Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.99287
Revised: November 7, 2024
Accepted: January 11, 2025
Published online: June 18, 2025
Processing time: 217 Days and 13.3 Hours
Marginal donation after circulatory death (DCD) liver grafts are carefully used to combat the constant shortage of donors. Clinically, the worst outcomes are mainly related to severe ischemia-reperfusion-injury and the dangerous effect of various inflammatory cytokines (CK). The machine perfusion (MP) is a promising device to rescue these grafts.
To analyze the role of MP connected to a sorbent cartridge (PerSorb®) and used for very damaged DCD pig livers.
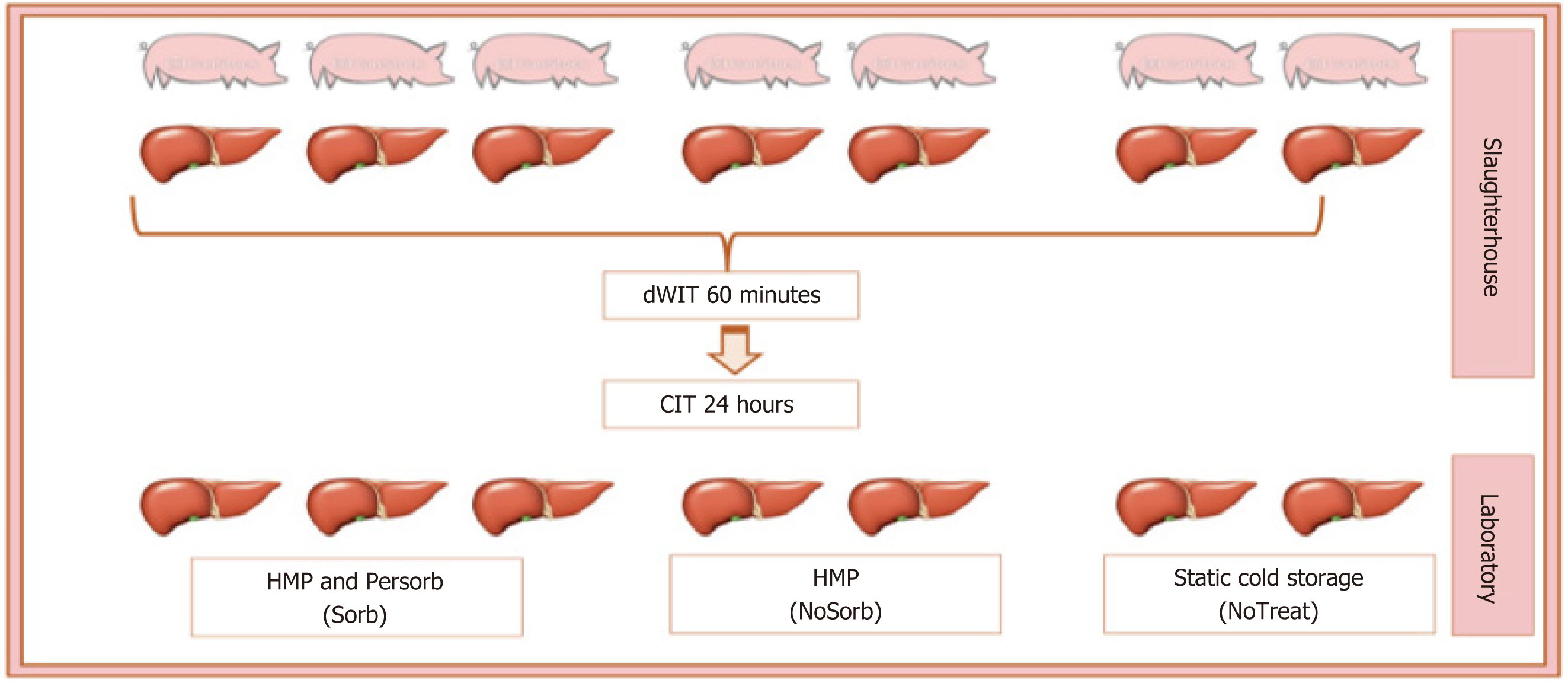
Seven grafts were procured from pigs from a slaughterhouse. Grafts were made very marginal with at least 60 minutes of donor warm ischemia time and 24 hours of static-cold ischemia time: (1) 3 grafts were perfused in hypothermic MP with PerSorb (Sorb); (2) 2 other grafts in hypothermic MP (HMP) without the cartridge (NoSorb); and (3) The other 2 livers stored in the ice box (NoTreat). The CK were measured at HMP start (T0) and at the end (Tend). Biopsies were taken at T0 and Tend.
All 5 grafts treated with HMP had a negative lactate trend after 3 hours of treatment (8.83 at T0 vs 6.4 at Tend of Sorb; 15 at T0 vs 5.45 at Tend for NoSorb, P value > 0.05). At Tend, both Sorb and NoSorb groups had better hemodynamic parameters, comparable between the two groups. Enzyme-linked immunosorbent assay analysis showed a reduction of monocyte chemotactic protein-1, tumor necrosis factor-alpha and interleukin-1β for NoSorb group at Tend and a complete downregulation to physiological levels of the same CK in Sorb livers after 3 hours of treatment. Biopsies showed a reduction of the perisinusoidal edema for the Sorb grafts compared with the NoSorb livers.
These data suggest a potential protective role of treatment of grafts with MP and sorbent cartridge in reducing the inflammatory response after a severe ischemic injury.
Core Tip: Marginal donation after circulatory death liver grafts are carefully used to fight the constant shortage of donors. Clinically, the worst outcomes are mainly related to severe ischemia-reperfusion-injury and the dangerous effect of various inflammatory cytokines (CK). The machine perfusion (MP) is a promising device to rescue these grafts. Recently, a new ex-situ MP device incorporating a filter cartridge to the machine has been considered. It was tested on pigs, and it appears to reduce the CK during the treatment of standard liver grafts. The purpose of the study was the analysis of the role of the CK cartridge connected to the MP and used to preserve extremely marginal pig grafts during processing in the MP. The results were analyzed biochemically and histologically.
- Citation: Scalera I, Franzin R, Stasi A, Castellaneta A, Fischetti E, Morelli G, Raele M, Panetta E, Kurevija A, Pulga W, Atti M, Gesualdo L. Haemoadsorption cartridge connected to the machine perfusion for donation after circulatory death porcine liver marginal grafts. World J Transplant 2025; 15(2): 99287
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/99287.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.99287
Ischemia-reperfusion-injury (IRI) is the cornerstone of the pathogenesis of major post-transplant complications. Increased severity of IRI correlates with inferior short-term graft outcomes[1]. Many donors related risk factors have been related to the severity of IRI. Severe steatosis, prolonged static-cold ischemia time (S-CIT), donation after circulatory death (DCD) are strong predictors of poor post-transplant liver function and donors with these characteristics have been classified as “marginal”[2].
Nowadays these grafts are used more often as the tremendous shortage of organs almost imposes to do to fight the mortality of the patients in waiting list for a transplant[3].
The IRI pathway is complex: Many cell types such as hepatocytes, Kupffer cells, neutrophils, platelets and many others interact with an intricate mechanism. These cells act through numerous inflammatory cytokines (CK) that cause cell death. Reperfusion injury is characterized by a loss of endothelial cell viability associated with activation of Kupffer cell. Proinflammatory CK, especially tumor necrosis factor-alpha (TNF-α), are presumed to have a key role in this process. Elevated levels of CK have been reported after liver transplantation, as well as after other types of surgery[4].
Machine perfusion (MP) has been used to overcome the IRI damage that starts from the time of organ retrieval and manifest with clinical outcomes in the recipient during and after the transplantation[5]. So far, this device has been helpful to preserve marginal grafts and to combat the shortage of organs. Many MP technologies have been developed to treat grafts before the implantation[3]. Recently, a new ex-situ MP device incorporating a filter cartridge to the machine has been considered. It was tested on pigs, and it appears to reduce the CK during the treatment of standard liver grafts.
Persorb (Sorb) is a sorbent cartridge based on well-known highly biocompatible polystyrene divinylbenzene copo
The purpose of the study was the analysis of the role of the CK cartridge connected to the MP and used to preserve very marginal pig grafts during processing in the MP. The results were analyzed biochemically and histologically.
The study was conducted using livers from slaughter pigs and in accordance with national rules for experimental studies and slaughtered for commercial purpose. All necessary standard measures were taken to preserve the animal welfare, as required by European legislation (EC 1099/2009). The retrieval was handled as already described in literature[9]. In a nutshell, the pigs were sacrificed by causing intracranial hemorrhage followed by the usual exsanguination. The slaughterhouse technician performed a rapid thoraco-laparotomy, and all abdominal organs were retrieved en-block, without interfering with the food production. The abdominal block was transferred to a nearby room to be cold washed by directly canulating the celiac trunk (or splenic artery) and portal vein (PV) and using StoreProtect® solution, (Store
The interval from the induction of the death until the start of the cold flushing was recorded and considered as donor warm ischemia time (dWIT). The time from cold flushing until the start of the MP treatment was referred to as S-CIT.
Seven livers were obtained from this experimental model of DCD and, after the slaughter, were made very marginal. They were subjected to at least 60 minutes of dWIT and then flushed and stored in an ice box for at least 24 hours (32 hours ± 15 hours). The livers were transported to a research laboratory for the treatment. On the day after retrieval, 3 livers were treated in MP, in hypothermic mode with dual perfusion and with Sorb (CytoSorbents INC, New Jersey, United States) connected (Sorb group); 2 other grafts in hypothermic MP without cartridge (NoSorb) and the other 2 livers underwent no treatment after the S-CIT (NoTreat) (Figure 1).
The machine used was PerLife® with PerLiver™ operational mode (PerLife®, Aferetica srl, Bologna, Italy), which has been described in the literature[10]. It was operated at temperature of 8-12 °C and with an average oxygenation flow of 2000 mL/minute at 100%. The pressure was 25 mmHg for the hepatic artery (HA) and 5 mmHg for the PV. The duration of treatment was 3 hours. Sorb cartridge was use for the Sorb group. The perfusion fluid used during the treatment was PumpProtect® (PumpProtect® solution, Carnamedinca, Warsaw, Poland).
For Sorb and NoSorb groups, during all the machine treatments, the pressure, flow and resistance of the arterial and portal circuit were monitored continuously, and the values were recorded at the beginning (T0), after two hours (T2) and at the end (Tend). A comparison between the final and initial parameters and between Tend of Sorb and Tend of NoSorb group was performed.
Perfusion fluid samples were taken at T0, T2 and Tend for Sorb and NoSorb and at the end of the S-CIT for NoTreat group. Lactate concentration was measured by a standard blood gas analysis and compared before and after the treatment for Sorb and NoSorb groups; the 3 levels of lactate at Tend were also compared.
The elapsed time from induction of the death to cold perfusion was considered as dWIT. The elapsed time from cold preservation to the start of the treatment in the MP was considered as S-CIT.
TNF-α, interleukin (IL)-6, IL-1β and monocyte chemotactic protein-1 (MCP-1) levels were measured by enzyme-linked immunosorbent assay (R and D Systems, Minneapolis MN, United States) as well as IL-1β and MCP-1 (MyBioSource, San Diego CA, United States) in liver perfusates at T0, T2 and Tend.
A liver biopsy was taken at the beginning of experimental procedure (T0). Additional biopsies were then obtained at different intervals during hypothermic MP (HMP) treatment (T2, Tend). A portion of each biopsy specimen was immediately placed in RNA-later and stored at 4 °C overnight, then at -80 °C. Another portion was fixed in buffered formalin (4%) for 12 hours and embedded in paraffin using standard hematoxylin and eosin (HE) (Millipore Sigma) and Periodic Acid Shift staining procedures. Digital slides were acquired and analyzed using the AperioScanScope CS2 device (Aperio, Vista, CA, United States) as previously described. HE staining was performed to assess histological damage. Liver injury was semi-quantitatively scored and assigned by two blinded observers. The score index in each animal was expressed as a mean value of all scores obtained. Hepatic injury was calculated as the extent of lobular damage, inflammatory cells infiltration, hemorrhage and interstitial necrosis. As indicated in a previously published study[11], the score ranging from 1 to 5 was reported. The pathological score for each group was expressed as mean ± SEM.
Statistical analysis was performed using the Wilcoxon test when To and Tend values of the same group were compared; a Mann Whitney test (unpaired, non-parametric tests) was used when Tend were analyzed among the three groups (Sorb, NoSorb and NoTreat) and for the two groups (Sorb and NoSorb). The significance level was set at 0.05 IBM® Statistical Package for the Social Sciences (version 24) was used for the analyses.
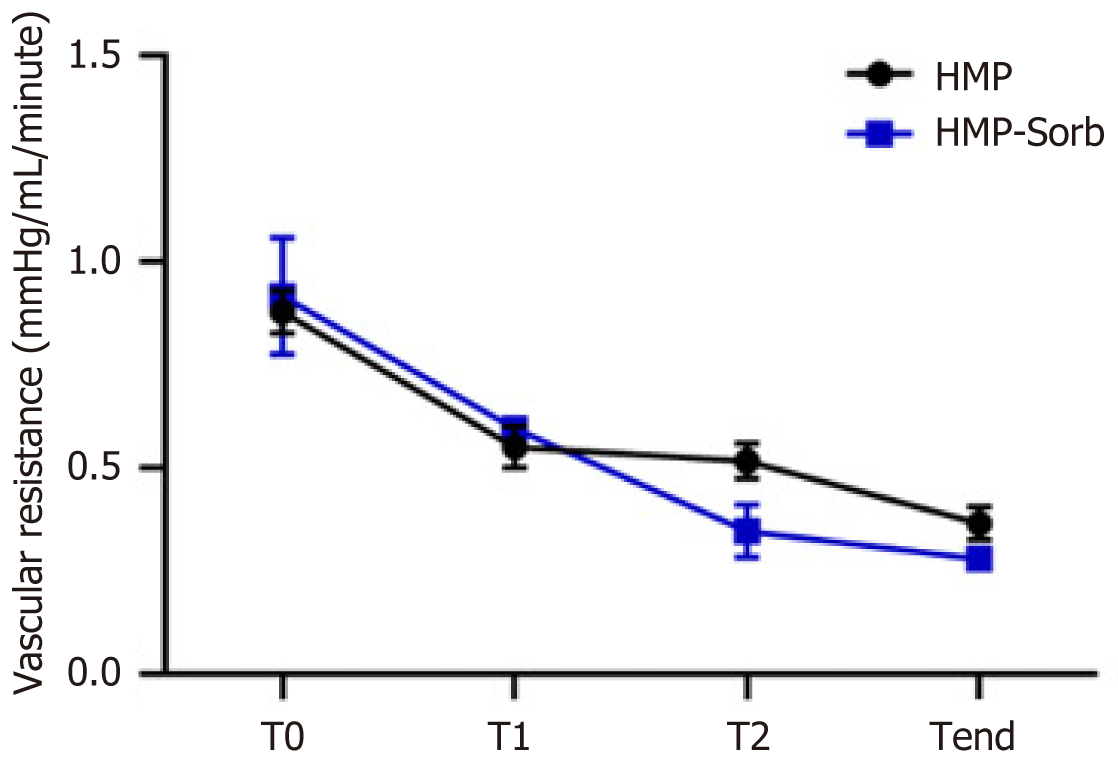
Seven grafts were procured from purebred pigs, weighing 190-210 kg. The MP lasted 180 minutes. Table 1 summarizes all parameters and lactate results expressed in means. HA and PV hemodynamic parameters improved during the MP cycle for the treated groups (Sorb and NoSorb), although the results were not statistically significant. Lactate concentration decreased steadily toward a clinically acceptable level from T0 to Tend of each group, but there was no statistical difference between lactate at T0 and Tend. (Table 1, Figure 2)
| Parameters | Groups | T0 | Tend | P value (Tend vs T0) |
| Hepatic artery resistance (mmHg/mL/minute) (mean ± SD) | Sorb | 0.62 ± 0.17 | 0.48 ± 0.07 | 0.11 |
| NoSorb | 0.76 ± 0.25 | 0.56 ± 0.01 | 0.18 | |
| Tend P value | 1 | |||
| Hepatic artery flow (mL/minute) (mean ± SD) | Sorb | 66 ± 33 | 85 ± 9 | 0.11 |
| NoSorb | 56 ± 41 | 75 ± 28 | 0.18 | |
| Tend P value | 0.4 | |||
| Portal vein flow (mL/minute) (mean ± SD) | Sorb | 345 ± 78 | 359 ± 97 | 0.28 |
| NoSorb | 321 ± 89 | 317 ± 146 | 0.65 | |
| Tend P value | 1 | |||
| Lactate (mg/mL) (mean ± SD) | Sorb | 8.83 ± 2.11 | 6.4 ± 0.88 | 0.11 |
| NoSorb | 15 ± 0.00 | 5.45 ± 2.05 | 0.18 | |
| NoTreat | 15 | |||
| Tend P value | 0.13 |
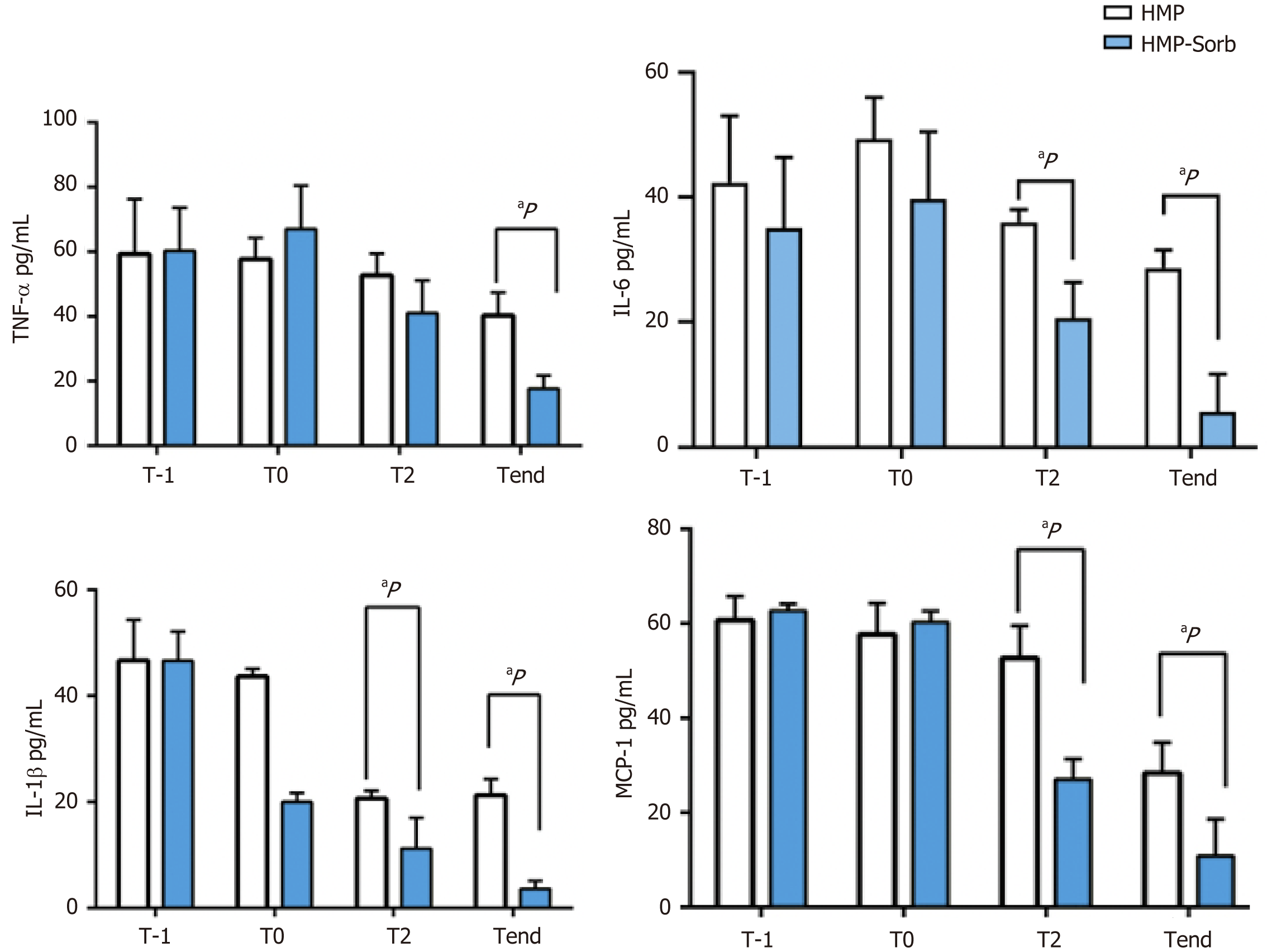
Baseline CK levels (T0) were similar between the two groups (P > 0.005). TNF-α level decreased during the 3 -hour perfusion (Tend) and the downregulation in Sorb group was statistically significant compared with the NoSorb group, although modulation was observed compared with T0 (P < 0.005). A similar trend was observed for IL-6, IL-1β and MCP-1 with a significant modulation as early as after 2 hours of perfusion (P < 0.005) (Figure 3).
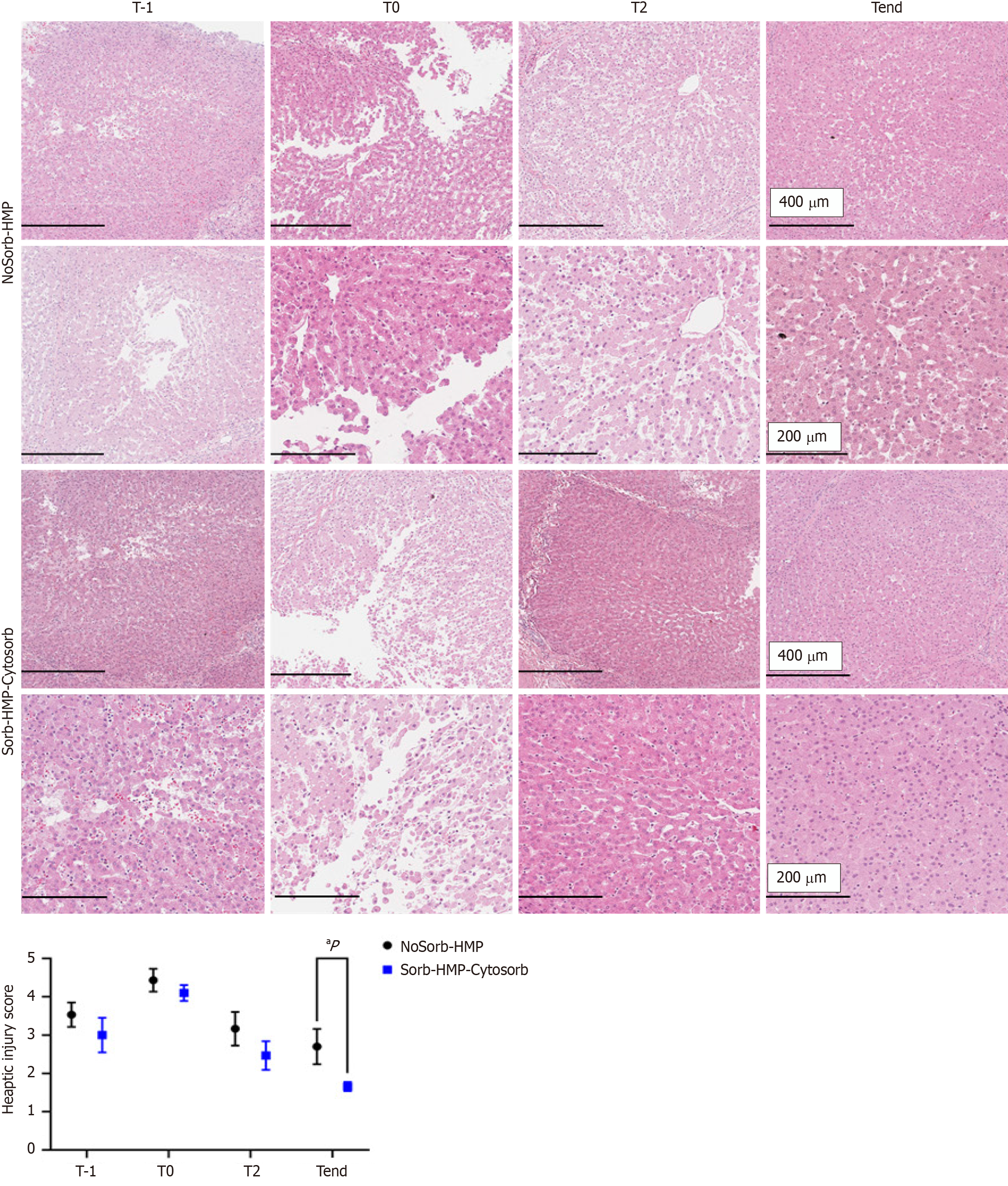
Histological changes were examined in all grafts. To detect the effect of static cold storage injury, liver biopsies were analyzed, thereby retrieved at the slaughterhouse immediately after the dWIT (T-1) and the cold flush and T0 liver biopsies, collected after long-time of cold static storage in ice. T-1 liver biopsies showed a moderate/high of tissue damage with dilation of sinusoids in the lobule. T0 biopsies showed reduced liver architecture, with dilation sinusoids, congestion, portal inflammation, microvacuolization, and infiltrating inflammatory cells. Hepatocytes appeared swollen and the hepatic cordae were disorganized. After treatment with HMP, the interlobular septa of pig livers repaired, and tissue architecture were improved.
The importance of controlling CK storm in liver transplantation has been well described, especially in DCD organs, steatotic livers, and grafts subjected to prolonged warm or cold storage[12]. Many strategies have been used to rescue these grafts and, many devices have been implemented to warm and to oxygenate the livers ex situ with good results.
The simultaneous use of MP and adsorption cartridge in the same device was recently tested in an animal model with a standard DCD liver graft[10]. The present study aimed to save very marginal DCD livers. This was achieved with a prolonged dWIT and S-CIT, then with a double severe ischemic hit to the already damaged grafts. Notably, all procedures were performed in accordance with the national experimental animal model law. All the grafts were treated after the slaughter technician interventions.
During the treatment in the MP, the flows and resistances of Sorb and NoSorb groups improved, although the final parameters were not statistically significant. (Table 1). This effect parallelled with the improvement in lactate trend. Although this trend was also not significant, it was noted an end level that in a clinical setting would refer to a suitable level of functioning graft. Moreover, the T0 level was often the highest value that the machine could detect (15 mg/dL), but not the actual value that could have been even higher, therefore the actual trend could be statistically significant. It was not possible to correlate this trend to other quality factors, as all the pigs were from the same weight, age and they suffer of the same ischemic times. Therefore, the basic risk factors were similar among the grafts. Notably, the negative lactate trend could not be associated to the direct effect of the cartridge, since the cartridge absorbs hydrophobic molecules, such as CK, and not hydrophilic component, such us lactate[10]. Therefore, the lactate levels at Tend could be explained by a regeneration of the liver function during the MP treatment.
This restoration could be explained by the reduction of CKs involved in the IRI, as observed for IL-1β, TNF-α and MCP-1 (Figure 3). IL-1β is an early response CK that promotes the process of IRI inducing TNF-α expression. The TNF-α is well known as the most important mediator that propagates the inflammatory response by upregulating adhesion molecules on endothelial cells and promoting expression of CK by hepatocytes. The neutrophil recruitment in the liver is facilitated and it leads to necrotic cell death[13].
These results were mirrored by histological specimens. Tend biopsies showed a regenerated graft, considering the very damaged conditions before the treatment, although significant changes between HMP and HMP-Sorb were detectable in only a few livers (Figure 4). T-1 liver biopsies retrieved at the slaughterhouse showed low/absent liver damage. T0 liver biopsies, collected after long-time of cold static storage in ice showed diminished liver architecture, with sinusoid dilation, congestion, portal inflammation, microvacuolization and infiltrating inflammatory cells. The hepatocytes appeared swollen and the hepatic cords were disorganized, furthermore exhibited patchy subcapsular hepatocyte necrosis (black arrows). After HMP treatments, repaired interlobular septa of pig livers and improved tissue architecture were detected. Significant histological alteration was detected at Tend in HMP and HMP Sorb group. All these results were graphically summarized with the trend of the hepatic injury score, showed in Figure 4.
This model may encourage to use safely grafts that suffer of IRI, such as livers with macro-steatosis > 30%. These livers indeed, are associated with inferior post-transplant outcomes[14], due to their high susceptibility to oxidative stress, impairment of circulation and all the inflammatory processes, mediated by CK[15].
The real challenge of this study was encountered in using such damaged grafts, after a very long period of ischemic times that in a clinical setting was not be thinkable to use. The 60 minutes of dWIT added to 24 hours of S-CIT are a very long times and so far, all the efforts are to reduce these timings, rather than to try to use them as they are. Implanting these grafts might expand the donors pool and plan the transplant.
The present work has some limitations: (1) It is an experimental and preliminary study; and (2) It does not have the clinical arm to demonstrate a correlation with post-transplant outcome. In addition, the samples are small. The research was performed during a normal working day for the slaughter, and it was not possible to obtain more livers.
Importantly, the livers included in the study were severely damaged and in a human clinical setting would never have met the transplant criteria prior to perfusion. However, using these slaughterhouse livers, serial wedge biopsies could be taken to characterize the parenchymal tissue and level of injury. Interestingly, such damaged livers showed the ability to clear elevated levels of lactates down to a very low level. The increased CK concentrations observed during whole perfusion suggested that decrease in lactate levels during the MP is not only due to potential dilution alone but may instead suggest preservation of hepatic metabolism[16]. Also, for the first time, heavily damaged livers were used and treated in MP and CK cartridge, showing an important reduction in CK level that could be very significant in limiting the IRI in liver transplantation.
Acknowledgements to Aferetica team to have supported all the research and the logistics.
| 1. | Ito T, Naini BV, Markovic D, Aziz A, Younan S, Lu M, Hirao H, Kadono K, Kojima H, DiNorcia J 3rd, Agopian VG, Yersiz H, Farmer DG, Busuttil RW, Kupiec-Weglinski JW, Kaldas FM. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am J Transplant. 2021;21:614-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39:788-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 3. | Resch T, Cardini B, Oberhuber R, Weissenbacher A, Dumfarth J, Krapf C, Boesmueller C, Oefner D, Grimm M, Schneeberger S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front Immunol. 2020;11:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Assadiasl S, Mooney N, Nicknam MH. Cytokines in Liver Transplantation. Cytokine. 2021;148:155705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ghinolfi D, Lai Q, Dondossola D, De Carlis R, Zanierato M, Patrono D, Baroni S, Bassi D, Ferla F, Lauterio A, Lazzeri C, Magistri P, Melandro F, Pagano D, Pezzati D, Ravaioli M, Rreka E, Toti L, Zanella A, Burra P, Petta S, Rossi M, Dutkowski P, Jassem W, Muiesan P, Quintini C, Selzner M, Cillo U. Machine Perfusions in Liver Transplantation: The Evidence-Based Position Paper of the Italian Society of Organ and Tissue Transplantation. Liver Transpl. 2020;26:1298-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Ankawi G, Xie Y, Yang B, Xie Y, Xie P, Ronco C. What Have We Learned about the Use of Cytosorb Adsorption Columns? Blood Purif. 2019;48:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Hosgood SA, Moore T, Kleverlaan T, Adams T, Nicholson ML. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J Transl Med. 2017;15:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Ravaioli M, De Pace V, Angeletti A, Comai G, Vasuri F, Baldassarre M, Maroni L, Odaldi F, Fallani G, Caraceni P, Germinario G, Donadei C, Malvi D, Del Gaudio M, Bertuzzo VR, Siniscalchi A, Ranieri VM, D'Errico A, Pasquinelli G, Morelli MC, Pinna AD, Cescon M, La Manna G. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci Rep. 2020;10:6063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Dondossola D, De Falco S, Kersik A, Maggioni M, Di Girolamo L, Biancolilli O, Busana M, Lonati C, Carù F, Zanella A, Gatti S. Procurement and ex-situ perfusion of isolated slaughterhouse-derived livers as a model of donors after circulatory death. ALTEX. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Ghinolfi D, Melandro F, Patrono D, Lai Q, De Carlis R, Camagni S, Gambella A, Ruberto F, De Simone P. A new ex-situ machine perfusion device. A preliminary evaluation using a model of donors after circulatory death pig livers. Artif Organs. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Zhang M, Ueki S, Kimura S, Yoshida O, Castellaneta A, Ozaki KS, Demetris AJ, Ross M, Vodovotz Y, Thomson AW, B Stolz D, Geller DA, Murase N. Roles of dendritic cells in murine hepatic warm and liver transplantation-induced cold ischemia/reperfusion injury. Hepatology. 2013;57:1585-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 665] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 13. | Konishi T, Lentsch AB. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr. 2017;17:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 14. | Jackson KR, Long J, Philosophe B, Garonzik-Wang J. Liver Transplantation Using Steatotic Grafts. Clin Liver Dis (Hoboken). 2019;14:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Jiménez-Castro MB, Cornide-Petronio ME, Gracia-Sancho J, Peralta C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells. 2019;8:1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 16. | Lee ACH, Edobor A, Lysandrou M, Mirle V, Sadek A, Johnston L, Piech R, Rose R, Hart J, Amundsen B, Jendrisak M, Millis JM, Donington J, Madariaga ML, Barth RN, di Sabato D, Shanmugarajah K, Fung J. The Effect of Normothermic Machine Perfusion on the Immune Profile of Donor Liver. Front Immunol. 2022;13:788935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |