Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.100065
Revised: December 20, 2024
Accepted: January 7, 2025
Published online: June 18, 2025
Processing time: 193 Days and 16.7 Hours
Bone marrow transplantation (BMT) is a breakthrough procedure for patients with hematological and oncological conditions, particularly when all other treat
To assess the clinical characteristics of patients who underwent BMT overseas and analyze the factors affecting their survival outcomes.
We conducted a retrospective cohort study of all Bahraini pediatric patients who underwent BMT between 2013 and 2024. Medical records from Salmaniya Medical Complex and Overseas Treatment Office were reviewed. Patient demographics, transplant indications, donor type, transplantation type, overseas centers, complications, and outcomes (overall and 5-year survival rates) were analyzed. Clinical characteristics and outcomes were compared using χ2 test, Student’s t-test, Mann–Whitney U test, and Kaplan–Meier method. Univariate and multivariate analyses were used to estimate survival predictors.
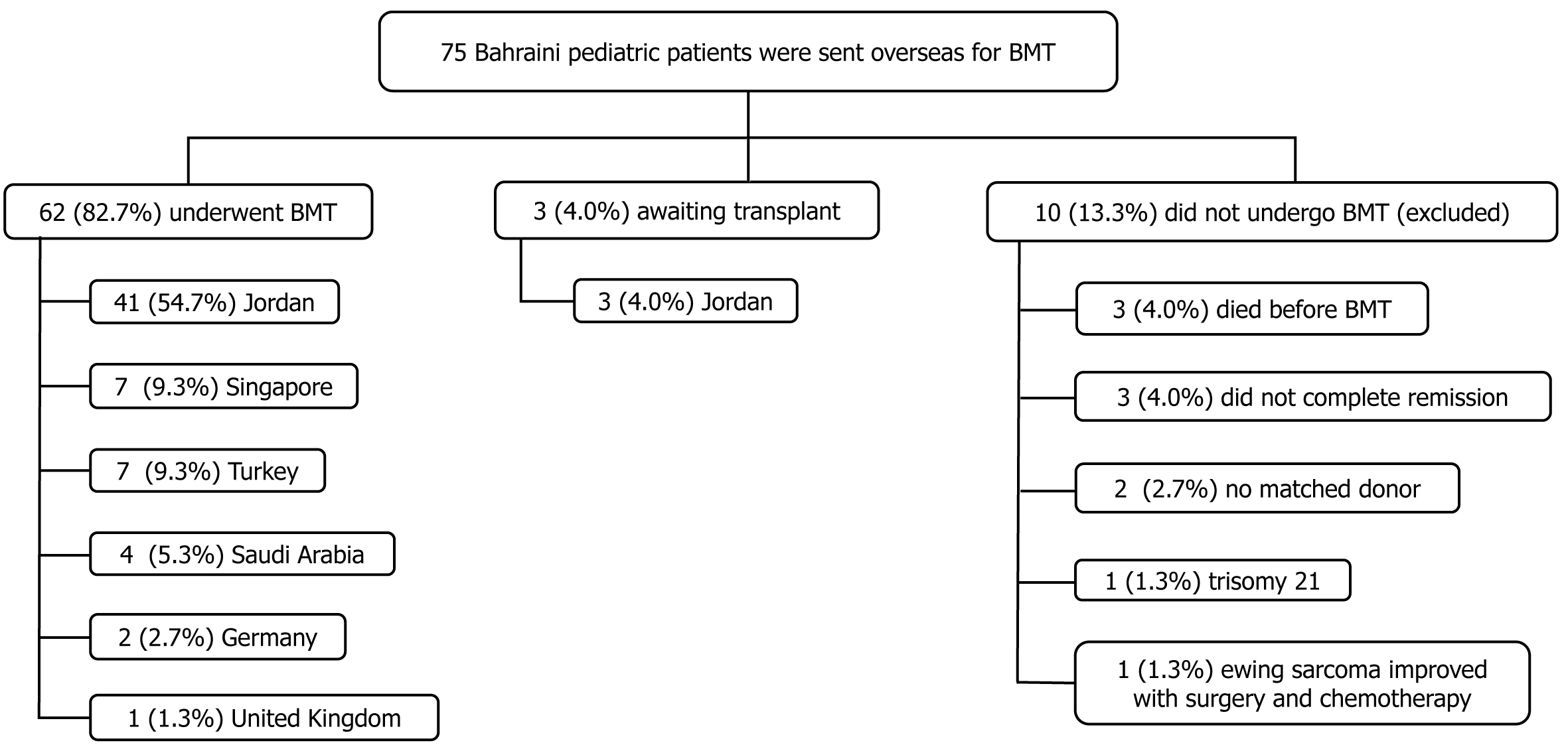
Of the 75 listed patients, 62 (82.7%) underwent BMT and were included, 10 (13.3%) did not, and 3 (4.0%) were awaiting transplantation. Most patients were male (n = 33, 53.2%). The mean age at transplantation was 7.8 ± 4.9 years. The main indication for treatment was acute myeloid leukemia (AML) (n = 15, 36.6%). Six patients (9.7%) required re-transplantation. Of the 68 transplants, 60 (88.2%) involved conditioning, mostly a combination of fludarabine and total body irradiation (n = 7, 11.7%). Most patients underwent allogeneic transplantation (n = 48, 77.4%), primarily from related donors (n = 47/48, 97.9%). The most common complication was infection (n = 51, 79.7%). Follow-up averaged 3.3 ± 2.5 years. The overall survival rate was 77.4%. Survival odds were better for non-AML patients and Middle Eastern centers (P = 0.015 and P = 0.032, respectively).
Bahraini males with AML primarily underwent allogeneic BMT. Non-AML patients and those transplanted in the Middle East had better survival rates, despite high complication rates.
Core Tip: The clinical characteristics and outcomes of Bahraini children who underwent overseas bone marrow trans
- Citation: Isa HM, Bucheeri ST, Aldoseri JY, Redha AA, Mubarak AF, Altamimi SA, AlOraibi AA, Alshaikh MI. Characteristics and outcomes of Bahraini pediatric patients sent abroad for bone marrow transplantation: A ten-year retrospective cohort study. World J Transplant 2025; 15(2): 100065
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/100065.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.100065
Hematopoietic stem cell transplantation (HSCT) was first performed in 1957 by Donnell Thomas to treat acute leukemia[1]. Since then, HSCT has served as a breakthrough therapeutic approach in the fields of oncology and hematology[2]. It is used to treat both malignant and non-malignant conditions, such as sickle cell anemia, thalassemia, and several autoimmune diseases[3].
HSCT, also called bone marrow transplantation (BMT), involves the infusion of healthy immune cells (graft) from a donor, resulting in the replacement of the recipient’s unhealthy bone marrow cells[2]. Generally, BMT can be classified as either autologous or allogeneic transplantation[3]. Autologous transplantation involves obtaining stem cells from the recipient, whereas allogeneic transplantation involves collecting stem cells from another person or from umbilical cord units[2]. Allogeneic transplantation is further categorized as having a matched related donor (i.e., a family member), matched unrelated donor, mismatched related (haploidentical) donor, or mismatched unrelated (haploidentical) donor[3]. Human leukocyte antigen (HLA) genes, including HLA class I (HLA-A, HLA-B, and HLA-C) and class II molecules (HLA-DR, HLA-DQ, and HLA-DP), are considered when matching donors and recipients[3,4].
The most common indication for BMT is hematological malignancies[2]. Of the malignancies, acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) are the leading indications for allogeneic BMT, whereas lymphoma and multiple myeloma are the main indications for autologous BMT[5,6]. BMT can also be used to treat solid tumors, such as neuroblastoma, and remains the standard first-line treatment for acquired severe aplastic anemia[3].
Although BMT serves as a potential cure for many diseases, it can lead to many complications apart from relapse of the condition itself. These complications are classified according to the engraftment period. Infections are the most common complication during the pre-engraftment period[7,8]. The major non-relapse complications associated with significant morbidity and mortality are acute and chronic graft-versus-host diseases (GvHD)[2,9,10]. Autologous BMT is associated with a significantly lower risk of complications than allogeneic BMT[11].
BMT is one of the procedures that is not performed in the Kingdom of Bahrain; hence, patients are sent abroad to receive the necessary treatment at transplant centers in different countries such as Jordan, Turkey, and Saudi Arabia. In countries where transplantation facilities are lacking, we hypothesized that sending patients overseas to undergo BMT might be a safe and effective alternative to leaving the patient to face eventual disease morbidity or even mortality if the procedure is not performed locally. However, studies evaluating BMT abroad are scarce.
Globally, multiple centers in different countries have published numerous reports on BMT experiences[12-16]. However, data on BMT in the Middle East and Gulf regions are rare[12-15]. Moreover, no studies have been conducted on Bahraini pediatric patients sent overseas for BMT. In addition, the literature has identified several research gaps in pediatric BMT. Studies have highlighted the need for safer and less harmful conditioning regimens, particularly for fragile pediatric patients[17]. The lack of genetic diversity in donor registries has a major effect on the access to matched donors for children from underrepresented populations[18]. Furthermore, research on stem cell collection, HLA typing, graft manipulation, ethical issues, survivorship, complications such as late effects, quality of life, and psychological support is limited[19]. These knowledge gaps prompted us to investigate the overseas BMT experiences of Bahraini pediatric patients. Accordingly, the main objective of this study was to assess the clinical characteristics, indications, conditioning regimens, complications, and outcomes of Bahraini pediatric patients who underwent BMT overseas, and to identify survival predictors.
This study was a retrospective cohort review conducted on all pediatric patients from the Kingdom of Bahrain who were sent overseas for BMT between October 1, 2013 and June 30, 2024. These patients were listed by the Department of Pediatrics at Salmaniya Medical Complex (SMC), Government Hospitals, and were registered at the Overseas Treatment Office, Ministry of Health. SMC is the main referral center for pediatric oncology, hematology, and immunology cases in the entire country. Post-BMT patients are also followed up at SMC. At SMC, four general pediatric wards and one daycare unit are available to provide services for patients with hematological diseases. For patients with oncological diseases, a specialized oncology unit consisting of eight single isolation rooms connected to a 24-hour daycare unit with five beds is available. In addition, we have daily hematology/oncology outpatient clinics and one immunology clinic (twice a week). The service is run by three senior consultants, one chief resident, and one senior resident, along with two to three nursing staff members per shift. The patients are assessed by their caring consultant who determines their requirement for BMT.
All patients aged 0-18 years who underwent BMT for hematological, oncological, or immunological diseases were included in the study. The exclusion criteria included all individuals above the age of 18 years, those awaiting BMT, those who died before BMT, and those with missing relevant data.
BMT donors can be patients themselves (autologous), close relatives, or unrelated donors (allogeneic). In the case of allogeneic BMT, according to our protocol, the acceptance of a potential donor requires that the donor must be between 1 and 55 years of age, in good health, and have appropriate body weight for age and height to ensure sufficient bone marrow volume collection and there should be compatible donor-recipient blood type, donor-recipient HLA type matching, and negative infectious disease screening, along with the donor or the child’s parents/guardian willingness to donate[20].
Initially, the caring consultant meets the donor, takes a full history, and performs a thorough physical examination to rule out any comorbidities. After obtaining a satisfactory history and physical examination, the consultant checks the serology results of both the patient and donor and decides on their blood group and HLA matching. If the donor matches the recipient, the donor is subjected to multiple tests, including complete blood count; peripheral blood smear; hemoglo
Once the donor is ready, the treating consultant submits an overseas request letter to the Supreme Committee for Treatment Abroad, which is responsible for sending Bahraini citizens overseas. This request includes a detailed patient medical report, along with all necessary investigations of the patient and donor. Once the case is approved by the committee, it is forwarded immediately to an overseas BMT center. Once accepted, the patient, donor, and two family members are sent to the overseas center.
Data were collected by medically trained research investigators who had received Health Insurance Portability and Accountability Act training. Data collection was facilitated using a uniform electronic data spreadsheet in Microsoft Excel. Patient data were collected from paper-based and electronic medical records at SMC and Overseas Treatment Office.
Patient demographics included sex, area of residence, age at diagnosis, age at transplantation, weight, height, body mass index (BMI) at BMT, presence of associated diseases, previous bone marrow biopsy, family history of similar conditions, follow-up duration, and number of overseas visits. BMT indications, BMT type (autologous or allogeneic; identical or haploidentical), and donor type were also recorded. BMT indications included the following: (1) Oncological conditions: AML, ALL, neuroblastoma, Hodgkin's lymphoma, Wilms’ tumor, mixed germ cell tumor, and hemophagocytic lymphohistiocytosis; (2) Hematological conditions: Aplastic anemia, beta-thalassemia major, Fanconi anemia, myelodysplastic syndrome, Diamond-Blackfan anemia, hemoglobin H disease, and sickle cell disease; and (3) Immunological conditions: Severe combined immunodeficiency, chronic granulomatous disease, and X-linked agammaglobulinemia.
The conditioning regimen administered to patients before undergoing BMT was also documented. Patients were administered conditioning regimens based on their case. These included the following: (1) Chemotherapeutic agents: Fludarabine, cyclophosphamide, busulfan, thiotepa, melphalan, etoposide, carboplatin, ifosfamide, carmustine, cytarabine, and treosulfan; (2) Immunotherapeutic agents: Antithymocyte globulin and alemtuzumab; and (3) Radiation therapy: Total body irradiation (TBI). The conditioning regimens were classified into two types: Chemotherapy- and TBI-based. Chemotherapy-based regimens include chemotherapy and immunotherapy, whereas TBI-based regimens include chemotherapy, TBI, and immunotherapy[19].
Complications that developed post-BMT were grouped into infectious and non-infectious complications. Infectious complications included viral, bacterial, fungal, parasitic, respiratory, gastrointestinal, systemic, mucocutaneous, ophthalmological, otolaryngological, dental, oral, urological, lymphatic, and musculoskeletal complications. Non-infectious complications included hematological, oncological, mucocutaneous, gastrointestinal, endocrine, dental, oral, respiratory, urological, otolaryngological, reproductive, cardiovascular, neurological, musculoskeletal, and ophthalmological complications.
The date of the first BMT, death date or end of the study date, and follow-up duration were recorded. Patient outcomes including overall survival, 5-year survival, and mortality rates were evaluated.
Statistical analyses were performed using the SPSS version 21 (IBM SPSS Statistics for Windows, Version 21.0. IBM Corp., Armonk, NY, United States). Categorical variables were expressed as frequencies and percentages. Continuous data were described as mean and standard deviation for normally distributed variables or as median and interquartile range (IQR) for non-normally distributed variables. Fisher’s exact test or Pearson’s χ2 test was used to investigate associations between categorical variables like patient’s sex, area of residence, age group at BMT, presence of associated diseases, graft type, donor-recipient relationship, BMT indications, post-BMT complications, and BMT center. Continuous variables, including age, weight, height, and BMI at transplantation, were compared using Student’s t-test or Mann–Whitney U test according to distribution normality. Kaplan–Meier curves were used to estimate two outcomes: Survival probability (%) and time after BMT in years. Univariate and multivariate logistic regression analyses were used to identify the clinical predictors of survival. Data were presented as odds ratio and confidence interval (CI). The CI was set at 95%, and P-values < 0.05 were considered statistically significant.
The study was conducted according to the principles of the Declaration of Helsinki of 1975 (revised 2013) and was approved by the Research and Research Ethics Committee, SMC, Government Hospitals, Kingdom of Bahrain (IRB number: 110311023; November 6, 2023). Patient confidentiality and anonymity were maintained. Informed consent was not required because the study was retrospective in nature and did not include patient data.
Until June 2024, 75 Bahraini pediatric patients were listed for possible BMT; 62 (82.7%) underwent BMT and were included in the study, while 13 (17.3%) were excluded (Figure 1). Most patients were males (n = 33, 53.2%) and the mean age at BMT was 7.8 ± 4.9 years (Table 1 and Supplementary Table 1). There was no significant difference between the sexes in terms of mean age at transplantation (P = 0.772). Forty-eight (77.4%) patients underwent primary allogeneic BMT, 13 (21.0%) underwent autologous BMT, and one (1.6%) patient’s BMT type was unknown. There were no signi
| Patient demography | Total, n = 62 (100) | Allogeneic, n = 48/61 (77.4) | Autologous, n = 13/61 (21.0) | P value (95%CI) |
| Sex | 0.5471 | |||
| Male | 33 (53.2) | 27 (56.3) | 6 (46.2) | |
| Female | 29 (46.8) | 21 (43.8) | 7 (53.8) | |
| Governorate | 0.3772 | |||
| Northern | 26 (41.9) | 17 (35.4) | 8 (61.5) | |
| Capital | 15 (24.2) | 13 (27.1) | 2 (15.4) | |
| Muharraq | 11 (17.7) | 9 (18.8) | 2 (15.4) | |
| Southern | 10 (16.1) | 9 (18.8) | 1 (7.7) | |
| Age at diagnosis (year) | 5.6 ± 4.8 | 5.4 ± 5.0 | 5.8 ± 4.0 | 0.7873 (-2.6, 3.4) |
| Age group at diagnosis (year) | 0.8472 | |||
| 0-5 | 33 (53.2) | 27 (56.3) | 6 (46.2) | |
| 6-10 | 20 (32.3) | 14 (29.2) | 5 (38.5) | |
| 11-15 | 8 (12.9) | 6 (12.5) | 2 (15.4) | |
| > 15 | 1 (1.6) | 1 (2.1) | 0 (0.0) | |
| Age at transplant (year) | 7.8 ± 4.9 | 7.8 ± 5.1 | 7.2 ± 4.3 | 0.6933 (-3.7, 2.5) |
| Age group at transplant (year) | 0.5742 | |||
| 0-5 | 25 (40.3) | 21 (43.8) | 4 (30.8) | |
| 6-10 | 21 (33.9) | 15 (31.3) | 6 (46.2) | |
| 11-15 | 13 (21.0) | 9 (18.8) | 3 (23.1) | |
| > 15 | 3 (4.8) | 3 (6.3) | 0 (0.0) | |
| Weight at transplant (kg), (n = 48) | 19.1 (12.0-29.8) | 19.1 (12.3-29.7) | 20.6 (10.7-27.6) | 0.7754 |
| Height at transplant (cm), (n = 47) | 115.8 ± 30.1 | 116.6 ± 29.1 | 113.5 ± 34.1 | 0.7623 (-23.6, 17.4) |
| Body mass index, (n = 49) | 16.0 (14.4-18.0) | 15.9 (14.1-17.9) | 16.6 (15.1-17.0) | 0.4154 |
| Presence of associated diseases5 | 29 (46.8) | 21 (43.8) | 7 (53.8) | 0.5471 |
| Previous bone marrow biopsy | 35 (56.5) | 25 (52.0) | 10 (76.9) | 0.1281 |
| Number of bone marrow aspirate, (n = 35) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 2.0 (1.0-2.0) | 0.1623 |
| Family history of similar condition, (n = 34) | 14 (41.2) | 13 (27.1) | 1 (7.7) | 0.1021 |
| Follow-up duration (year) | 3.3 ± 2.5 | 3.41 ± 2.50 | 2.98 ± 2.42 | 0.5773 (-1.9, 1.1) |
| Number of overseas visits | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 0.9494 |
The most common indication for BMT was oncological (n = 41, 66.1%), followed by hematological (n = 21, 33.9%) and immunological indications (n = 7, 11.3%) (Table 2). The main oncological indication was AML (n = 15, 36.6%). Based on the French-American-British (FAB) classification of AML[21], four (26.7%) patients were classified as subtype M0, two (13.3%) as subtypes M1 and M5 each, one (6.7%) as subtypes M2, M6, and M7 each, one (6.7%) as a combination of M1 and M2 subtypes, and three (20.0%) patients’ subtype was unknown. Aplastic anemia was the main hematological indication (n = 9, 42.3%), whereas severe combined immunodeficiency was the main immunological indication (n = 5, 71.4%). Patients with oncological diseases received more autologous BMT than allogeneic BMT (P = 0.002), whereas those with hematological and immunological diseases received allogeneic grafts only.
| Indications of bone marrow transplantation1 | Total, n = 62 (100.0) | Allogeneic, n = 48/61 (77.4) | Autologous, n = 13/61 (21.0) | P value1 |
| Oncological indications | 41 (66.1) | 27 (56.3) | 13 (100.0) | 0.002 |
| Acute myeloid leukemia | 15 (36.6) | 14 (29.2) | 0 (0.0) | 0.028 |
| Acute lymphoblastic leukemia | 11 (26.8) | 11 (22.9) | 0 (0.0) | 0.099 |
| Neuroblastoma | 7 (17.1) | 0 (0.0) | 7 (53.8) | < 0.0001 |
| Hodgkin’s lymphoma | 4 (9.8) | 1 (2.1) | 3 (23.1) | 0.028 |
| Wilms tumor | 2 (4.9) | 0 (0.0) | 2 (15.4) | 0.043 |
| Mixed germ cell tumor | 1 (2.4) | 0 (0.0) | 1 (7.7) | 0.213 |
| Hemophagocytic lymphohistiocytosis | 1 (2.4) | 1 (2.1) | 0 (0.0) | 1.000 |
| Hematological indications | 21 (33.9) | 19 (39.6) | 0 (0.0) | 0.006 |
| Aplastic anemia | 9 (42.3) | 8 (16.7) | 0 (0.0) | 0.184 |
| Beta thalassemia major | 5 (23.8) | 5 (10.4) | 0 (0.0) | 0.575 |
| Myelodysplastic syndrome | 2 (9.5) | 2 (4.2) | 0 (0.0) | 1.000 |
| Fanconi anemia | 2 (9.5) | 1 (2.1) | 0 (0.0) | 1.000 |
| Diamond Blackfan anemia | 1 (4.8) | 1 (2.1) | 0 (0.0) | 1.000 |
| Hemoglobin H disease | 1 (4.8) | 1 (2.1) | 0 (0.0) | 1.000 |
| Sickle cell disease | 1 (4.8) | 1 (2.1) | 0 (0.0) | 1.000 |
| Immunological indications | 7 (11.3) | 7 (14.6) | 0 (0.0) | 0.328 |
| Severe combined immunodeficiency | 5 (71.4) | 5 (10.4) | 0 (0.0) | 0.575 |
| Chronic granulomatous disease | 1 (14.3) | 1 (2.1) | 0 (0.0) | 1.000 |
| X-linked agammaglobulinemia | 1 (14.3) | 1 (2.1) | 0 (0.0) | 1.000 |
Six patients (9.7%) underwent a second BMT, of whom three (50.0%) underwent re-transplantation after 2 years, two (33.3%) after 1 year, and one (16.7%) after 2 months. The main indication for re-transplantation was disease relapse (n = 4, 66.7%). One patient’s (16.7%) white blood cell count remained low after the first BMT; hence, an emergency re-transplantation was required. One patient (16.7%) responded well after the primary BMT but underwent a second autologous BMT as part of the treatment plan.
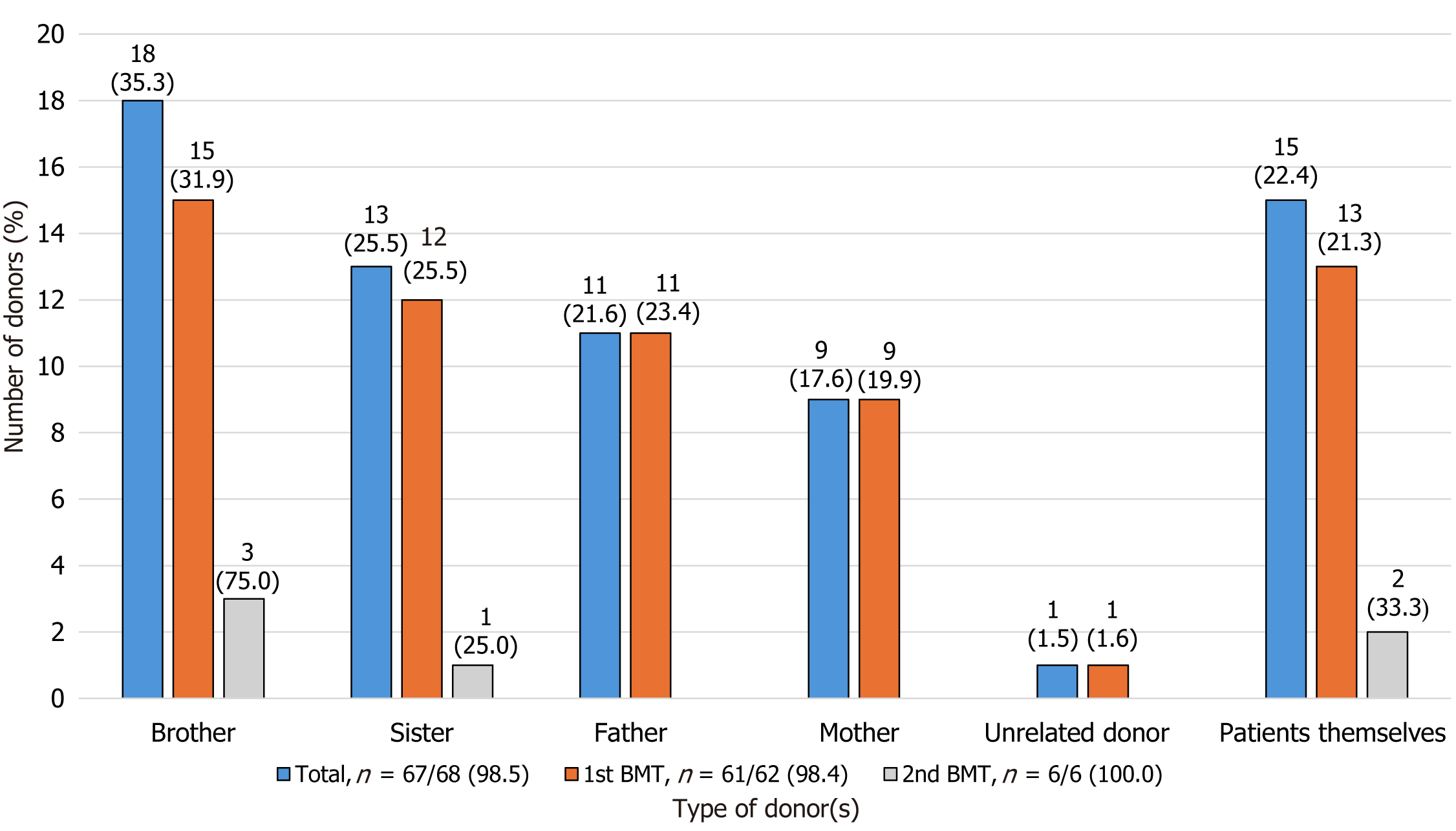
Of the 68 BMTs, data regarding donor type were available for 67 (98.5%) patients, with 61 (91.0%) being first BMTs and six (9.1%) second BMTs (Figure 2). Allogeneic donors were the most common (n = 52/67, 77.6%), with related first-degree donors being the most frequent (n = 51/67, 76.1%); fifteen (22.4%) patients underwent autologous BMT. Parental consanguinity was found in 38.2% (n = 13/34) of patients. Of the 48 (71.6%) first allogeneic transplants, 26 (54.2%) were HLA-haploidentical donors [18 (37.5%) parents, 7 (14.6%) siblings, and 1 (2.1%) unrelated donor], 20 (41.7%) were HLA-identical donors [19 (39.6%) siblings and 1 (2.1%) parent], and two (4.2%) were unknown. As for the six (9.1%) second BMTs, of the four (66.7%) allogeneic transplants, two (50.0%) patients had the same donor, one (25.0%) patient had an autologous primary BMT, and one (25.0%) patient had an allogeneic transplant from a different related donor. Two patients (33.3%) who underwent a second autologous BMT underwent a primary autologous BMT. Donor age at first BMT showed that autologous BMT recipients were younger than allogeneic BMT donors, with a median age of 7.4 (IQR: 2.5-8.8) vs 13.0 (IQR: 8.0-33.3) years, respectively (P = 0.003).
As for the source of stem cells for the first 62 BMTs, 35 (56.5%) patients received peripheral blood stem cells, 14 (22.6%) received bone marrow stem cells, 12 (19.4%) had unknown stem cell sources, and one (1.6%) received both peripheral and bone marrow stem cells. Regarding the second BMT, two (33.3%) patients received bone marrow stem cells, two (33.3%) received peripheral stem cells, and the stem cell sources of two (33.3%) patients were unknown.
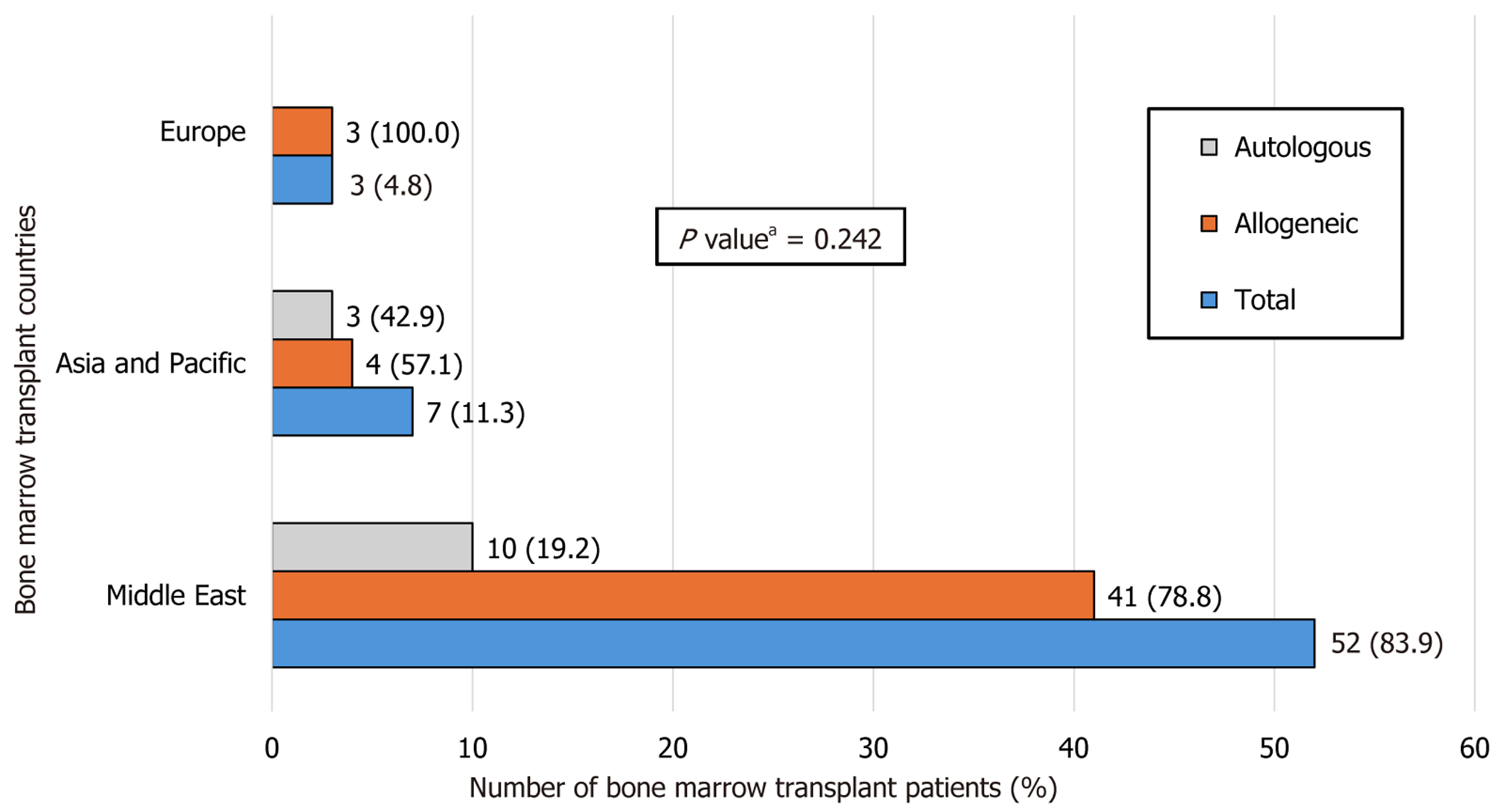
Most patients underwent BMT in Jordan (n = 41, 66.2%), followed by Turkey and Singapore (n = 7, 11.3%), Saudi Arabia (n = 4, 6.5%), Germany (n = 2, 3.2%), and the United Kingdom (n = 1, 1.6%). There was no significant difference between the BMT center locations with respect to the graft type (P = 0.242) (Figure 3).
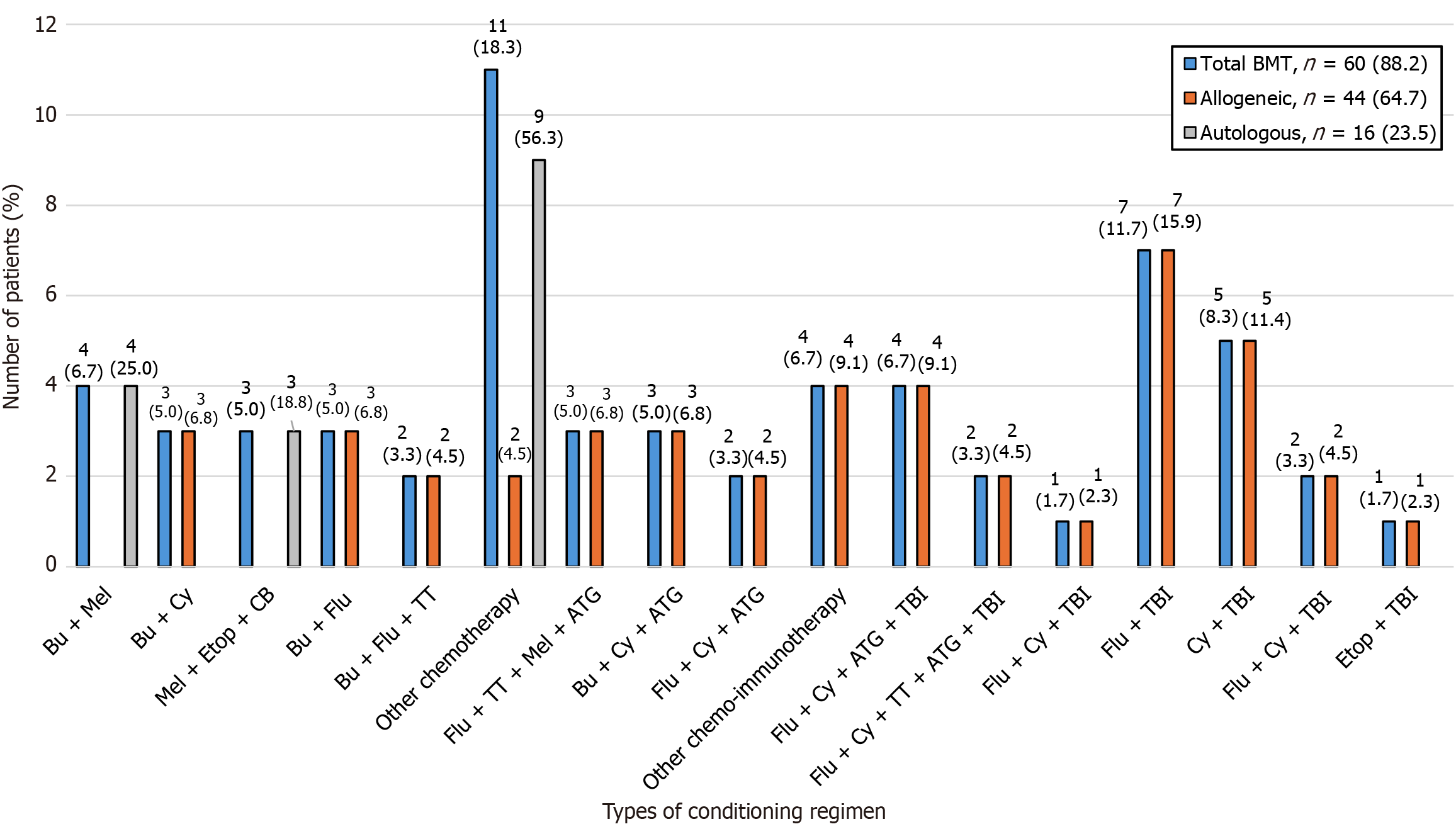
Of the 68 BMTs, 60 (88.2%) were preceded by a conditioning regimen, 7 (10.3%) had missing data and 1 (1.5%) did not receive any conditioning regimen. Thirty-eight (63.3%) patients received chemotherapy-based regimens and 22 (36.7%) received TBI-based regimens. The type of conditioning regimen administered was based on each patient’s case. A combination of fludarabine and TBI was the most prescribed regimen (n = 7, 11.7%), followed by cyclophosphamide and TBI (n = 5, 8.3%) (Figure 4 and Supplementary Table 2).
In terms of GvHD prophylaxis for the first BMTs, 50 (80.6%) patients had available data, of which 25 (50.0%) received tacrolimus, 21 (42.0%) received cyclophosphamide, 19 (38.0%) received mycophenolate mofetil, 14 (28.0%) received methotrexate, and 10 (20.0%) received cyclosporine. Most patients received at least two GvHD prophylactic medications
The total nucleated cell count for the primary BMT was available in 27 (43.5%) patients, with a median infused dose of 6.6 × 108/kg (IQR: 5.3-9.6 × 108/kg). The cluster of differentiation (CD) 34+ cell count was available for 46 (74.2%) patients, with a median infused dose of 7.7 × 106/kg (IQR: 5.1-9.6 × 106/kg). The CD3+ cell dose was available for 26 (41.9%) patients, with a median infused dose of 18.5 × 107/kg (IQR: 6.5-34.6 × 107/kg). CD3+, CD4+, CD8+, and CD16+ cells were infused in one (1.6%) patient at doses of 1.1 × 109/kg, 0.39 × 109/kg, 0.68 × 109/kg, 0.051 × 109/kg, respectively. Another (1.6%) patient received an infusion of mononucleated cells at a dose of 26.6 × 108/kg. As for the second BMTs, data regarding the number of infused cells were available for one (16.7%) patient, who received a total nucleated cell dose of 2.15 × 108/kg and CD34+ cell dose of 5.97 × 106/kg.
The median length of hospital stay post-BMT was 35 (IQR: 35.5-93) days (n = 37/62, 59.7%). The median neutrophil engraftment period for the primary BMT was 13.0 (IQR: 12.0-15.0) days (n = 46/62, 74.2%), while for the second BMT, the mean was 17.0 ± 6.9 days (n = 4/6, 66.7%). The mean platelet engraftment period for the primary BMT was 15.9 ± 7.9 (n = 12, 19.4%) and 16.6 ± 4.9 (n = 2, 33.3%) days for the second BMT. The median chimerism level after the first BMT was 100% (IQR: 97%-100%) (n = 33, 53.2%), with a median of 33 days (IQR: 28.5-169.0 days) (n = 26, 41.9%). For the second BMT, only one (16.7%) patient had a chimerism of 12% in 47 days (about one and a half months).
Post-BMT complications were documented in 94.1% (n = 64/68) of all BMTs. Patients who received allogeneic primary grafts experienced higher complication rates than those who received autologous grafts [47/48 (97.9%) vs 11/13 (84.6%), respectively] (P = 0.112). The most common complications were infections (n = 51/64, 79.7%), followed by mucositis (n = 37, 57.8%), acute GvHD (aGvHD), and neutropenia (n = 20, 31.3% each), while disease relapse was noted in 28.1% of patients (n = 18/64 BMTs) (Table 3, Supplementary Table 3 and 4). Upper respiratory tract infection was the most common infection (n = 36/51, 70.6%), with a median of four (IQR: 1.0-5.0) episodes, followed by cytomegalovirus reactivation and gastroenteritis (n = 16/51, 31.4% each). Gastroenteritis had a median of two (IQR: 1.0-2.0) episodes. The mucositis type was available for 25/36 (69.4%) patients, with the most common types being oral (n = 22, 88.0%), perianal, and skin and gut (n = 1, 4.0% each) mucositis. The grade of mucositis was available for 52 (83.9%) patients [grade 0 (n = 22, 42.3%), grade I-II (n = 11, 21.2%), grade II-III (n = 6, 11.5%), grade II (n = 5, 9.6%), and grade I and III (n = 4, 7.7% each)].
| Complications2 | Total BMTs, n = 64/68 (94.1) | Allogeneic, n = 51/52 (79.7) | Autologous, n = 13/15 (86.7) | P value1 |
| Infectious | ||||
| URTI | 36 (56.3) | 31 (60.8) | 5 (38.5) | 0.086 |
| CMV reactivation | 16 (25.0) | 15 (29.4) | 1 (7.7) | 0.095 |
| Gastroenteritis | 16 (25.0) | 12 (23.5) | 4 (30.8) | 0.743 |
| Sepsis | 14 (21.9) | 12 (23.5) | 2 (15.4) | 0.719 |
| Colitis | 11 (17.2) | 8 (15.7) | 3 (23.1) | 0.699 |
| Cytokine release syndrome | 9 (14.1) | 9 (17.7) | 0 (0.0) | 0.191 |
| Conjunctivitis | 9 (14.1) | 7 (13.7) | 2 (15.4) | 1.000 |
| Coronavirus | 7 (10.9) | 6 (11.8) | 1 (7.7) | 1.000 |
| Otitis media | 7 (10.9) | 4 (7.8) | 3 (23.1) | 0.181 |
| Tonsilitis | 6 (9.4) | 5 (9.8) | 1 (7.7) | 1.000 |
| BK virus-induced hemorrhagic cystitis | 6 (9.4) | 6 (11.8) | 0 (0.0) | 0.325 |
| Epstein-Barr virus reactivation | 5 (7.8) | 5 (9.8) | 0 (0.0) | 0.580 |
| Pneumonia | 5 (7.8) | 3 (5.9) | 2 (15.4) | 0.310 |
| Viral warts | 5 (7.8) | 4 (7.8) | 1 (7.7) | 1.000 |
| Otitis externa | 4 (6.3) | 3 (5.9) | 1 (7.7) | 1.000 |
| Adenovirus reactivation | 4 (6.3) | 3 (5.9) | 1 (7.7) | 1.000 |
| Sinusitis | 4 (6.3) | 4 (7.8) | 0 (0.0) | 0.568 |
| Cellulitis | 3 (4.7) | 3 (5.9) | 0 (0.0) | 1.000 |
| Chronic gingivitis | 3 (4.7) | 3 (5.9) | 0 (0.0) | 1.000 |
| Acute gingivitis | 3 (4.7) | 3 (5.9) | 0 (0.0) | 1.000 |
| Herpes zoster | 3 (4.7) | 2 (3.9) | 1 (7.7) | 0.539 |
| Others3 | 39 (60.9) | 35 (68.6) | 4 (30.8) | 0.007 |
| Non-infectious | ||||
| Mucositis | 37 (57.8) | 29 (56.9) | 8 (61.5) | 1.000 |
| Acute GvHD | 20 (31.3) | 20 (39.2) | 0 (0.0) | 0.003 |
| Neutropenia | 20 (31.3) | 12 (23.5) | 8 (61.5) | 0.051 |
| Disease relapse | 18 (28.1) | 11 (21.6) | 7 (53.8) | 0.094 |
| Chronic GvHD | 12 (18.8) | 12 (23.5) | 0 (0.0) | 0.055 |
| Skin rash | 9 (14.1) | 9 (17.7) | 0 (0.0) | 0.191 |
| Unspecified skin changes/disorder | 9 (14.1) | 8 (15.7) | 1 (7.7) | 0.671 |
| Gastritis | 9 (14.1) | 8 (15.7) | 1 (7.7) | 0.671 |
| Acute kidney injury | 8 (12.5) | 8 (15.7) | 0 (0.0) | 0.183 |
| Electrolyte imbalance | 7 (10.9) | 5 (9.8) | 2 (15.4) | 0.649 |
| Dental caries | 7 (10.9) | 7 (13.7) | 0 (0.0) | 0.335 |
| New malignancy | 5 (7.8) | 4 (7.8) | 1 (7.7) | 1.000 |
| Leukopenia | 4 (6.3) | 4 (7.8) | 0 (0.0) | 0.568 |
| Thrombocytopenia | 4 (6.3) | 4 (7.8) | 0 (0.0) | 0.568 |
| Impetigo | 4 (6.3) | 4 (7.8) | 0 (0.0) | 0.568 |
| Amenorrhea | 3 (4.7) | 3 (5.9) | 0 (0.0) | 1.000 |
| Appendicitis | 3 (4.7) | 3 (5.9) | 0 (0.0) | 1.000 |
| Cushing’s syndrome | 3 (4.7) | 2 (3.9) | 1 (7.7) | 0.539 |
| Short stature | 3 (4.7) | 3 (5.9) | 0 (0.0) | 1.000 |
| Allergic rhinitis | 3 (4.7) | 1 (1.9) | 2 (15.4) | 0.123 |
| Hypertension | 3 (4.7) | 3 (5.9) | 0 (0.0) | 1.000 |
| Recurrent oral aphthae | 3 (4.7) | 2 (3.9) | 1 (7.7) | 0.539 |
| Others4 | 61 (95.3) | 48 (94.1) | 13 (100.0) | 0.609 |
aGvHD was documented in 20/56 (35.7%) patients and involved the skin (n = 15, 75.0%), skin and gut (n = 2, 10.0%), oral mucosa (n = 1, 5.0%), and unknown sites (n = 2, 10.0%). Chronic GvHD (cGvHD) was documented in 12/56 (21.4%) patients and involved the oral mucosa (n = 6, 50.0%), skin (n = 3, 25.0%), skin and gut, oral and soft tissues, and muscle and soft tissues (n = 1, 8.3% each). The cGvHD grade was available for one (9.1%) patient (grade I). Four patients (33.3%) with cGvHD had not previously acquired aGvHD.
Five (8.1%) patients developed new malignancies [ALL, acute erythroid leukemia, malignant neoplasm of the adrenal gland, myeloid sarcoma, combined myeloid sarcoma, and diffuse large B-cell lymphoma (n = 1, 1.6% each)].
Following their BMT abroad, patients attended pediatric outpatient clinics within 2 weeks of the initial follow-up. During the first 6 months, they were closely monitored. Thereafter, follow-up appointments were scheduled every month during the first year and every 2 months in the second year. The mean follow-up time was 3.3 ± 2.5 years, and the median number of overseas follow-up visits was one (IQR: 1–2) per year.
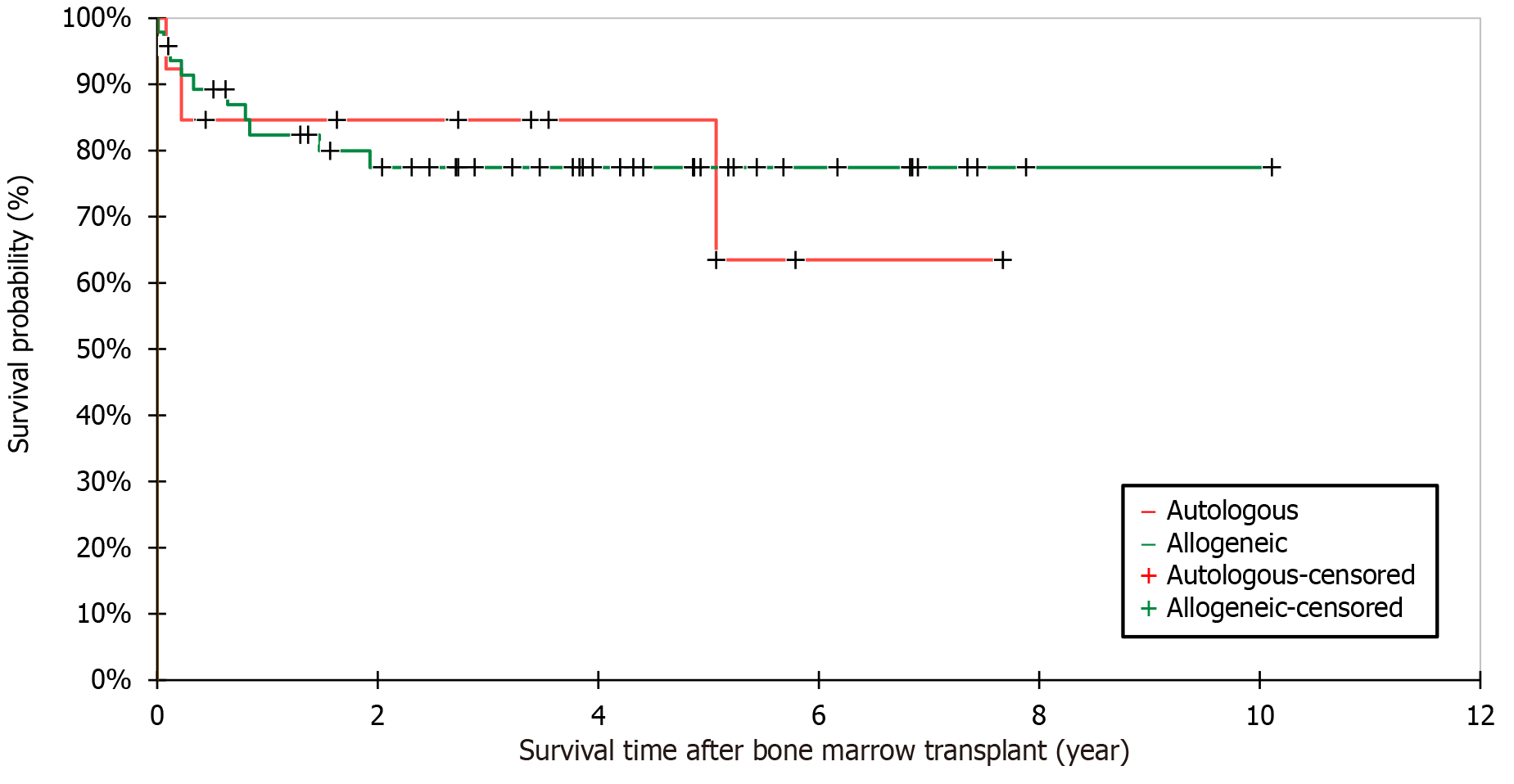
The Kaplan–Meier method was used to calculate the survival rate post-BMT (Figure 5). The overall survival rate was 77.4% (n = 48/62) and the 5-year survival rate was 79.0% (n = 49/62). There was no significant difference between allogeneic and autologous BMT recipients regarding the mean survival period post-BMT (3.4 ± 2.5 vs 2.9 ± 2.4 years, respectively; P = 0.577, 95%CI: 1.9-1.1). The overall mortality rate was 22.6% (n = 14/62). The mean age at death was 7.9 ± 4.3 years. The causes of death were disease relapse (n = 8, 57.1%), bronchopneumonia, significant major infections secondary to severe myelosuppression, disease refractoriness, sepsis, capillary leak syndrome (n = 1, 7.1% each), and unknown causes (n = 3, 2.1%).
Patients with AML had worse survival outcomes than those with other indications for BMT (P = 0.028) (Table 4). Although patients with ALL had better survival rates than those with AML [7/10 (70.0%) vs 8/15 (53.3%), respectively], the difference was not significant (P = 0.678). Patients who underwent BMT in Middle Eastern centers had a higher overall survival rate than those who underwent BMT in other centers (P = 0.038). Among the Middle Eastern centers, the survival rate was higher in Saudi Arabia (n = 4/4, 100%) than in Jordan (n = 34/41, 82.9%) and Turkey (n = 5/7, 71.4%); however, this difference was not statistically significant (P = 0.159). The follow-up period of survivors was significantly longer than that of those who died (P < 0.0001). In univariate analysis (Table 5), patients with indications other than AML and those who underwent BMT in Middle Eastern centers had significantly better odds of survival than other patients (P = 0.015 and P = 0.032, respectively). There was no effect of conditioning regimens, chemotherapy- or TBI-based regimens, on patient survival [27/33 (81.8%) vs 17/21 (81.0%), respectively, P = 1.000]. Regarding donor age, older donors were associated with better recipient survival [12 (7.0-28.0) vs 9.4 (2.5-28.0) years]; however, this finding was not statistically significant (P = 0.450). After classifying the donors into adults and children (< 18 years), despite being statistically insignificant, younger adult donors [35.1 (28.0-42.0) vs 37.0 (28.0-44.0), P = 0.859] and older pediatric donors [8 (6.2-12.0) vs 7.4 (2.5-11.0), P = 0.263] showed better recipient survival. The survival rate was not significantly affected by other variables such as sex, area of residence, age, weight, height, BMI, presence of associated diseases, graft type, and post-BMT complications. Specifically, GvHD patients had no significant difference in survival rate compared with those without GvHD [19/24 (79.2%) vs 24/33 (72.7%), respectively, P = 1.000]. This finding involved both aGvHD [16/21 (76.2%) vs 29/36 (80.6%), respectively, P = 0.744] and cGvHD [9/11 (81.8%) vs 35/45 (77.8%), respectively, P = 0.715)].
| Variable | Survived, n = 48 (77.4) | Died, n = 14 (22.6) | P value (95%CI) |
| Sex | 1.0001 | ||
| Male | 26 (54.2) | 7 (50) | |
| Female | 22 (45.8) | 7 (50) | |
| Area of residence | 0.1142 | ||
| Northern | 17 (35.4) | 9 (64.3) | |
| Capital | 14 (29.2) | 1 (7.1) | |
| Southern | 9 (18.8) | 1 (7.1) | |
| Muharraq | 8 (16.7) | 3 (21.4) | |
| Age at BMT (year) | 7.9 ± 5.0 | 7.4 ± 4.6 | 0.772 (-2.6-3.4)3 |
| Age group at BMT | 0.8202 | ||
| 0-5 | 19 (76.0) | 6 (24.0) | |
| 6-10 | 16 (76.2) | 5 (23.8) | |
| 11-15 | 10 (76.9) | 3 (23.1) | |
| > 15 | 3 (100) | 0 (0.0) | |
| Weight at transplant (kg), (n = 48) | 21.2 (12.3-29.7) | 18.1 (8.8-24.6) | 0.2804 |
| Height at transplant (cm), (n = 47) | 116.8 ± 28.8 | 110.9 ± 37.8 | 0.619 (-17.8-29.6)3 |
| Body mass index, (n = 49) | 16.0 (14.4-18.6) | 16.0 (15.4-17.0) | 0.5854 |
| Presence of associated diseases | 24 (50.0) | 5 (35.7) | 0.3801 |
| Type of graft (n = 61) | 1.0001 | ||
| Autologous | 10 (20.8) | 3 (23.1) | |
| Allogeneic | 38 (79.2) | 10 (76.9) | |
| Donor-recipient relationship (n = 61) | 0.8632 | ||
| Related donors | 37 (77.1) | 10 (76.9) | |
| Patients themselves | 10 (20.8) | 3 (23.1) | |
| Unrelated donors | 1 (2.1) | 0 (0.0) | |
| Indications of BMT | |||
| Acute myeloid leukemia | 8 (16.7) | 7 (50.0) | 0.0281 |
| Other indications | 40 (83.3) | 7 (50.0) | |
| Post-BMT complications (n = 58) | 45 (95.7) | 13 (100.0) | 1.0001 |
| BMT countries | 0.0382 | ||
| Middle East | 43 (89.6) | 9 (64.3) | |
| Other countries | 5 (10.4) | 5 (35.7) | |
| Follow-up duration (year) | 3.9 ± 2.3 | 0.8 ± 1.3 | < 0.0001 (2.1-4.1)3 |
| Variable | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Male sex | 0.846 (0.257-2.786) | 0.783 | 0.819 (0.093-7.184) | 0.857 |
| Governorate (Northern vs others) | 3.282 (0.947-11.376) | 0.061 | 4.415 (0.248-78.607) | 0.312 |
| Age at BMT (year) | 0.821 (0.411-1.460) | 0.577 | 1.481 (0.658-3.331) | 0.342 |
| Age group (0-5 years vs others) | 1.145 (0.343-3.825) | 0.826 | 1.081 (0.013-93.501) | 0.973 |
| Weight at BMT (kg) | 0.965 (0.900-1.034) | 0.307 | 0.863 (0.512-1.455) | 0.581 |
| Height at BMT (cm) | 0.993 (0.968-1.019) | 0.611 | 0.982 (0.819-1.179) | 0.849 |
| Body mass index (kg/m2) | 0.858 (0.692-1.064) | 0.164 | 0.850 (0.324-2.230) | 0.741 |
| Presence of associated diseases | 1.800 (0.526-6.164) | 0.349 | 2.841 (0.343-23.518) | 0.333 |
| Type of graft (allogeneic vs autologous) | 1.140 (0.263-4.940) | 0.861 | 4.532 (0.330-62.245) | 0.258 |
| Indication (AML vs others) | 5.000 (1.371-18.232) | 0.015 | 21.556 (0.719-646.242) | 0.077 |
| Presence of complications | 1.154 (0.111-12.048) | 0.905 | 0.000 (0.000-0.000) | 0.999 |
| BMT countries (Middle East vs others) | 4.778 (1.140-20.020) | 0.032 | 0.463 (0.034-6.300) | 0.563 |
This study demonstrated that most pediatric patients who underwent BMT abroad were males (53.2%). This is similar to the results of a study conducted by Kaphan et al, who reported a male predominance of 55.5%[22]. Other studies have also reported similar findings, where the male predominance ranged from 51.2% to 67.5%[12-15,23-25]. However, most published studies have combined adult and pediatric populations. The male predominance could be attributed to the higher incidence of certain hematologic disorders, such as AML, which is more common in males, and genetic disorders, such as thalassemia[26,27].
The mean age at transplant in this study was 7.8 ± 4.9 years. This finding is comparable to those of studies conducted in Saudi Arabia and Turkey, which reported a median age of 7.46 and 7 years, respectively[12,24]. This similarity may reflect common regional practices and guidelines regarding the timing of BMT in pediatric patients. However, a notable deviation was observed in another study from Saudi Arabia, which reported a significantly lower median age of 2.5 years in pediatric patients[25]. This could be explained by the specific patient disease profile in that study, as most patients had metabolic and immune deficiency disorders that are often identified at an early age, and undergoing early BMT would improve their outcomes[28,29].
In this study, AML was the main indication for BMT (36.6%), followed by ALL (26.8%). Similarly, a study in Saudi Arabia conducted by Solaiman et al[23] reported that the main indication for pediatric BMT was AML (30.0%), followed by ALL (30.0%). This pattern is consistent with findings from other studies conducted in the Middle East and North Africa, such as in Jordan, Oman, and Algeria, as well as in regions beyond Europe and China. Studies have reported a range of 22.0% to 67.4% for AML and 18.0% to 45.6% for ALL[13,16,30-32]. This high prevalence of AML as an indication for BMT might be due to the aggressive nature of the disease and poorer prognosis compared with other hematologic malignancies, often necessitating BMT as a curative approach[33]. In contrast, a large survey (n = 39093) conducted by Passweg et al[32] in Europe up to 2020 showed that the main indications for BMT were lymphoid malignancies (64%), followed by myeloid malignancies (25%) and non-malignant disorders (6%). Moreover, a study from Saudi Arabia reported that immune deficiency disorders were the most common indications[25]. The high rate of consanguineous marriages in Saudi Arabia (57.7%) may have contributed to the higher incidence of inherited immunodeficiency disorders[25,34]. Our study also showed a high parental consanguinity rate of 38.2%.
Most of the patients in our study received allogeneic grafts (77.4%). Similarly, a study from Oman conducted by Dennison et al[30] also showed a predominance of patients undergoing primary allogeneic BMT, but with a higher percentage of 97%. Nonetheless, our findings align with the trends observed in studies from Jordan, Algeria, and Saudi Arabia, which ranged from 62.0% to 74.0%[13,35,36]. The predominance of allogeneic transplants in our study and other regions is likely because BMT is the standard of care for a wide range of hematological diseases[37]. Regarding the source of stem cells, 56.5% of our patients received peripheral blood stem cells, which is concordant with a study carried out by Yesilipek et al[14] who reported a percentage of 55.9%.
Most allogeneic transplants in our study were from HLA-identical siblings (39.6%). Likewise, studies from Jordan, Saudi Arabia, Turkey, Tunisia, Oman, and Algeria reported that most donors were HLA-identical. However, this contrasts with a significantly higher range of 82.6% to 97.0%[13,23,24,30,31,35,36,38]. This could be due to the larger family size, which increases the probability of finding an HLA-matched donor within the family.
Although allogeneic grafts were most commonly used (77.6%), mainly from related first-degree donors (76.1%), donor registries were not available in Bahrain. Even in countries with donor registries available, challenges may arise in offering suitable donors. According to studies from the United States, donors of European ancestry typically dominate donor registries, resulting in disproportionately lower match rates for people of other ethnic origins, such as African, non-Black Hispanic, and Asian populations[39,40]. For young patients who frequently need quick access to donors for life-threatening diseases, this discrepancy is especially troubling.
Conditioning regimens are a critical component of BMT aimed at suppressing the immune system and preparing for engraftment[17]. Most patients in the current study received a conditioning regimen before the first and second BMT (n = 60/68, 88.2%). This finding aligns with other studies that have reported the use of conditioning regimens ranging from 85% to 100%, depending on the specific center and the patient’s case[12,21,23,24]. The most common conditioning regimen in our study was a combination of fludarabine and TBI (11.7%). However, studies conducted in Kuwait and Turkey reported that the combination of busulfan and cyclophosphamide was the most common regimen, reported in 47.6% and 38.8% of patients, respectively[14,15]. The variations in the choice of conditioning regimens across centers could be attributed to the differences in institutional protocols, patient demographics, underlying diseases, and available resources. Traditional regimens often involving high-dose chemotherapy or TBI can lead to significant toxicities, particularly in pediatric patients[17]. Gyurkocza et al[17] have reported that higher doses of TBI led to more frequent and fatal gastrointestinal, hepatic, and pulmonary toxicities, secondary malignancies, and stunted growth and development in children, even though they decreased the chance of relapse. Toxicities that occur later in life, such as infertility, have also been reported[41]. Accordingly, a standardized conditioning regimen for different types of indications should be explored by comparing myeloablative, reduced-intensity, and non-myeloablative regimens.
In this study, many patients developed various complications post-BMT (94.1%). Similarly, a study conducted in Germany reported a postoperative complication rate of 74.2%[11]. The most common complication in our study was infection (79.7%). Similarly, a multicenter trial[8] found that infections were the most common post-BMT complication (77.0%). This could be due to the various risk factors that lead to the development of complications such as comorbidities, source of stem cells, type of donor, usage of prophylaxis, and intensity of the conditioning regimen[42]. In this study, upper respiratory tract infection was the most common infectious complication (56.3%), followed by cytomegalovirus reactivation, gastroenteritis (25.0% each), and sepsis (21.9%). A multicenter study reported that most viral infections were caused by cytomegalovirus (23.6%)[8]. Another study from Lebanon discussed the reasons for emergency department presentation after BMT discharge and found that most patients developed pneumonia (29.6%), followed by gastroenteritis (20.0%) and sepsis (18.3%)[43]. Even though gastroenteritis and sepsis were the main complications in our study, the finding of pneumonia being the main complication contrasts with our study findings, in which pneumonia accounted for only 7.8% of our patients. The most common non-infectious complications in our study were mucositis (57.8%) and aGvHD (31.3%), while disease relapse was noted in 28.1% of patients. However, Chaudhry et al[44] reported an overall rate of any type of mucositis as high as 82.4%. GvHD was another frequent complication reported in numerous studies, in which aGvHD ranged from 13.5% to 54.4%, which is similar to that in our study, and cGvHD ranged from 9.0% to 43.4%[14,22,23,25,30,45]. Moreover, in parallel with our study, a French study reported a disease relapse of 33.8%[22]. The variation in post-BMT complication rates could be due to differences in the conditioning regimens prescribed, donor matching, and prophylactic measures used across different centers.
The mean follow-up time in this study was 3.3 ± 2.5 years, which is comparable to that of a study from Tunisia that reported a median follow-up of 3.5 years[38]. In contrast, other studies documented longer median follow-up periods ranging from 3.9 to 5.2 years[12,14,24,30]. The shorter follow-up period in our study could be attributed to logistical challenges associated with patients undergoing BMT overseas and then returning to our hospital for follow-up care.
We observed an overall survival rate of 77.4%. In contrast, a study in the United States reported a 30-year overall survival rate of 57.5%, highlighting a significant difference in the outcomes[46]. Similarly, a study from Jordan reported an overall survival rate of 65.0%, which was also lower than the rates observed in our study[35]. The higher overall survival rate in this study could be due to the high utilization of related donors in allogeneic transplants, an opportunity for re-transplantation, and conducting BMT in reputable centers worldwide. Additionally, this study reported a 5-year survival rate of 79.0%, which falls within the range of 52.0% to 92.3% reported in other studies[12,14,24,25]. However, a study in Saudi Arabia reported a 5-year survival rate of 17%[23]. This could be due to the study’s unique cohort of patients who required admission to an intensive care unit[23].
Several factors affect the survival of patients. Our study showed that non-AML patients and those who underwent transplantation in Middle Eastern centers had better survival rates. Conversely, Arab Borzu et al[47] showed that AML patients had better survival than those with ALL, with a lower mortality risk by 0.38 times (hazard ratio: 0.62, 95%CI: 0.29-0.7). Other factors such as donor age, conditioning regimen, and GvHD complications may also affect survival. Regarding donor age, like this study, Kollman et al[48] found that younger adult donors had improved overall survival and better disease-free survival in BMT recipients. This emphasizes the importance of prioritizing younger donors in clinical practice. Given the presence of several conditioning regimens tailored to different types of malignancies, studying the effects of different conditioning regimens on patient survival was challenging. Although our study did not find a difference between the two types of conditioning regimens, chemotherapy- and TBI-based, in terms of survival, van der Stoep et al[49] mentioned that one of the main causes of transplant-related mortality was the toxicity of the conditioning regimen. Moreover, Juric et al[4] showed that reduced intensive conditioning (less chemotherapy and radiation) leads to a better survival rate than myeloablative conditioning (use of alkylating agents and TBI). Therefore, the use of less harmful and safer conditioning regimens is recommended[17]. Although our study did not show an effect of GvHD development on patient survival, Yu et al[9] reported that a lower rate of GvHD post-BMT was associated with improved survival rates and reduced hospital stay.
Regarding long-term survivorship and quality of life of patients post-BMT, studies have demonstrated that BMT survivors suffer a significantly higher burden of severe chronic diseases and impairments affecting almost every organ system and overall quality of life than non-transplanted childhood cancer survivors[17,39-41,49,50]. However, these aspects require further investigation.
Considering the retrospective nature of our study, most limitations were related to data inaccessibility, such as missing patient data, including surgical history, anthropometric data during BMT, and medications used. This study also had a limited sample size; however, this could be attributed to the relatively small population of Bahrain. According to Bahrain's June 2024 population statistics, the total population was 1588670 and the population at risk (up to the age of 19 years) was only 275791 (203445 of them were Bahraini children)[51]. Given the size of Bahrain’s population, the number of pediatric patients requiring BMT each year is limited. Therefore, this study included all eligible patients during the study period, making it a comprehensive reflection of the available cohort. Additionally, although an extensive literature search was conducted, we could not find studies from countries without BMT facilities to compare with our study. Despite these limitations, the findings of this study are important as they could inform clinical practice by improving the pre-transplant evaluation process, patient selection, and follow-up care strategies for pediatric BMT patients. Moreover, these findings point to areas for future research, such as exploring the long-term health outcomes of pediatric patients post-BMT and investigating factors that may influence the success of transplantation. Furthermore, this study is significant as it is the first to focus on Bahraini pediatric patients who underwent BMT overseas. The results of this study contribute to the literature by highlighting the effectiveness of BMT in a range of pediatric conditions. The findings of this study can further benefit centers with no BMT facilities.
BMT is widely regarded as a life-saving procedure for oncological, hematological, and immunological diseases. However, the development of BMT facilities can be costly and challenging for several reasons. These include: (1) Availability of facilities and resources; (2) Expense of transplantation and drug costs; (3) Managing legal and regulatory requirements; (4) Recruiting and training a collaborative multidisciplinary team; and (5) Collaborating with other BMT centers. Although these challenges are daunting, many countries have established effective BMT facilities. On December 7, 2018, the first BMT procedure was performed in Bahrain at King Hamad University Hospital in a 55-year-old male headed by a team from Turkey[52]. However, none of these pediatric patients underwent transplantation in Bahrain. Although pre-BMT care, donor selection, and post-BMT management are available locally, the lack of a pediatric BMT unit necessitates conducting transplantations abroad. To enhance healthcare standards, Bahrain invites highly qualified visiting specialists, including pediatric hematologists and oncologists, to provide advanced patient care, share knowledge, conduct training sessions, and introduce cutting-edge treatment protocols. This collaborative effort reflects Bahrain's dedication to delivering world-class healthcare services. Moreover, the Bahrain Oncology Center (BOC) at King Hamad University Hospital, another hospital in Bahrain that was inaugurated on February 5, 2019, currently provides state-of-the-art services for adult oncology, hematology, BMT, and radiation therapy. This center collaborates with SMC on patient management through weekly multidisciplinary team meetings. It is worth mentioning that the BOC continues to aim for further expansion and a state-of-the-art pediatric BMT unit is expected to open soon. The planned pediatric BMT unit represents a critical milestone, reflecting the center’s commitment to address unmet needs within the community. Once started, the pediatric BMT unit will gradually reduce dependence on international centers for BMT procedures, enhance Bahrain’s reputation as a comprehensive healthcare provider, and strengthen local expertise through continuous training and partnerships with leading global institutions.
This study found that male patients with AML were the leading indicators for BMT in Bahrain. Most patients received chemotherapy-based conditioning regimens and underwent allogeneic transplantation, mostly from HLA-identical siblings. This study also demonstrated a high prevalence of post-BMT complications, with infectious complications being the most common. Mucositis was the most frequent non-infectious complication. Despite these complications, the overall and 5-year survival rates of 77.4% and 79.0%, respectively, highlighted the effectiveness of BMT as a life-saving procedure. Moreover, non-AML patients and those who underwent BMT in Middle Eastern countries showed better survival outcomes. In comparison, we found that Bahraini pediatric patients had clinical characteristics comparable to those reported from different countries, including neighboring and international countries. Although Bahrain has advanced medical services, it still lacks a pediatric BMT unit; therefore, pursuing BMT overseas can present patients with advanced diseases with considerable hope when a compatible donor is provided. The main priority should be to identify high-risk patients and manage early overseas BMT to deliver ideal patient care. Moreover, establishing a pediatric BMT unit in Bahrain presents a strategic opportunity to enhance accessibility, reduce logistical challenges, and improve patient outcomes through localized multidisciplinary care. A multicenter prospective study should be conducted to determine the cost-effectiveness of overseas BMTs in countries without this essential facility. Moreover, further studies are needed to explore specific measures to reduce post-BMT complications, such as post-transplant vaccination, to reduce episodes of infections.
The authors extend their deep gratitude to all medical staff in the Department of Pediatrics, Salmaniya Medical Complex, Government Hospitals, the Supreme Committee for Treatment Abroad, and the Overseas Treatment Office at the Ministry of Health, Manama, Kingdom of Bahrain, for their devoted care of bone marrow transplant patients.
| 1. | Thomas ED, Lochte HL Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 621] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Bazinet A, Popradi G. A general practitioner's guide to hematopoietic stem-cell transplantation. Curr Oncol. 2019;26:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, Dolstra H, Duarte RF, Glass B, Greco R, Lankester AC, Mohty M, Neven B, de Latour RP, Pedrazzoli P, Peric Z, Yakoub-Agha I, Sureda A, Kröger N; European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 190] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 4. | Juric MK, Ghimire S, Ogonek J, Weissinger EM, Holler E, van Rood JJ, Oudshoorn M, Dickinson A, Greinix HT. Milestones of Hematopoietic Stem Cell Transplantation - From First Human Studies to Current Developments. Front Immunol. 2016;7:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, Giralt SA, LeMaistre CF, Marks DI, Omel JL, Orchard PJ, Palmer J, Saber W, Veys PA, Carpenter PA, Hamadani M. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 6. | Passweg JR, Baldomero H, Chabannon C, Corbacioglu S, de la Cámara R, Dolstra H, Glass B, Greco R, Mohty M, Neven B, Peffault de Latour R, Perić Z, Snowden JA, Yakoub-Agha I, Sureda A, Kröger N; European Society for Blood and Marrow Transplantation (EBMT). Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 2022;57:742-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Scales DC, Thiruchelvam D, Kiss A, Sibbald WJ, Redelmeier DA. Intensive care outcomes in bone marrow transplant recipients: a population-based cohort analysis. Crit Care. 2008;12:R77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Young JH, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, Knust K, Horowitz MM, Confer DL, Dubberke ER, Pergam SA, Marty FM, Strasfeld LM, Brown JWM, Langston AA, Schuster MG, Kaul DR, Martin SI, Anasetti C; Blood and Marrow Transplant Clinical Trials Network Trial 0201. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol Blood Marrow Transplant. 2016;22:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Yu J, Parasuraman S, Shah A, Weisdorf D. Mortality, length of stay and costs associated with acute graft-versus-host disease during hospitalization for allogeneic hematopoietic stem cell transplantation. Curr Med Res Opin. 2019;35:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Li Z, Rubinstein SM, Thota R, Savani M, Brissot E, Shaw BE, Majhail NS, Mohty M, Savani BN. Immune-Mediated Complications after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2016;22:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Hierlmeier S, Eyrich M, Wölfl M, Schlegel PG, Wiegering V. Early and late complications following hematopoietic stem cell transplantation in pediatric patients - A retrospective analysis over 11 years. PLoS One. 2018;13:e0204914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Al-Sweedan S, Al-Seraihy A, Al-Ahmari A, Al-Jefri A, Mohammed V, Jafri R, Siddiqui K, Ayas M. Factors Determining the Outcome of Hematopoietic Stem Cell Transplantation in Patients With Acute Lymphoblastic Leukemia at King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia. J Pediatr Hematol Oncol. 2017;39:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Halahleh K, Sarhan MM, Peter Gale R, Hashem H, Khattab E, Da'na W, Ma'koseh M, Abu Shanab M, Yousef M, Khalil L, Hussein N, Sharma S, Abujazar H, Rihani R, Tbakhi A, Al Abadi A. Hematopoietic cell transplants in Jordan: different indications from the US and EU. Bone Marrow Transplant. 2019;54:1379-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Yesilipek MA, Ertem M, Cetin M, Öniz H, Kansoy S, Tanyeli A, Anak S, Kurekci E, Hazar V. HLA-matched family hematopoetic stem cell transplantation in children with beta thalassemia major: the experience of the Turkish Pediatric Bone Marrow Transplantation Group. Pediatr Transplant. 2012;16:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Al Shemmari SH, Ameen R. Kuwait bone marrow transplantation activities. Hematol Oncol Stem Cell Ther. 2017;10:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Xu LP, Lu DP, Wu DP, Jiang EL, Liu DH, Huang H, Sun ZM, Li NN, Liu QF, Zhang X, Lai YR, Song YP, Song XM, Liu SX, Zhang YC, Luo CJ, Xia LH, Niu T, Yu Y, Zhang XH, Tang XW, Luo Y, Huang XJ; Chinese Blood and Marrow Transplantation Registry Group. Hematopoietic Stem Cell Transplantation Activity in China 2020-2021 During the SARS-CoV-2 Pandemic: A Report From the Chinese Blood and Marrow Transplantation Registry Group. Transplant Cell Ther. 2023;29:136.e1-136.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 17. | Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 18. | Madbouly A, Bolon YT. Race, ethnicity, ancestry, and aspects that impact HLA data and matching for transplant. Front Genet. 2024;15:1375352. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Shimoni A, Radici V, Nagler A. Conditioning. 2024 Apr 11. In: The EBMT Handbook: Hematopoietic Cell Transplantation and Cellular Therapies [Internet]. Cham (CH): Springer, 2024. [PubMed] |
| 20. | Cleaver SA, Warren P, Kern M, Hurley CK, Raffoux C, Keller J, Kiesel U, Koza V, Marry E, Mitterschiffthaler A, Nakamura M, Okah CT, Persson U, Radde-Stepaniak T, Ranson L, Raymond J, do Rosario Sancho M, Varla-Leftherioti M, Wiegand T, Winterhager JM, Woodfield DG. Donor work-up and transport of bone marrow--recommendations and requirements for a standardized practice throughout the world from the Donor Registries and Quality Assurance Working Groups of the World Marrow Donor Association (WMDA). Bone Marrow Transplant. 1997;20:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Vakiti A, Reynolds SB, Mewawalla P. Acute Myeloid Leukemia. 2024 Apr 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 22. | Kaphan E, Bettega F, Forcade E, Labussière-Wallet H, Fegueux N, Robin M, Peffault De Latour R, Huynh A, Lapierre L, Berceanu A, Marcais A, Debureaux PE, Vanlangendonck N, Bulabois CE, Magro L, Daniel A, Galtier J, Lioure B, Chevallier P, Antier C, Loschi M, Guillerm G, Mear JB, Chantepie S, Cornillon J, Rey G, Poire X, Bazarbachi A, Rubio MT, Contentin N, Orvain C, Dulery R, Bay JO, Croizier C, Beguin Y, Charbonnier A, Skrzypczak C, Desmier D, Villate A, Carré M, Thiebaut-Bertrand A. Late relapse after hematopoietic stem cell transplantation for acute leukemia: a retrospective study by SFGM-TC. Transplant Cell Ther. 2023;29:362.e1-362.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Solaiman OM, Elhassan T, Fakih RE, Mannan A, Alduhailib Z, Mahdali AA, Alzahrani H, Jamil M, Chaudhri N, Elhazmi A, Kolko M, Al-Sharif FZ, Alrbiaan A, Shaban M, Shaheen M, Salahuddin N, Alfraih FA, Altarifi AS, Hassanein M, Hosaini S, Alhashim N, Mohamed AA, Hanbali A, Aljanoubi AH, Al-Obaidi NR, Rasheed W, Maghrabi K, Almohareb F, Soubani A, Aljurf M, Ahmed SO. Outcomes and Long-Term Survival of Adolescent and Young Adult Patients Admitted to the Intensive Care Unit Following Allogeneic Hematopoietic Stem Cell Transplantation: A Single-Center Experience of 152 Patients. Hematol Oncol Stem Cell Ther. 2024;17:110-119. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Yesilipek MA, Uygun V, Kupesiz A, Karasu G, Ozturk G, Ertem M, Şaşmaz İ, Daloğlu H, Güler E, Hazar V, Fisgin T, Sezgin G, Kansoy S, Kuşkonmaz B, Akıncı B, Özbek N, İnce EÜ, Öztürkmen S, Küpesiz FT, Yalçın K, Anak S, Bozkurt C, Karakükçü M, Küpeli S, Albayrak D, Öniz H, Aksoylar S, Okur FV, Albayrak C, Yenigürbüz FD, Bozkaya İO, İleri T, Gürsel O, Karagün BŞ, Kintrup GT, Çelen S, Elli M, Aksoy BA, Yılmaz E, Tanyeli A, Akyol ŞT, Siviş ZÖ, Özek G, Uçkan D, Kartal İ, Atay D, Akyay A, Bilir ÖA, Çakmaklı HF, Kürekçi E, Malbora B, Akbayram S, Demir HA, Kılıç SÇ, Güneş AM, Zengin E, Özmen S, Antmen AB. Thalassemia-free and graft-versus-host-free survival: outcomes of hematopoietic stem cell transplantation for thalassemia major, Turkish experience. Bone Marrow Transplant. 2022;57:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Ayas M, Al-Seraihi A, Al-Jefri A, Al-Ahmari A, Al-Mahr M, Al-Ghonaium A, Al-Muhsen S, Al-Mousa H, Al-Dhekri H, Alsaud B, Eldali A, Mohamad A, Al-Humaidan H, Chadrawi A, Al-Kaff M, Al-Hassnan Z, El-Solh H. Unrelated cord blood transplantation in pediatric patients: a report from Saudi Arabia. Bone Marrow Transplant. 2010;45:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Amini M, Sharma R, Jani C. Gender differences in leukemia outcomes based on health care expenditures using estimates from the GLOBOCAN 2020. Arch Public Health. 2023;81:151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Tuo Y, Li Y, Li Y, Ma J, Yang X, Wu S, Jin J, He Z. Global, regional, and national burden of thalassemia, 1990-2021: a systematic analysis for the global burden of disease study 2021. EClinicalMedicine. 2024;72:102619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 28. | Yabe H. Allogeneic hematopoietic stem cell transplantation for inherited metabolic disorders. Int J Hematol. 2022;116:28-40. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Castagnoli R, Delmonte OM, Calzoni E, Notarangelo LD. Hematopoietic Stem Cell Transplantation in Primary Immunodeficiency Diseases: Current Status and Future Perspectives. Front Pediatr. 2019;7:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 30. | Dennison D, Al Kindi S, Pathare A, Daar S, Nusrat N, Ur Rehman J, Zia F, Khan H, Khan MI, Alghazaly A, Al Zadjali S, Tauro M, Al Lawatia A, Ganguly S. Hematopoietic stem cell transplantation in Oman. Bone Marrow Transplant. 2008;42 Suppl 1:S109-S113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Benakli M, Ahmed Nacer R, Mehdid F, Belhadj R, Talbi A, Rahmoune N, Niederwieser C, Baazizi M, Akhrouf S, Ait Ouali D, Bouarab H, Zerkout S, Abderahim I, Harieche F, Hamladji RM. Two decades of experience in a combined adult/pediatric allogeneic hematopoietic stem cell transplantation center in Algiers, Algeria. Ann Hematol. 2020;99:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Cámara R, Corbacioglu S, Dolstra H, Duarte R, Glass B, Greco R, Lankester AC, Mohty M, Peffault de Latour R, Snowden JA, Yakoub-Agha I, Kröger N; European Society for Blood and Marrow Transplantation (EBMT). Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56:1651-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 252] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 33. | Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1592] [Article Influence: 144.7] [Reference Citation Analysis (1)] |
| 34. | el-Hazmi MA, al-Swailem AR, Warsy AS, al-Swailem AM, Sulaimani R, al-Meshari AA. Consanguinity among the Saudi Arabian population. J Med Genet. 1995;32:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 275] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Abdel-Rahman F, Hussein A, Rihani R, Hlalah O, El Taani H, Sharma S, Nserat T, Sarhan M. Bone marrow and stem cell transplantation at King Hussein cancer center. Bone Marrow Transplant. 2008;42 Suppl 1:S89-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Saudi Society of Blood and Marrow Transplantation (SSBMT). Hematopoietic cell transplantation and cell therapy activity landscape survey in the Kingdom of Saudi Arabia; a report from the Saudi Society of Blood and Marrow Transplantation (SSBMT). Bone Marrow Transplant. 2024;59:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, Omel JL, Orchard PJ, Palmer J, Saber W, Savani BN, Veys PA, Bredeson CN, Giralt SA, LeMaistre CF. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 38. | Ben Othman T, Torjemane L, Abdelkefi A, Lakhal A, Ladeb S, Ben Hamed L, Slama H, Ben Abdeladhim A. Allogeneic hematopoietic stem cell transplantation in Tunisia. Bone Marrow Transplant. 2008;42 Suppl 1:S139-S141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Fingrut WB, Davis E, Archer A, Brown S, Devlin S, Nhaissi M, Rapoport C, Chinapen S, Kelly A, Wells D, Scaradavou A, Gyurkocza B, Papadopoulos E, Politikos I, Shaffer BC, Barker JN. Racial/ethnic disparities in availability of volunteer unrelated donors for allogeneic transplantation. Blood Adv. 2024;8:2753-2764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 40. | Ustun C, Bachanova V, Shanley R, MacMillan ML, Majhail NS, Arora M, Brunstein C, Wagner JE, Weisdorf DJ. Importance of donor ethnicity/race matching in unrelated adult and cord blood allogeneic hematopoietic cell transplant. Leuk Lymphoma. 2014;55:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Diesch-Furlanetto T, Gabriel M, Zajac-Spychala O, Cattoni A, Hoeben BAW, Balduzzi A. Late Effects After Haematopoietic Stem Cell Transplantation in ALL, Long-Term Follow-Up and Transition: A Step Into Adult Life. Front Pediatr. 2021;9:773895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Cho SY, Lee HJ, Lee DG. Infectious complications after hematopoietic stem cell transplantation: current status and future perspectives in Korea. Korean J Intern Med. 2018;33:256-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | El Majzoub I, Cheaito RA, Cheaito MA, Bazarbachi A, Sweidan K, Sarieddine A, Al Chami F, Tamim H, El Cheikh J. Clinical characteristics and outcomes of bone marrow transplantation patients presenting to the ED of a tertiary care center. Am J Emerg Med. 2021;46:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Chaudhry HM, Bruce AJ, Wolf RC, Litzow MR, Hogan WJ, Patnaik MS, Kremers WK, Phillips GL, Hashmi SK. The Incidence and Severity of Oral Mucositis among Allogeneic Hematopoietic Stem Cell Transplantation Patients: A Systematic Review. Biol Blood Marrow Transplant. 2016;22:605-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 45. | Khan S, Siddiqui K, ElSolh H, AlJefri A, AlAhmari A, Ghemlas I, AlSaedi H, AlEnazi A, AlSeraihi A, Ayas M. Outcomes of blood and marrow transplantation in children less than 2-years of age: 23 years of experience at a single center. Int J Pediatr Adolesc Med. 2022;9:190-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Bhatia S, Dai C, Landier W, Hageman L, Wu J, Schlichting E, Siler A, Funk E, Hicks J, Lim S, Balas N, Bosworth A, Te HS, Francisco L, Bhatia R, Salzman D, Goldman FD, Forman SJ, Weisdorf DJ, Wong FL, Armenian SH, Arora M. Trends in Late Mortality and Life Expectancy After Autologous Blood or Marrow Transplantation Over Three Decades: A BMTSS Report. J Clin Oncol. 2022;40:1991-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 47. | Arab Borzu Z, Khadembashi N, Hajifathali A, Baghestani A, Roshandel E. Survival Rate and Prognostic Factors Among Patients Undergoing Hematopoietic Stem Cell Transplantation: Using the Joint Model. Int J Cancer Manag. 2021;14:e106846. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, Hegland J, Kamani N, Kernan NA, King R, Ratanatharathorn V, Weisdorf D, Confer DL. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 542] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 49. | van der Stoep MYEC, Oostenbrink LVE, Bredius RGM, Moes DJAR, Guchelaar HJ, Zwaveling J, Lankester AC. Therapeutic Drug Monitoring of Conditioning Agents in Pediatric Allogeneic Stem Cell Transplantation; Where do We Stand? Front Pharmacol. 2022;13:826004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 50. | Chow EJ, Anderson L, Baker KS, Bhatia S, Guilcher GM, Huang JT, Pelletier W, Perkins JL, Rivard LS, Schechter T, Shah AJ, Wilson KD, Wong K, Grewal SS, Armenian SH, Meacham LR, Mulrooney DA, Castellino SM. Late Effects Surveillance Recommendations among Survivors of Childhood Hematopoietic Cell Transplantation: A Children's Oncology Group Report. Biol Blood Marrow Transplant. 2016;22:782-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 52. | Gulf Daily News. Bahrain's first bone marrow transplant operation a success. Available from: https://www.gdnonline.com/Details/468746/Bahrain. |