Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.101997
Revised: February 12, 2025
Accepted: February 21, 2025
Published online: September 18, 2025
Processing time: 195 Days and 20.2 Hours
Metabolic dysfunction-associated steatohepatitis (MASH) is increasingly common, as is hepatocellular carcinoma (HCC) in the background of MASH. Liver transplantation (LT) provides superior long-term survival for patients with unresectable MASH-HCC, but not all patients have equal access to transplant. MASH-HCC disproportionately affects Hispanic patients, but minorities are less likely to undergo LT for HCC. Additionally, females also undergo LT at lower rates than males.
To investigate whether race/ethnicity and sex affect LT waitlist outcomes.
Records of adults with MASH-HCC in the United States Organ Procurement and Transplantation Network database listed for LT between 1/2015 and 12/2021 were analyzed.
Most of the 3810 patients waitlisted for LT for MASH-HCC were non-Hispanic (NH) white (71.2%) or Hispanic (23.4%), with only 49 (1.1%) NH Black candidates. Hispanics underwent LT at lower rates than NH whites (71.6% vs 78.4%, P < 0.001), but race/ethnicity did not affect waitlist mortality (P = 0.06). Patients with Hispanic [hazard ratio (HR) = 0.85, 95%CI: 0.77-0.95, P = 0.002] or Asian (HR = 0.79, 95%CI: 0.63-0.98, P = 0.04) race/ethnicity were less likely to undergo LT. Women were also less likely to receive LT (male: HR = 1.16, 95%CI: 1.04-1.29, P = 0.01). Patients in regions 1 and 9 were less likely to be transplanted as well (P = 0.07).
Hispanic patients are less likely to undergo LT for MASH-HCC, concerning given their susceptibility to MASH and HCC. There were very few NH Black candidates. Disparities were also unequal across regions, which is particularly concerning in states where at-risk populations have rising cancer incidence. Additional research is needed to identify strategies for mitigating these differences in access to LT for MASH-HCC.
Core Tip: Ethnicity, sex, and geographic region affect access to liver transplantation for hepatocellular carcinoma in the background of metabolic dysfunction-associated steatohepatitis, highlighting the need for strategies to address these discrepancies.
- Citation: Victor DW, Kodali S, Noureddin M, Brombosz EW, Lopez A, Basra T, Graviss EA, Nguyen DT, Saharia A, Connor AA, Abdelrahim M, Cheah YL, Simon CJ, Hobeika MJ, Mobley CM, Ghobrial RM. Disparities in liver transplantation for metabolic dysfunction-associated steatohepatitis-associated hepatocellular carcinoma. World J Transplant 2025; 15(3): 101997
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/101997.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.101997
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly non-alcoholic fatty liver disease and steatohepatitis [metabolic dysfunction-associated steatohepatitis (MASH), formerly nonalcoholic steatohepatitis (NASH)], are increasing in prevalence worldwide, affecting an estimated 30% of the global population[1]. MASH is also the second most common etiology of non-malignant liver disease of new patients on the United States liver transplantation (LT) waitlist[2]. Having MASH is a risk factor for developing hepatocellular carcinoma (HCC)[3], and the increasing prevalence of MASH is expected to lead to a concurrent increase in HCC[4,5]. The rise in patients waitlisted for MASH-HCC is much larger than for HCC with other etiologies[2].
Unfortunately, these increases in MASH-HCC prevalence have affected some communities more than others. MASH-HCC rates are much higher in Hispanic patients than other ethnicities[4], surpassing the prevalence of hepatitis C virus-associated HCC in 2019[2,4,6]. Hispanics are also disproportionally predisposed to developing MASH[6-9] and HCC[10,11] in general, thought to be due to a combination of genetic[12,13] and environmental factors[14]. In the United States, Black patients with HCC often have reduced survival relative to patients of other races[15]. Many members of racial minorities in the United States also have reduced access to treatment for HCC, including potentially curative treatments such as resection and LT[16-20]. Minorities are also more likely to present with advanced HCC[21], which can place them outside criteria for United States Organ Procurement and Transplantation Network (OPTN) model for end-stage liver disease (MELD) exception points for LT.
Although studies have shown that minorities are less likely to receive LT for HCC[17,22,23], the effect of etiology on access to transplant has not been fully addressed. As described above, race/ethnicity can affect the likelihood of developing MASH-HCC, but it is unclear whether minorities are waitlisted and transplanted at lower-than-expected rates. The goal of this study was to determine whether race/ethnicity affects the likelihood of receiving LT for MASH-HCC in the US, and if so, to better understand potential barriers in access to transplantation for this indication. We analyze national-level predictors to determine whether patient race, ethnicity, or sex affect liver allocation, an important component of overall access to LT. Given that LT provides patients with unresectable HCC the best chance of long-term survival[24,25], it is important to identify any differences in access to LT for the growing indication of MASH-HCC.
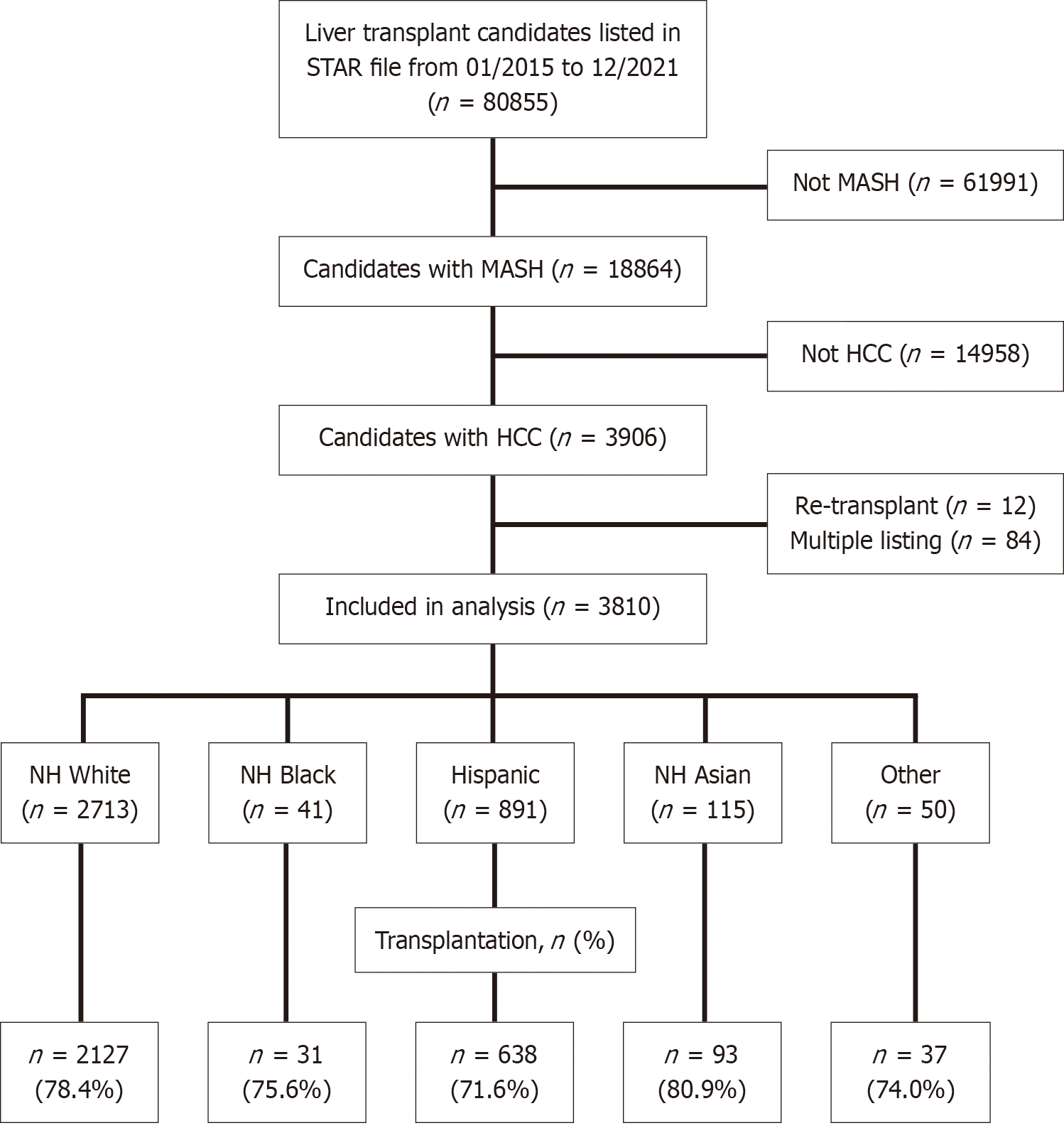
Adults with MASH-HCC waitlisted for LT in the direct-acting antiviral era between 1/2015 and 12/2021 were identified in the United States OPTN Standard Transplant Analysis and Research file. This data set was chosen for this project because it includes transplant-specific outcomes, including waitlist outcomes, a major focus of the study. The transplants and therefore diagnoses were made prior to the 2023 MASH nomenclature change[26]. Patients with HCC were identified by having a primary or secondary diagnosis of HCC. Patients with MASH were identified by: (1) NASH as a primary or secondary diagnosis; and (2) A diagnosis of cryptogenic/idiopathic cirrhosis and body mass index ≥ 30 kg/m2[27]. Multi-organ transplantation or retransplantation listings were excluded from the analysis. United Network for Organ Sharing (UNOS) region was included as a proxy for geographic regional differences in racial and ethnic diversity as an additional method for studying the association between race/ethnicity and LT.
Demographic and clinical characteristics were reported as frequencies and proportions for categorical variables and median and interquartile range (IQR) for continuous variables. Differences between racial/ethnic groups were compared using χ² or Fisher’s exact tests for categorical variables and Kruskal Wallis test for continuous variables, as appropriate. Differences between sub-groups (race/ethnicity, UNOS region) were compared using the χ² test with Bonferroni correction for multiple comparisons where appropriate.
Cox regression modeling was performed to determine characteristics associated with receiving LT. Covariates used in the multivariable models were selected based on the clinical importance and least absolute shrinkage and selection operator method with the cross-validation selection option[28,29]. The model discrimination was determined by the C-statistic. All the analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX, United States). A P value of < 0.05 was considered statistically significant.
Of the 3810 LT candidates with MASH-HCC, the majority (2713, 71.2%) were non-Hispanic (NH) white (Figure 1). Only 41 (1.1%) were Black, 891 (23.4%) were Hispanic, and 115 (3.0%) were Asian. NH Black patients spent the shortest time on the liver transplant waitlist, at a median of 190 days (IQR: 59-317), whereas Asians were on the waitlist the longest (median: 311 days; IQR: 195-496) (Table 1). There were no differences in the proportion of patients receiving HCC exception points among the racial/ethnic groups (P = 0.1) (Table 1). Listings pre-median (on or before 5/31/2019) and post-median MELD at Transplant (MMAT) (on or after 6/1/2019) were similar between races/ethnicities (P = 0.24) (Table 1).
| Non-Hispanic White (n = 2713) | Non-Hispanic Black (n = 41) | Hispanic (n = 891) | Asian (n = 115) | Other (n = 50) | Overall P value | |
| Age, median (IQR) (years) | 64.0 (59.0, 68.0) | 62.0 (56.0, 68.0) | 63.0 (59.0, 67.0) | 65.0 (60.0, 68.0) | 63.0 (58.0, 67.0) | 0.002 |
| Male sex | 1945 (71.7) | 20 (48.8) | 465 (52.2) | 65 (56.5) | 30 (60.0) | < 0.001 |
| Body mass index, median (IQR) (kg/m2) | 32.6 (29.0, 36.6) | 32.6 (28.7, 37.7) | 31.6 (28.1, 35.2) | 27.5 (25.0, 30.5) | 34.8 (29.4, 37.7) | < 0.001 |
| Days on wait list, median (IQR) | 217 (100, 355) | 190 (59, 317) | 265 (140, 424) | 311 (195, 496) | 206.5 (102, 360) | < 0.001 |
| History of diabetes at listing | 1845 (68.1) | 20 (48.8) | 603 (68.1) | 81 (70.4) | 32 (64.0) | 0.11 |
| Encephalopathy at listing | 1339 (49.4) | 15 (36.6) | 422 (47.4) | 37 (32.2) | 26 (52.0) | 0.003 |
| Ascites at listing | 1666 (61.4) | 24 (58.5) | 511 (57.4) | 53 (46.1) | 26 (52.0) | 0.004 |
| Laboratory model for end-stage liver disease at listing, median (IQR) | 12.0 (9.0, 17.0) | 13.0 (8.0, 18.0) | 12.0 (9.0, 17.0) | 10.0 (8.0, 15.0) | 13.0 (9.0, 20.0) | 0.01 |
| Albumin at listing, median (IQR) | 3.3 (2.9, 3.7) | 3.1 (2.5, 3.8) | 3.2 (2.8, 3.6) | 3.3 (2.9, 3.8) | 3.2 (2.6, 3.6) | < 0.001 |
| Creatinine at listing, median (IQR) (mg/dL) | 0.9 (0.8, 1.2) | 1.0 (0.8, 1.2) | 0.8 (0.7, 1.1) | 0.8 (0.7, 1.1) | 1.0 (0.8, 1.4) | < 0.001 |
| Bilirubin at listing, median (IQR) (mg/dL) | 1.6 (1.0, 2.7) | 1.8 (0.7, 4.3) | 1.6 (1.0, 2.8) | 1.5 (0.8, 2.1) | 1.6 (1.1, 3.6) | 0.17 |
| Received hepatocellular carcinoma exception points | 2034 (75.0) | 28 (68.3) | 703 (78.9) | 91 (79.1) | 37 (74.0) | 0.10 |
| Functional status at listing | < 0.001 | |||||
| Unknown | 68 (2.5) | 0 (0.0) | 6 (0.7) | 0 (0.0) | 0 (0.0) | |
| 10 | 3 (0.1) | 0 (0.0) | 3 (0.3) | 0 (0.0) | 0 (0.0) | |
| 20 | 36 (1.3) | 1 (2.4) | 20 (2.2) | 3 (2.6) | 1 (2.0) | |
| 30 | 57 (2.1) | 2 (4.9) | 30 (3.4) | 0 (0.0) | 3 (6.0) | |
| 40 | 195 (7.2) | 0 (0.0) | 34 (3.8) | 4 (3.5) | 4 (8.0) | |
| 50 | 299 (11.0) | 10 (24.4) | 85 (9.5) | 8 (7.0) | 6 (12.0) | |
| 60 | 398 (14.7) | 7 (17.1) | 149 (16.7) | 17 (14.8) | 7 (14.0) | |
| 70 | 626 (23.1) | 7 (17.1) | 231 (25.9) | 26 (22.6) | 6 (12.0) | |
| 80 | 615 (22.7) | 8 (19.5) | 198 (22.2) | 26 (22.6) | 11 (22.0) | |
| 90 | 338 (12.5) | 5 (12.2) | 106 (11.9) | 23 (20.0) | 8 (16.0) | |
| 100 | 78 (2.9) | 1 (2.4) | 29 (3.3) | 8 (7.0) | 4 (8.0) | |
| Working for income at registration | 774 (28.8) | 12 (29.3) | 195 (22.1) | 38 (33.0) | 20 (40.0) | < 0.001 |
| Listing era | 0.24 | |||||
| Pre-MMAT era (1/1/2015-5/31/2019) | 1653 (60.9) | 27 (65.9) | 517 (58.0) | 75 (65.2) | 26 (52.0) | |
| Post-MMAT era (6/1/2019-12/31/2021) | 1060 (39.1) | 14 (34.1) | 374 (42.0) | 40 (34.8) | 24 (48.0) | |
| Candidate final status | 0.01 | |||||
| Died | 132 (4.9) | 2 (4.9) | 66 (7.4) | 6 (5.2) | 4 (8.0) | |
| Transplanted | 2127 (78.4) | 31 (75.6) | 638 (71.6) | 93 (80.9) | 37 (74.0) | |
| Other | 454 (16.7) | 8 (19.5) | 187 (21.0) | 16 (13.9) | 9 (18.0) |
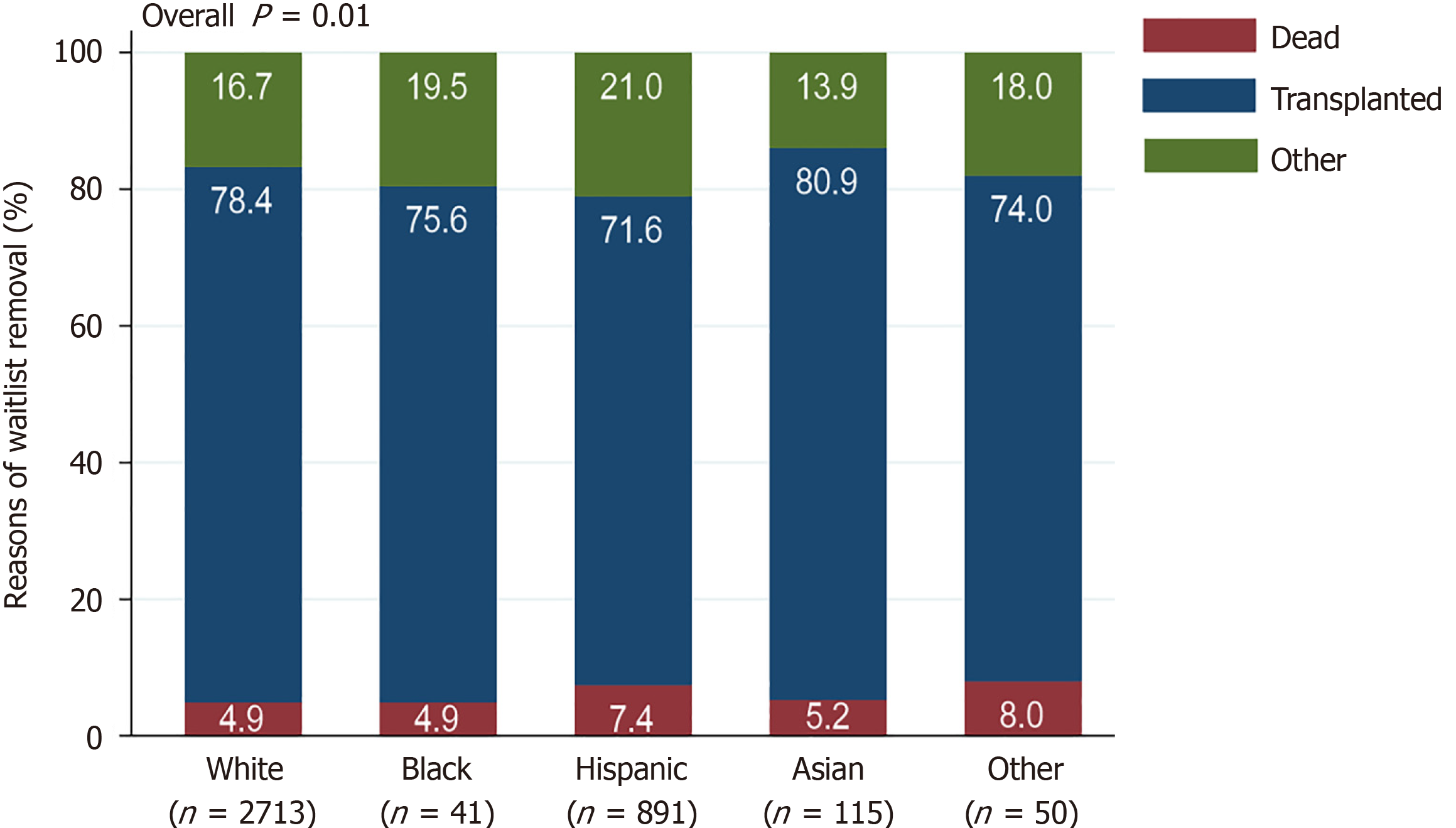
Most candidates went on to undergo LT, although the proportions of patients receiving LT among races/ethnicities were significantly different (P = 0.01) (Figure 2). In pairwise comparisons, Hispanics underwent LT at significantly lower rates than NH whites (P < 0.001) (Supplementary Table 1). Other groups received LT at similar proportions (all P > 0.05) (Supplementary Table 1). Waitlist mortality rates were not significantly different among races/ethnicities (P = 0.06) (Figure 2, Supplementary Figure 1).
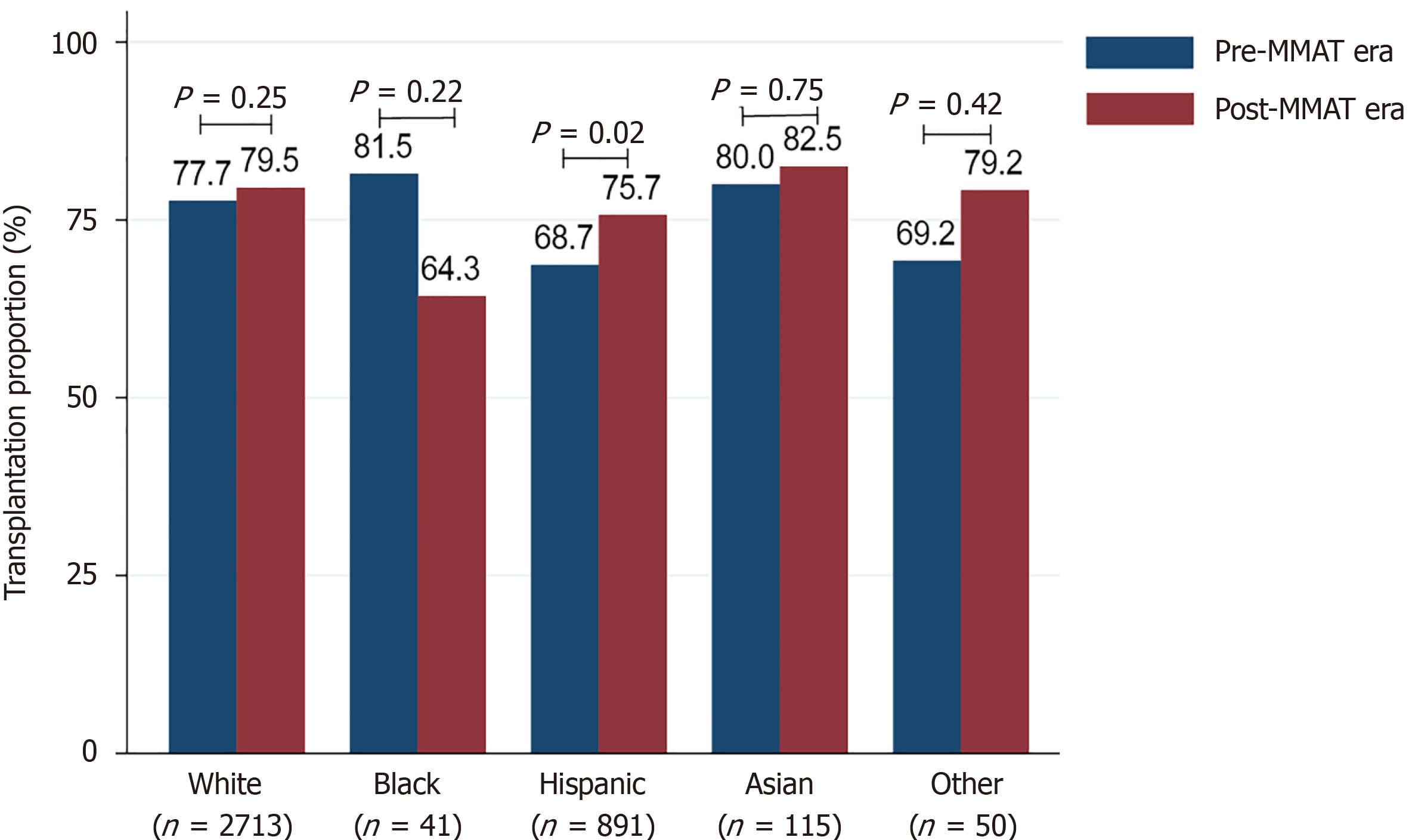
Hispanics received LT at significantly higher rates in the post-MMAT era than in the pre-MMAT era (P = 0.02) (Figure 3). MMAT era did not impact the proportion of patients undergoing LT for other races/ethnicities (all P > 0.05). Although not statistically significant, the proportion of NH Black patients receiving LT decreased from 81.5% pre-MMAT to 64.3% post-MMAT (P = 0.22) (Figure 3).
In multivariable Cox regression analysis, Hispanic [hazard ratio (HR) = 0.85, 95%CI: 0.77-0.95, P = 0.002] and Asian (HR = 0.79, 95%CI: 0.63-0.98, P = 0.04) patients were significantly less likely to receive LT than NH white patients (Table 2). Male sex was also significantly associated with receiving LT (HR = 1.16, 95%CI: 1.04-1.29, P = 0.01). Interestingly, receiving MELD exception points for HCC was associated with a lower chance of undergoing LT (HR = 0.39, 95%CI: 0.34-0.44, P < 0.001). Being listed in the post-MMAT era was also significantly associated with receiving LT (HR = 1.26, 95%CI: 1.16-1.36, P < 0.001).
| Univariable | Multivariable | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | 1.00 (0.99, 1.00) | 0.12 | 1.00 (1.00, 1.01) | 0.18 |
| Male sex | 1.17 (1.08, 1.26) | < 0.001 | 1.16 (1.04, 1.29) | 0.01 |
| Race/ethnicity | ||||
| White | (Reference) | (Reference) | ||
| Black | 1.07 (0.75, 1.52) | 0.71 | 0.78 (0.54, 1.11) | 0.17 |
| Hispanic | 0.75 (0.69, 0.82) | < 0.001 | 0.85 (0.77, 0.95) | 0.003 |
| Asian | 0.67 (0.54, 0.83) | < 0.001 | 0.79 (0.63, 0.98) | 0.04 |
| Other | 0.93 (0.67, 1.28) | 0.65 | 0.98 (0.70, 1.36) | 0.89 |
| Height (cm) | 1.01 (1.006, 1.01) | < 0.001 | 1.00 (1.00, 1.00) | 0.99 |
| Body mass index (kg/m2) | 1.00 (0.99, 1.00) | 0.15 | -- | -- |
| History of diabetes | 0.86 (0.80, 0.93) | < 0.001 | 0.97 (0.89, 1.05) | 0.41 |
| Encephalopathy | 1.31 (1.22, 1.41) | < 0.001 | 0.91 (0.83, 0.99) | 0.03 |
| Ascites | 1.36 (1.26, 1.46) | < 0.001 | 1.02 (0.93, 1.12) | 0.69 |
| Laboratory model for end-stage liver disease score | 1.08 (1.08, 1.09) | < 0.001 | 1.12 (1.11, 1.14) | < 0.001 |
| Albumin (g/dL) | 0.82 (0.77, 0.87) | < 0.001 | 1.03 (0.96, 1.10) | 0.45 |
| Serum creatinine (mg/dL) | 1.13 (1.07, 1.18) | < 0.001 | 0.87 (0.81, 0.95) | 0.001 |
| Serum bilirubin (mg/dL) | 1.14 (1.13, 1.15) | < 0.001 | 1.03 (1.01, 1.04) | 0.001 |
| Receiving hepatocellular carcinoma exception points | 0.40 (0.37, 0.43) | < 0.001 | 0.39 (0.34, 0.44) | < 0.001 |
| Functional status | ||||
| Unknown | 1.40 (1.00, 1.95) | 0.049 | 0.84 (0.59, 1.18) | 0.31 |
| 10 | 18.76 (7.59, 46.38) | < 0.001 | 1.67 (0.40, 7.07) | 0.48 |
| 20 | 6.12 (4.35, 8.60) | < 0.001 | 1.66 (1.13, 2.43) | 0.01 |
| 30 | 4.28 (3.17, 5.78) | < 0.001 | 1.31 (0.95, 1.82) | 0.10 |
| 40 | 1.80 (1.40, 2.31) | < 0.001 | 1.07 (0.82, 1.39) | 0.64 |
| 50 | 1.58 (1.25, 1.99) | < 0.001 | 1.09 (0.85, 1.40) | 0.48 |
| 60 | 1.33 (1.06, 1.67) | 0.01 | 1.01 (0.79, 1.28) | 0.96 |
| 70 | 1.33 (1.06, 1.65) | 0.01 | 1.08 (0.86, 1.35) | 0.53 |
| 80 | 1.08 (0.87, 1.35) | 0.49 | 0.96 (0.76, 1.20) | 0.70 |
| 90 | 1.05 (0.83, 1.32) | 0.71 | 0.91 (0.72, 1.15) | 0.45 |
| 100 | (Reference) | (Reference) | ||
| On mechanical ventilation | 16.54 (7.39, 37.04) | < 0.001 | 1.25 (0.34, 4.61) | 0.74 |
| International normalized ratio | 1.31 (1.28, 1.35) | < 0.001 | 0.96 (0.89, 1.04) | 0.32 |
| Working for income | 0.97 (0.90, 1.05) | 0.51 | -- | -- |
| United Network for Organ Sharing region | ||||
| 1 | (Reference) | (Reference) | ||
| 2 | 1.75 (1.42, 2.16) | < 0.001 | 1.74 (1.42, 2.15) | < 0.001 |
| 3 | 2.62 (2.15, 3.20) | < 0.001 | 2.57 (2.10, 3.15) | < 0.001 |
| 4 | 1.53 (1.25, 1.89) | < 0.001 | 1.55 (1.25, 1.92) | < 0.001 |
| 5 | 1.32 (1.08, 1.60) | 0.01 | 1.37 (1.11, 1.68) | 0.003 |
| 6 | 1.82 (1.35, 2.46) | < 0.001 | 1.95 (1.44, 2.65) | < 0.001 |
| 7 | 1.73 (1.39, 2.14) | < 0.001 | 1.72 (1.39, 2.14) | < 0.001 |
| 8 | 2.01 (1.58, 2.54) | < 0.001 | 1.74 (1.37, 2.22) | < 0.001 |
| 9 | 1.25 (0.99, 1.59) | 0.06 | 1.25 (0.98, 1.59) | 0.07 |
| 10 | 2.54 (2.05, 3.15) | < 0.001 | 2.44 (1.96, 3.04) | < 0.001 |
| 11 | 2.41 (1.96, 2.97) | < 0.001 | 2.20 (1.78, 2.72) | < 0.001 |
| Listing era | ||||
| Pre-MMAT era (1/1/2015-5/31/2019) | (Reference) | (Reference) | ||
| Post-MMAT era (6/1/2019-12/31/2021) | 1.32 (1.22, 1.42) | < 0.001 | 1.26 (1.16, 1.36) | < 0.001 |
| C-statistic = 0.70 | ||||
The effects of severity of illness on likelihood to receive LT were mixed. Encephalopathy (HR = 0.91, 95%CI: 0.83-0.99, P = 0.03) and high serum creatinine levels (HR = 0.87, 95%CI: 0.81-0.95, P = 0.001) made patients less likely to receive LT (Table 2). However, patients with high bilirubin levels (HR = 1.03, 95%CI: 1.01-1.04, P = 0.001) and MELD scores (HR = 1.12, 95%CI: 1.11-1.14, P < 0.001) were more likely to undergo LT. Functional status (Karnofsky score) largely did not affect likelihood of proceeding to LT—only a score of 20 was significantly associated with LT (HR = 1.66, 95%CI: 1.13-2.43, P = 0.01).
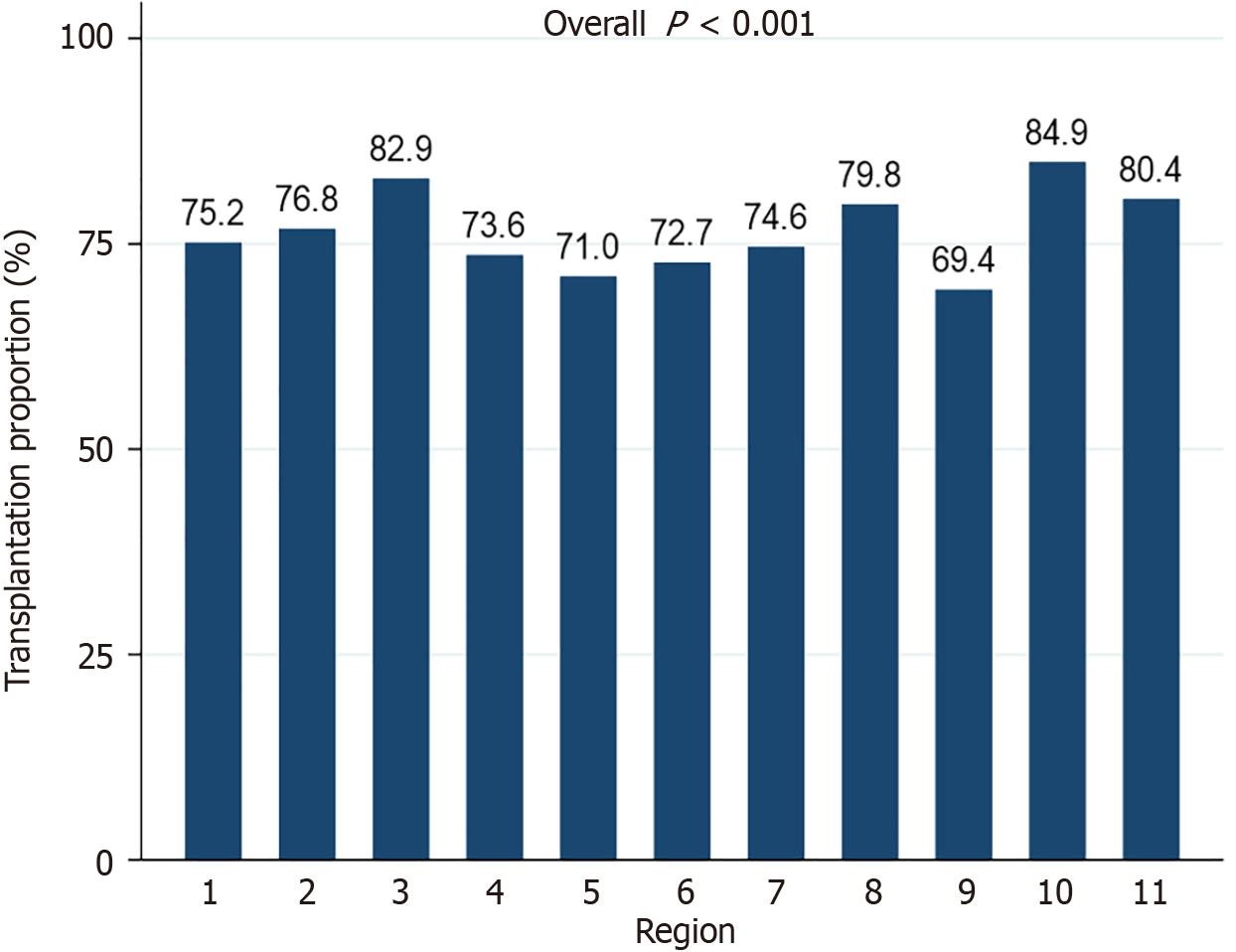
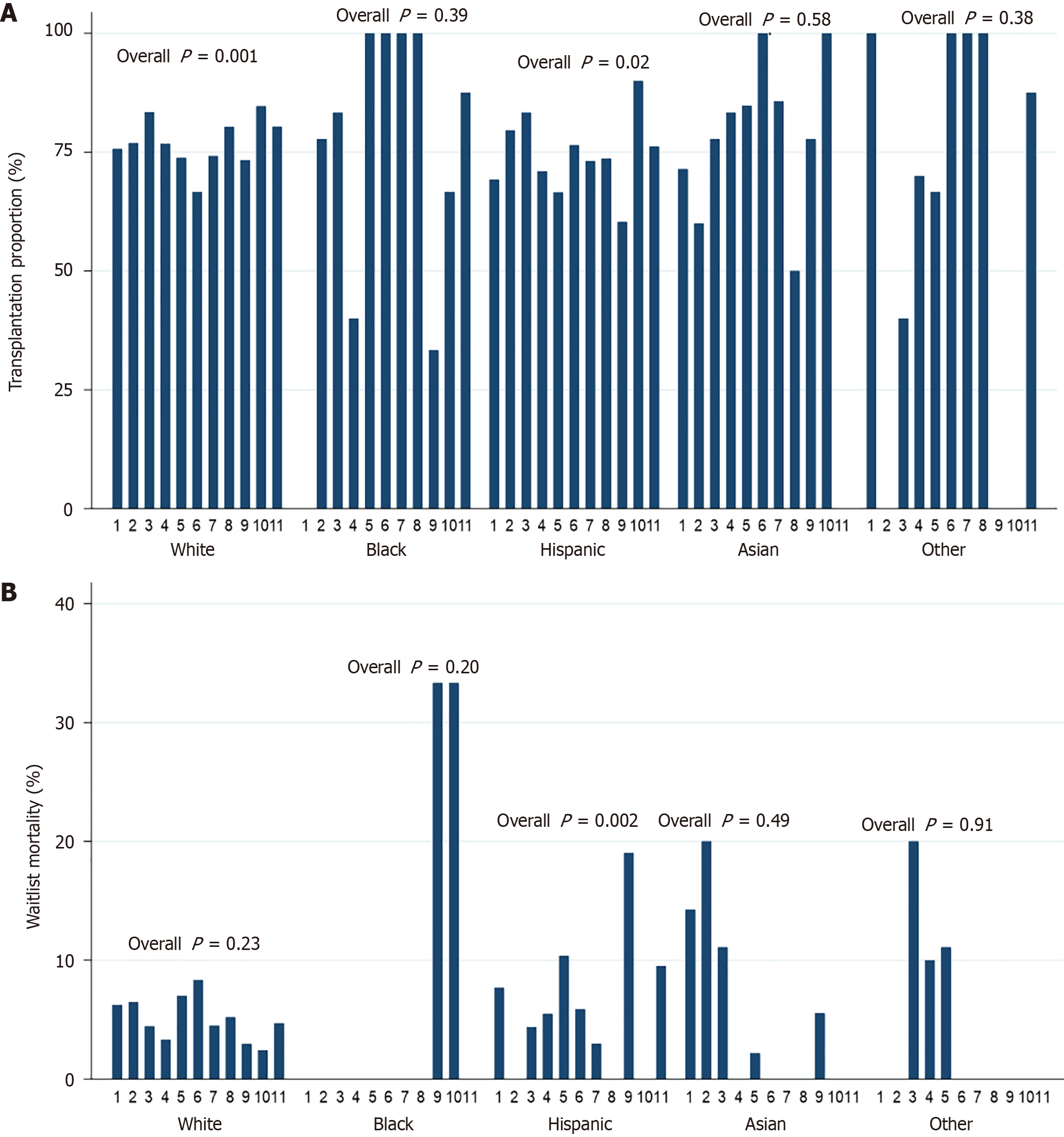
The proportion of waitlisted patients receiving LT was significantly different among the 11 UNOS regions (P < 0.001) (Figure 4). Region 9 had the lowest percentage of patients undergoing transplantation (69.4%). Region 10 had the highest percentage (84.9%). In NH whites and Hispanics, the proportion of patients receiving LT varied significantly across UNOS regions (P = 0.001 and P = 0.02, respectively) (Figure 5A). Waitlist mortality rates were significantly different across UNOS regions for Hispanics (P = 0.002), but not other races/ethnicities (all P > 0.05) (Figure 5B).
In multivariable analysis, patients in Region 1 and Region 9 were similarly likely to receive LT (HR = 1.25, 95%CI: 0.98-1.59, P = 0.07). Patients in all other regions were more likely to undergo LT than in region 1 (all P < 0.05) (Table 2). Candidates in region 3 were the most likely to receive LT for MASH-HCC (HR = 2.57, 95%CI: 2.10-3.15, P < 0.001), followed by region 10 (HR = 2.44, 95%CI: 1.96-3.04, P < 0.001) and region 11 (HR = 2.20, 95%CI: 1.16-1.36, P < 0.001).
This study highlighted several disparities in access to LT for MASH-HCC, a concerning result given the expected rise in both MASH and MASH-HCC in coming years. Studies utilizing the United States national Surveillance, Epidemiology and End-Results (SEER) registry show that Hispanic patients have very high HCC rates, and that incidence is increasing in this group[11,17,30]. Cohort studies have reported between 42% and 56% of patients with MASH-HCC are of Hispanic ethnicity[31-33]. Similarly, VoPham et al[34] showed using United States Veteran’s Affairs Corporate Data Warehouse data that Hispanics were 32% more likely to be diagnosed with HCC. Hispanics are less likely to receive LT for this indication than other races/ethnicities[17]. Our results echo differences other authors have found in access to LT for HCC of all etiologies[17] and MASLD/MASH without HCC[18]. Although other studies have highlighted how Hispanic patients are less likely to undergo LT for MASH or HCC separately[18,31], it is important to understand the specific factors affecting access for patients with MASH-HCC. Many risk factors for MASH also have an additive effect on the risk of developing HCC. It is therefore important to investigate whether high-risk populations, such as Hispanic patients, experience difficulties accessing transplant for this specific indication to identify whether interventions are needed. Improved HCC screening in high-risk Hispanic patients with MASLD or MASH and targeted outreach programs to improve transplant referrals[35] may also help mitigate access disparities.
Encouragingly, a significantly greater proportion of Hispanic patients received LT in the post-MMAT era, suggesting that efforts to improve their access to transplant are working and should be continued. Other work has shown that other allocation policy changes such as Share 35 have also increased minority access to LT. Specifically, in the post-Share 35 period, Hispanic patients were 15% more likely and Asian patients were 11% more likely to undergo LT relative to NH White patients[36]. However, it is important to note that a greater percentage of Hispanic patients with MASH-HCC underwent LT after MMAT, approximately 3 times as many NH White patients received LT for MASH-HCC each month during the post-MMAT era. Cox proportional hazards analysis also identified Asian race as an independent predictor of time to receive LT, likely due to their significantly longer time spent on the waitlist.
While is it well-described that NH Black patients are less likely to have cirrhosis from MASH[37], the proportion of Black patients waitlisted for MASH-HCC LT (1.1%) was lower than reported in other studies. For example, a recent analysis of patients with MASLD-HCC in the SEER–Medicare database found 3.2% of patients with MASLD-HCC were NH Black[38], more than double the present study. This may be due, in part, to a lower genetic propensity to develop MASLD and MASH[39], or it could be an artifact of the data set utilized. However, low overall incidence of MASH-HCC does not explain why the approximately 3-fold difference between NH Black patients with MASH-HCC in the SEER and OPTN databases. The proportion of NH Black patients undergoing LT for MASH-HCC was also lower in the post-MMAT era (64.3%) than the pre-MMAT era (81.5%), although the difference was not significant. The lack of statistical significance may have been due to the small number of NH Black patients eligible for inclusion in the study (n = 41). Additional research is needed to determine whether NH Black patients are diagnosed at lower rates than other races/ethnicities, or whether they are truly less prone to developing MASH-HCC[38].
Interestingly, receiving MELD exception points for HCC was associated with a lower chance of undergoing LT. One possible explanation is the high rates of incidental HCC seen in the explanted livers of MASH patients. Additionally, LT recipients in need of a transplant without exception points are likely to have a higher MELD overall, increasing their chances of being allocated an organ. However, further study is needed to determine if patients transplanted for MASH-HCC are more likely to be transplanted due to their biologic MELD. This may again speak to the limited access in these underserved racial communities.
Women also appear to have reduced access to transplant for MASH-HCC. Given that MASH is the most common indication for LT in women[27] and MASH is a risk factor for developing HCC, this result is concerning. Other studies have shown that women have reduced access to LT overall[40], suggesting this problem is not unique to women with MASH-HCC but other indications for LT as well. Some of the difficulties in access to LT for women may have been a result of the MELD-Na score, likely due to differences in body size and the accuracy of serum creatinine as a measure of kidney function[41]. Differences in liver disease severity and etiology, the prevalence of frailty, and psychosocial factors may also contribute to sex-based disparities[42]. Future studies should investigate the ability of the upcoming MELD 3.0 system to address these sex-based disparities[43].
Patients waitlisted for MASH-HCC in regions 5 and 9 appear to be at a disadvantage relative to patients in other UNOS regions. Given the growing incidence of obesity-related cancers such as MASH-HCC in New York (region 9)[44], among other states, this result is concerning. In particular, low proportions of NH Black and Hispanic patients received LT in region 9, and Hispanic patients experienced significantly higher mortality. Prior studies show that region 9 transplants the highest proportion of MASH patients with HCC (Supplementary Figure 2)[45]. High demand may have affected access to LT in this region, which in turn means that more resources and work are needed in these underserved areas for minorities. Waitlist mortality rates were also high for Hispanic patients in region 5, states with large Hispanic populations and growing obesity-related cancer incidence[44]. Conversely, region 3 patients were most likely to receive LT, where other studies have shown that patients of all etiologies have shorter wait times[46] and higher survival rates after listing[47]. Changes in allocation policy have affected regions and donor service areas unequally, and these differences have particularly affected waitlisted patients with HCC[48]. Our results suggest that Hispanic and NH Black patients may be disproportionately affected by regional variations in access to transplantation. Further investigation into the interactions of race/ethnicity with regional access disparities is warranted, as well as disparities within regions. Future research might also include neighborhood-level factors influencing access to LT for MASH-HCC.
This study was limited by its retrospective nature and the accuracy of data reported to UNOS/OPTN. Additionally, we are unable to draw any conclusions about disparities in access to transplant listing utilizing the OPTN database. Getting referred and evaluated for LT is a major hurdle for many patients, particularly patients of low socioeconomic status[49,50]. Black patients, Hispanic patients, and women are also less likely to be referred and evaluated for LT[17,51,52]. Reduced referral rates restrict the ability of racial/ethnic minorities and women to proceed to LT. Potential barriers to referral and evaluation in racial/ethnic minorities and potential steps for mitigating those barriers warrant further attention. Investigators should also consider using mixed methods studies to investigate race/ethnicity- and sex-based barriers in access to LT in greater depth.
In summary, patient race/ethnicity does affect ability to undergo LT for the growing indication of MASH-HCC. Females were also less likely to undergo LT, emphasizing the importance of mitigating sex-based barriers to transplant. Our study further highlights the need for improved access to LT for minorities and females as the incidence of MASH-HCC continues to rise.
| 1. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1417] [Article Influence: 708.5] [Reference Citation Analysis (2)] |
| 2. | Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, Ahmed A, Racila A, Henry L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19:580-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 362] [Article Influence: 90.5] [Reference Citation Analysis (1)] |
| 3. | Degasperi E, Colombo M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2016;1:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Zarrinpar A, Faltermeier CM, Agopian VG, Naini BV, Harlander-Locke MP, Kaldas FM, Farmer DG, Busuttil RW. Metabolic factors affecting hepatocellular carcinoma in steatohepatitis. Liver Int. 2019;39:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Koh JH, Ng CH, Nah B, Tan DJH, Loomba R, Huang DQ; Nash HCC Transplant Collaborative. NASH is the Leading Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2024;22:197-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Rich NE, Noureddin M, Kanwal F, Singal AG. Racial and ethnic disparities in non-alcoholic fatty liver disease in the USA. Lancet Gastroenterol Hepatol. 2021;6:422-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, Singal AG. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:198-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 8. | Morrill KE, Wightman P, Cruz A, Batai K, Block GD, Hsu CH, Garcia DO. Disparities in hepatocellular carcinoma incidence among Hispanic and non-Hispanic adults in Arizona: Trends between 2009-2017. Ann Epidemiol. 2024;96:48-52. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Ochoa-Allemant P, Marrero JA, Serper M. Racial and ethnic differences and the role of unfavorable social determinants of health across steatotic liver disease subtypes in the United States. Hepatol Commun. 2023;7:e0324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (1)] |
| 11. | Ha J, Yan M, Aguilar M, Bhuket T, Tana MM, Liu B, Gish RG, Wong RJ. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer. 2016;122:2512-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Thrift AP, Kanwal F, Liu Y, Khaderi S, Singal AG, Marrero JA, Loo N, Asrani SK, Luster M, Al-Sarraj A, Ning J, Tsavachidis S, Gu X, Amos CI, El-Serag HB. Risk stratification for hepatocellular cancer among patients with cirrhosis using a hepatic fat polygenic risk score. PLoS One. 2023;18:e0282309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Kanda T, Goto T, Hirotsu Y, Masuzaki R, Moriyama M, Omata M. Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma. Int J Mol Sci. 2020;21:1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Johnson NM, Qian G, Xu L, Tietze D, Marroquin-Cardona A, Robinson A, Rodriguez M, Kaufman L, Cunningham K, Wittmer J, Guerra F, Donnelly KC, Williams JH, Wang JS, Phillips TD. Aflatoxin and PAH exposure biomarkers in a U.S. population with a high incidence of hepatocellular carcinoma. Sci Total Environ. 2010;408:6027-6031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, Wagman LD, Nissen N, Colquhoun SD, Kim J. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Alawadi ZM, Phatak UR, Kao LS, Ko TC, Wray CJ. Race not rural residency is predictive of surgical treatment for hepatocellular carcinoma: Analysis of the Texas Cancer Registry. J Surg Oncol. 2016;113:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Robinson A, Ohri A, Liu B, Bhuket T, Wong RJ. One in five hepatocellular carcinoma patients in the United States are Hispanic while less than 40% were eligible for liver transplantation. World J Hepatol. 2018;10:956-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Lim WH, Yong JN, Ong CEY, Ng CH, Tan DJH, Zeng RW, Chung CH, Kaewdech A, Chee D, Tseng M, Wijarnpreecha K, Syn N, Bonney GK, Kow A, Huang DQ, Noureddin M, Muthiah M, Tan E, Siddiqui MS. Ethnic disparities in waitlist outcomes of patients with nonalcoholic steatohepatitis listed for liver transplantation in the US. Liver Transpl. 2023;29:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 19. | Beltrán Ponce S, Gokun Y, Douglass F, Dawson L, Miller E, Thomas CR Jr, Pitter K, Conteh L, Diaz DA. Disparities in outcomes and access to therapy options in hepatocellular carcinoma. J Natl Cancer Inst. 2024;116:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Agudile EP, Vega EA, Salirrosas O, Agudile UM, Chirban AM, Lathan C, Sorescu GP, Odisio BC, Panettieri E, Conrad C. Temporal trends of health disparity in the utilization of curative-intent treatments for hepatocellular carcinoma: are we making progress? J Gastrointest Surg. 2024;28:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Robinson A, Tavakoli H, Liu B, Bhuket T, Wong RJ. Advanced Hepatocellular Carcinoma Tumor Stage at Diagnosis in the 1945-1965 Birth Cohort Reflects Poor Use of Hepatocellular Carcinoma Screening. Hepatol Commun. 2018;2:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Yu JC, Neugut AI, Wang S, Jacobson JS, Ferrante L, Khungar V, Lim E, Hershman DL, Brown RS Jr, Siegel AB. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Di Sandro S, Sposito C, Ravaioli M, Lauterio A, Magistri P, Bongini M, Odaldi F, De Carlis R, Botta F, Centonze L, Maroni L, Citterio D, Guidetti C, Bagnardi V, De Carlis L, Cescon M, Mazzaferro V, Di Benedetto F; HV-HCC-MRT-group. Surgical Treatment of Hepatocellular Carcinoma: Multicenter Competing-risk Analysis of Tumor-related Death Following Liver Resection and Transplantation Under an Intention-to-treat Perspective. Transplantation. 2023;107:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Koh JH, Tan DJH, Ong Y, Lim WH, Ng CH, Tay PWL, Yong JN, Muthiah MD, Tan EX, Pang NQ, Kim BK, Syn N, Kow A, Goh BKP, Huang DQ. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg Nutr. 2022;11:78-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1212] [Cited by in RCA: 1304] [Article Influence: 652.0] [Reference Citation Analysis (0)] |
| 27. | Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 28. | O'Brien CM. Statistical Learning with Sparsity: The Lasso and Generalizations. In: Bura E, Markatou M, editor. International Statistical Review. United States: Wiley, 2016; 84: 156-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | StataCorp. Stata Base Reference Manual. United States: Stata Press, 2011. |
| 30. | Alvarez CS, Petrick JL, Parisi D, McMahon BJ, Graubard BI, McGlynn KA. Racial/ethnic disparities in hepatocellular carcinoma incidence and mortality rates in the United States, 1992-2018. Hepatology. 2022;76:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, Yopp AC, Singal AG. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2019;17:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 32. | Venepalli NK, Modayil MV, Berg SA, Nair TD, Parepally M, Rajaram P, Gaba RC, Bui JT, Huang Y, Cotler SJ. Features of hepatocellular carcinoma in Hispanics differ from African Americans and non-Hispanic Whites. World J Hepatol. 2017;9:391-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Hester CA, Rich NE, Singal AG, Yopp AC. Comparative Analysis of Nonalcoholic Steatohepatitis- Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2019;17:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | VoPham T, Cravero A, Feld LD, Green P, Feng Z, Berry K, Kim NJ, Vutien P, Mendoza JA, Ioannou GN. Associations of Race and Ethnicity with Hepatocellular Carcinoma, Decompensation, and Mortality in US Veterans with Cirrhosis. Cancer Epidemiol Biomarkers Prev. 2023;32:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Kodali S, Mobley CM, Brombosz EW, Lopez A, Graves R, Ontiveros J, Velazquez M, Saharia A, Cheah YL, Simon CJ, Valverde C, Brown A, Corkrean J, Moore LW, Graviss EA, Victor DW 3rd, Maresh K, Hobeika MJ, Egwim C, Ghobrial RM. Effect of a Hispanic outreach program on referral and liver transplantation volume at a single center. Transpl Immunol. 2024;84:102034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 36. | Zhang Y. The Impact of the Share 35 Policy on Racial and Ethnic Disparities in Access to Liver Transplantation for Patients with End Stage Liver Disease in the United States: An Analysis from UNOS Database. Int J Equity Health. 2017;16:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Qayed E, Migdal AL, Jagannathan R, Miller LS, Pasquel FJ. Characteristics and Outcomes of Black and White Patients Hospitalized With Nonalcoholic Steatohepatitis: A Nationwide Analysis. J Clin Gastroenterol. 2023;57:508-514. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Karim MA, Singal AG, Kum HC, Lee YT, Park S, Rich NE, Noureddin M, Yang JD. Clinical Characteristics and Outcomes of Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma in the United States. Clin Gastroenterol Hepatol. 2023;21:670-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 39. | Wijarnpreecha K, Scribani M, Raymond P, Harnois DM, Keaveny AP, Ahmed A, Kim D. PNPLA3 gene polymorphism and overall and cardiovascular mortality in the United States. J Gastroenterol Hepatol. 2020;35:1789-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation. 2018;102:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 41. | Locke JE, Shelton BA, Olthoff KM, Pomfret EA, Forde KA, Sawinski D, Gray M, Ascher NL. Quantifying Sex-Based Disparities in Liver Allocation. JAMA Surg. 2020;155:e201129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 42. | Hundt MA, Tien C, Kahn JA. Addressing sex-based disparities in liver transplantation. Curr Opin Organ Transplant. 2023;28:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, Wood NL, Gentry SE, Kwong AJ. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021;161:1887-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 337] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 44. | Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, Puckett M, Richardson LC. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2017;66:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 45. | Yuan L, Hanlon CL, Terrault N, Alqahtani S, Tamim H, Lai M, Saberi B. Portrait of Regional Trends in Liver Transplantation for Nonalcoholic Steatohepatitis in the United States. Am J Gastroenterol. 2022;117:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol. 2018;31:84-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Rana A, Riaz IB, Gruessner AC, Gruessner RW. Geographic inequity results in disparate mortality: a multivariate intent-to-treat analysis of liver transplant data. Clin Transplant. 2015;29:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Shah RH, Chyou D, Goldberg DS. Impact of major hepatocellular carcinoma policy changes on liver transplantation for hepatocellular carcinoma in the United States. Liver Transpl. 2022;28:1857-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Ng YH, Pankratz VS, Leyva Y, Ford CG, Pleis JR, Kendall K, Croswell E, Dew MA, Shapiro R, Switzer GE, Unruh ML, Myaskovsky L. Does Racial Disparity in Kidney Transplant Waitlisting Persist After Accounting for Social Determinants of Health? Transplantation. 2020;104:1445-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 50. | Strauss AT, Moughames E, Jackson JW, Malinsky D, Segev DL, Hamilton JP, Garonzik-Wang J, Gurakar A, Cameron A, Dean L, Klein E, Levin S, Purnell TS. Critical interactions between race and the highly granular area deprivation index in liver transplant evaluation. Clin Transplant. 2023;37:e14938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Park C, Jones MM, Kaplan S, Koller FL, Wilder JM, Boulware LE, McElroy LM. A scoping review of inequities in access to organ transplant in the United States. Int J Equity Health. 2022;21:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 52. | Yakovchenko V, Chang MF, Hernaez R, Awad JA, Anwar J, Nobbe A, McCurdy H, Sharma P, Spoutz P, Murugavel M, Wilson MA, Dominitz JA, Patton HM, Adams MA, Morgan TR, Rogal SS. Access to Evaluation for Liver Transplantation in the Veterans Health Administration. Dig Dis Sci. 2025;70:552-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |