Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.102798
Revised: February 2, 2025
Accepted: February 25, 2025
Published online: September 18, 2025
Processing time: 167 Days and 10.7 Hours
Hyperspectral imaging (HSI) offers useful information on organ quality and has already been successfully used in kidney and liver transplantation to assess transplanted organs. Up to now, there is no case report in the literature describing HSI for quality assessment of a machine perfused donor liver. The allocated liver from a 49-year-old female donor (161 cm, 70 kg) was perfused with the OrganOx® normothermic machine perfusion system in the recommended way. Organ quality assessment was performed based on laboratory values at defined time points. In addition, the final evaluation of the liver comprised macroscopic fi
The donor liver’s size (29 cm × 17 cm × 11 cm) and weight of 2180 g posed challenges for adequate placement within the organ container. Baseline biopsy of the liver revealed no evidence of fibrosis, steatosis or inflammation. An hour after perfusion start, measurements of the perfusate indicated a pH of 7.18, a glucose level of 404 mg/dL, and a lactate level of 1.7 mmol/L. Throughout perfusion, a significant decline in glucose levels began at the fourth hour, reaching a nadir of 20 mg/dL after eight hours. Concurrently, lactate levels steadily rose, peaking at 4.9 mmol/L after the total perfusion time of 12 hours. Macroscopic alterations (signs of congestion and reduced blood circulation) on the liver’s surface were noted, particularly pronounced in segments 2, 3, and 8. HSI of these areas un
This case report describes the integration of HSI in the decision making of the decline of a 49-year-old machine perfused donor liver. HSI offered useful information concerning the tissue morphology and graft viability and could therefore be a useful additional tool in assessing donor liver quality before transplantation.
Core Tip: This case report highlights the potential of hyperspectral imaging (HSI) as a valuable tool for assessing machine-perfused donor liver viability. By integrating HSI with laboratory and histological data, this method provides non-invasive insights into tissue oxygenation and morphology, aiding in organ quality evaluation and supporting decisions on transplantation suitability. This case underscores HSI’s potential as a valuable tool in pre-transplant liver evaluation.
- Citation: El-Mahrouk M, Langner C, Sucher R, Kniepeiss D. Introducing hyperspectral imaging as a novel tool for assessing donor liver quality during machine perfusion: A case report. World J Transplant 2025; 15(3): 102798
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/102798.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.102798
Hyperspectral imaging (HSI) captures detailed spectral data across a wide range of wavelengths, offering more information than traditional imaging. Initially used in remote sensing and astronomy, HSI is now being applied in medicine to provide non-invasive biochemical and physiological insights during surgery[1]. Recent studies have explored HIS’s potential in assessing organ quality in normothermically ex vivo perfused livers. While the findings are promising, the evidence is limited due to the small number of cases in these studies[2]. The global shortage of liver organs for transplantation is a growing challenge, sparking interest in machine perfusion for marginal liver grafts over traditional cold storage. Normothermic machine perfusion (NMP) preserves livers at body temperature, keeping them metabolically active, reducing ischemic damage, and extending viability. It also enables repeat real-time assessment of the liver’s condition, improving the evaluation of marginal organs. In that sense, NMP could help address the liver shortage, reduce waiting times, and improve transplant outcomes[3-5]. The aim of this case report is to explore HSI as a promising, innovative method for assessing donor livers during machine perfusion. Due to its non-invasive and rapid capabilities, it holds potential to become an essential supplementary tool in donor liver evaluation in the future.
A prominent example of HSI technology in surgery is the TIVITA® Tissue System, developed by Diaspective Vision. TIVITA® captures hyperspectral images (wavelength between 500-1000 nm), during surgery and provides real-time feedback on tissue oxygenation, perfusion, and water content. These parameters are crucial for assessing tissue viability and health[1]. The OrganOx metra® is a state-of-the-art normothermic perfusion device for liver transplantation. OrganOx significantly extends the organ’s viability outside the body for up to 24 hours. This capability not only enhances the evaluation of the organ’s viability but also increases the logistical flexibility for transplant procedures[6-8].
On March 18, 2024, a 49-year-old female patient (161 cm, 70 kg) passed away from a subarachnoid hemorrhage. Following confirmation of brain death, organ retrieval proceeded smoothly at our clinic on March 19, 2024. The macroscopic assessment revealed less than 20% steatosis and fibrosis grade 1 or lower. A baseline biopsy of the liver revealed no evidence of fibrosis, severe steatosis (under 5%), or inflammation. The liver was allocated for a recipient at our center. Due to the fact that the organ has been classified as marginal, we decided to attach the liver to NMP in order to perform further assessments of the organ's functionality.
Significantly, the donor’s liver presented challenges due to its size (29 cm × 17 cm × 11 cm) and weight of 2180 g. Due to its size, the liver segments 2, 3, and 8 were inverted to fit into the organ container. Once appropriately positioned, perfusion was monitored using regular laboratory controls. Successful perfusion of the liver was observed within minutes of initiating machine perfusion. Initial buffering was performed with 40 mL bicarbonate 8.4%, and further buffering was performed with 20 mL every two hours. An hour after perfusion started, measurements of the perfusate indicated a pH of 7.18, a glucose level of 404 mg/dL, and a lactate level of 1.7 mmol/L. Throughout perfusion, a significant decline in glucose levels began at the fourth hour, reaching a nadir of 20 mg/dL after eight hours. Concurrently, lactate levels steadily rose, peaking at 4.9 mmol/L after the total perfusion time of 12 hours. Macroscopic alterations on the liver’s surface were noted, particularly pronounced in segments 2, 3, and 8, possibly due to spatial constraints and impaired microcirculation within the liver in the container. HSI of these areas unveiled reduced oxy
This is a case report on a machine-perfused liver from a deceased donor; therefore, a history of present illness is not applicable.
A history of past illness is not applicable.
The personal and family medical history is not applicable.
A physical examination is not applicable.
Table 1 presents the laboratory values of the machine-perfused liver over time. Due to the increasing lactate levels and reduced oxygenation observed in the HSI, the organ was discarded.
| March 19, 22: 58 | March 20, 00: 01 | March 20, 01: 10 | March 20, 02: 00 | March 20,04: 01 | March 20, 04: 39 | March 20, 05: 27 | March 20, 06: 59 | March 20, 08: 15 | March 20, 09: 57 | |
| Natrium, mmol/L | 105 | 109 | 118 | 121 | 127 | 140 | 138 | 132 | 130 | 134 |
| Potassium, mmol/L | 13.60 | 14.9 | 13.3 | 13.5 | 13.5 | 12 | 13.6 | 16.5 | 17.3 | 16.6 |
| Chloride, mmol/L | 88.00 | 91 | 92 | 93 | 93 | 0.63 | 89 | 86 | 85 | 82 |
| Calcium, mmol/L | 0.64 | 0.67 | 0.67 | 0.68 | 0.68 | 90 | 0.6 | 0.58 | 0.58 | 0.56 |
| Glucose, mg/dL | 404.00 | 195 | 125 | 95 | 36 | 23 | 21 | 32 | 71 | 173 |
| Lactate, mmol/L | 1.70 | 1.6 | 2.4 | 2.3 | 2.4 | 2.5 | 2.7 | 3 | 3.4 | 4.9 |
| pO2 arterial, mmHg | 113.00 | 102 | 125 | 114 | 112 | 98.6 | 92.7 | 97.6 | 111 | 102 |
| pH arterial | 7.18 | 7.06 | 7.12 | 7.04 | 7.05 | 7.33 | 7.27 | 7.18 | 7.1 | 7.16 |
| pCO2 arterial, mmHg | 35.30 | 37.6 | 32.5 | 37.3 | 9.9 | 34.6 | 37 | 35 | 37.2 | 12.5 |
| Actual HCO3 arterial, mmol/L | 13.10 | 10.7 | 10.5 | 10 | 35.5 | 18.2 | 16.8 | 13.2 | 11.5 | 35.2 |
| Standard HCO3 arterial, mmol/L | 13.30 | 10.6 | 11 | 9.9 | 10 | 18.5 | 16.8 | 13.4 | 11.4 | 12.7 |
| Base excess arterial, mmol/L | -14.00 | -18 | -17.3 | -19.2 | -19 | -7.1 | -9.3 | -13.9 | -16.7 | -19.9 |
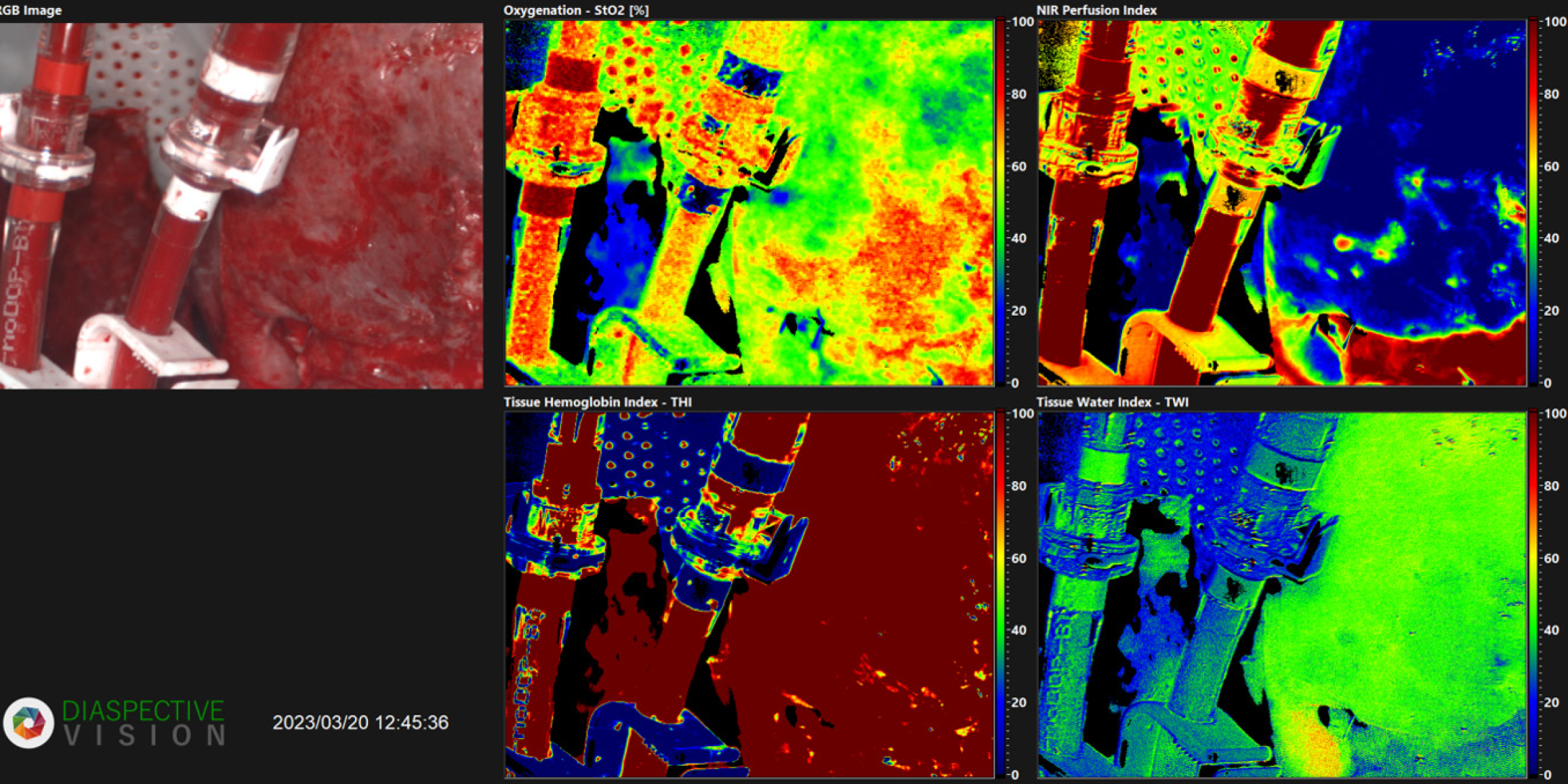
The HIS shows the hyperspectral image of the perfused liver, which demonstrates a reduced oxygenation (Figure 1).
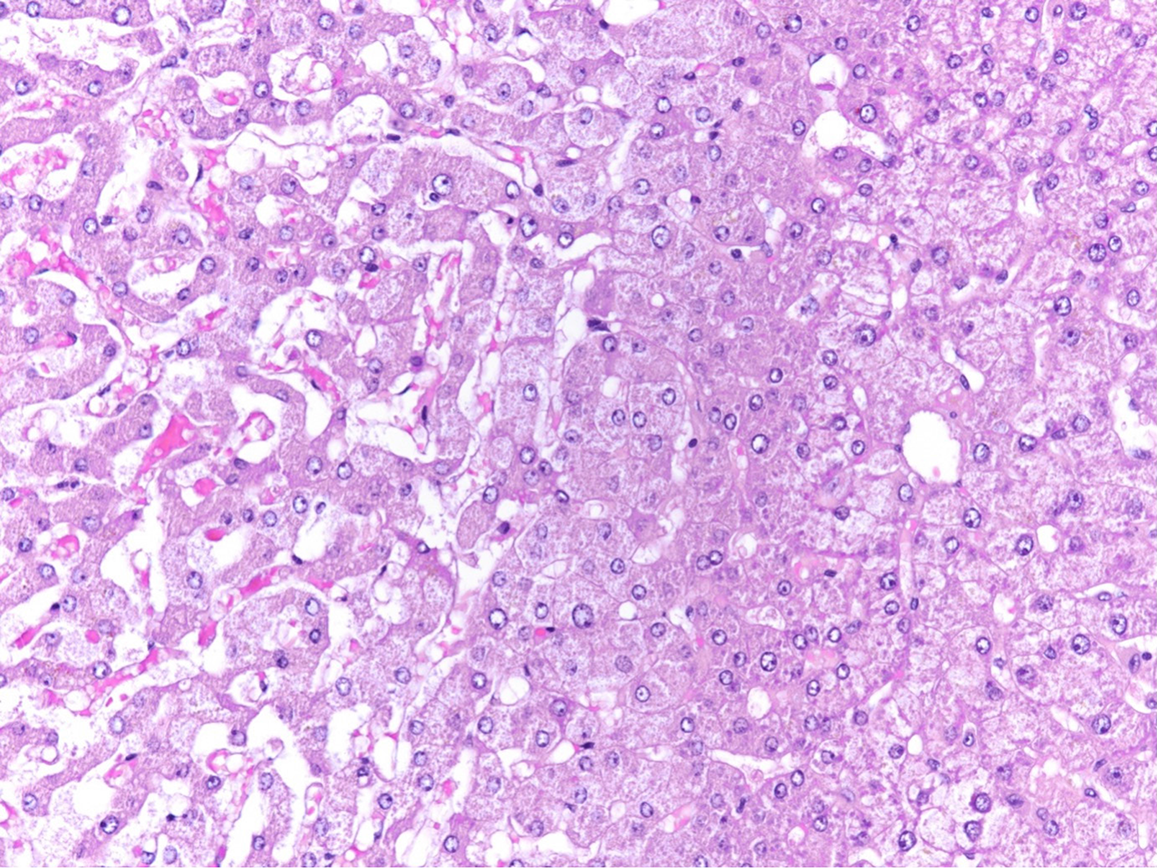
The final histological diagnosis of the discarded liver is sinusoidal dilatation and congestion, accompanied by hepatocyte atrophy and hypoxic injury (Figure 2).
This case report details a machine-perfused liver from a deceased donor that was ultimately discarded. As no transplantation took place, no treatment is applicable in this case.
The organ was discarded due to the rising lactate levels and reduced oxygenation observed in the HSI.
In light of the severe organ shortage, all available options must be exhausted today to keep the number of discarded organs as low as possible. Machine perfusion represents a promising advancement in this regard. It allows for a thorough functional assessment of organs initially deemed unsuitable. Nevertheless, not every organ is ultimately usable, and the decision for or against transplantation can be extremely difficult. The examination of the perfusate solution is crucial for assessing the condition of an organ during normothermic perfusion. However, there is a significant lack of studies that establish definitive cut-off laboratory values to determine when an organ should be discarded[3,4]. HSI can be an effective, non-invasive, and rapid method for assessing organ perfusion. This has already been demonstrated in kidney transplants, where organs with poor perfusion in HSI showed delayed graft function[1]. Additionally, recent studies investigated HSI in discarded machine-perfused livers, showing that it is a practical technology for the evaluation of organ function and viability[2,9].
Due to these promising results, we used HSI in this case report to assess organ perfusion. Macroscopic examination of the liver revealed areas of increased brightness compared to normal tissue (Figure 1). HSI identified these brighter regions, coded green, as having low oxygen saturation (40%-50%). Conversely, areas with normal perfusion appeared red in the hyperspectral image with an oxygenation of 90%-100% (Figure 1). Furthermore, deeper layers with a penetration depth of 4 to 6 millimeters were examined using near-infrared perfusion. In the under perfused areas, a significantly reduced oxygen saturation ranging from 0% to 20% was observed, depicted in blue, which indicates impaired microcirculation in the liver (Figure 1). As a result, it can be deduced that there are dysfunctions in those segments. Additionally, the lactate level rose steadily with a peak level of 4.9 mmol/L (Table 1). This suggests that a comprehensive analysis of the perfusion solution could reveal significant dysfunction of the transplant organ, necessitating its rejection. Based on the laboratory chemical findings and HSI, the decision was made to discard the organ. Subsequent histological examination confirmed these findings, indicating the presence of not only imaging clues but also histological correlations. Therefore, in our case, HIS was incorporated into the decision-making process of organ acceptance or discharge.
On closer examination of the histological and HSI images, it is noticeable that the areas of sinusoidal dilatation and congestion with atrophy and hypoxic injury of hepatocytes have occurred on the inverted segments of the liver. One possible explanation might be a perfusion impairment or an outflow obstruction because of the size discrepancy between the organ and its container. Therefore, it might cautiously be concluded that large organs are not suitable for NMP due to the size mismatch. Preliminary evidence suggests that HSI offers a promising non-invasive modality for evaluating organ function. However, a prospective study is warranted to rigorously assess the utility of this technique in organs undergoing machine perfusion. Consequently, a correlation of HSI data with traditional biochemical markers may provide a more comprehensive assessment of organ viability.
HSI is a promising tool for assessing organ viability for transplantation by providing a rapid, non-invasive method to evaluate organ function. However, the current limitations of the size constraints may restrict its application in bigger organs.
We acknowledge support from the Medical University of Graz within the program of Open Access Publishing.
| 1. | Sucher R, Wagner T, Köhler H, Sucher E, Quice H, Recknagel S, Lederer A, Hau HM, Rademacher S, Schneeberger S, Brandacher G, Gockel I, Seehofer D. Hyperspectral Imaging (HSI) of Human Kidney Allografts. Ann Surg. 2022;276:e48-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Kneifel F, Wagner T, Flammang I, Vogt F, Katou S, Vogel T, Houben P, Becker F, Wahl P, Pascher A, Radunz S. Hyperspectral Imaging for Viability Assessment of Human Liver Allografts During Normothermic Machine Perfusion. Transplant Direct. 2022;8:e1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Sousa Da Silva RX, Weber A, Dutkowski P, Clavien PA. Machine perfusion in liver transplantation. Hepatology. 2022;76:1531-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 4. | Parente A, Tirotta F, Pini A, Eden J, Dondossola D, Manzia TM, Dutkowski P, Schlegel A. Machine perfusion techniques for liver transplantation - A meta-analysis of the first seven randomized-controlled trials. J Hepatol. 2023;79:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 5. | Goldaracena N, Cullen JM, Kim DS, Ekser B, Halazun KJ. Expanding the donor pool for liver transplantation with marginal donors. Int J Surg. 2020;82S:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 6. | Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, Barton D, Curbishley S, Wilkhu M, Neil DAH, Hübscher SG, Muiesan P, Isaac JR, Roberts KJ, Abradelo M, Schlegel A, Ferguson J, Cilliers H, Bion J, Adams DH, Morris C, Friend PJ, Yap C, Afford SC, Mirza DF. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 7. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 856] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 8. | Javanbakht M, Mashayekhi A, Trevor M, Branagan-Harris M, Atkinson J. Cost-utility analysis of normothermic liver perfusion with the OrganOx metra compared to static cold storage in the United Kingdom. J Med Econ. 2020;23:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Fodor M, Lanser L, Hofmann J, Otarashvili G, Pühringer M, Cardini B, Oberhuber R, Resch T, Weissenbacher A, Maglione M, Margreiter C, Zelger P, Pallua JD, Öfner D, Sucher R, Hautz T, Schneeberger S. Hyperspectral Imaging as a Tool for Viability Assessment During Normothermic Machine Perfusion of Human Livers: A Proof of Concept Pilot Study. Transpl Int. 2022;35:10355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |