Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.103247
Revised: December 17, 2024
Accepted: January 2, 2025
Published online: June 18, 2025
Processing time: 98 Days and 18.6 Hours
Tacrolimus (TAC) is metabolized primarily by the CYP3A-encoded enzyme family (CYP3A4, CYP3A5, and CYP3A7). Individuals expressing the CYP3A51 allele are considered fast metabolizers and generally require higher TAC doses to reach therapeutic levels.
To evaluate the predictive value of the TAC concentration-to-dose (C0/D) ratio for identifying CYP3A5 poly
Eighty-six de novo kidney transplant recipients with TAC-based immunosuppression from the Department of Nephrology and Dialysis at Military Hospital 103 (Hanoi, Vietnam) were included in this retrospective study. Blood samples were collected within the first week post-transplantation to monitor TAC levels and to perform genotyping for CYP3A5 genetic polymorphisms.
The CYP3A53/3 genotype was identified in 37 patients (43%), CYP3A51/3 in 40 patients (46.5%), and CYP3A51/1 in 9 patients (10.5%). Patients carrying the CYP3A51/3 or CYP3A51/1 genotype, classified as fast metabolizers (CYP3A5 expressers), had significantly lower TAC C0 concentrations and C0/D ratios compared to slow meta
This study demonstrates that the TAC C0/D ratio provides a reliable predictive value for CYP3A5 polymorphisms, which can be used to individualize TAC dosing in renal transplant recipients in Vietnam and other low-income countries.
Core Tip: We included in this single-center retrospective study 86 de novo kidney transplant recipients with tacrolimus (TAC)-based immunosuppression. This study aims to evaluate the predictive value of the TAC concentration-to-dose (C0/D) ratio for identifying CYP3A5 polymorphisms within the first week after kidney transplantation. We found that the TAC C0/D ratio provides a reliable predictive value for CYP3A5 polymorphisms, which can be used to individualize TAC dosing in kidney transplant recipients in low-income countries.
- Citation: Dung PT, Su HX, Tue NC, Ben NH, Phuong NM, Tran TN, Nghia PB, Van DT, Dung NTT, Vinh HT, Rostaing L, Toan PQ. Predictive value of tacrolimus concentration/dose ratio in first post-transplant week for CYP3A5-polymorphism in kidney-transplant recipients. World J Transplant 2025; 15(2): 103247
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/103247.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.103247
Tacrolimus (TAC), a calcineurin inhibitor, is widely used to prevent acute rejection and improve clinical outcomes in kidney transplant recipients[1]. However, optimizing the clinical dosing strategy for TAC remains a significant challenge due to its narrow therapeutic index and substantial pharmacokinetic variability. Consequently, monitoring TAC concentrations in whole blood during the early post-transplant period is crucial for dose adjustment to achieve therapeutic levels and avoid nephrotoxicity associated with excessive TAC exposure[2]. TAC is metabolized primarily by the CYP3A-encoded enzyme family, which includes three functional members: CYP3A4, CYP3A5, and CYP3A7, all located on chromosome 7q22.1. CYP3A5 polymorphisms, particularly CYP3A53 (6986A/G, rs776746), significantly influence TAC metabolism. Individuals expressing the CYP3A51 allele are considered fast metabolizers and generally require higher TAC doses to reach therapeutic levels, while slow metabolizers, predominantly those with the CYP3A53/3 genotype, achieve higher TAC levels and require lower doses to maintain the same therapeutic range[3,4]. It is recommended that identifying CYP3A5 polymorphisms can support the personalization of TAC dosing, in combination with frequent therapeutic drug monitoring[5]. However, in resource-limited settings, routine genetic testing is not always feasible. As an alternative, early monitoring of the TAC trough concentration relative to the daily dose [concentration-to-dose (C0/D) ratio] has emerged as a useful marker of TAC metabolism[6]. This study aims to assess the value of the TAC trough concentration/dose ratio during the first week post-transplantation as a predictive marker for CYP3A5 polymorphisms among renal transplant recipients in Vietnam.
A retrospective study analyzed data from 86 patients who underwent kidney transplantation and received TAC therapy were enrolled in this study, conducted at the Department of Nephrology and Dialysis, Military Hospital 103, Vietnam Military Medical University, between November 2017 and December 2019; these objects were randomly selected. Blood samples were collected for routine post-operative laboratory testing and remaining specimens were frozen for subsequent DNA extraction to genotype CYP3A5 polymorphisms. Clinical data and laboratory parameters were recorded throughout the follow-up period. The study’s purpose was explained to all participants, and informed consent was obtained. The study protocol was reviewed and approved by the local ethics committee (No. November 25th, 2022).
Patients received the standard immunosuppressive drug regimen followed at our hospital. TAC was initiated at a dose of 0.1 mg/kg/day, with subsequent adjustments to achieve a target therapeutic range of 8 ng/mL to 12 ng/mL in the first three months, as per current guidelines for patients undergoing induction therapy of basiliximab (D0 and D4)[2]. TAC concentrations in whole blood were monitored on various days during the first week post-transplantation, using chemiluminescent microparticle immunoassay on the Architect i2000 system (Abbott Diagnostics Laboratories, Abbott, Unite Sates). In addition, all kidney transplant patients were on 2 g mycophenolate mofetil/day and IV methylprednison with a dose of 500 mg on the first day and 250 mg on the second and third days; then taper the steroid doses day by day to the dose of 40 mg on the seventh day.
DNA was extracted from whole blood samples using the GeneAll DNA Viral Kit (GeneAll Biotechnology Co, Seoul, Korea) according to the manufacturer’s instructions. Samples were stored at -70 °C until analysis. Genotyping of the CYP3A5 polymorphisms was performed using polymerase chain reaction-restriction fragment length polymorphism as described previously[7], and a subset of samples was randomly selected for confirmation by DNA sequencing. Based on their CYP3A5 genotype, patients were classified as fast metabolizers (CYP3A51/1 and CYP3A51/3) or slow metabolizers
Statistical analyses were performed using Stata version 18 (Unite Sates). Categorical variables were analyzed using the χ2 test or Fisher’s exact test, as appropriate. Continuous data were assessed for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Comparisons between the two groups were conducted using the Students’ t-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. A P value < 0.05 was considered statistically significant.
The baseline characteristics of the 86 kidney transplant recipients are presented in Table 1. The mean age was 36.13 ± 8.71 years, with 61 males (70.9%) and 25 females (29.1%). The primary causes of end-stage renal disease were chronic glomerulonephritis (83 patients, 96.5%) and pyelonephritis (3 patients, 3.5%). All patients received transplants from living donors. Post-transplantation, one patient experienced acute rejection, one had delayed graft function, and the majority exhibited a trend of decreasing serum creatinine levels, with an improvement in estimated glomerular filtration rate (eGFR) to near-normal levels. The mean creatinine levels on day 3 and day 7 after transplantation were 179.59 ± 118.19 (μmol/L) and 104.67 ± 46.09 (μmol/L), whereas corresponding mean eGFR on day 3 and day 7 were 45.11 ± 16.57 (mL/minute) and 69.13 ± 17.83 (mL/minute), respectively. Among the 86 recipients, the CYP3A53/3 genotype was present in 37 patients (43%), CYP3A51/3 in 40 patients (46.5%), and CYP3A51/1 in 9 patients (10.5%). Based on these genotypes, patients were classified into two groups: The CYP3A5 expressers group (CYP3A51/3 or CYP3A51/1, considered fast metabolizers) and the CYP3A5 non-expressers group (CYP3A53/3, considered slow metabolizers).
| Characteristics | Values, range | |
| Age (years) | 36.13 ± 8.71 (18-63) | |
| Gender, n (%) | Male | 61 (70.9) |
| Female | 25 (29.1) | |
| Donor type, n (%) | Living related | 11 (12.8) |
| Living unrelated | 75 (87.2) | |
| Causes of end-stage renal disease, n (%) | Chronic glomerulonephritis | 83 (96.5) |
| Pyelonephritis | 3 (3.5) | |
| HLA mismatch | 3.38 ± 1.12 (1-6) | |
| Height (cm) | 163.76 ± 6.44 (145-176) | |
| Weight (kg) | 54.15 ± 8.38 (40-89) | |
| BMI (kg/m2) | 20.16 ± 2.71 (15.6-34.5) | |
| Creatinine pre-transplantation (μmol/L) (n = 85) | 847.46 ± 541.29 (375.00-5444.70) | |
| Creatinine post-transplantation (μmol/L) | ||
| Day 1 (n = 80) | 630.33 ± 191.09 (232.30-1216.15) | |
| Day 2 (n = 86) | 362.83 ± 159.12 (124.80-893.71) | |
| Day 3 (n = 84) | 179.59 ± 118.19 (68.60-653.88) | |
| Day 4 (n = 83) | 136.35 ± 82.07 (51.30-542.60) | |
| Day 5 (n = 68) | 125.38 ± 76.48 (59.90-499.02) | |
| Day 6 (n = 69) | 116.43 ± 66.98 (58.60-491.73) | |
| Day 7 (n = 83) | 104.67 ± 46.09 (53.50-337.06) | |
| eGFR pre-transplantation (mL/minute) (n = 85) | 8.70 ± 2.64 (1.15-18.88) | |
| eGFR post-transplantation (mL/minute) | ||
| Day 1 (n = 80) | 11.49 ± 4.19 (5.38-25.53) | |
| Day 2 (n = 86) | 21.16 ± 9.13 (8.23-61.43) | |
| Day 3 (n = 84) | 45.11 ± 16.57 (10.84-84.24) | |
| Day 4 (n = 83) | 55.83 ± 16.89 (13.07-103.74) | |
| Day 5 (mL/minute) (n = 68) | 60.06 ± 16.91 (14.77-109.33) | |
| Day 6 (mL/minute) (n = 69) | 63.39 ± 16.01 (14.42-102.14) | |
| Day 7 (mL/minute) (n = 83) | 69.13 ± 17.83 (25.15-141.30) | |
| Delayed graft function, n (%) | 1 (1.2) | |
| Clinical acute rejection, n (%) | Yes | 1 (1.2) |
| No | 85 (98.8) | |
| CYP3A5, n (%) | CYP3A51/1 | 9 (10.5) |
| CYP3A51/3 | 40 (46.5) | |
| CYP3A53/3 | 37 (43.0) | |
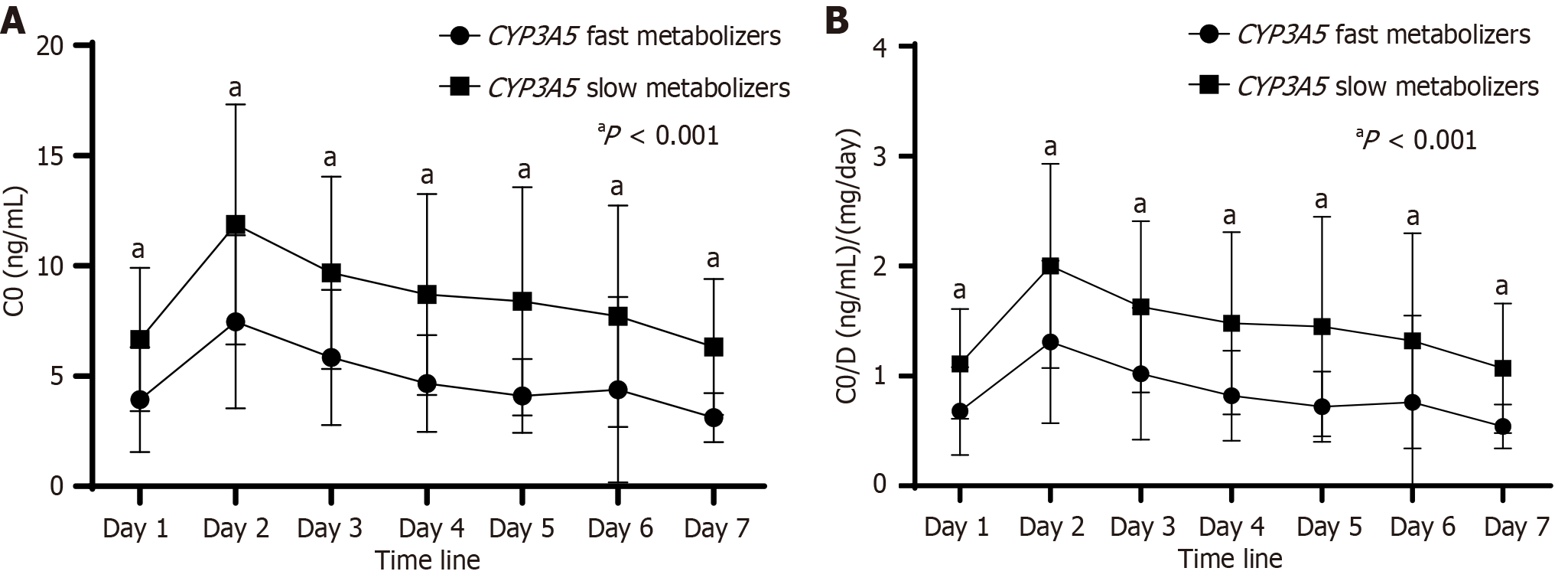
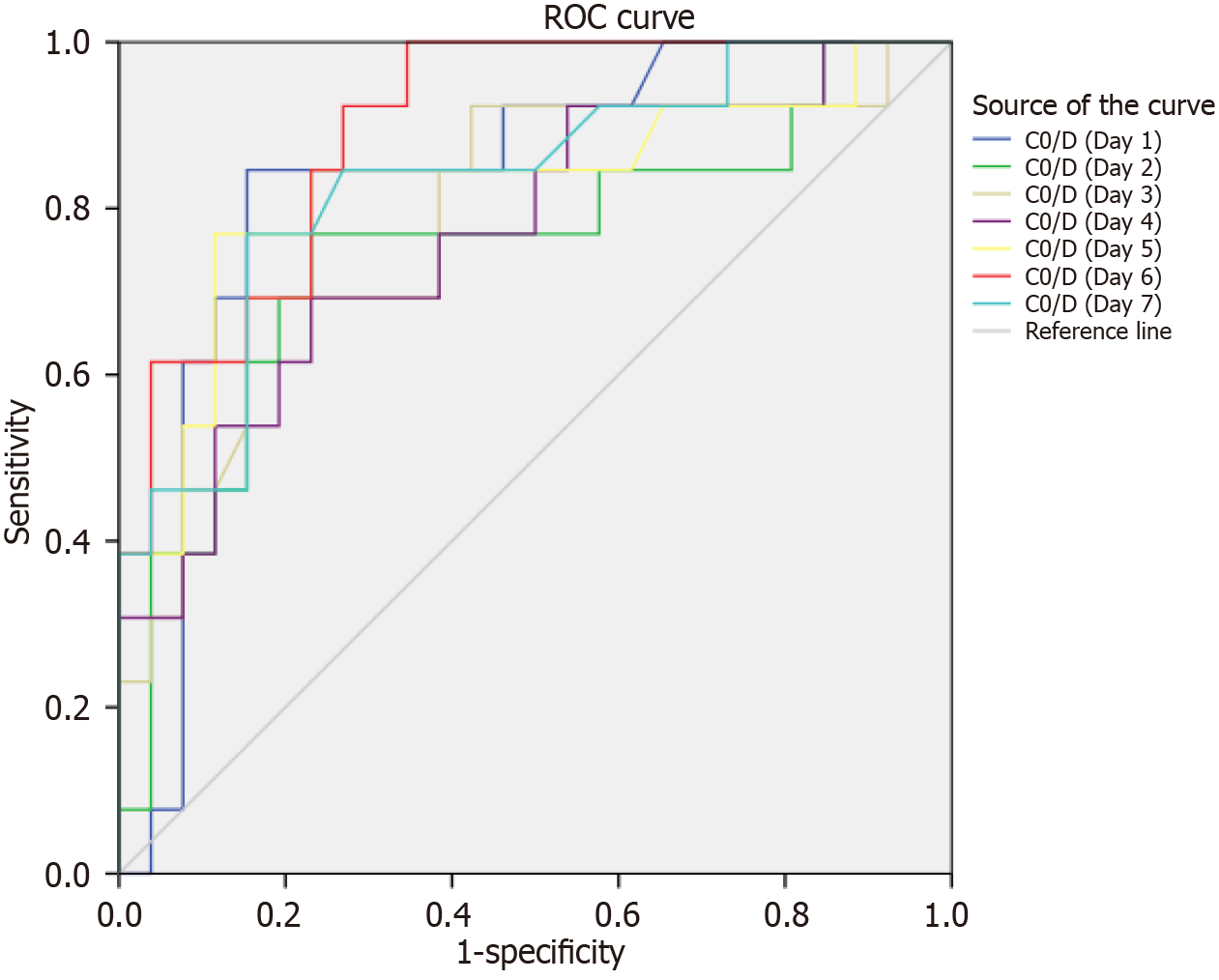
TAC concentrations and corresponding TAC doses were analyzed at various time points during the first week post-transplantation. The findings are summarized in Table 2 and Figure 1A. No significant differences were observed in initial or adjusted TAC doses between the two groups. However, TAC concentrations in fast metabolizers (CYP3A5 expressers) were consistently lower than those in slow metabolizers (CYP3A5 non-expressers) at all time points (3.93 ± 2.38 vs 6.66 ± 3.25; 7.46 ± 3.93 vs 11.88 ± 5.45; 5.84 ± 3.07 vs 9.68 ± 4.37; 4.66 ± 2.19 vs 8.70 ± 4.56; 4.10 ± 1.68 vs 8.39 ± 5.17; 4.38 ± 4.72 vs 7.72 ± 5.03; 3.11 ± 1.11 vs 6.32 ± 3.08, all values P < 0.001). Similar trends were observed when comparing TAC metabolism rates using the TAC C0/D ratio. As shown in Table 2 and Figure 1B, the C0/D ratio was significantly lower in fast metabolizers compared to slow metabolizers across all time points (0.68 ± 0.40 vs 1.11 ± 0.50; 1.31 ± 0.74 vs 2.00 ± 0.93; 1.02 ± 0.60 vs 1.63 ± 0.78; 0.82 ± 0.41 vs 1.48 ± 0.83; 0.72 ± 0.32 vs 1.45 ± 1.00; 0.76 ± 0.79 vs 1.32 ± 0.98; 0.54 ± 0.20 vs 1.07 ± 0.59, all values P < 0.001). Furthermore, a receiver operating characteristic curve analysis was performed to assess the predictive value of the TAC C0/D ratio for distinguishing between CYP3A5 metabolizers. The analysis revealed that a C0/D ratio cut-off value of 0.91 on the first day post-transplantation (area under the curve: 0.839) provided acceptable discrimination between fast and slow metabolizers (Figure 2, Table 3), with a sensitivity and specificity of 84.6%.
| Parameters | CYP3A5 fast metabolizers | CYP3A5 slow metabolizers | P value |
| Dose (mg/day) | |||
| Initial dose (n = 86) | 5.84 ± 0.72 (5.0-8.0) | 6.00 ± 0.67 (5.0-8.0) | 0.192 |
| Adjusted dose (n = 86) | 6.52 ± 1.18 (5.0-10.0) | 6.08 ± 0.86 (4.0-8.0) | 0.149 |
| Dose (mg/kg/day) | |||
| Initial dose (n = 86) | 0.11 ± 0.01 (0.09-0.14) | 0.11 ± 0.01 (0.09-0.15) | 0.236 |
| Adjusted dose (n = 86) | 0.12 ± 0.02 (0.09-0.19) | 0.11 ± 0.02 (0.07 ± 0.16) | 0.091 |
| Co (ng/mL) | |||
| Day 1 (n = 85) | 3.93 ± 2.38 (1.1-15.1) | 6.66 ± 3.25 (1.5-15.5) | < 0.001 |
| Day 2 (n = 84) | 7.46 ± 3.93 (2.5-18.7) | 11.88 ± 5.45 (4.2-30.1) | < 0.001 |
| Day 3 (n = 84) | 5.84 ± 3.07 (2.0-20.4) | 9.68 ± 4.37 (2.1-21.5) | < 0.001 |
| Day 4 (n = 76) | 4.66 ± 2.19 (2.2-11.1) | 8.70 ± 4.56 (2.2-20.0) | < 0.001 |
| Day 5 (n = 55) | 4.10 ± 1.68 (1.9-9.5) | 8.39 ± 5.17 (1.2-24.5) | < 0.001 |
| Day 6 (n = 64) | 4.38 ± 4.72 (1.5-31.0) | 7.72 ± 5.03 (1.7-26.5) | < 0.001 |
| Day 7 (n = 81) | 3.11 ± 1.11 (1.1-6.3) | 6.32 ± 3.08 (2.7-17.4) | < 0.001 |
| C0/D (ng/mL)/(mg/day) | |||
| Day 1 (n = 85) | 0.68 ± 0.40 (0.18-2.52) | 1.11 ± 0.50 (0.21-2.21) | < 0.001 |
| Day 2 (n = 84) | 1.31 ± 0.74 (0.40-3.74) | 2.00 ± 0.93 (0.61-5.02) | < 0.001 |
| Day 3 (n = 84) | 1.02 ± 0.60 (0.40-4.08) | 1.63 ± 0.78 (0.34-3.66) | < 0.001 |
| Day 4 (n = 76) | 0.82 ± 0.41 (0.30-1.98) | 1.48 ± 0.83 (0.31-4.00) | < 0.001 |
| Day 5 (n = 55) | 0.72 ± 0.32 (0.30-1.66) | 1.45 ± 1.00 (0.17-4.90) | < 0.001 |
| Day 6 (n = 64) | 0.76 ± 0.79 (0.30-5.17) | 1.32 ± 0.98 (0.24-5.30) | < 0.001 |
| Day 7 (n = 81) | 0.54 ± 0.20 (0.22-1.05) | 1.07 ± 0.59 (0.48-3.48) | < 0.001 |
| Post-transplantation (days) | AUC | Cut-off | Sensitivity (%) | Specificity (%) |
| Day 1 (n = 85) | 0.839 | 0.92 | 84.6 | 84.6 |
| Day 2 (n = 84) | 0.751 | 1.79 | 76.9 | 76.9 |
| Day 3 (n = 84) | 0.788 | 1.45 | 69.2 | 84.6 |
| Day 4 (n = 76) | 0.769 | 1.08 | 69.2 | 76.9 |
| Day 5 (n = 55) | 0.827 | 1.16 | 76.9 | 88.5 |
| Day 6 (n = 64) | 0.896 | 0.61 | 100.0 | 65.4 |
| Day 7 (n = 81) | 0.833 | 0.79 | 76.9 | 84.6 |
In this study, we retrospectively analyzed TAC concentrations to evaluate the predictive value of the C0/D ratio in the early post-transplant period for identifying CYP3A5 polymorphisms in kidney transplant recipients. Our findings confirm a significant association between CYP3A5 genetic polymorphisms and TAC concentrations at various early follow-up time points. Specifically, fast metabolizers exhibited significantly lower mean C0 concentrations compared to slow metabolizers across the different time points investigated (Figure 1A, Table 2). Additionally, the C0/D ratio was significantly lower in fast metabolizers than in the slow metabolizer group (Figure 1B, Table 2). These results are consistent with previous reports in the literature[8-11]. It has been demonstrated that the C0/D ratio is associated with long-term kidney transplant outcomes[12]. Patients with fast metabolism had significantly decreased graft function compared with those of slow metabolism and an optimal cut-off value of 1.05 was identified for distinguishing between fast metabolizers and slow metabolizers[6]. This is a simple and cost-effective tool for identifying patients at high risk of nephrotoxicity and needed indication biopsies in context of clinical situation. Nowicka and colleagues analyzed data from 101 kidney transplant patients at 3 months, 6 months, 12 months, and 24 months after kidney transplant, showing that fast metabolizers (C0/D ratio < 1.47 ng/mL × 1 mg) presented with significantly worse graft function throughout the whole study period (P < 0.05 at each timepoint) and were significantly less likely to develop good graft function (eGFR ≥ 45 mL/minute/1.73 m2) than slow metabolizers[13].
The observed effects of the CYP3A53 polymorphism on TAC metabolism underscore the importance of genotyping for tailoring therapeutic decisions. Understanding a patient’s CYP3A5 genotype can assist clinicians in determining optimal starting doses and achieving therapeutic target levels of TAC, thus enhancing monitoring and management strategies for renal transplant patients. However, routine genetic analysis is not easily accessible in many clinical laboratories, which may pose challenges due to ethical concerns and associated costs. In clinical practice, the initial TAC dose is typically set at 0.10 mg/kg, as recommended by current guidelines[2]. Monitoring the trough concentration at 24 hours is easy and essential for adjusting the dose appropriately to prevent overdose and minimize side effects. The predictability of TAC metabolism using the C0/D ratio is particularly crucial in clinical settings[14]. Our study identified a cut-off value of 0.91 for the C0/D ratio on the first day post-transplantation, which predicted CYP3A5 polymorphism with a sensitivity and specificity of 84.6%.
Although a C0/D ratio cut-off of 0.61 on day 6 yielded the highest sensitivity (100%), it had a lower specificity of 65.4%. Conversely, the highest specificity (88.5%) was observed on day 5, though with a sensitivity of 76.9% (area under the curve: 0.827) (Figure 2, Table 3). Based on these findings, we propose utilizing the C0/D ratio obtained on the first day post-transplantation as an optimal measure of TAC metabolism in kidney transplant patients, stratified by CYP3A5 genotypes. This approach could provide a practical alternative for clinicians, particularly in settings where genetic testing is not feasible. In contrast, our study has several limitations: This was a single-center retrospective study with a relative small population and short period of follow up; the C0/D had the limitation of requiring data to be gathered during the monitoring of patients after renal transplantation. Additionally, the present study focused on monitoring of TAC concentrations within the first week; the study did not have sufficient statistical power to detect any difference of clinical outcome of renal transplant recipients in relation to TAC metabolism.
This study demonstrated the value of the C0/D ratio on the first day post-transplantation as a predictor of CYP3A5 polymorphism, facilitating the individualization of TAC dosing to optimize allograft function in renal transplant recipients in Vietnam.
The authors would like to thank all the patients who participated in this study.
| 1. | Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 1043] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 2. | Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, Lemaitre F, Marquet P, Seger C, Shipkova M, Vinks A, Wallemacq P, Wieland E, Woillard JB, Barten MJ, Budde K, Colom H, Dieterlen MT, Elens L, Johnson-Davis KL, Kunicki PK, MacPhee I, Masuda S, Mathew BS, Millán O, Mizuno T, Moes DAR, Monchaud C, Noceti O, Pawinski T, Picard N, van Schaik R, Sommerer C, Vethe NT, de Winter B, Christians U, Bergan S. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther Drug Monit. 2019;41:261-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 3. | Yanik MV, Seifert ME, Locke JE, Hauptfeld-Dolejsek V, Crowley MR, Cutter GR, Mannon RB, Feig DI, Limdi NA. CYP3A5 genotype affects time to therapeutic tacrolimus level in pediatric kidney transplant recipients. Pediatr Transplant. 2019;23:e13494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Khan AR, Raza A, Firasat S, Abid A. CYP3A5 gene polymorphisms and their impact on dosage and trough concentration of tacrolimus among kidney transplant patients: a systematic review and meta-analysis. Pharmacogenomics J. 2020;20:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ, Leeder JS, van Schaik R, Thummel KE, Klein TE, Caudle KE, MacPhee IA. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 2015;98:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 528] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 6. | Thölking G, Fortmann C, Koch R, Gerth HU, Pabst D, Pavenstädt H, Kabar I, Hüsing A, Wolters H, Reuter S, Suwelack B. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS One. 2014;9:e111128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Rong G, Jing L, Deng-Qing L, Hong-Shan Z, Shai-Hong Z, Xin-Min N. Influence of CYP3A5 and MDR1(ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in Chinese renal transplant recipients. Transplant Proc. 2010;42:3455-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Vannaprasaht S, Reungjui S, Supanya D, Sirivongs D, Pongskul C, Avihingsanon Y, Tassaneeyakul W. Personalized tacrolimus doses determined by CYP3A5 genotype for induction and maintenance phases of kidney transplantation. Clin Ther. 2013;35:1762-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Yaowakulpatana K, Vadcharavivad S, Ingsathit A, Areepium N, Kantachuvesiri S, Phakdeekitcharoen B, Sukasem C, Sra-Ium S, Sumethkul V, Kitiyakara C. Impact of CYP3A5 polymorphism on trough concentrations and outcomes of tacrolimus minimization during the early period after kidney transplantation. Eur J Clin Pharmacol. 2016;72:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Zhang X, Liu L, Tong W. Value of CYP3A5 genotyping on determining initial dosages of tacrolimus for Chinese renal transplant recipients. Transplant Proc. 2010;42:3459-3464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Chen P, Li J, Li J, Deng R, Fu Q, Chen J, Huang M, Chen X, Wang C. Dynamic effects of CYP3A5 polymorphism on dose requirement and trough concentration of tacrolimus in renal transplant recipients. J Clin Pharm Ther. 2017;42:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Jouve T, Fonrose X, Noble J, Janbon B, Fiard G, Malvezzi P, Stanke-Labesque F, Rostaing L. The TOMATO Study (Tacrolimus Metabolization in Kidney Transplantation): Impact of the Concentration-Dose Ratio on Death-censored Graft Survival. Transplantation. 2020;104:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Nowicka M, Górska M, Nowicka Z, Edyko K, Edyko P, Wiślicki S, Zawiasa-Bryszewska A, Strzelczyk J, Matych J, Kurnatowska I. Tacrolimus: Influence of the Posttransplant Concentration/Dose Ratio on Kidney Graft Function in a Two-Year Follow-Up. Kidney Blood Press Res. 2019;44:1075-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Vidal-Alabró A, Colom H, Fontova P, Cerezo G, Melilli E, Montero N, Coloma A, Manonelles A, Favà A, Cruzado JM, Torras J, Grinyó JM, Lloberas N. Tools for a personalized tacrolimus dose adjustment in the follow-up of renal transplant recipients. Metabolizing phenotype according to CYP3A genetic polymorphisms versus concentration-dose ratio. Nefrologia (Engl Ed). 2024;44:204-216. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |