Published online Dec 18, 2024. doi: 10.5500/wjt.v14.i4.98797
Revised: August 21, 2024
Accepted: September 2, 2024
Published online: December 18, 2024
Processing time: 75 Days and 10.9 Hours
Prostaglandin E1 (PGE1), or alprostadil, is a potent vasodilator that improves hepatic blood flow and reduces ischemia-reperfusion injury post-liver trans
To assess the impact of PGE1 administration on renal function in patients who underwent liver or liver-kidney transplant.
This retrospective study included all patients who underwent liver or liver-kidney transplant at our institution from January, 2011 to December, 2021. Patients were classified based on whether they received PGE1. PGE1 was administered post-LT to those with transaminases > 1000 U/L in the immediate postoperative period. Demo
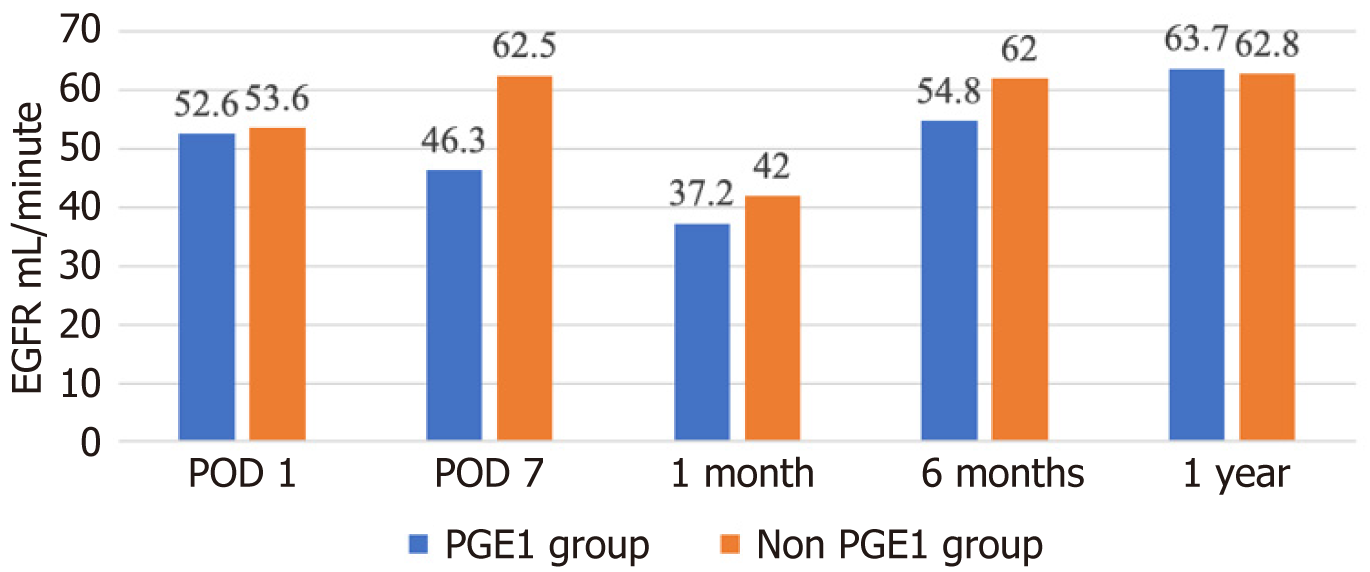
A total of 145 patients underwent LT, with 44 (30%) receiving PGE1. Baseline patient characteristics were comparable, except the PGE1 group had significantly higher aspartate aminotransferase (AST) (1961.9 U/L ± 1862.3 U/L vs 878 U/L ± 741.4 U/L, P = 0.000), alanine aminotransferase (1070.6 U/L ± 895 U/L vs 547.7 U/L ± 410 U/L, P = 0.000), international normalized ratio on post-LT day 1 (2 ± 0.74 vs 1.8 ± 0.4, P = 0.03), a longer intensive care unit stay (8.1 days ± 11.8 days vs 3.8 days ± 4.6 days, P = 0.003), more vasopressor use (55.53 hours ± 111 hours vs 16.33 hours ± 26.3 hours, P = 0.002), and higher immediate postoperative complications (18.6% vs 4.9%, P = 0.04). The PGE1 group also had a significantly higher 90-day readmission rate (29.6% vs 13.1%, P = 0.02) and lower 1-year liver graft survival (87.5% vs 98.9%, P = 0.005). However, 30-day readmission (31.6% vs 27.4%, P = 0.64), LT complications (hepatic artery thrombosis, biliary complications, rejection of liver graft, cardiomyopathy), 1-year patient survival (96.9% vs 97.8%, P = 0.77), overall liver graft survival, and overall patient survival were similar between the two groups (95.4% vs 93.9%, P = 0.74 and 88.4% vs 86.9%, P = 0.81 respectively). Although the PGE1 group had a significantly lower glomerular filtration rate (eGFR) on post-LT day 7 (46.3 mL/minute ± 26.7 mL/minute vs 62.5 mL/minute ± 34 mL/minute, P = 0.009), the eventual need for renal replacement therapy (13.6% vs 5.9%, P = 0.09), the number of dialysis sessions (0.91 vs 0.27, P = 0.13), and eGFR at 1-month (37.2 mL/minute ± 35.9 mL/minute vs 42 mL/minute ± 36.9 mL/minute, P = 0.49), 6-months (54.8 mL/minute ± 21.6 mL/minute vs 62 mL/minute ± 21.4 mL/minute, P = 0.09), and 12-months (63.7 mL/minute ± 20.7 mL/minute vs 62.8 mL/minute ± 20.3 mL/minute, P = 0.85) post-LT were similar to those in the non-PGE1 group.
In patients who received PGE1 for ischemia-reperfusion injury, despite immediate acute renal injury post-LT, the renal function at 1-month, 6-months, and 12-months post-LT was similar compared to those without ischemia-reperfusion injury. Prospective clinical trials are needed to further elucidate the benefits of PGE1 use in renal function.
Core Tip: This retrospective study examines the renal outcomes of prostaglandin E1 (PGE1) administration following liver transplantation (LT). Despite the PGE1 group experiencing initial acute renal injury and higher postoperative complications, their long-term renal function (up to 12 months) was comparable to those not receiving PGE1. The findings suggest that PGE1 may offer renoprotective benefits, warranting further prospective clinical trials to evaluate its potential in mitigating renal dysfunction post-LT.
- Citation: Jahagirdar V, Ahmed M, Fatima I, Ali H, Alba L, Helzberg JH, Cummings LS, Wilkinson M, Forster J, Likhitsup A. Prostaglandin E1 administration post liver transplantation and renal outcomes: A retrospective single center experience. World J Transplant 2024; 14(4): 98797
- URL: https://www.wjgnet.com/2220-3230/full/v14/i4/98797.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i4.98797
Liver transplantation (LT) is a lifesaving procedure for patients with acute and chronic end-stage liver disease. The number of LT performed annually in the United States has steadily increased, from 6291 in 2019 to 9528 in 2022[1]. Given the scarcity of donor organs and the high mortality while on the waitlist, maximizing graft function and recipient survival after LT is crucial.
Registry data indicate that the 5-year graft-survival and patient-survival post-LT are approximately 72.8% and 77% respectively[1,2]. Advancements in immunosuppression, management of rejection, infection prevention, and surgical techniques have improved short-term mortality after LT[3]. However, long-term mortality has not changed significantly in recent years[4]. The main causes of late death after LT are graft failure, malignancy, cardiovascular disease, and renal failure[5].
Renal dysfunction, whether before or after LT, significantly impacts several outcomes, including healthcare cost, graft function, and patient survival[3]. Depending on the definitions and assessment methods used, the incidence of perioperative renal dysfunction varies widely, ranging from 11% to 94%[6,7]. Risk factors for perioperative kidney dysfunction include sepsis, nephrotoxic medications, and impaired renal perfusion due to hemodynamic instability during surgery[8]. Long-term implications of post-LT kidney dysfunction have also been observed. Watt et al[5] analysed 798 LT recipients over a median follow-up period of ten years and found that the presence of renal insufficiency increased the risk of death from any cause in these patients. Studies have shown that patients who develop chronic kidney disease (CKD) after LT have a significantly higher risk of mortality, and renal failure is a leading cause of death among liver transplant recipients[9,10]. Renal impairment following LT can severely diminish a patient’s quality of life. CKD can lead to the need for dialysis, which not only affects physical health but also imposes a significant emotional and financial burden on patients and their families. The presence of renal dysfunction post-transplantation is associated with lower health-related quality of life scores, underscoring the importance of preserving renal function[11]. The need for dialysis, hospital readmissions, and potential retransplantation are significant economic burdens on healthcare systems. Managing renal complications in liver transplant patients significantly adds to the overall cost of care[12].
Hence interventions that can prevent or mitigate renal dysfunction could have profound benefits for patient outcomes and healthcare systems.
Ischemic-reperfusion injury (IRI) refers to tissue damage that occurs when an organ’s blood supply is interrupted and then restored. Cold IRI, which occurs during organ preservation, is caused by injury to hepatic sinusoidal endothelial cell, while warm IRI develops in situ during the LT and leads to hepatocellular damage[13]. The local innate immune system plays a crucial role in IRI. The lack of oxygen combined with adenosine triphosphate depletion leads to initial liver parenchymal cell death and ischemic injury. Reactive oxygen species, produced by the activation of Kupffer cells and sinusoidal endothelial cells, cause early reperfusion injury. The subsequent influx of neutrophils and CD4+ T-lymphocytes, along with their release of cytotoxic enzymes in the late phase of reperfusion, contributes to cellular degradation[14].
Hepatic IRI can detrimentally affect numerous distant organs, including the lungs, pancreas, and adrenals, thus precipitating multi-organ dysfunction[15]. Hepatic IRI is most commonly associated with kidney dysfunction[16,17]. There is a significant correlation between severe IRI and graft failure, including primary nonfunction (PNF), early allograft dysfunction, and the need for 90-day re-transplantation[18].
Alprostadil, a synthetic form of prostaglandin E1 (PGE1), interacts with G-protein-coupled receptors to increase adenosine levels, which in turn cause vasodilation, reduced leukocyte activity, and inhibition of platelet aggregation[19]. The hepatoprotective properties of prostaglandin (PG) were first discovered in animal models of liver injury caused by dimethyl nitrosamine, carbon tetrachloride, and IRI[20-22]. It is hypothesized that PGE1 increases hepatic blood flow and reduces cell-mediated cytotoxicity against liver cells[23-25].
PGE1 has shown potential benefits in improving graft survival in patients with PNF[26]. Randomized control trials have demonstrated that PGE1 use in LT recipients can reduce the need for immediate renal replacement in the perioperative period[27,28]. A randomized control trial (RCT) from India reported decreased incidence of immediate post-transplant renal dysfunction in patients undergoing living-donor LT who received perioperative PGE1[29]. However, data on its role in renal dysfunction among those undergoing deceased-donor LT are limited. This study aims to compare renal outcomes up to 1-year post-LT in patients who received PGE1 with those who did not.
We identified 145 consecutive patients who underwent LT at Saint Luke’s Hospital of Kansas City between January 1, 2011, and December 31, 2021. Clinical data were collected retrospectively from the electronic medical records and securely stored. We recorded and analyzed patients’ demographic details, post-LT treatments received, complications (hepatic artery thrombosis, biliary complications, rejection of liver graft, and cardiomyopathy), renal function, and survival. The sodium-model for end-stage liver disease (Na-MELD) scores were calculated using laboratory data immediately prior to LT. Patients were grouped based on whether or not they received PGE1. At our institution, PGE1 infusion was administered post-LT to patients who developed elevated transaminases [alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST)] > 1000 U/L after 24 hours.
The mean follow-up period was 2 years. Three specific time points were arbitrarily chosen to assess and compare renal function between the two groups, namely 1 month, 6-months and 12-months post-transplant.
Data are expressed as means ± SD. Categorical variables were compared using χ² and Fisher exact tests and continuous variables using t tests. Multivariable logistic regression analysis was performed using Stata/SE 16.1 9 (Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). A two-tailed P value < 0.05 was considered statistically significant. The study underwent IRB review and was approved.
A total of 145 patients were identified, who underwent LT at the institution between January 1, 2011, and December 31, 2021. Seventeen patients (12%) underwent simultaneous liver and kidney transplant. Thirty percent (n = 44) received PGE1, while 70% (n = 101) did not. The PGE1 group received an average of 2250 (range: 1566-2997) mcg of the drug over 47 hours (range: 5-78 hours). The mean age of the patients receiving PGE1 was 58 years ± 11.9 years, and majority were Caucasian males. Demographic characteristics between the two groups were comparable and are listed in Table 1.
| Variable | PGE1 (n = 44) | Non-PGE1 (n = 101) | P value |
| Age (years; mean ± SD) | 58 ± 11.9 | 56.7 ± 12.2 | 0.57 |
| Male | 54.6 | 61.4 | 0.44 |
| White | 84.1 | 83.2 | 0.78 |
| Indication for transplant | 0.3 | ||
| Nonalcoholic fatty liver disease | 31.8 | 28.7 | |
| Alcoholic liver disease | 25 | 29.7 | |
| Hepatitis C virus infection | 11.4 | 15.8 | |
| Others | 31.8 | 25.8 | |
| Model for end-stage liver disease score at transplant | 23.8 ± 10 | 25.2 ± 8 | 0.4 |
| Hepatorenal syndrome pre-transplant | 59.1 | 64.4 | 0.55 |
The leading indication for LT in the both groups was non-alcoholic fatty liver disease, followed by alcoholic liver disease and hepatitis C virus infection. The Na-MELD score at transplant was similar between the two groups (23.8 ± 10 in the PGE1 group, and 25.2 ± 8 in the non-PGE1 group). The prevalence of the hepato-renal syndrome was comparable between the groups (56.1% in the PGE1 and 64.4% in the non-PGE1 group respectively). Pre-LT characteristics are listed in Table 1.
On post-operative day 1, the PGE1 group had significantly higher AST (1961.9 U/L ± 1862.3 U/L vs 878 U/L ± 741.4 U/L, P = 0.000), ALT (1070.6 U/L ± 895 U/L vs 547.7 U/L ± 410 U/L, P = 0.000) and international normalized ratio (2 ± 0.74 vs 1.8 ± 0.4, P = 0.03). There was no significant difference in the glomerular filtration rate (eGFR) (52.6 mL/minute ± 27.6 7 mL/minute vs 53.6 mL/minute ± 29.8 mL/minute, P = 0.86) and total bilirubin (5.3 mg/dL ± 3.3 mg/dL vs 4.6 mg/dL ± 3.2 mg/dL, P = 0.19).
The PGE1 group had a longer intensive care unit (ICU) stay (8.1 days ± 11.8 days vs 3.8 days ± 4.6 days, P = 0.003) and a more prolonged vasopressor requirement (55.53 hours ± 111 hours vs 16.33 hours ± 26.3 hours, P = 0.002). The incidence of post-operative complications within 48 hours, including immediate post-operative bleeding with or without re-operation, was higher in the PGE1 group (18.6% vs 4.9%, P = 0.04).
On post-operative day 7, the PGE1 group showed a significantly higher total bilirubin (3.1 mg/dL ± 2.8 mg/dL vs 2 mg/dL ± 1.7 mg/dL, P = 0.002), ALT (214 U/L ± 122.6 U/L vs 146.7 U/L ± 164.1 U/L, P = 0.02) and lower eGFR (46.3 mL/minute ± 26.7 mL/minute vs 62.5 mL/minute ± 34 mL/minute, P = 0.009). There was no significant difference in AST levels between the two groups (82.3 U/L ± 105.1 U/L vs 60.2 U/L ± 111.5 U/L, P = 0.27).
The need for renal replacement therapy (RRT) during hospitalisation was not statistically different between the two groups (13.6% vs 5.9%, P = 0.09), with a comparable number of dialysis sessions in the PGE1 and non-PGE1 group (0.91 vs 0.27, P = 0.13).
The incidence of LT complications was similar between the two groups. These included hepatic artery thrombosis (4.6% in PGE1 group vs 1% in non PGE1 group, P = 0.17), biliary complications (27.3% vs 23%, P = 0.58), rejection of the liver graft (18.2% vs 13%, P = 0.42), and cardiomyopathy (0% vs 3%, P = 0.25).
Post-LT, there was no significant difference in the eGFR between the two groups at 1 month (37.2 mL/minute ± 35.9 mL/minute vs 42 mL/minute ± 36.9 mL/minute, P = 0.49), 6 months (54.8 mL/minute ± 21.6 mL/minute vs 62 mL/minute ± 21.4 mL/minute, P = 0.09), and 1 year (63.7 mL/minute ± 20.7 mL/minute vs 62.8 mL/minute ± 20.3 mL/minute, P = 0.85) (Figure 1). The 30-day readmission rates were similar (31.6% vs 27.4%, P = 0.64), although the PGE1 group experienced a significantly higher 90-day readmission rate (29.6% vs 13.1%, P = 0.02) (Table 2).
| Variable | PGE1 (n = 44) | Non-PGE1 (n = 101) | P value |
| Intensive care unit length of stay (days) | 8.1 ± 11.8 | 3.8 ± 4.6 | 0.003 |
| Post-operative complication within 48 hours1 | 18.6 | 4.9 | 0.04 |
| EGFR day 1 (mL/minute/1.73 m2) | 52.6 ± 27.6 | 53.6 ± 29.8 | 0.86 |
| AST day 1 (U/L) | 1961.9 ± 1862.3 | 878 ± 741.4 | 0.0000 |
| ALT day 1 (U/L) | 1070.6 ± 895 | 547.7 ± 410 | 0.0000 |
| Total bilirubin day 1 (mg/dL) | 5.3 ± 3.3 | 4.6 ± 3.2 | 0.19 |
| International normalized ratio day 1 | 2 ± 0.74 | 1.8 ± 0.4 | 0.03 |
| EGFR day 7 (mL/minute) | 46.3 ± 26.7 | 62.5 ± 34 | 0.009 |
| AST day 7 (U/L) | 82.3 ± 105.1 | 60.2 ± 111.5 | 0.27 |
| ALT day 7 (U/L) | 214 ± 122.6 | 146.7 ± 164.1 | 0.02 |
| Total bilirubin day 7 | 3.1 ± 2.8 | 2 ± 1.7 | 0.002 |
| Renal replacement therapy required | 13.6 | 5.9 | 0.09 |
| Dialysis (number of sessions) | 0.91 | 0.27 | 0.13 |
Multivariable analysis for eGFR at 1-month, 6-months, and 12-months post LT, adjusted for ICU duration, post-operative complications, 90-day readmission, and follow-up duration, showed no significant difference between the PGE1 and non-PGE1 group.
One-year graft survival was significantly lower in the PGE1 group (87.5% vs 98.9%, P = 0.005) although one-year patient survival was comparable (96.9% vs 97.8%, P = 0.77). The PGE1 group had a significantly shorter follow-up duration (615.3 days ± 569.4 days vs 1092.8 days ± 877.7 days, P = 0.002). Nonetheless, overall liver-graft survival and overall patient survival were similar between the two groups (95.4% vs 93.9%, P = 0.74 and 88.4% vs 86.9%, P = 0.81 respectively) (Table 3). Multivariable analysis, adjusted for factors including ICU stay duration, post-operative complications within 48 hours, eGFR at day 7, bilirubin at day 7, 90-day readmission, and follow-up duration, revealed no significant association with eGFR at 1 month, 6 months, and 12 months post-LT (Table 4).
| Variable | PGE1 (n = 44) | Non-PGE1 (n = 101) | P value |
| EGFR at 6 months (mL/minute) (mean ± SD) | 54.8 ± 21.6 | 62 ± 21.4 | 0.09 |
| EGFR at 1 year (mL/minute) (mean ± SD) | 63.7 ± 20.7 | 62.8 ± 20.3 | 0.85 |
| The 30-day readmission | 31.6 | 27.4 | 0.64 |
| The 90-day readmission | 29.6 | 13.1 | 0.02 |
| Liver transplant complications | |||
| Hepatic artery thrombosis | 4.6 | 1 | 0.17 |
| Biliary complications | 27.3 | 23 | 0.58 |
| Rejection of liver graft | 18.2 | 13 | 0.42 |
| Cardiomyopathy | 0 | 3 | 0.25 |
| Liver graft survival at 1 year | 87.5 | 98.9 | 0.005 |
| Patient survival at 1 year | 96.9 | 97.8 | 0.77 |
| Follow up duration (days) (mean ± SD) | 615.3 ± 569.4 | 1092.8 ± 877.7 | 0.002 |
| Overall liver graft survival | 95.4 | 93.9 | 0.74 |
| Overall patient survival | 88.4 | 86.9 | 0.81 |
| Parameter | Coefficient | Standard error | P value |
| eGFR at 1-month | 0.00055 | 0.0012 | 0.65 |
| eGFR at 6-months | -0.0011 | 0.002 | 0.58 |
| eGFR at 12-months | 0.0019 | 0.002 | 0.39 |
In this retrospective study, those who received PGE1 were a sicker group. We observed that these patients with elevated aminotransferases post-LT experienced longer ICU stay, had more post-operative complications, and had a lower eGFR one-week post-LT. However, by the one-year mark, these patients exhibited renal outcomes comparable to those who did not receive PGE1, in terms of need for RRT, the number of dialysis sessions, and eGFR at 1 month, 6 months, and 12 months. The fact that the two groups of patients had similar kidney function suggests that PGE1 improves or protects the kidneys.
A pilot study investigating the role of PGI2 provided initial evidence suggesting that PGI2 may have a positive impact on liver graft dysfunction[30]. Additionally, a recently published Cochrane review assessing various outcomes associated with PG use in LT found that it could significantly reduce the incidence of acute kidney failure (relative risk 0.42, 95%CI, 0.24-0.73)[31]. However, there remains a gap in the literature regarding the long-term renal outcomes associated with PGE1 administration during the peri-operative period, as well as the specific criteria for its use.
Previous RCTs align with the findings of the current study. Klein et al[28] randomized 118 patients into PGE1 and non-PGE1 groups and reported improved early renal function and fewer dialysis treatments in the PGE1 group. Similarly, Henley et al[27], who administered PGE1 intraoperatively and continued up to 21 days post-operation, noted reduced needs for renal support and shorter duration of hospitalization and ICU stays in the PGE1 group.
In the current study, the administration of PGE1 post-operatively was associated with longer ICU stays and increased vasopressor requirements, possibly due to graft dysfunction or the vasodilatory effects of PGE1. Vasopressor administration, performed exclusively in the ICU at our institution, likely contributed to extended ICU durations.
Various factors contribute to kidney dysfunction post-LT. The use of the Na-MELD score for organ allocation has increased the proportion of candidates with elevated creatine and/or those requiring RRT on the LT waitlist[32]. Sharma et al[11] reported that out of 221 patients who underwent LT at their institute, 51% had eGFR < 60 mL/minute, and 6.3% were on dialysis prior to LT. Similarly, close to two thirds of the present study population exhibited pre-transplant renal dysfunction. Subsequently, post-LT, various factors such as hypotension, renal ischemia, and drug-nephrotoxicity may worsen kidney function.
In animal models, cyclosporine-induced nephrotoxicity is primarily linked to endothelial activation and afferent arteriolar vasoconstriction. Research by Makowka et al[33] found that PG treatment could normalise renal function and improve survival in rodents suffering from cyclosporine nephrotoxicity. Similarly, PGE1, known for its vasodilatory and immunosuppressive properties, has been shown to mitigate renal dysfunction in post-LT IRI by down-regulating cytokine storms[34]. In the present study, there was no significant difference in need for RRT or in the renal function 1 month, 6 months, and 12 months post-LT between the two study groups, supporting the renoprotective properties of PGE1.
Watt et al[5] reported that development of renal insufficiency up to five years post-LT is associated with hazard ratio of 6.58 for mortality, indicating that the timing of development of renal insufficiency is a predictor of long-term mortality post-LT. In the present analysis, the PGE1 group exhibited more severe renal insufficiency immediately post-transplant. Despite this, the 1-year patient survival, overall liver-graft survival, and overall patient survival were comparable to those who did not receive PGE1, spanning a mean follow up period of 2 years.
The exact mechanism by which PGE1 may contribute to improved renal outcomes post LT is yet to be fully elicited. PGE1 works by binding to specific PG receptors on vascular smooth muscle cells, leading to increased levels of cyclic adenosine monophosphate, which induces smooth muscle relaxation and vasodilation. This effect is particularly important in maintaining adequate renal perfusion during and after the LT procedure, thereby preventing acute kidney injury. It has been suggested that restoring PG levels in vulnerable vascular areas, such as the renal vasculature, after ischemic events may help decrease the occurrence of renal dysfunction[35]. PGE1 has anti-inflammatory properties that reduce the extent of ischemia-reperfusion injury by inhibiting the production of pro-inflammatory cytokines and reducing oxidative stress[36].
While alprostadil has promising benefits for renal protection during LT, its use is associated with potential risks that must be carefully weighed against its benefits. Alprostadil is a potent vasodilator, which can lead to significant hypotension during surgery.
Common side effects of alprostadil include flushing, headache, and gastrointestinal disturbances. Close monitoring and individualized patient care are essential to mitigate these risks.
The main limitation of this study is its retrospective nature, which precludes a matched control group, and may allow for unmeasured confounders that limit causal assessment. The study population was from a single institution, predominantly managed by two transplant surgeons. Donor data, preservation methods, as details on surgical technique (piggy-back vs cava replacement), and post-transplant immunosuppression were not accounted for in the analysis.
A major strength of this study is its extended follow up period, which exceeds those of similar studies by Klein et al[28]. Additionally, the demographic characteristics of the study population closely mirror the United States national liver transplant recipient cohort per Organ Procurement and Transplant Network data, enhancing the generalizability of the findings.
This study demonstrated that in patients receiving PGE1 for elevated transaminases, despite renal dysfunction immediately post LT, that renal function up to 1 year post transplant was comparable to those without transaminase elevation. This study has important implications, as it suggests that PGE1 may be reno-protective and can be utilized in other settings of renal dysfunction, especially in the transplant setting. Post-transplant early renal dysfunction is a known risk factor for CKD development. Hence, prospective, multi-center trials should explore the potential of PGE1 to mitigate the progression of kidney dysfunction in the long term, and explore different dosages and administration protocols to optimize its clinical use.
| 1. | Lentine KL, Dew MA, Xiao H, Wisniewski A, Levan ML, Al Ammary F, Sharfuddin A, Axelrod DA, Waterman AD, Kasiske B. Factors enabling transplant program participation in the Scientific Registry of Transplant Recipients (SRTR) Living Donor Collective: A national survey. Clin Transplant. 2023;37:e14908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Robinson AM, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant. 2019;19 Suppl 2:184-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 3. | Shachar M, Benishti N, Cohen S. Effects of mechanical stimulation induced by compression and medium perfusion on cardiac tissue engineering. Biotechnol Prog. 2012;28:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Durand F. How to improve long-term outcome after liver transplantation? Liver Int. 2018;38 Suppl 1:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 586] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 6. | Caragata R, Wyssusek KH, Kruger P. Acute kidney injury following liver transplantation: a systematic review of published predictive models. Anaesth Intensive Care. 2016;44:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | McCauley J, Van Thiel DH, Starzl TE, Puschett JB. Acute and chronic renal failure in liver transplantation. Nephron. 1990;55:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Nevens F, Pirenne J. Renal disease in the allograft recipient. Best Pract Res Clin Gastroenterol. 2020;46-47:101690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | VanWagner LB, Holl JL, Montag S, Gregory D, Connolly S, Kosirog M, Campbell P, Pine S, Daud A, Finn D, Ladner D, Skaro AI, Levitsky J, Lloyd-Jones DM. Blood pressure control according to clinical practice guidelines is associated with decreased mortality and cardiovascular events among liver transplant recipients. Am J Transplant. 2020;20:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 11. | Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Sharma P, Bari K. Chronic Kidney Disease and Related Long-Term Complications After Liver Transplantation. Adv Chronic Kidney Dis. 2015;22:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 669] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 14. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 311] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Nastos C, Kalimeris K, Papoutsidakis N, Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V, Arkadopoulos N. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. 2014;2014:906965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | DeInnocentes P, Perry AL, Graff EC, Lutful Kabir FM, Curtis Bird R. Characterization of HOX gene expression in canine mammary tumour cell lines from spontaneous tumours. Vet Comp Oncol. 2015;13:322-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68-78. [PubMed] |
| 18. | Ali JM, Davies SE, Brais RJ, Randle LV, Klinck JR, Allison ME, Chen Y, Pasea L, Harper SF, Pettigrew GJ. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver Transpl. 2015;21:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Hsieh CC, Hsieh SC, Chiu JH, Wu YL. Protective Effects of N-acetylcysteine and a Prostaglandin E1 Analog, Alprostadil, Against Hepatic Ischemia: Reperfusion Injury in Rats. J Tradit Complement Med. 2014;4:64-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Suzuki A, Hagino M, Yasuda N, Sagawa K, Terawaki T, Ogawa M, Kondo K, Hamanaka N, Tanaka M, Aze Y. [Inhibitory effects of prostaglandin E1.alpha-cyclodextrin (PGE1.CD) on dimethylnitrosamine-induced acute liver damage in rats]. Nihon Yakurigaku Zasshi. 1995;105:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Quiroga J, Prieto J. Liver cytoprotection by prostaglandins. Pharmacol Ther. 1993;58:67-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Natori S, Fujii Y, Kurosawa H, Nakano A, Shimada H. Prostaglandin E1 protects against ischemia-reperfusion injury of the liver by inhibition of neutrophil adherence to endothelial cells. Transplantation. 1997;64:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Anderson RE, Williams WL, Tokuda S. Effect of low-dose irradiation upon T cell subsets involved in the response of primed A/J mice to SaI cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Gatta A, Dante A, Del Gaudio M, Pinna AD, Ravaioli M, Riganello I, Volta G, Faenza S. The use of prostaglandins in the immediate postsurgical liver transplant period. Transplant Proc. 2006;38:1092-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Shin M, Song SH, Kim JM, Kim SJ, Joh JW, Lee SK, Kwon CH. Effectiveness of intraportal prostaglandin E1 administration after liver transplantation. Transplant Proc. 2012;44:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Greig PD, Woolf GM, Sinclair SB, Abecassis M, Strasberg SM, Taylor BR, Blendis LM, Superina RA, Glynn MF, Langer B. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation. 1989;48:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 185] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Henley KS, Lucey MR, Normolle DP, Merion RM, McLaren ID, Crider BA, Mackie DS, Shieck VL, Nostrant TT, Brown KA. A double-blind, randomized, placebo-controlled trial of prostaglandin E1 in liver transplantation. Hepatology. 1995;21:366-372. [PubMed] |
| 28. | Klein AS, Cofer JB, Pruett TL, Thuluvath PJ, McGory R, Uber L, Stevenson WC, Baliga P, Burdick JF. Prostaglandin E1 administration following orthotopic liver transplantation: a randomized prospective multicenter trial. Gastroenterology. 1996;111:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Bharathan VK, Chandran B, Gopalakrishnan U, Varghese CT, Menon RN, Balakrishnan D, Sudheer OV, Dhar P, Surendran S. Perioperative prostaglandin e1 infusion in living donor liver transplantation: A double-blind, placebo-controlled randomized trial. Liver Transpl. 2016;22:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Bärthel E, Rauchfuss F, Hoyer H, Breternitz M, Jandt K, Settmacher U. The PRAISE study: a prospective, multi-center, randomized, double blinded, placebo-controlled study for the evaluation of iloprost in the early postoperative period after liver transplantation (ISRCTN12622749). BMC Surg. 2013;13:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Mohamed ZU, Varghese CT, Sudhakar A, Kumar L, Gopalakrishnan U, Balakrishnan D, Narayanamenon R, Sudhindran S. Prostaglandins for adult liver transplanted recipients. Cochrane Database Syst Rev. 2023;8:CD006006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Makowka L, Lopatin W, Gilas T, Falk J, Phillips MJ, Falk R. Prevention of cyclosporine (CyA) nephrotoxicity by synthetic prostaglandins. Clin Nephrol. 1986;25 Suppl 1:S89-S94. [PubMed] |
| 34. | Pollak R, Dumble LJ, Wiederkehr JC, Maddux MS, Moran M. The immunosuppressive properties of new oral prostaglandin E1 analogs. Transplantation. 1990;50:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Tobimatsu M, Konomi K, Saito S, Tsumagari T. Protective effect of prostaglandin E1 on ischemia-induced acute renal failure in dogs. Surgery. 1985;98:45-53. [PubMed] |
| 36. | Snyder DS, Beller DI, Unanue ER. Prostaglandins modulate macrophage Ia expression. Nature. 1982;299:163-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 391] [Article Influence: 9.1] [Reference Citation Analysis (0)] |