Published online Sep 18, 2024. doi: 10.5500/wjt.v14.i3.93561
Revised: May 27, 2024
Accepted: June 13, 2024
Published online: September 18, 2024
Processing time: 152 Days and 14.5 Hours
Recipient functional status prior to transplantation has been found to impact post-transplant outcomes in heart, liver and kidney transplants. However, information on how functional status, before and after transplant impacts post-transplant survival outcomes is lacking.
To investigate the impact of recipient functional status on short and long term intestinal transplant outcomes in United States adults.
We conducted a retrospective cohort study on 1254 adults who underwent first-time intestinal transplantation from 2005 to 2022. The primary outcome was mortality. Using the Karnofsky Performance Status, functional impairment was categorized as severe, moderate and normal. Analyses were conducted using Kaplan-Meier curves and multivariable Cox regression.
The median age was 41 years, majority (53.4%) were women. Severe impairment was present in 28.3% of recipients. The median survival time was 906.6 days. The median survival time was 1331 and 560 days for patients with normal and severe functional impairment respectively. Recipients with severe impairment had a 56% higher risk of mortality at one year [Hazard ratio (HR) = 1.56; 95%CI: 1.23–1.98; P < 0.001] and 58% at five years (HR = 1.58; 95%CI: 1.24–2.00; P < 0.001) compared to patients with no functional impairment. Recipients with worse functional status after transplant also had poor survival outcomes.
Pre- and post-transplant recipient functional status is an important prognostic indicator for short- and long-term intestinal transplant outcomes.
Core Tip: Pre- and post-transplant assessment of recipient functional status using the Karnofsky Performance Status scale is crucial for predicting short- and long-term outcomes in intestinal transplantation. Severe impairment significantly increases the risk of mortality at one- and five-years post-transplant, emphasizing the importance of comprehensive pre-transplant evaluation and potential interventions to optimize functional status in recipients undergoing intestinal transplantation in the United States.
- Citation: Boateng S, Ameyaw P, Gyabaah S, Adjepong Y, Njei B. Recipient functional status impacts on short and long-term intestinal transplant outcomes in United States adults. World J Transplant 2024; 14(3): 93561
- URL: https://www.wjgnet.com/2220-3230/full/v14/i3/93561.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i3.93561
Intestinal transplantation is the mainstay treatment option for eligible patients with severe gastrointestinal diseases, including inflammatory bowel diseases, acute mesenteric infarction, and refractory end-stage intestinal failure[1]. Over the last three years, there have been a rise in the number of intestinal transplantations in the United States, with an average of 90 transplants a year since 2019[2]. While there have been significant advancements in surgical techniques, immunosuppression, and patient management, as high as 44% of intestinal transplant recipients still die within 5-10 years of transplant in the United States[3]. The process of identifying appropriate candidates for intestinal transplants is multifaceted and focuses on classifying potential recipients based on their present clinical condition, nutritional support requirements, and overall intestinal function[4]. Thus, identifying reliable prognostic indicators for post-transplant outcomes is paramount in optimizing patient selection, pre-transplant management, and patient counseling[5]. Recipient functional status (RFS) has emerged as an influential factor impacting post-transplant outcomes across various solid organ transplantations, such as heart, liver, and kidney transplants[6-9]. The Karnofsky Performance Status (KPS) scale is a widely accepted and validated measure of RFS in clinical practice and research[10]. It has been employed as a prognostic tool in liver, kidney, and lung transplantation[11-13]. However, there is limited research on the impact of RFS on short-term and long-term outcomes in intestinal transplantation.
In this study, our objective was to investigate the association of RFS, assessed using the KPS scale, with one and five-year intestinal transplant outcomes among adult recipients in the United States. We hypothesize that lower functional status prior to transplantation is associated with poorer one and five-year post-transplant outcomes. Further, we hypothesize that reduced post-transplant KPS is associated with poorer post-transplant survival[14]. To evaluate these hypotheses, we performed a retrospective cohort study of all adult patients who underwent a first-time intestinal transplantation in the United States between 2005 and 2022 using the United Network for Organ Sharing (UNOS) database. The UNOS records information about all transplants performed in the US, since its establishment in 1988[15]. By examining the relationship of RFS with intestinal transplant outcomes, our study aims to offer valuable insights for clinicians in prognostic counseling, patient selection, and pre-transplant management. Ultimately, this study will contribute to the ongoing efforts to improve outcomes for intestinal transplant recipients.
This retrospective cohort study utilized the UNOS dataset, which contains information on all intestinal transplantations performed in the United States from 1988 to date[15]. We obtained the fully anonymized data from the UNOS database via a request submitted to the Organ Procurement and Transplant Network. UNOS is provided with the patient data at the point of patient registration (listing) and updated it during transplantation. The database is updated annually with post-transplant outcomes information. Since the UNOS database is a public resource and was already deidentified before we obtained it, the data was deemed exempt from Institutional Review Board supervision by the Yale institutional review board. The datasets utilized in this study can be found on the UNOS website at https://unos.org/[15]. Patients were followed up to the time of death or loss to follow -up, whichever occurred first. We included adult patients (≥ 18 years) who underwent a first-time intestinal transplantation between January 1, 2005 and December 31, 2022.
Exclusion criteria: (1) Pediatric transplants (< 18 years); (2) Multi-organ transplants; and (3) Missing data on functional status prior to transplant.
The primary exposure of interest was RFS at transplant, assessed using the KPS scale (Table 1). The KPS quantifies functional status using an 11-point scale, ranging from 100% (normal, without complaints or signs of disease) to 0% (deceased), with increments of 10% and three corresponding categories[10,16]. We utilized the three-tiered version of the scale due to its higher inter-rater reliability scores[9,10]. As adopted by Dolgin et al[7] the three categories of functional status were based on the level of functional impairment/disability: (1) None/normal (function) (80%-100%); (2) Moderate (impairment in function) (50%-70%); and (3) Severe (functional impairment/disability) (10%-40%).
| Functional level | Score range (%) | Patient capabilities and care requirements |
| High function | 80-100 | 100%: Fully active, no symptoms of disease |
| 90%: Minor symptoms, fully active | ||
| 80%: Some symptoms, normal activity with effort | ||
| Moderate function | 50-70 | 70%: Manages self-care, limited activity |
| 60%: Occasionally needs help but manages most personal needs | ||
| 50%: Requires regular assistance and medical care | ||
| Low function | 0-40 | 40%: Disabled, requires special assistance |
| 30%: Severely disabled, frequent hospital care | ||
| 20%: Very ill, active medical intervention needed | ||
| 10%: Terminally ill, rapid disease progression | ||
| 0%: Deceased |
The primary outcomes of interest were one and five-year all-cause mortality. We examined the hazard ratios of post-transplant outcomes according to pre-transplant RFS. We also assessed the KPS at follow-up to examine post-transplant changes in KPS.
Descriptive statistics were used to summarize baseline recipient, donor, and clinical characteristics (covariates) across the functional status categories. Recipient clinical characteristics at transplant considered for inclusion in the analysis were based on clinical plausibility or prior literature on organ transplantation. Specifically, the covariates assessed were recipient age, sex, race/ethnicity, donor age, and clinical factors including smoking history, illicit drug use, cancer history, hypertension or diabetes, recipient diagnosis, the presence of any form of life support such as invasive ventilation (mechanical or extracorporeal membrane oxygenation) or non-invasive artificial ventilation (such as Bilevel positive airway pressure or continuous positive airway pressure) , creatinine levels (an indicator of kidney failure)[17], bilirubin levels (an indicator of liver disease)[18], ischemic time (the duration between graft harvest and reperfusion in the recipient)[19], weight mismatch (donor-recipient weight ratio < 0.8), Cytomegalovirus (CMV) mismatch (presence of positive CMV IgG serology in the donor and negative CMV serology in the recipient), ABO blood group incompatibility (differing ABO blood groups between donor and recipient), and Human Leukocyte Antigen mismatch (≥ 4 mismatched antigens)[20]. Further, we conducted a subgroup analysis according to different time periods, categorized as 2003-2014 and 2014-2022. Univariate cox regression analysis was performed to evaluate the effect of each covariate on one and five-year mortality. Those covariates that were associated with one or five-year mortality in univariate analysis (P < 0.2) were included in a multivariable cox regression model.
We utilized multivariable Cox regression models to estimate the adjusted hazard ratios in one and five-year post-transplant outcomes according to pre-transplant RFS. Models were adjusted for covariates on recipient and donor characteristics as well as clinical information (listed above).
We additionally conducted a predictive analysis of the prognostic factors accounting for poor KPS after transplant using multivariable cox regression analysis. A landmark time of 30 days was used for the univariable and multivariable cox regression analysis.
To assess the effect of change in KPS on survival outcomes, survival probabilities, based on changes in KPS, were calculated separately for the three groups of baseline KPS using Kaplan-Meier method. KPS scores from the initial three months post-transplant were excluded to avoid the recovery phase, focusing on the 3–12 month period for patient stratification. The criteria for KPS improvement varied across groups. High KPS patients (80%–100%) were divided based on a KPS score decrease of at least 10% or any improvement. Intermediate KPS patients (50%–70%) were grouped into no improvement (≤ 0%), an improvement of 10%–20%, or ≥ 30%. Low KPS patients (≤ 40%) were categorized into no improvement (≤ 0%), improvements of 10%–40%, 50%–70%, or ≥ 80%. This stratification aimed to reflect potential variances in KPS changes among the groups. We estimated the post-transplant survival probability at specific times, from one to five years, and median survival times across the different categories. This stratification has been previously used in assessing KPS changes in post liver transplants by Thuluvath et al[14].
All statistical comparisons of continuous data were made using one-way analysis of variance, and the chi-square test was used for categorical data. All data were presented as mean ± SD or number with percentage. Hazard ratios were presented with 95%CI. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, United States). A two-sided P-value of less than 0.05 was considered statistically significant.
To evaluate the robustness of our study findings and to quantify the potential impact of unmeasured confounding, we conducted a sensitivity analysis using the E-value method. The E-value is a statistical tool that provides a measure of the minimum strength of association, on the hazard ratio scale, that an unmeasured confounder would need to have with both the treatment and the outcome, above and beyond the measured confounders, to fully explain away a specific treatment–outcome association[19]. It provides an evidence-based approach to discuss the possible impact of unmeasured confounding on the observed study results. For our analysis, we computed the E-values for both the observed hazard ratios and their lower confidence limits. A larger E-value indicates that greater unmeasured confounding would be needed to explain away the treatment–outcome association, providing stronger evidence for a causal association. We computed the E-values using the formulas provided by VanderWeele and Ding[21].
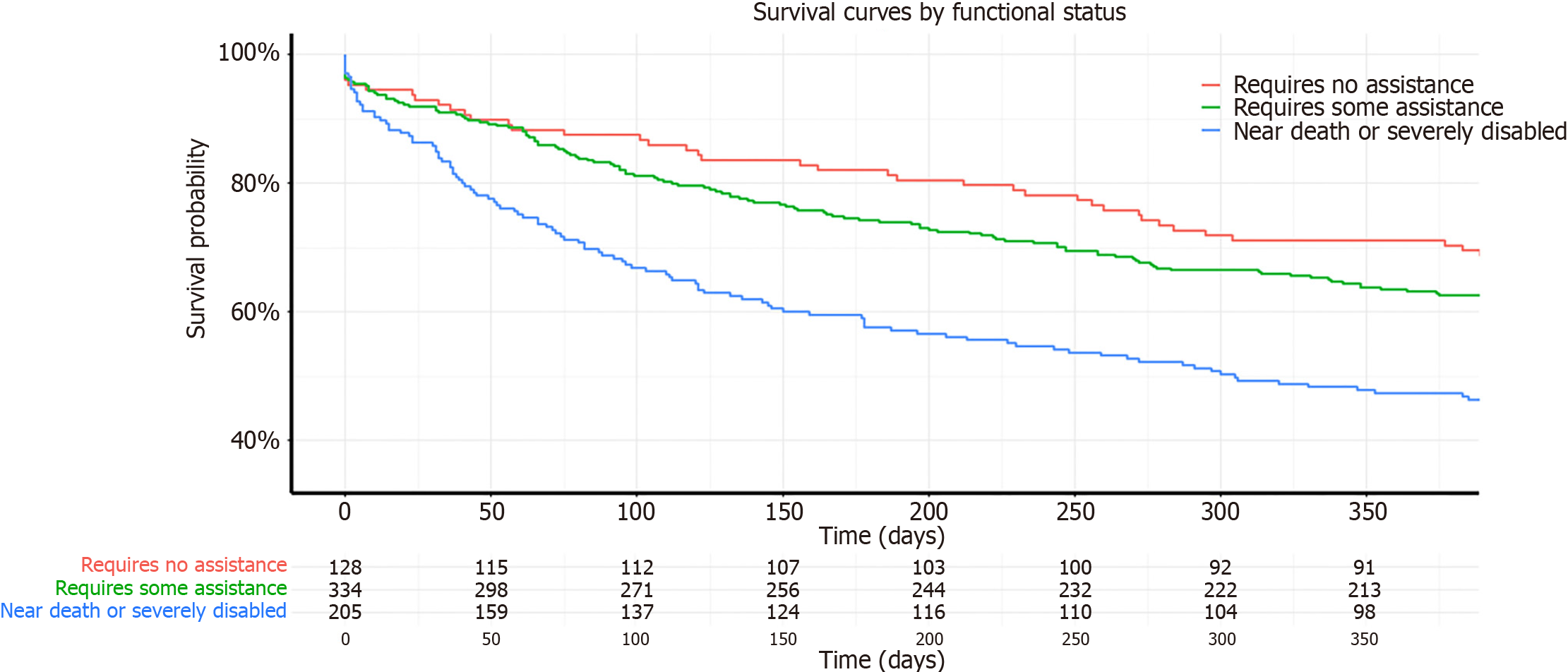
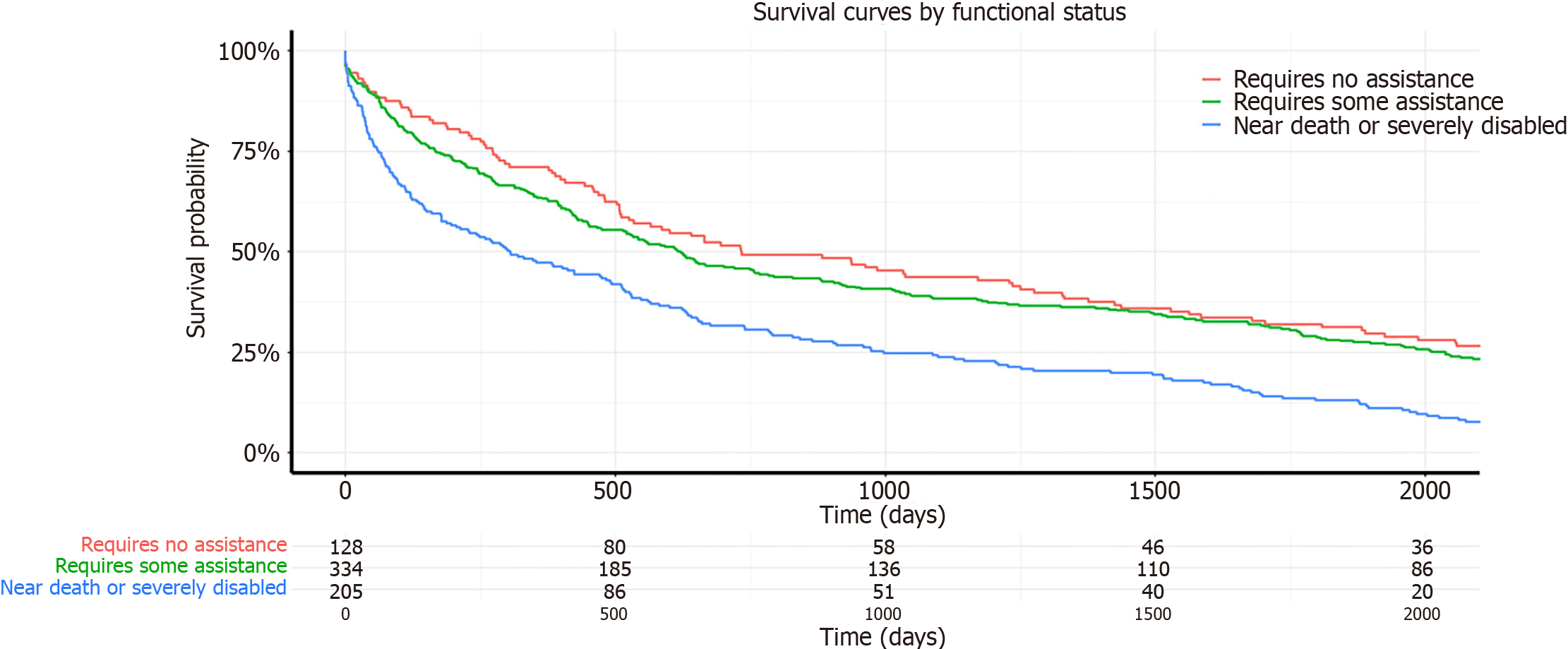
A total of 2379 first time intestinal transplants were performed between January 1, 2005 and December 31, 2022. Of these, 1254 intestinal transplants were included in the study. The recipients ranged in age from 18 to 73 years (mean 40.9 years). Overall, there were 53.4% females, 11.4% Blacks, 78.5% Whites and 8.1% Hispanics. In terms of the RFS, 28.3% were classified as near death or severely disabled using the KPS, 51.7% as requiring some assistance, and 20% as requiring no assistance. In the majority of recipients, the indication for transplant was short gut syndrome (62.1%). The median survival time was 906.6 days (mean 1526 days). The median survival time was 1331 days for recipients with normal KPS, 1078 days for those with moderate impairment and 560 days for those with severe impairment. Table 2 presents the baseline characteristics of the patients in each functional status group, considering all intestinal transplants without any time periods. No significant differences were observed among the three groups for recipient age (P = 0.22), and race (P = 0.79). However, significant differences were found between the functional status groups for recipient gender (P = 0.034), patients on life support at the time of transplant (P value = 0.004), patients in the intensive care unit (ICU) (P < 0.001), and total ischemic time (P = 0.01).
| Functional status at transplant | |||||
| | Requires no assistance (n = 251) | Requires some assistance (n = 648) | Near death or severely disabled (n = 355) | Total (n = 1254) | P value |
| Gender | 0.0341 | ||||
| Female | 130 (51.8) | 368 (56.8) | 172 (48.5) | 670 (53.4) | |
| Male | 121 (48.2) | 280 (43.2) | 183 (51.5) | 584 (46.6) | |
| Age (Year) | 0.222 | ||||
| n | 251 | 648 | 355 | 1254 | |
| Mean (SD) | 40.9 (14.18) | 42.7 (13.04) | 42.1 (12.79) | 42.1 (13.21) | |
| Median | 41.0 | 44.0 | 42.0 | 43.0 | |
| Range | 18.0-73.0 | 18.0-72.0 | 18.0-70.0 | 18.0-73.0 | |
| Race | 0.791 | ||||
| Hispanic | 22 (8.8) | 49 (7.6) | 31 (8.7) | 102 (8.1) | |
| Non-Hispanic Asian | 3 (1.2) | 11 (1.7) | 5 (1.4) | 19 (1.5) | |
| Non-Hispanic Black | 29 (11.6) | 71 (11.0) | 43 (12.1) | 143 (11.4) | |
| Non-Hispanic White | 194 (77.3) | 515 (79.5) | 275 (77.5) | 984 (78.5) | |
| Other/Multiracial | 3 (1.2) | 2 (0.3) | 1 (0.3) | 6 (0.5) | |
| Patients on life support | 0.00402 | ||||
| No | 242 (96.4) | 602 (92.9) | 317 (89.3) | 1161 (92.6) | |
| Yes | 9 (3.6) | 46 (7.1) | 38 (10.7) | 93 (7.4) | |
| Sex match | 0.161 | ||||
| No | 125 (49.8) | 288 (44.4) | 177 (49.9) | 590 (47.0) | |
| Yes | 126 (50.2) | 360 (55.6) | 178 (50.1) | 664 (53.0) | |
| Recipient total bilirubin at transplantation (mg/dL) | 0.00062 | ||||
| n | 249 | 644 | 354 | 1247 | |
| Mean (SD) | 2.2 (5.36) | 2.3 (4.57) | 4.6 (8.44) | 2.9 (6.15) | |
| Median | 0.8 | 0.8 | 1.1 | 0.9 | |
| Range | 0.1-50.0 | 0.1-43.0 | 0.1-48.2 | 0.1-50.0 | |
| Serum creatinine at time of transplantation (mg/dL) | 0.361 | ||||
| n | 251 | 645 | 353 | 1249 | |
| Mean (SD) | 1.0 (0.72) | 1.1 (0.96) | 1.2 (1.04) | 1.1 (0.94) | |
| Median | 0.9 | 0.9 | 0.9 | 0.9 | |
| Range | 0.3-7.6 | 0.3-10.1 | 0.1-9.0 | 0.1-10.1 | |
| Total ischemic time (sec) | 0.0102 | ||||
| n | 222 | 619 | 347 | 1188 | |
| Mean (SD) | 6.9 (1.88) | 7.5 (2.32) | 7.5 (2.28) | 7.4 (2.24) | |
| Median | 7.0 | 7.3 | 7.4 | 7.2 | |
| Range | 1.0-11.8 | 0.4-31.5 | 0.9-31.7 | 0.4-31.7 | |
| Recipient's primary diagnosis | 0.010411 | ||||
| Short gut syndrome | 154 (19.8) | 409 (52.5) | 216 (27.7) | 779 (62.1) | |
| Functional bowel problem | 27 (28.1) | 51 (53.1) | 18 (18.8) | 96 (7.7) | |
| Retransplant | 4 (6.2) | 39 (60.9) | 21 (32.8) | 64 (5.1) | |
| Others | 66 (21.0) | 149 (47.3) | 100 (31.7) | 315 (25.1) | |
| Donor age (years) | 0.362 | ||||
| n | 251 | 648 | 355 | 1254 | |
| Mean (SD) | 22.4 (10.79) | 21.1 (10.54) | 21.3 (11.47) | 21.4 (10.86) | |
| Median | 20.0 | 19.0 | 20.0 | 19.0 | |
| Range | 4.0-56.0 | 2.0-52.0 | 1.0-59.0 | 1.0-59.0 | |
| Race match | 0.431 | ||||
| No | 119 (47.4) | 278 (42.9) | 152 (42.8) | 549 (43.8) | |
| Yes | 132 (52.6) | 370 (57.1) | 203 (57.2) | 705 (56.2) | |
| Weight mismatch | 0.00691 | ||||
| NA | 4 (1.6) | 7 (1.1) | 0 (0.0) | 11 (0.9) | |
| No mismatch | 170 (67.7) | 397 (61.3) | 201 (56.6) | 768 (61.2) | |
| Weight mismatch | 77 (30.7) | 244 (37.7) | 154 (43.4) | 475 (37.9) | |
| History of diabetes | 0.0821 | ||||
| Diabetes | 21 (8.4) | 68 (10.5) | 49 (13.9) | 138 (11.0) | |
| Not diabetes | 230 (91.6) | 580 (89.5) | 303 (86.1) | 1113 (89.0) | |
| Liver function | < 0.00011 | ||||
| Liver dysfunction | 62 (24.9) | 148 (23.0) | 126 (35.6) | 336 (26.9) | |
| No dysfunction | 187 (75.1) | 496 (77.0) | 228 (64.4) | 911 (73.1) | |
| Renal function | 0.0381 | ||||
| No dysfunction | 244 (97.2) | 617 (95.7) | 328 (92.9) | 1189 (95.2) | |
| Renal dysfunction | 7 (2.8) | 28 (4.3) | 25 (7.1) | 60 (4.8) | |
| HLA mismatch | 0.201 | ||||
| High HLA mismatch | 208 (87.0) | 522 (82.6) | 293 (85.7) | 1023 (84.3) | |
| Low HLA mismatch | 31 (13.0) | 110 (17.4) | 49 (14.3) | 190 (15.7) | |
| Recipient's weight (kg) | 0.0572 | ||||
| n | 247 | 641 | 355 | 1243 | |
| Mean (SD) | 68.0 (17.35) | 66.8 (17.16) | 69.2 (17.83) | 67.7 (17.41) | |
| Median | 65.8 | 64.0 | 66.8 | 65.1 | |
| Range | 34.0-130.0 | 36.3-140.1 | 27.4-122.0 | 27.4-140.1 | |
| Calculated body mass index of recipients (kg/m2) | 0.282 | ||||
| n | 246 | 635 | 355 | 1236 | |
| Mean (SD) | 23.9 (5.25) | 23.4 (4.83) | 23.9 (5.28) | 23.7 (5.05) | |
| Median | 23.2 | 22.8 | 23.0 | 23.0 | |
| Range | 15.0-40.6 | 15.1-41.9 | 15.1-44.2 | 15.0-44.2 | |
In the unadjusted analysis at one year post-transplant (Table 3), compared to patients with no functional limitations, the hazard risk of death for patients who required some assistance was 1.03 times those with mild/no functional limitation (95%CI: 0.84-1.28; P = 0.77), and for those near death or severely disabled, it was 1.24 times compared to those with mild/no functional limitation (95%CI: 0.99–1.57; P < 0.001). At five years post-transplant, compared to recipients with no functional limitations, the hazard risk of death for patients who required some assistance was 1.03 times (95%CI: 0.83–1.27; P = 0.35), and for those near death or severely disabled, it was 1.27 times the risk of those with mild/no functional limitation (95%CI: 1.01–2.32; P < 0.042). This data aligns with the Kaplan-Meier survival curves depicted in Figures 1 and 2, illustrating that recipients with severe disability consistently demonstrated the lowest survival rates at both one-year and five-year follow-up intervals. In the adjusted analysis at one year post-transplant (Table 3), compared to patients with no functional limitations, the adjusted hazard risk of death for patients who required some assistance was 1.10 times those with mild/no functional limitation (95%CI: 0.90–1.37; P = 0.36), and for those near death or severely disabled, it was 1.56 times that of recipients with mild/no functional limitation at transplant (95%CI: 1.23–1.98; P < 0.001). At five years post-transplant, the adjusted risk of death for patients who required some assistance was 1.09 times (95%CI: 0.89–1.36; P = 0.38) and for those near death or severely disabled, it was 1.58 times (95%CI: 1.24–2.00; P < 0.001) compared to those with mild/no functional limitation.
| Variable | Timepoints | Unadjusted HR | Adjusted HR1 | ||||
| Functional status | HR | 95%CI | P value | HR | 95%CI | P value | |
| None (Ref.) | 1 year | 1.00 | 1.00 | ||||
| Moderate | 1.03 | 0.84-1.28 | 0.77 | 1.10 | 0.90–1.37 | 0.36 | |
| Severe | 1.24 | 0.99–1.57 | 0.066 | 1.56 | 1.23–1.98 | 0.00029 | |
| None (Ref.) | 5 years | 1.00 | 1.00 | ||||
| Moderate | 1.03 | 0.83–1.27 | 0.78 | 1.09 | 0.89–1.36 | 0.38 | |
| Severe | 1.27 | 1.00–1.61 | 0.042 | 1.58 | 1.24–2.00 | 0.00019 | |
When patients were stratified by time period of transplant, the adjusted hazard ratios at one and five years, for severe impairment vs mild impairment remained significant in both the 2005- 2014 [ (one year- a (hazard ratio) HR: 1.64; 95%CI: 1.19–2.24; P = 0.0021); (five years- aHR: 1.70; 95%CI: 1.24–2.32; P < 0.0001)] and 2014-2022 groups [ (one year- aHR: 1.53; 95%CI: 1.03–2.26; P = 0.034); (five years- aHR: 1.53; 95%CI: 1.04–2.26; P = 0.035)] These results are displayed in Tables 4 and 5.
| Variables | Timepoints | Unadjusted HR | Adjusted HR1 | ||||
| Functional status | HR | 95%CI | P value | HR | 95%CI | P value | |
| None (Ref.) | 1 year | 1.00 | 1.00 | ||||
| Moderate | 1.11 | 0.86-1.44 | 0.4247 | 1.09 | 0.84–1.41 | 0.52 | |
| Severe | 1.63 | 1.20–2.23 | 0.002 | 1.64 | 1.19–2.24 | 0.002 | |
| None (Ref.) | 5 years | 1.00 | 1.00 | ||||
| Moderate | 1.10 | 0.85–1.42 | 0.487 | 1.08 | 0.83–1.39 | 0.57 | |
| Severe | 1.69 | 1.24–2.30 | < 0.0001 | 1.70 | 1.24–2.32 | < 0.0001 | |
| Variables | Timepoints | Unadjusted HR | Adjusted HR1 | ||||
| Functional status | HR | 95%CI | P value | HR | 95%CI | P value | |
| None (Ref.) | 1 year | 1.00 | 1.00 | ||||
| Moderate | 1.12 | 0.77–1.64 | 0.56 | 1.14 | 0.78–1.68 | 0.49 | |
| Severe | 1.48 | 1.00–2.18 | 0.049 | 1.53 | 1.03–2.26 | 0.034 | |
| None (Ref.) | 5 years | 1.00 | 1.00 | ||||
| Moderate | 1.11 | 0.76–1.62 | 0.59 | 1.13 | 0.77–1.66 | 0.54 | |
| Severe | 1.48 | 1.00–2.19 | 0.046 | 1.53 | 1.04–2.26 | 0.035 | |
Among recipients with severe functional impairment, the predictive prognostic factors associated with poor outcomes are displayed in Table 6. Notably, older age, Black race, ICU stay, and baseline liver dysfunction were associated with worse survival outcomes, among recipients with severe impairment (Table 6).
| Variable | HR | 95%CI | P value |
| Age | 1.02 | 1.00-1.03 | 0.001 |
| Race | |||
| Hispanic | 1.21 | 0.66-2.23 | 0.53 |
| Non-Hispanic Asian | 1.20 | 0.287-4.98 | 0.81 |
| Non-Hispanic Black | 1.98 | 1.27-3.08 | 0.003 |
| Other/Multiracial | 2.77 | 0.38-20.46 | 0.32 |
| Non-Hispanic White (Ref.) | 1.00 | ||
| Female gender | 0.85 | 0.62-1.17 | 0.32 |
| Donor age | 1.02 | 1.01-1.04 | 0.008 |
| Weight matched | 0.78 | 0.55-1.09 | 0.008 |
| ICU stay | 2.13 | 1.36-3.38 | 0.001 |
| Total bilirubin | 1.02 | 1.01-1.03 | 0.04 |
| Total ischemic time | 1.05 | 0.99-1.10 | 0.09 |
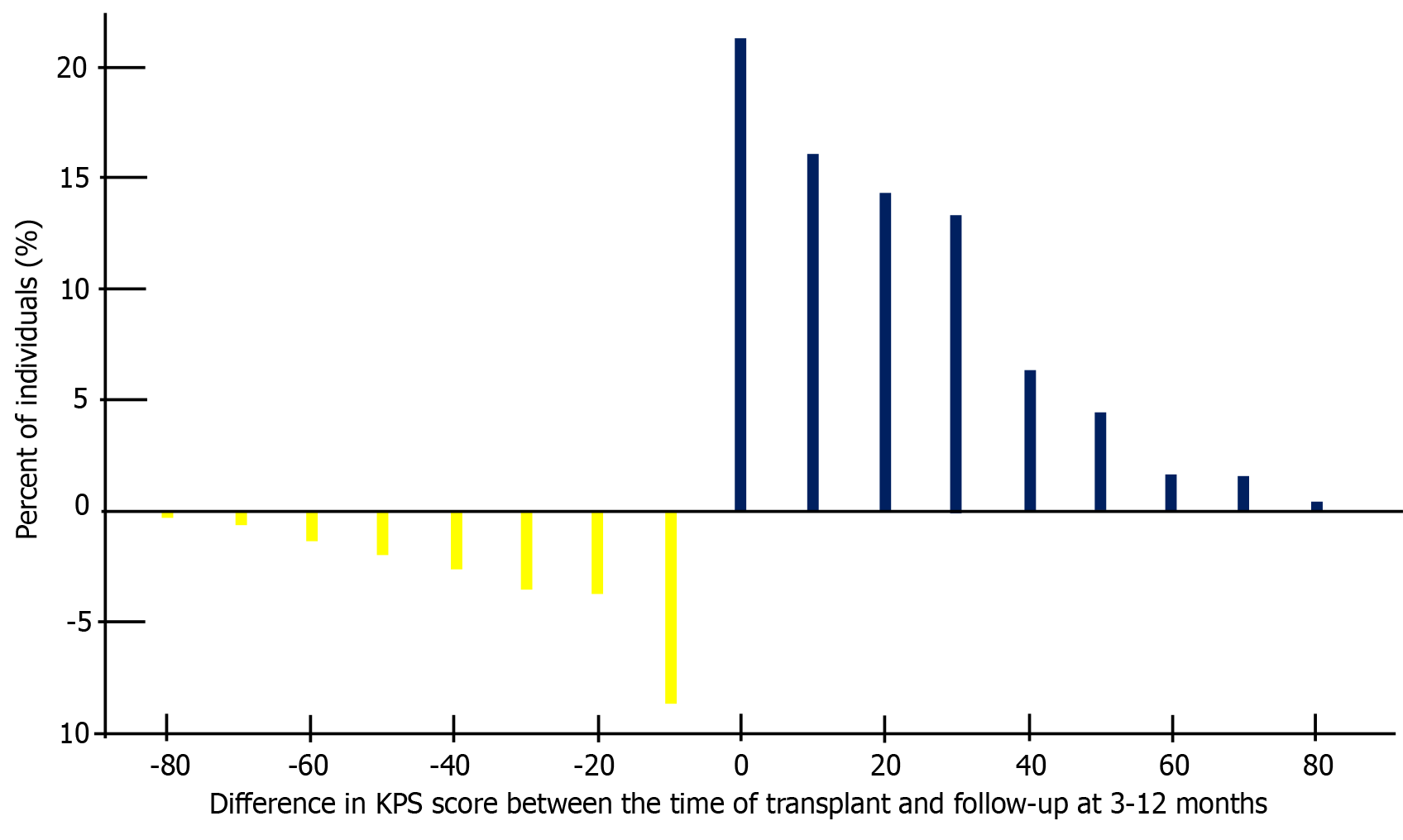
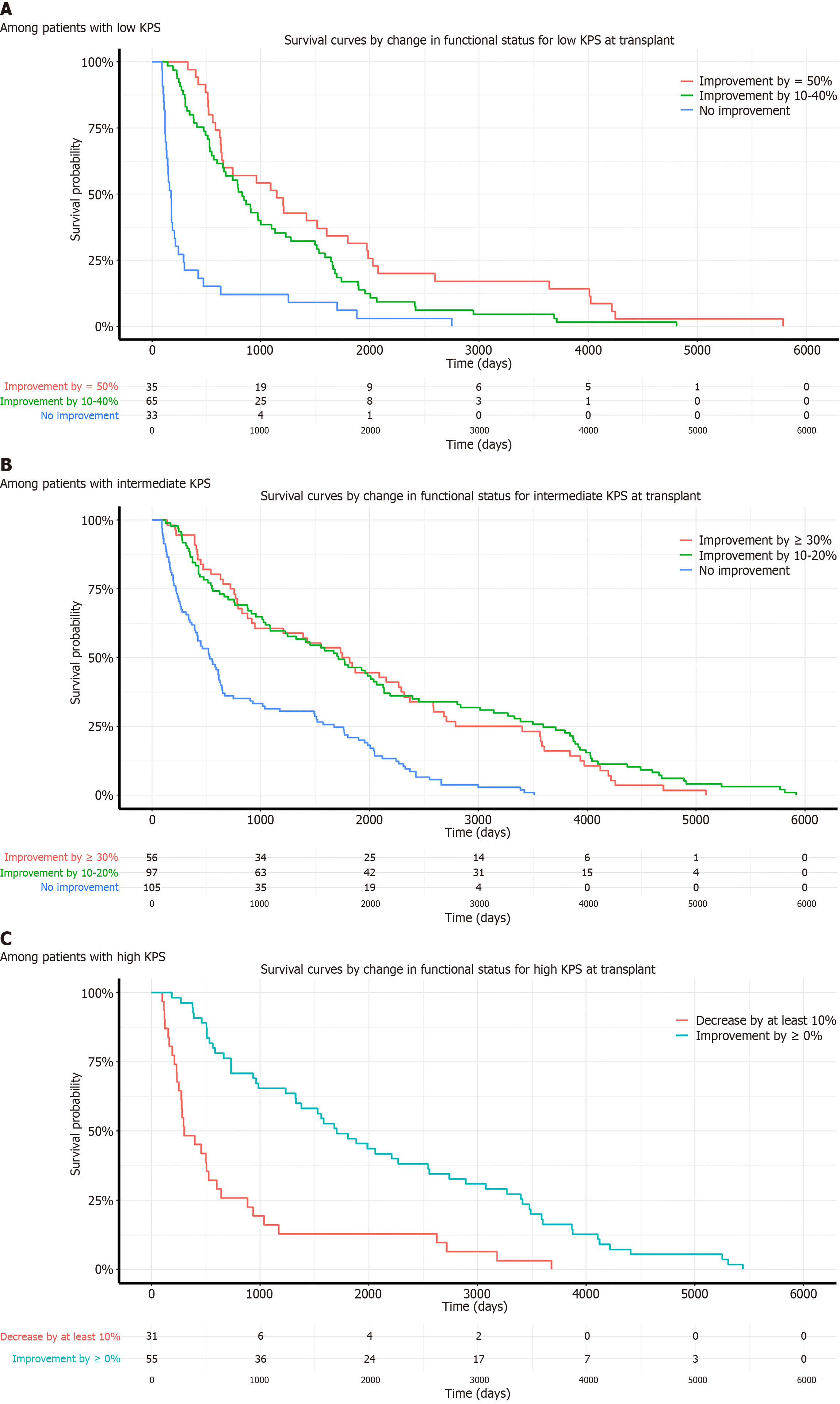
As shown in Figure 3, majority of the transplant recipients had an improvement in KPS score after intestinal transplant. For patients with a low baseline KPS, those with no improvement in KPS scores post-transplant experienced a markedly lower one-year survival rate of 39%. This is in stark contrast to those who demonstrated improved KPS scores, where the survival rates soared between 98% to 100%. Similarly, in the intermediate KPS group, patients who did not show any improvement had a one-year survival of 81%, compared to those with improved KPS scores, who exhibited survival rates of 98%.
Even among patients with a high baseline KPS, the survival outcomes varied based on KPS changes. Those with a decrease in KPS experienced a one-year survival rate of 88%, while those with stable or improved KPS maintained a notably higher survival rate of 100%. These results are shown in Table 7 and Figure 4.
| Time since transplant | |||||
| Changes in KPS | 6 months | 1 year | 2 years | 3 years | 5 years |
| Low KPS at transplant | |||||
| Improvement by ≥ 50% (%) | 100 (100-100) | 97 (92-100) | 60 (46-79) | 51 (37-71) | 31 (19-51) |
| Improvement by 10%–40% (%) | 98 (96-100) | 80 (71-90) | 57 (46-70) | 38 (28-52) | 17 (9.9-29) |
| No improvement (%) | 39 (26-60) | 21 (11-41) | 12 (4.8-30) | 12 (4.8-30) | 6.1 (1.6-23) |
| Intermediate KPS at transplant | |||||
| Improvement by ≥ 30% (%) | 98 (95-100) | 95 (89-100) | 75 (64-87) | 61 (49-75) | 48 (37-63) |
| Improvement by 10%–20% (%) | 98 (95-100) | 87 (80-94) | 71 (63-81) | 60 (51-70) | 46 (37-57) |
| No improvement (%) | 81 (74-89) | 62 (53-72) | 36 (28-47) | 31 (24-42) | 21 (14-30) |
| High KPS at transplant | |||||
| Decrease by at least 10% (%) | 81 (68-96) | 48 (34-70) | 26 (14-47) | 16 (7.2-36) | 13 (5.2-32) |
| Improvement by ≥ 0% (%) | 100 (100-100) | 96 (92-100) | 76 (66-88) | 65 (54-79) | 47 (36-62) |
Median survival times further substantiated these observations (Table 8). Low KPS patients at transplant with significant improvement (≥ 50%) had a median survival of 1146 days, whereas those with minimal or no improvement had markedly shorter median survival times of 177 days. Intermediate KPS patients showing significant improvement had median survivals exceeding 1700 days, whereas those with no improvement had much shorter survival times of 533 days. High KPS patients who experienced a decrease in performance had the shortest median survival times compared to those with stable or improved KPS.
| Characteristic | Median survival |
| Low KPS at transplant | |
| Improvement by = 50% | 1146 (641-1971) |
| Improvement by 10%–40% | 828 (651-1128) |
| No improvement | 177 (136-243) |
| Intermediate KPS at transplant | |
| Improvement by = 30% | 1783 (945-2369) |
| Improvement by 10%–20% | 1712 (1226-2132) |
| No improvement | 533 (415-641) |
| High KPS at transplant | |
| Decrease by at least 10% | 304 (251-641) |
| Improvement by = 0% | 1703 (1331-2556) |
In the sensitivity analysis using the E-value method (Table 9), the E-value for the point estimate of the hazard ratio for moderate functional impairment at 1 year was 1.43, and for the lower confidence interval, it was 1. For severe functional impairment at one year, the E-value for the point estimate was 2.49, and for the lower confidence interval, it was 1.76. This suggests that an unmeasured confounder would need to be associated with both functional impairment and one year mortality by a risk ratio of at least 1.43-fold above and beyond the measured confounders to explain away the observed association for moderate impairment, and by a risk ratio of at least 2.49-fold for severe impairment.
At the five year mark, the E-value for the point estimate of the hazard ratio for moderate functional impairment was 1.40, and for the lower confidence interval, it was 1. For severe functional impairment at five years, the E-value for the point estimate was 2.54, and for the lower confidence interval, it was 1.79. This indicates that an unmeasured confounder would need to be associated with both functional impairment and five year mortality by a risk ratio of at least 1.4-times the measured confounders to explain away the observed association for moderate impairment, and by a risk ratio of at least 2.54-fold for severe impairment.
In this study, we aimed to delve deeper into the significance of pre-transplant functional status, as measured by the KPS and categorized into three groups as a prognostic indicator for post-operative mortality in intestinal transplant patients. Our findings revealed that, recipients with severe impairments had a 56% higher risk of mortality at one year (95%CI: 1.23–1.98; P < 0.001) and 58% higher risk of mortality at five years, when compared to recipients with no impairments (95%CI: 1.24–2.00; P < 0.001).
Additionally, our study showed that recipients with improved KPS after transplant have better outcomes, with up to six times longer median survival times, compared with recipients with worsening KPS after transplant. This finding that lower KPS categories pretransplant, and worsening KPS after transplant are linked to increased post-operative mortality in intestinal transplant patients, is consistent with findings with heart, liver, and kidney transplant recipients[8,12,22,23]. Grimm et al[22] conducted a comparable analysis of the UNOS database, demonstrating that a lower preoperative KPS score in lung transplant patients was linked to a higher mortality rate at one year[22]. Similarly, Shaw and Pickett[24] demonstrated that functional status serves as a potent predictor of survival at 30 days and one year following heart transplantation[24]. In the context of liver transplantation, Thuluvath et al[12] analyzed transplant patients in the UNOS database and found that both graft and patient survival rates were notably lower for liver transplant recipients with lower KPS scores[12]. Thuluvath et al[14] also found that, recipients with decreased KPS after liver transplantation had significantly worse survival outcomes. In kidney transplants, Bui et al[23] also discovered that deceased donor kidney transplant recipients with higher KPS scores had a greater likelihood of survival, even when receiving lower-quality organs, as determined by the kidney donor profile index[23]. Our investigation supports these findings and expands their applicability to intestinal transplantation.
The significant relationship between pretransplant functional status and posttransplant survival can be attributed to various factors. Patients requiring complete assistance before surgery are more likely to be weakened and possess less physiological reserve than those who do not need assistance[25]. Our comparison of baseline characteristics showed that individuals with lower functional status had a significantly higher likelihood of being on life support before transplantation. Moreover, approximately 13% of the patients with lower functional status also needed intensive care unit stay before undergoing intestinal transplantation.
Gerlach et al[26] in a multicenter study found that early identification and transplantation of patients with Crohn's disease and intestinal failure was associated with an increase in post-transplant KPS, patient and graft survival. They utilized a modified American Gastroenterology Association scoring system for Intestinal transplantation that enhances the identification and early referral of patients with severe and transplant-eligible CD[26]. Our findings also show that transplantation results in an improvement in post-transplant KPS and survival outcomes in the majority of eligible patients, highlighting the need for appropriate selection of candidates to maximize outcome benefits.
Although the concentration of these high-risk variables in the group with lower functional status undoubtedly played a role in the observed association with poorer survival outcomes, multivariable analysis indicated that the influence of functional status on outcomes persisted even after accounting for these factors, implying a likely independent relationship. Furthermore, the sensitivity analyses suggest that our findings are relatively robust to potential unmeasured confounding. The E-values indicate that a substantial level of confounding would be needed to explain away the observed associations between functional impairment and post-transplant mortality, particularly for severe impairment at the 1-year mark.
The E-value for the point estimate of severe functional impairment and mortality at 1 year was larger than the risk ratios reported in two previous studies by O,Súilleabháin et al[27] and Sáenz et al[28] that estimated the risk of reduced functional status on mortality to be 1.3 and 2.16, respectively. This suggests that even if an unmeasured confounder associated with both functional impairment and mortality was present, it would need to have a stronger association than those reported in these previous studies to fully account for our findings.
These results highlight the potential importance of assessing functional status in patients undergoing intestinal transplant and suggest that interventions to improve functional status could potentially improve post-transplant outcomes. However, further research is needed to investigate the mechanisms underlying these associations and to develop effective interventions. Future studies with the ability to measure additional potential confounders could help to further clarify these associations.
Our observations, combined with the ease of calculating the KPS, highlight the potential value of preoperative functional status as a risk assessment tool for candidates undergoing intestinal transplantation.
As the demand for intestinal transplants continues to rise, it becomes increasingly important to identify factors that can be optimized to improve patient outcomes. Our results are meant to reiterate the potential for preoperative intervention and optimization in patients with low functional status, particularly when the causes of declining KPS scores can be identified and addressed. This highlights the need for a multidisciplinary approach that involves surgeons, transplant teams, physical therapists, and nutritionists to develop tailored pre-transplant rehabilitation programs.
The impact of functional status on post-transplant outcomes extends beyond survival rates. It also has implications for the patient's quality of life, recovery time, and long-term morbidity[25,29,30]. By improving functional status, transplant centers can not only improve survival rates but also enhance the overall well-being of intestinal transplant patients[25,29,30]. Moreover, understanding a patient's pre-transplant functional status can help transplant teams anticipate postoperative care requirements, facilitate communication among the multidisciplinary team, and coordinate resources for postoperative critical care, rehabilitation, and potential longer-term occupational therapy[31-35].
Our study also contributes to the growing body of evidence supporting the potential benefits of prehabilitation in transplant patients. Prehabilitation, which involves physical therapy, nutritional support, and other interventions designed to improve functional status before surgery, has been shown to be effective in improving postoperative recovery and outcomes in major abdominal surgery[36,37]. Further research on prehabilitation specific to intestinal transplant patients is warranted to determine its effectiveness in this population.
Our study has several limitations that should be considered when interpreting the results. First, as this is an observational study, our findings may be subject to unmeasured confounding. However, we adjusted for a comprehensive list of potential confounders in our analyses, including donor age, sex, ethnicity, ischemic time, as well as recipient age, sex, and ethnicity. Thus, although we acknowledge the potential for residual confounding, we have made every effort to minimize its impact on our findings. Second, the extended time frame over which data was collected could introduce issues such as changes in allocation policies, eligibility criteria, and follow-up periods, all of which may affect the study outcomes. Third, one potential limitation of our study is the selection bias inherent in the database, as the functional status pre-transplant KPS can directly impact the likelihood of a patient being selected for transplantation as well as the post-transplant outcomes. Such bias might obscure the independent effect of the KPS on post-transplant outcomes, which is the primary interest of our study. However, it is important to note that this bias is inherent in all observational studies using secondary data and is not unique to our study. To address this potential limitation, we conducted a sensitivity analysis using the E-value method to assess the robustness of our results to potential unmeasured confounding. The E-value provides an estimate of the minimum strength of association that an unmeasured confounder would need to have with both the exposure (KPS) and the outcome (post-transplant survival), above and beyond the measured confounders, to fully explain away the observed associations. Our E-value analysis demonstrated that the associations we found between pre-transplant functional status and post-transplant mortality are robust to potential unmeasured confounding, at least for some categories of functional status. Despite these limitations, our study possesses several notable strengths that lend credibility to our conclusions. First, we used a dataset comprising the most extensive cohort of intestinal transplant patients, which allowed for increased statistical power and precision. Second, our study is the first to examine the impact of RFS on short and long-term intestinal transplant outcomes in United States adults, which represents an innovative and critical area of research with potential implications for clinical decision-making and patient outcome improvement. By utilizing data from the UNOS database between 2005 and 2022, our study offers a comprehensive understanding of the influence of RFS on survival outcomes in adult intestinal transplant patients and the factors associated with them.
Our study highlights the importance of pre- and post-transplant functional status in determining post-operative outcomes in intestinal transplant patients, as evidenced by the increased risk of mortality at one and five years in severe functionally impaired patients compared to those with mild/no functional impairment, as well in those with poor post transplant survival outcomes and emphasizes the need for preoperative optimization and intervention to improve patients' functional status. This comprehensive approach to patient care has the potential to significantly enhance not only survival rates but also the overall quality of life for intestinal transplant recipients. Future studies should continue to investigate the impact of functional status on outcomes and explore the potential of rehabilitation in the context of intestinal transplantation, with the aim of informing the development of more effective allocation policies and pre-transplant rehabilitation strategies.
The authors gratefully acknowledge the UNOS for providing the data used in this study.
| 1. | Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, Farmer DG, Lacaille F, Iyer K, Fishbein T; Intestinal Transplant Association. Intestinal transplant registry report: global activity and trends. Am J Transplant. 2015;15:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 2. | OPTN/SRTR Annual Data Report. Statistics on donation and transplantation in the United States. Available from: https://www.srtr.org/reports/optnsrtr-annual-data-report/. |
| 3. | Cunha-melo JR, Costa G. Intestinal transplantation: evolution and current status. Medical Express. 2014;1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Am I eligible for an intestinal transplant? Intestine Transplant Eligibility Transplant Nebraska Medicine. Available from: https://www.nebraskamed.com/transplant/intestine/eligibility. |
| 5. | Grant D, Abu-Elmagd K, Reyes J, Tzakis A, Langnas A, Fishbein T, Goulet O, Farmer D; Intestine Transplant Registry. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 323] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | Kobashigawa J, Dadhania D, Bhorade S, Adey D, Berger J, Bhat G, Budev M, Duarte-Rojo A, Dunn M, Hall S, Harhay MN, Johansen KL, Joseph S, Kennedy CC, Kransdorf E, Lentine KL, Lynch RJ, McAdams-DeMarco M, Nagai S, Olymbios M, Patel J, Pinney S, Schaenman J, Segev DL, Shah P, Singer LG, Singer JP, Sonnenday C, Tandon P, Tapper E, Tullius SG, Wilson M, Zamora M, Lai JC. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19:984-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 7. | Dolgin NH, Movahedi B, Anderson FA, Brüggenwirth IM, Martins PN, Bozorgzadeh A. Impact of recipient functional status on 1-year liver transplant outcomes. World J Transplant. 2019;9:145-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 8. | Shaw TB, Blitzer D, Carter KT, Lirette S, Mohammed A, Copeland J, Baran DA, Copeland H. Functional status of heart transplant recipients predicts survival. Clin Transplant. 2022;36:e14748. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Kilic A, Beaty CA, Merlo CA, Conte JV, Shah AS. Functional status is highly predictive of outcomes after redo lung transplantation: an analysis of 390 cases in the modern era. Ann Thorac Surg. 2013;96:1804-11; discussion 1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak. 2013;13:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Weiss ES, Allen JG, Arnaoutakis GJ, George TJ, Russell SD, Shah AS, Conte JV. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg. 2011;92:914-21; discussion 921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, Grover FL. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 538] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 14. | Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky performance status before and after liver transplantation predicts graft and patient survival. J Hepatol. 2018;69:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | OPTN: Organ Procurement and Transplantation Network - OPTN. Available from: https://optn.transplant.hrsa.gov/. |
| 16. | Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Kashani K, Rosner MH, Ostermann M. Creatinine: From physiology to clinical application. Eur J Intern Med. 2020;72:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 18. | Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. 2009;3:17. [PubMed] |
| 19. | Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly JF, Stern M, Loirat P, Lesèche G, Bertocchi M, Mornex JF, Haloun A, Despins P, Pison C, Blin D, Reynaud-Gaubert M. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Wayda B, Clemons A, Givens RC, Takeda K, Takayama H, Latif F, Restaino S, Naka Y, Farr MA, Colombo PC, Topkara VK. Socioeconomic Disparities in Adherence and Outcomes After Heart Transplant: A UNOS (United Network for Organ Sharing) Registry Analysis. Circ Heart Fail. 2018;11:e004173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2080] [Cited by in RCA: 3408] [Article Influence: 426.0] [Reference Citation Analysis (0)] |
| 22. | Grimm JC, Valero V 3rd, Kilic A, Crawford TC, Conte JV, Merlo CA, Shah PD, Shah AS. Preoperative performance status impacts perioperative morbidity and mortality after lung transplantation. Ann Thorac Surg. 2015;99:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Bui K, Kilambi V, Rodrigue JR, Mehrotra S. Patient Functional Status at Transplant and Its Impact on Posttransplant Survival of Adult Deceased-donor Kidney Recipients. Transplantation. 2019;103:1051-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Shaw RJ, Pickett KE. The health benefits of Hispanic communities for non-Hispanic mothers and infants: another Hispanic paradox. Am J Public Health. 2013;103:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Tepas JJ 3rd. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206:818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Gerlach UA, Vrakas G, Reddy S, Baumgart DC, Neuhaus P, Friend PJ, Pascher A, Vaidya A. Chronic intestinal failure after Crohn disease: when to perform transplantation. JAMA Surg. 2014;149:1060-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | OʼSúilleabháin PS, Gallagher S, Steptoe A. Loneliness, Living Alone, and All-Cause Mortality: The Role of Emotional and Social Loneliness in the Elderly During 19 Years of Follow-Up. Psychosom Med. 2019;81:521-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Sáenz V, Zuljevic N, Elizondo C, Martin Lesende I, Caruso D. Baseline Functional Status and One-year Mortality After Hospital Admission in Elderly Patients: a prospective cohort study. Rev Fac Cien Med Univ Nac Cordoba. 2020;77:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Reuben DB, Rubenstein LV, Hirsch SH, Hays RD. Value of functional status as a predictor of mortality: results of a prospective study. Am J Med. 1992;93:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 263] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Dy SM, Pfoh ER, Salive ME, Boyd CM. Health-related quality of life and functional status quality indicators for older persons with multiple chronic conditions. J Am Geriatr Soc. 2013;61:2120-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Balentine CJ, Naik AD, Berger DH, Chen H, Anaya DA, Kennedy GD. Postacute Care After Major Abdominal Surgery in Elderly Patients: Intersection of Age, Functional Status, and Postoperative Complications. JAMA Surg. 2016;151:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Clark F, Azen SP, Carlson M, Mandel D, LaBree L, Hay J, Zemke R, Jackson J, Lipson L. Embedding health-promoting changes into the daily lives of independent-living older adults: long-term follow-up of occupational therapy intervention. J Gerontol B Psychol Sci Soc Sci. 2001;56:P60-P63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Taberna M, Gil Moncayo F, Jané-Salas E, Antonio M, Arribas L, Vilajosana E, Peralvez Torres E, Mesía R. The Multidisciplinary Team (MDT) Approach and Quality of Care. Front Oncol. 2020;10:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 34. | Stepkovitch K. Geriatric Functional Assessment. Decis Mak Med. 2010;. [DOI] [Full Text] |
| 35. | Kwon S, Symons R, Yukawa M, Dasher N, Legner V, Flum DR. Evaluating the association of preoperative functional status and postoperative functional decline in older patients undergoing major surgery. Am Surg. 2012;78:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, Scott S, Mayo NE. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 37. | Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, Liberman AS, Stein B, Charlebois P, Feldman LS, Carli F. Prehabilitation vs rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 558] [Article Influence: 50.7] [Reference Citation Analysis (0)] |