3% of cases.
There is no universal standard for reporting MFI values; different laboratories use different cutoffs. This has considerable implications for patient selection, induction immunosuppression, and the maintenance immunosuppression[24]. If the threshold is set too low, false positive results preclude potential recipients from transplant, or if the threshold is set too high, there may be an increased risk for ABMR. Singh et al[25] found poor graft survival in patients with extremely low DSA levels (100 MFIs), while Phanish[26] found excellent graft survival across extremely high DSA values (> 10000 MFIs).
A question that remains unanswered from all the studies is which MFI should be used for induction therapy. A systematic review and meta-analysis by Mohan et al[27] of seven retrospective cohort studies comprising 1119 patients showed that DSAs double the risk of ABMR and graft failure. Many of the studies did not mention cutoff values for MFI, while others used induction if values were > 500, > 1000, or > 1500[27]. In another study, Lefaucheur et al[28] indicated that patients with MFI values > 6000 exhibited > 100-fold risk of ABMR than patients with MFI values < 500. Similarly, Reinsmoen et al[29] indicated that pretransplant DSA MFI values > 10000 had an augmented risk of ABMR. In our study, the use of induction therapy and the fact that 62.5% of our patients had no DSA at the time of rejection perhaps suggests induction beyond MFI values of > 1000[30].
Our present study has both strengths and limitations. The strength of this study is the fairly large number of patients with low-level preformed HLA-DSAs in a live-related kidney transplant setup. We transplanted patients with low levels of MFIs and induced all recipients with ATG. We also used more potent maintenance immunosuppression comprising of a Tac-mycophenolate mofetil combination. These factors explain the low AR rates, which were mostly of the TCMR type and of mild-to-moderate degree according to the Banff classification.
There are a number of limitations to this study as well. This is a single center and retrospective study. The HLA-DP locus is not typed at our laboratory. Therefore, we could not detect DSAs against this locus. The presence of anti-HLA-DP DSAs in our patients could not be entirely excluded. We did not do a routine surveillance antibody screen. Antibody testing was done at the time of the for-cause biopsy only. This might have given us a clearer understanding of the trends of these preformed DSAs and changes in MFIs after immunosuppression as we utilized ATG induction in all recipients. We also acknowledge that while the overall cohort is large, the small percentage of patients with low-level DSAs (2.9%) limits the generalizability of the findings.
The presence of low-level, preformed DSAs detected by the Luminex system with a negative CDC and FCXM should not be a contraindication for transplantation. However, these patients require induction with ATG and the use of potent immunosuppressants for better outcomes. It is imperative to create an MFI risk profile for live donor kidney transplants and to assess the status of their DSAs after transplantation to identify the patients at risk of ABMR and graft failure in the long-term follow-up.
| 1. | Jordan SC, Choi J, Vo A. Kidney transplantation in highly sensitized patients. Br Med Bull. 2015;114:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Noble J, Jouve T, Malvezzi P, Rostaing L. Desensitization in Crossmatch-positive Kidney Transplant Candidates. Transplantation. 2023;107:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 3. | Marfo K, Ajaimy M, Colovai A, Kayler L, Greenstein S, Lubetzky M, Gupta A, Kamal L, de Boccardo G, Masiakos P, Kinkhabwala M, Akalin E. Pretransplant immunologic risk assessment of kidney transplant recipients with donor-specific anti-human leukocyte antigen antibodies. Transplantation. 2014;98:1082-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1088] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Konvalinka A, Tinckam K. Utility of HLA Antibody Testing in Kidney Transplantation. J Am Soc Nephrol. 2015;26:1489-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Frischknecht L, Deng Y, Wehmeier C, de Rougemont O, Villard J, Ferrari-Lacraz S, Golshayan D, Gannagé M, Binet I, Wirthmueller U, Sidler D, Schachtner T, Schaub S, Nilsson J; Swiss Transplant Cohort Study. The impact of pre-transplant donor specific antibodies on the outcome of kidney transplantation - Data from the Swiss transplant cohort study. Front Immunol. 2022;13:1005790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 7. | Parajuli S, Bath NM, Hidalgo L, Leverson G, Garg N, R Redfield R 3rd, Mandelbrot DA. Impact of low-level pretransplant donor-specific antibodies on outcomes after kidney transplantation. Immun Inflamm Dis. 2021;9:1508-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Wisse BW, Kamburova EG, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, Baas MC, Spierings E, Hack CE, van Reekum FE, van Zuilen AD, Verhaar MC, Bots ML, Drop ACAD, Plaisier L, Seelen MAJ, Stephan Sanders J, Hepkema BG, Lambeck AJA, Bungener LB, Roozendaal C, Tilanus MGJ, Voorter CE, Wieten L, van Duijnhoven EM, Gelens MACJ, Christiaans MHL, van Ittersum FJ, Nurmohamed SA, Lardy NM, Swelsen W, van der Pant KAMI, van der Weerd NC, Ten Berge IJM, Bemelman FJ, Hoitsma AJ, van der Boog PJM, de Fijter JW, Betjes MGH, Heidt S, Roelen DL, Claas FH, Otten HG. Toward a Sensible Single-antigen Bead Cutoff Based on Kidney Graft Survival. Transplantation. 2019;103:789-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Louis K, Lefaucheur C. DSA in solid organ transplantation: is it a matter of specificity, amount, or functional characteristics? Curr Opin Organ Transplant. 2022;27:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87:1681-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Lee H, Lee H, Eum SH, Ko EJ, Min JW, Oh EJ, Yang CW, Chung BH. Impact of Low-Level Donor-Specific Anti-HLA Antibody on Posttransplant Clinical Outcomes in Kidney Transplant Recipients. Ann Lab Med. 2023;43:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transpl Immunol. 2013;28:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Tian J, Li D, Alberghini TV, Rewinski M, Guo N, Bow LM. Pre-transplant low level HLA antibody shows a composite poor outcome in long-term outcome of renal transplant recipients. Ren Fail. 2015;37:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Abbas K, Aziz T, Musharaf W, Mubarak M, Zafar MN. Impact of pre-transplant donor specific antibodies detected by Luminex with negative microlymphocytotoxicity assay and flow crossmatch in live-related renal transplant recipients. Clin Transplant. 2023;37:e14935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Adebiyi OO, Gralla J, Klem P, Freed B, Davis S, Wiseman AC, Cooper JE. Clinical Significance of Pretransplant Donor-Specific Antibodies in the Setting of Negative Cell-Based Flow Cytometry Crossmatching in Kidney Transplant Recipients. Am J Transplant. 2016;16:3458-3467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Gupta A, Iveson V, Varagunam M, Bodger S, Sinnott P, Thuraisingham RC. Pretransplant donor-specific antibodies in cytotoxic negative crossmatch kidney transplants: are they relevant? Transplantation. 2008;85:1200-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20112] [Article Influence: 1257.0] [Reference Citation Analysis (0)] |
| 18. | Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, Mengel M. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318-2331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 587] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 19. | Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, D'Agati VD, Cohen DJ, Ratner LE, Suciu-Foca N. Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol. 2009;70:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Aubert V, Venetz JP, Pantaleo G, Pascual M. Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum Immunol. 2009;70:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Parajuli S, Joachim E, Alagusundaramoorthy S, Aziz F, Blazel J, Garg N, Muth B, Mohamed M, Redfield RR, Mandelbrot DA, Zhong W, Djamali A. Donor-Specific Antibodies in the Absence of Rejection Are Not a Risk Factor for Allograft Failure. Kidney Int Rep. 2019;4:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Betjes MGH, Sablik KS, Otten HG, Roelen DL, Claas FH, de Weerd A. Pretransplant Donor-Specific Anti-HLA Antibodies and the Risk for Rejection-Related Graft Failure of Kidney Allografts. J Transplant. 2020;2020:5694670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Thammanichanond D, Ingsathit A, Mongkolsuk T, Rattanasiri S, Kantachuvesiri S, Sakhonrat C, Leenanupan C, Worawichawongs S, Kitpoka P. Pre-transplant donor specific antibody and its clinical significance in kidney transplantation. Asian Pac J Allergy Immunol. 2012;30:48-54. [PubMed] |
| 24. | Gibney EM, Cagle LR, Freed B, Warnell SE, Chan L, Wiseman AC. Detection of donor-specific antibodies using HLA-coated microspheres: another tool for kidney transplant risk stratification. Nephrol Dial Transplant. 2006;21:2625-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Singh N, Djamali A, Lorentzen D, Pirsch JD, Leverson G, Neidlinger N, Voss B, Torrealba JR, Hofmann RM, Odorico J, Fernandez LA, Sollinger HW, Samaniego M. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation. 2010;90:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Phanish MK. Immunological risk assessment and human leukocyte antigen antibody testing in kidney transplantation. Indian J Nephrol. 2016;26:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, Ratner LE, Cohen DJ, Radhakrishnan J. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23:2061-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 651] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 29. | Reinsmoen NL, Lai CH, Vo A, Cao K, Ong G, Naim M, Wang Q, Jordan SC. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation. 2008;86:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Dwivedi R, Shashikiran KB, Manuel S, Ansari FA, Madipalli RT, Golla A, Raju SB. Induction with rATG versus No-induction in Deceased Donor Renal Transplantation - A Retrospective Observational Study. Indian J Nephrol. 2022;32:423-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |