Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.89822
Peer-review started: November 13, 2023
First decision: November 29, 2023
Revised: December 11, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: March 18, 2024
Processing time: 122 Days and 12.7 Hours
There is shortage of organs, including kidneys, worldwide. Along with deceased kidney transplantation, there is a significant rise in live kidney donation. The prevalence of prediabetes (PD), including impaired fasting glucose and impaired glucose tolerance, is on the rise across the globe. Transplant teams frequently come across prediabetic kidney donors for evaluation. Prediabetics are at risk of diabetes, chronic kidney disease, cardiovascular events, stroke, neuropathy, retinopathy, dementia, depression and nonalcoholic liver disease along with increased risk of all-cause mortality. Unfortunately, most of the studies done in prediabetic kidney donors are retrospective in nature and have a short follow up period. There is lack of prospective long-term studies to know about the real risk of complications after donation. Furthermore, there are variations in recommendations from various guidelines across the globe for donations in prediabetics, leading to more confusion among clinicians. This increases the responsibility of transplant teams to take appropriate decisions in the best interest of both donors and recipients. This review focuses on pathophysiological changes of PD in kidneys, potential complications of PD, other risk factors for development of type 2 diabetes, a review of guidelines for kidney donation, the potential role of diabetes risk score and calculator in kidney donors and the way forward for the evaluation and selection of prediabetic kidney donors.
Core Tip: An increasing number of prediabetic kidney donors are encountered by transplant physicians. The decision to allow or to not allow these donors is always challenging. Prediabetics are prone to multiple complications in the future, including diabetes mellitus and chronic kidney disease. Variability in recommendations by various organizations and societies about kidney donation in prediabetics leads to even further confusion in decision making. This extensive review focuses on evidence from both the general population and kidney donors regarding kidney donation in prediabetics. This review will help clinicians to take well informed decisions and to identify a direction for further research and the need for a uniform position by international transplant societies like The Transplantation Society or International Society of Nephrology.
- Citation: Khalil MAM, Sadagah NM, Tan J, Syed FO, Chong VH, Al-Qurashi SH. Pros and cons of live kidney donation in prediabetics: A critical review and way forward. World J Transplant 2024; 14(1): 89822
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/89822.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.89822
Prediabetes (PD) is described as high blood glucose levels which do not satisfy the criteria for the diagnosis of diabetes mellitus (DM). A fasting plasma glucose level of 126 mg/dL (6.99 mmol/L) or greater, and glycated hemoglobin (HbA1c) level of 6.5% or greater, or a 2-h post prandial level of 200 mg/dL (11.1 mmol/L) or greater are consistent with the diagnosis of type 2 diabetes. On the other side, a fasting plasma glucose level of 100 to 125 mg/dL (5.55-6.94 mmol/L), an HbA1c level of 5.7% to 6.4%, or a 2-h post prandial glucose level of 140 to 199 mg/dL (7.77-11.04 mmol/L) are consistent with PD[1]. The World Health Organization (WHO) and numerous other diabetes organizations define the impaired fasting glucose (IFG) cutoff to be 110 mg/dL (6.1 mmol/L)[1]. The global prevalence of PD reported in literature has been variable due to a variety of reasons. Firstly, the definition of PD by WHO and the American Diabetes Association (ADA) has been different and as a result prevalence has varied among different studies depending on the definition being used. Secondly, studies used different parameters such as fasting glucose, glucose tolerance test or glycosylated hemoglobin to define PD, which could also have led to variable prevalence. Rooney et al[2] used the WHO definition of PD and reported the global prevalence of impaired glucose tolerance (IGT) in 2021 as 9.1% (464 million) and projected it to go up by 10% (638 million) in 2045. Similarly, the global prevalence of IFG in 2021 was 5.8% (298 million) and it was projected to increase by 6.5% (414 million) in 2045[2]. Bullard et al[3] used the ADA definition and reported the prevalence of PD in adults aged ≥ 18 years as 29.2% in 1999-2002, increasing to 36.2% in 2007-2010 in United States population[3]. A study from China used the ADA 2010 definition and reported prevalence at 50.1%[4]. Around 5%-10% of people with PD develop DM annually[5,6] although the conversion rate varies by population characteristics and the exact criteria used for the definition of PD. IFG is a predictor of cardiovascular mortality and it increases cardiovascular mortality by 20%[7,8].
Kidney transplantation (KT) is the treatment of choice for end stage renal disease (ESRD)[9]. KT improves quality of life and survival rates of patients with ESRD[10,11]. Living kidney donation leaves the kidney donor with a single kidney for the rest of their life, hence increasing their vulnerability to acquire kidney impairment in the future. Recent studies comparing donors to healthy non-donors found that kidney donation is related to a small but statistically significant increased risk of ESRD[12,13]. Prediabetic kidney donors have a seven-fold increased risk of DM (15.6%) compared to donors with normal glucose levels (2.2%)[14]. In view of this significant risk, it is important for KT physicians to carefully assess donors with PD for eligibility of donation.
Abnormal glomerular hemodynamic homeostasis has been proposed as an important factor in the pathogenesis of renal diseases. This is usually manifested as increased hyperfiltration leading to an increase glomerular filtration rate (GFR)[15]. PD has been shown to cause hyperfiltration and increased GFR in both animal and human studies. Experimental glucose infusion in dogs has been shown to cause a reactive increase in GFR[16]. Similarly, in human clinical studies, hy
The synergistic deleterious effects of PD and donor nephrectomy in the development of CKD in kidney donors is not well studied. Post-kidney donation often causes mild proteinuria and reduced GFR, with incidence of proteinuria ranging from less than 5% to more than 20%[27]. The proteinuria usually becomes more pronounced over a period of time[27]. Kidney donation is also associated with a 30%-35% dip of GFR in the earlier period[28], but compensatory hyperfiltration in the remaining kidney can lessen the expected GFR reduction.
Early CKD in kidney donors is mostly due to glomerulonephritis[13,29-32]. However late CKD in kidney donors is due to Denovo DM[13,29-31] and hypertensive nephrosclerosis[32]. PD has been implicated in hyperfiltration[16-18] and the development of microalbuminuria[21,22] in the general population, which are usually early manifestations of renal injury. Though the risk of conversion from PD to diabetes is higher in kidney donors (15.6%) when compared to healthy control (2.2%)[14], the real risk of CKD reported in few studies is minimal. Chandran et al[14] found that prediabetic patients are not at risk of developing CKD in the short term[14]. Similarly, a study from Japan compared donors with PD and diabetes with those having normal glucose and found no difference in surgical complications, mortality or risk of ESRD[33]. Hebert et al[34] and his colleagues also did not find increased risk of CKD in donors with PD[34]. The annual incidence rate of development of DM is 6%-11%. Around 70% of individuals with PD will eventually develop DM in their life time[35]. About 40% of diabetics will develop CKD in their life span[36]. Microalbuminuria and hyperfiltration develop 5-10 years after the initial diagnosis of DM (or PD). Macroalbuminuria develops in another 15 years and ESRD will ensue in 19 years from the diagnosis of diabetes[37]. Therefore, to know the real impact of PD we need long term studies of at least greater than 19 years to see the real sequalae of PD. Most of the studies done in prediabetic donors have a short period of follow up ranging from 88 months[33] to 10.4 years[14], which may miss out patients with late onset DM and diabetic kidney disease.
Many studies have been conducted on PD and the risk of CKD in the general population, with mixed findings. In the Framingham Heart Study, odds of developing CKD were 0.98 (95%CI: 0.67-1.45), 1.71 (95%CI: 0.83-3.55), and 1.93 (95%CI: 1.06-3.49) among those with IFG or IGT, newly diagnosed DM, or known DM when compared to those with a normal glucose level. The authors of this study proposed that cardiovascular disease risk factors explained much of the relationship between PD and the development of CKD[38]. With a mean follow-up of 14 years, study participants without baseline diabetes had glycosylated hemoglobin of 5.7%-6.4% and ≥ 6.5%, and < 5.7% were found to have a hazard ratio (HR) of 1.12 (0.94-1.34) and 1.39 (1.04-1.85) for development of CKD. Selvin et al[39] in their study with a mean follow-up of 14 years of study participants without baseline diabetes compared glycosylated hemoglobin of 5.7-6.4% and ≥ 6.5%, with < 5.7%, and found a HR of 1.12 (0.94-1.34) and 1.39 (1.04-1.85) for development of CKD. The corresponding HR for ESRD were 1.51 (0.82-2.76) and 1.98 (0.83-4.73), respectively[39]. In a study from Korea[20], the OR for microalbuminuria and CKD in an individual with PD having impaired fasting were 1.54 (95%CI: 1.02-2.33) and 1.58 (1.10-2.25). The OR significantly went up to 2.57 (1.31-5.06) in individuals having both IFG and IGT. The National Health and Nutrition Examination Survey study (1999-2006) showed that 17.7% of participants with PD had CKD as compared to 10.6% with no diabetes[40]. Redon et al[41] found that there was a close relationship between abnormal urinary albumin excretion and renal insufficiency in patients with essential hypertension, which was more pronounced in patients with the highest IFG (110-125.9 mg/dL).
However, there are also studies which did not find associations between PD and development of CKD. In a study from Germany, the prevalence of risk for CKD and the incidence of CKD were higher in subjects with PD than in subjects with euglycemia. However, the authors found that the increased risk did not persist after adjusting for established cardiovascular risk factors. After careful adjustments for established cardiovascular risk factors, the relative risk (RR) for IFG was 0.97 (95%CI: 0.75-1.25) and for HbA1c -defined PD was 1.03 (95%CI: 0.86-1.23). This led the authors to conclude that the higher incidence reduced kidney function in subjects with PD is most likely caused by increased cardiovascular risk factors[42]. In secondary analysis of the Systolic Blood Pressure Intervention Trial, where participants were followed for a median of 3.3 years, 41.8% had IFG but IFG was not associated with worsening kidney function or albuminuria[43]. Similarly, a study from Japan found an association of PD with the development of proteinuria but it failed to show any association between PD and CKD[44].
A meta-analysis of 9 cohort studies, the participants of which were mainly Asian and white, found increased risk of CKD in PD. Eight studies used the definition of impaired fasting as 6.1-6.9 mmol/L and after adjustment for established risk factors, the RR of CKD was 1.11 (95%CI: 1.02-1.21). One study in this meta-analysis used definition of IFG as 5.6-6.9 mmol/dL. Combining all studies together, the overall RR of CKD was 1.12 (95%CI: 1.02-1.21; Table 1)[45].
| Ref. | Journal/Year | Study type | Objective | Findings |
| Fox et al[38] | Diabetes Care/2005 | Follow up of Framingham Heart Study (1991-1995) after 75-gram oral glucose tolerance test | To study the impact of IFG and IGT on development of CKD | The odd of developing CKD was 0.98 (95%CI: 0.67-1.45), 1.71 (95%CI: 0.83-3.55) and 1.93 (95%CI: 1.06-3.49) among patients with IFG or IGT, newly diagnosed diabetes or known diabetes |
| Redon et al[41] | J Am Soc Nephrol/2006 | Prospective multicenter, cross-sectional study | To assess the relationship between UAE and glomerular filtration rate in patients with glucose metabolism abnormalities having hypertension | The prevalence of abnormal UAE, > or = 3.4 mg/mmol across the spectrum of glucose abnormalities were 39.7%, 46.2%, 48.6%, and 65.6% for normoglycemic, low-range, and high-range impaired fasting glucose and diabetes. Predictors of low GFR < 60 mL/min were UAE ≥ 3.4 mg/mmol (OR 1.87; 95%CI: 1.61 to 2.17), IFG and diabetes (OR 1.30; 95%CI: 1.05 to 1.62), and BP ≥ 140/90 mmHg, or ≥ 130/80 mmHg if diabetes (OR 1.23; 95%CI: 1.04 to 1.45) |
| Plantinga et al[40] | Clin J Am Soc Nephrol/2010 | Retrospective analysis of 1999-2006 national health and nutrition examination survey | To measure and compare the prevalence of CKD among people with diagnosed diabetes, undiagnosed diabetes, PD, or no diabetes | 39.6% of people with diagnosed and 41.7% with undiagnosed diabetes had CKD; 17.7% with PD and 10.6% without diabetes had CKD. Among those with CKD, 39.1% had undiagnosed or PD |
| Okamoto et al[33] | Transplantation/2010 | Retrospective study | To assess the indications for live kidney donation in glucose intolerance and to analyze perioperative complications associated with donor nephrectomies and its long-term consequences | Perioperative complications, survival rates and mortality were not significant between glucose intolerance and those with normal glucose tolerance |
| Selvin et al[39] | Diabetes/2011 | Prospective cohort and cross-sectional analyses of ARIC study | To examine association between 2010 American Diabetes Association diagnostic cut points for glycated hemoglobin and microvascular outcomes (CKD, ESRD and retinopathy) | Risk of CKD, with adjusted HRs of 1.12 (0.94-1.34) and 1.39 (1.04-1.85) was found for glycated hemoglobin 5.7%-6.4% and ≥ 6.5%, respectively, as compared with < 5.7% (P = 0.002). HR for ESRD were 1.51 (0.82-2.76) and 1.98 (0.83-4.73) |
| Schöttker et al[42] | Prev Med/2013 | Prospective study | (1) To determine the risk for incident reduced kidney function in participants with pre-diabetes; and (2) To determine dose-response relationships of fasting glucose and HbA1c with reduced kidney functions in subjects with manifest diabetes mellitus | Reduced kidney function risk factor prevalences and incidences were higher in participants with pre-diabetes than without PD. Increased risk did not persist after adjusting for established cardiovascular risk factors [RR (IFG): 0.97 (95%CI: 0.75-1.25) and RR (HbA1c-defined pre-diabetes): 1.03 (95%CI: 0.86-1.23)] |
| Chandran et al[14] | Transplantation/2014 | Retrospective study | To compare development of diabetes, the estimated glomerular filtration rate, and the level of albumin excretion in donors with IFG to matched controls with normal pre-donation fasting glucose | (1) Higher proportion of IFG donors had developed DM (15.56% vs 2.2%, P = 0.06); (2) eGFR at 10.4 years was 70.7 ± 16.1 vs 67.3 ± 16.6 mL/min/1.73 m2, P = 0.21) was similar between 2 groups; and (3) Urine albumin/creatinine 9.76 ± 23.6 vs 5.91 ± 11 mg/g, P = 0.29) was similar between 2 groups |
| Echouffo-Tcheugui et al[45] | Diabet Med/2016 | Metanalysis | To assess the effect of PD on the incidence of CKD | Relative risk of CKD after adjustment for established risk factors was 1.11 (95%CI: 1.02-1.21) when IFG was defined as 6.1-6.9 mmol/L |
| Bigotte Vieira et al[43] | J Clin Endocrinol Metab/2019 | Post hoc analysis of participants of the SPRINT trial | To find association of PD with adverse kidney outcomes | Impaired fasting glucose was not associated with higher rates of the composite outcome (HR: 0.97; 95%CI: 0.8 to 1.16), worsening kidney function (HR: 1.02; 95%CI: 0.75 to 1.37), or albuminuria (HR: 0.98; 95%CI: 0.78 to 1.23) |
| Furukawa et al[44] | Diabet Med/2021 | Retrospective analysis of health check-up in 2014 in Japan | To investigate the associations of PD with the proteinuria and eGFR decline | PD was independently associated with the proteinuria development (OR 1.233; 95%CI: 1.170-1.301). No association was found with eGFR decline (OR 0.981; 95%CI: 0.947-1.017) |
| Hebert et al[34] | Transplantation/2022 | Retrospective data analysis of The RELIVE study | To study mortality, proteinuria, and ESKD according to donation FPG: < 100 mg/dL, 100-125 mg/dL, and ≥ 1 26 mg/dL | IFG was associated with a higher diabetes risk (adjusted HR, 1.65; 95%CI: 1.18-2.30) and hypertension (adjusted HR 1.35; 95%CI: 1.10-1.65; P = 0.003 for both), but not higher risk of proteinuria or ESKD |
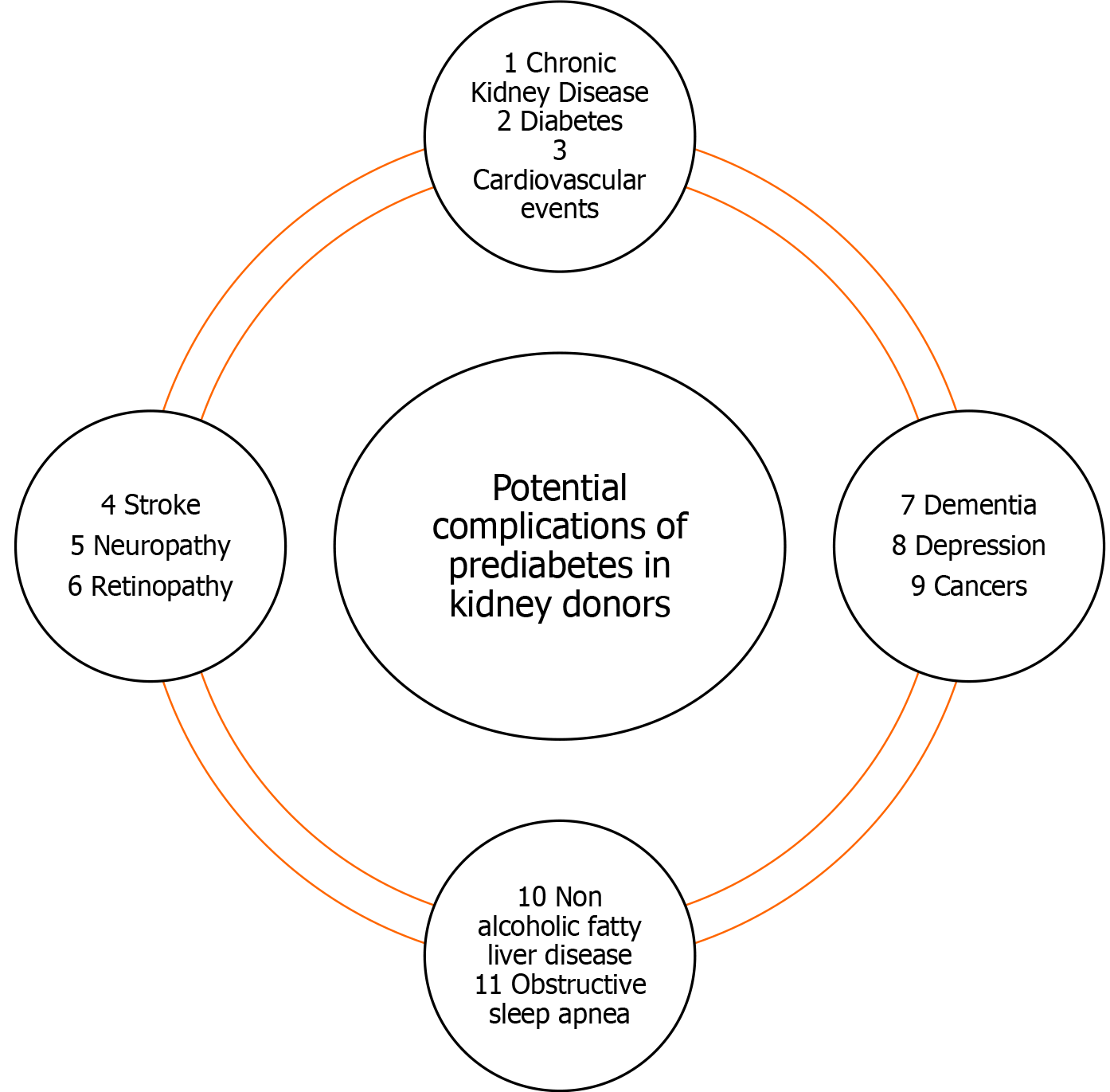
PD causes various other complications other than CKD. These complications should be kept in mind and should be taken into consideration before allowing a potential donor to donate. PD can cause overt DM, cardiovascular events, stroke, microvascular complications such as neuropathy and retinopathy and has been associated with dementia, depression, cancer and an increase in all-cause mortality[46,47].
Risk of progression from PD to diabetes varies widely due to differences in the definition of PD, heterogeneity of PD, and social and physical environment[48]. The lower cut-off point for IFG, which is still used by WHO, is 6.1 mmol/L[49]. In 2003, this cut-off point was lowered to 5.6 mmol/L by the ADA[50]. As a result, there is variability in the prevalence of PD and its subsequent progression to diabetes. Around 10%-50% of individuals will develop diabetes in next 5-10 years[35,51,52]. On the other hand, 30%-60% will revert to normoglycemia within 1-5 years[51].
The risk of progression of PD to diabetes is less well studied in kidney donors. Various studies done in kidney donors reported the incidence of diabetes as 1.5%-7.4%. However, most of these studies were cross sectional in nature, having a sampling bias with a lack of baseline glucose levels before donation[53-62]. The risk of diabetes in kidney donors with PD is 6 times more compared to donor without PD[14]. In a retrospective review with 1826 kidney donors, patients with IFG (100-126) were compared to those with normal blood glucose (< 100 mg/dL) and donors with a fasting glucose ≥ 126 mg/dL[34]. IFG was associated with a higher risk of diabetes and hypertension, but these patients were not found to be at higher risk of proteinuria or ESRD. Only 3.5% of donors from this cohort with normal glucose developed diabetes, at 15.4 ± 10.9 years, compared to 5.5% donors with IFG who developed diabetes 10.6 ± 8.8 years after donation.
Post kidney donation living donors are prone to high blood pressure and proteinuria[27,63-65]. Proteinuria, hypertension and reduced GFR are known risk factors for cardiovascular events[66-68]. There are mixed findings regarding the post donation risk of cardiovascular events. A recent long-term follow-up (11.3 years) of kidney donors showed that donors were at an increased risk of ischemic heart disease when compared with healthy controls[69]. Conversely, there are also studies which did not find an increased risk of cardiovascular events[70,71]. PD is a well-known risk for cardiovascular events. Unfortunately, there is paucity of data linking PD to cardiovascular events in kidney donors. However, most of the evidence linking PD to cardiovascular illness has been gathered from the general population. PD has been implicated as a risk factor for cardiovascular diseases in a range of studies[7,72,73]. PD shows a 20% higher risk of developing cardiovascular disease compared to those with normal blood sugar[74]. Insulin resistance, inflammation and endothelial dysfunction in PD are linked to more cardiovascular events[75]. IGT is more often associated with cardiovascular events than IFG[76-78], with an overall similar cardiovascular risk to type 2 DM in many landmark trials such as Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe[76], Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia[79] and Funagata Diabetes study[73]. Similarly, increase in glycosylated hemoglobin even within a normal range has been shown to cause more cardiovascular mortality. In the European Prospective Investigation into Cancer (EPIC) Norfolk study, even a small 1% increase in HbA1c within the normal range caused an increase in 10-year cardiovascular mortality[80]. Since PD causes insulin resistance, inflammation and endothelial dysfunction[75], KT physicians have to be more mindful on potential future cardiovascular risks.
Stroke is one of the macrovascular complications of PD. The prevalence of PD in patient with a recent ischemic stroke or transient ischemic attack (TIA) is around 37%[81]. Two-hour IGT is a stronger predictor of stroke and cardiovascular events compared to IFG[76,82,83]. IGT has also been implicated in recurrent ischemic stroke and TIA and it increases risk of recurrent TIA and minor stroke by 2 folds[82]. A meta-analysis of 15 prospective cohort studies found a positive association between PD and stroke. The authors, after excluding studies with undiagnosed diabetes, found that IGT or the combination of IFG and IGT were independent risk factors for stroke[84]. Unfortunately, the association of PD with stroke in kidney donors is not well studied and there is need to explore this group of individuals for risk of stroke.
Neuropathy is one of the microvascular complications. Around 35% of newly diagnosed type 2 diabetics have peripheral neuropathy indicating an early subclinical phase before the development of diabetes[85]. PD has been linked to the development of peripheral neuropathy in the general population, though its prevalence is varied in different studies. The 1999-2004 cohort from Katon et al[86] reported the RR of peripheral neuropathy of 1.1 in PD and 1.7 in diabetes[86]. A study from Germany reported significant peripheral neuropathy of 24% in individuals who have both IFG and IGT. However, isolated IFG or IGT in this study failed to show significance for development of peripheral neuropathy[87]. The MONICA/KORA study found that neuropathy was more common in patients with IGT when compared to control[88]. Authors of this study used Michigan Neuropathy Screening Instrument and found that neuropathy, predominantly involving small nerve fibers, were present in 13.3% of patients with diabetes, 8.7% of patients with IGT, 4.2% of patients with IFG and 1.2% of patients with normoglycemia[88]. The Prospective Metabolism and Islet Cell Evaluation study followed patients for peripheral neuropathy and at 3 years follow up. Authors found that prevalence was highest among individuals who progressed to diabetes (50%) and followed by those who developed PD (49%), compared to individuals with normoglycemia who have an incidence of 29%[89]. A meta-analysis found that there was a wide range of prevalence estimates from 2%-77%, but most studies included in this analysis reported a prevalence ≥ 10%[90]. Unfortunately, there is lack of data on peripheral neuropathy in prediabetic kidney donors.
The prevalence of retinopathy has been different in various studies. In an epidemiological study done in Pima Indians, retinopathy was reported in 12% of patients with IGT[91]. Diabetes Prevention Program study who had elevated blood glucose, but no history of diabetes, showed that retinopathy was present in 7.9% in patients with PD[92]. Post hoc analysis of a systematic review[93] showed lower median retinopathy in patients with a normal glucose tolerance of 3.2% (interquartile range 0.3%-7.3%) compared to 6.6% (interquartile range 1.9%-9.8%) in prediabetics. Reduced retinal arteriolar dilatation has been in implicated as manifestation of retinopathy in PD[94]. The Maastricht Study using spectral domain optical coherence tomography found that macular thickness is reduced in PD even before the onset of diabetic retinopathy. Hypertension, abdominal obesity and hyperglycemia were found to be predictors of incident retinopathy across all glucose levels from normoglycemia to PD and diabetes[95]. Though the association of retinopathy in the general population is strong, this is again not thoroughly investigated in kidney donors with PD.
Dementia has been a recognized complication of PD. Insulin and insulin-like growth factors have an important role in the vital functions of neurons including survival and neuron growth, gene expression, protein synthesis, myelin production and maintenance in oligodendrocytes, synapse formation and plasticity[96,97]. PD, like diabetes, is a state of hyperinsulinism with insulin resistance which affects the function of brain cells (neurons and glial cells) leading to neurodegeneration and dementia[98-100]. A study from Sweden has shown significant brain volume loss affecting predominantly white matter leading to progressive cognitive impairment over a period of 9 years in both PD and DM[101]. Similarly, another study in elderly women showed risk of the development of cognitive impairment among participants with IFG (OR 1.64) and DM (OR 1.79)[102]. Prediabetics in the Maastricht study participants were found to have more cerebral lacunar infarcts, white matter lesions and loss of brain volume when compared with normoglycemic participants[103]. Hyperglycemia is a continuum from normoglycemia to PD. Diabetes and increasing hyperglycemia across this spectrum in prediabetic and diabetics affected executive functions in the NHANES 2011-2014 cohort[104]. In another population-based study, PD and DM were associated with minor deficits in global cognitive function, processing speed and executive functioning and an inverse correlation between glucose level with cognitive abilities in non-diabetics was found[105].
PD has been linked to risk of depression in various studies[106,107], likely through insulin resistance. Insulin resistance in the brain induces mitochondrial and dopaminergic dysfunction leading to anxiety and depressive-like behaviors[108]. Two meta-analyses done on the association of PD with depression reported mixed findings; one metanalysis reported that the prevalence of depression is moderately increased in prediabetic and in undiagnosed diabetic patients[109] and the other found that prediabetics are not at a higher risk of depression[110]. Some studies have also shown that the combination of PD with depression increases the risk of progression to the development of diabetes[111-113]. Since anxiety, depression and regret have been reported in some kidney donors[114-116], therefore, it is important to understand the potential future neurological sequelae of PD.
PD has been reported to be associated with cancers in several studies[117-119]. A community-based study from China reported that glucose intolerance (PD & DM) was associated with stomach, colorectal, and kidney cancer in individuals aged < 65 year[120]. PD is associated with obesity and overweight, which are the recognized risk factors for cancer[121]. Hyperglycemia has been linked to the increased production of reactive oxygen species, reduced levels of antioxidant capacity, and increased levels of DNA damage which may be a potential mechanism of carcinogenesis in these patients[122]. A meta-analysis of 16 prospective studies found that PD was associated with an increased risk of cancer overall (RR 1.15; 95%CI: 1.06-1.23). The analysis also found that cancer of the stomach/colorectum, liver, pancreas, breast and endometrium were significantly associated with PD (P < 0.05). However, no association was found with cancer of the bronchus/Lung, prostate, ovary, kidney or bladder[121].
Kidney donors have a similar incidence of liver cancer, melanoma, breast cancer, and non-Hodgkin lymphoma 7 years post donation as compared to the general population. However, there is an increased incidence of colorectal cancer (adjusted incidence rate ratio 2.07, 95%CI: 154-2.79) and kidney cancer (2.97, 1.58-5.58) in kidney donors[123]. Given the evidence, kidney donors with PD, especially those who are overweight and are actively smoking, may be more prone to develop tumors post donation.
The prevalence of nonalcoholic fatty liver disease (NAFLD) is 48.25% in patients with PD[124]. In a study from United States, 44%-62% of the adults with PD had NAFLD[125] Prevalence in the general population is 26%, which is much lower than PD[126]. Obesity associated insulin resistance increases free fatty acid levels which leads to more storage of fat in the liver. This leads to more hepatic insulin resistance and activation of inflammatory pathways and oxidative stress, which promote fibrosis in liver[127]. Subclinical chronic hepatic inflammation and insulin resistance has been shown to cause NAFLD in PD[128]. NAFLD has been linked with reduced GFR[129]. Living kidney donors do not have underlying kidney disease but have reduced GFR as a result of nephrectomy. However, a study reported that reduced kidney function after kidney donation is not associated with increased incidence or progression of NAFLD[130], but that data on prediabetic kidney donors is lacking. Looking at data from the general population, it will be interesting to evaluate the association between NAFLD and PD in kidney donors.
PD has been linked to increased all-cause mortality[131]. A study from Japan showed that PD was significantly associated with increased risk of death from all causes and cancer but not cardiovascular diseases[132]. PD along with hypertension not only caused increased all-cause mortality but also increased cardiovascular mortality[8]. Another recent metanalysis of 16 studies found that PD was associated with an increased risk of all-cause mortality[46]. Inactivity and obesity are common among PDs. Physical activity is of utmost important in prediabetics. A recent study showed that conversion of euglycemia along with physically activity was associated with a lower risk of death compared with persistent PD and physical inactivity[133]. Keeping these facts in mind, it is important to fully educate prediabetic kidney donors about physical activity prior to donation. Figure 1 showed potential complications which can happen in a kidney donor.
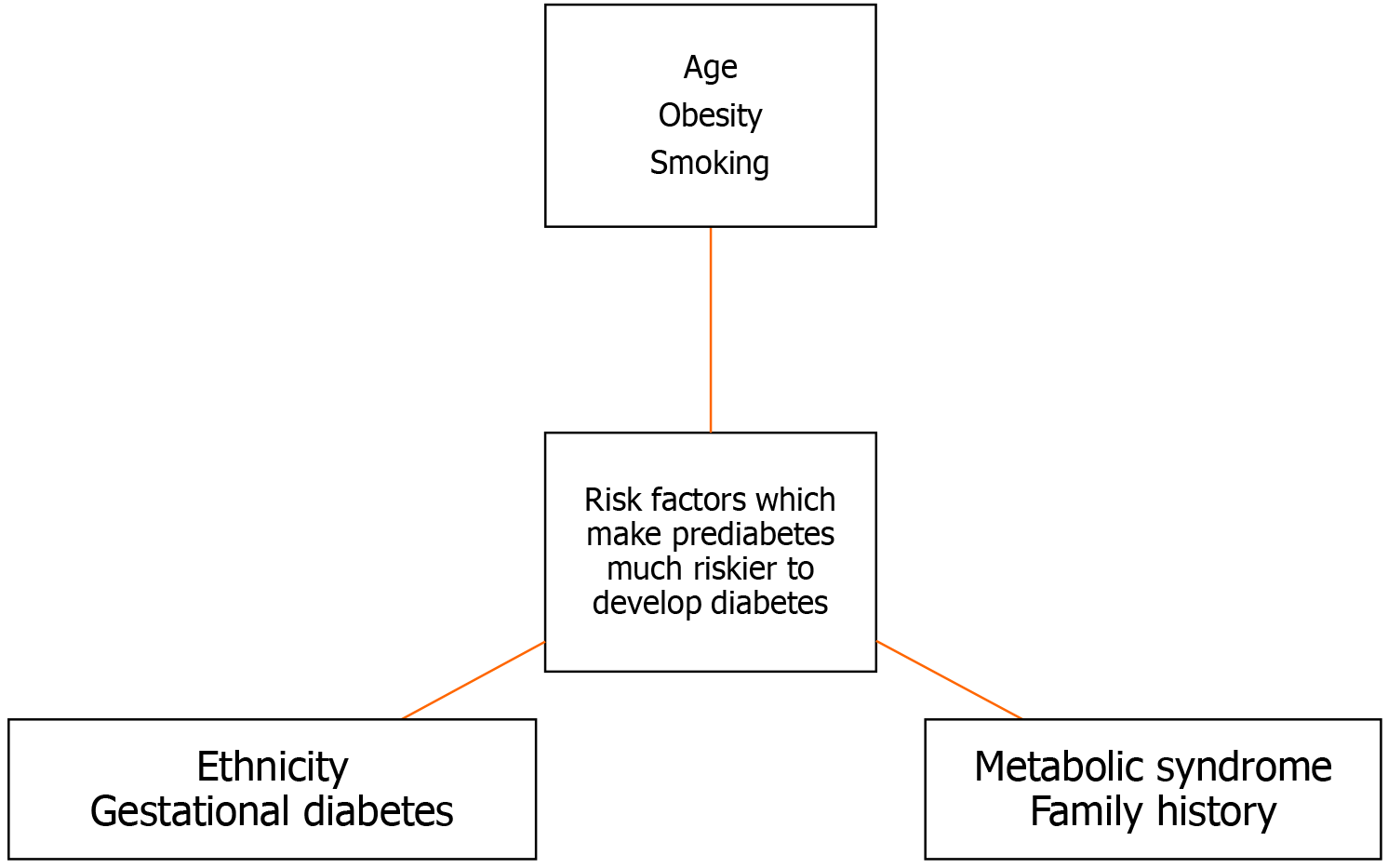
Various risk factors, when present in prediabetics, make them prone to develop diabetes. KT should be cognizant of these risk factors before allowing a prediabetic kidney donor to donate. These risk factors are as follows:
The elderly have a higher prevalence of diabetes and PD than young and middle-aged people[139,140]. Age is an important risk factor for the development of diabetes because of inflammation, mitochondrial dysfunction and abnormal lipid metabolism[141]. However, there are studies which showed that the majority of the PD either remained stable or reverted to normoglycemia[142,143]. Since PD is a continuous and cumulative risk, most transplant programs may discourage young prediabetics to donate.
Obesity is a potentially modifiable risk factor for diabetes[144]. Obesity is characterized by insulin resistance which is manifested by decreased insulin-stimulated glucose transport and metabolism in adipocytes and skeletal muscle and by impaired suppression of hepatic glucose output[145]. Individual adipose cell type composition, adipose mitochondrial gene expression and body fat percentage have been shown to predict insulin resistance in both prediabetics and obese individuals[146]. Excess visceral fat and insulin resistance, rather than general adiposity, were found to be associated with the development of PD and diabetes[147].
Kidney donors with BMI ≥ 25 kg/m2 at the time of donation are prone to develop significant weight gain over 1-year post-donation[148]. Praga et al[148] found that kidney donors with higher BMI had a greater risk for the development of proteinuria and renal dysfunction[149]. Similarly, another study also found a significant relationship between increasing BMI and the rate of kidney insufficiency after kidney donation[150]. Therefore, prediabetics with obesity should be evaluated carefully due to the risks of the development of diabetes, proteinuria and renal dysfunction.
Smoking has been shown to decrease insulin action and increased insulin resistance in experimental settings[150]. Coronary artery risk development in young adults (CARDIA-study) studied the effect of active and passive smoking on glucose intolerance. At a 15-year follow-up, glucose intolerance was highest among smokers (21.8%), followed by passive smokers who never smoked (17.2%) and ex-smokers (14.4%), compared to 11.5% in individuals who never smoked[151]. Another study found that 5-10 pack-years of smoking increased odds of PD by 2-fold, which is reversible with smoking cessation[152]. Smokers are 30% to 40% more likely to develop diabetes compared to non-smokers[153]. Various studies have shown strong associations between cigarette smoking and the development of DM[154-157]. Smoking is common in kidney donors, though pre-donation education usually reduces incidence of smoking[158]. Active or passive smoking in kidney donors may lead to higher serum creatinine compared to non-smokers[158,159]. Therefore, prediabetic kidney donors with a history of smoking should be advised to stop and be evaluated thoroughly for future risk of DM.
Certain ethnicities are more prone to developing diabetes and its complications. The United States is populated by multiple ethnic groups. The rate of diagnosis of diabetes is 14.5% in American Indian/Alaskan Natives, 12.1% in non-Hispanic blacks, 11.8% in Hispanics, 9.5% in Asian Americans and 7.4% in non-Hispanic whites. Among Asian Americans, 12.6% of Asian Indians have diabetes, followed by Filipinos (10.6%) and Chinese (5.6%). Among Hispanic adults, 14.4% have diabetes followed by 14.4% Puerto Ricans[160]. Similarly, in the United Kingdom, the prevalence of type 2 diabetes is indeed higher among Asian, Black and minority ethnic groups[161]. Health Survey for England found reported prevalence of type 2 diabetes in Black Caribbean (9.5% men, 7.6% women), Indian (9.2% men, 5.9% women), Pakistani (7.3% men, 8.4% women), and Bangladeshi (8.0% men, 4.5% women) people[161]. The percentage of change in the number of people with diabetes between years 2000 to 2030 has been 97% for Sub-Saharan Africa, 67% for Middle East, and 42% for Asia and Islands[162]. The propensity for development of diabetes among various ethnic groups should be kept in mind before allowing a pre-diabetic kidney donor to donate his kidney.
Gestational diabetes has been an important recognized risk factor for future development of diabetes. Insulin resistance along with pancreatic β-cell dysfunction has been proposed as a mechanism for gestational diabetes[163]. The risk of the development of diabetes is 7-10 times higher in women with gestational diabetes[164,165]. After the diagnosis of gestational diabetes, rapid conversion to overt diabetes is seen within 5 years, with a slower progression subsequently[166]. Furthermore, women with gestational diabetes are at higher risk of developing metabolic syndrome[167,168] and are at increased risk of cardiovascular events[167]. It should also be kept in mind that subsequent pregnancy post-donation makes female donors more prone to a higher risk of preeclampsia, gestational hypertension and preterm birth[169]. Therefore, female kidney donors with PD and a history of gestational diabetes should be thoroughly assessed for risk vs benefits.
The combination of glucose intolerance, hypertension, dyslipidemia and obesity is known as metabolic syndrome[170]. In the Beaver Dam study, the OR for the incidence of diabetes was 9.37 if three abnormalities of metabolic syndrome were present. The OR went up to 33.67 if four or more abnormalities were present[171]. In the Framingham Heart Study Offspring Study, the RR for type 2 diabetes increased with the number of metabolic syndrome components[171]. The West of Scotland Coronary Prevention Study used National Cholesterol Education Program definition for metabolic syndrome with or without the inclusion of C-reactive protein. The study found a RR for diabetes at 7.26 with three abnormalities of metabolic syndrome. The RR went up to 24.4 for four more abnormalities of metabolic syndrome[172]. The British Regional Heart study found the RR for diabetes to be at 4.56 for three abnormalities. The RR for the development of diabetes went up to 10.88 for four more abnormalities[173].
IFG is one of the components of metabolic syndrome. Various studies have shown that IFG is one of the strongest predictors of the development of diabetes compared to the other elements of metabolic syndrome. In a study from Finland[174], the HR for the development for IFG was 5.16, which was the highest when compared with obesity (HR 1.75), triglyceride (HR 1.34), High density liptein-cholesterol (HR 1.60) and blood pressure (HR 1.87). The Framingham Offspring Study showed that individuals with metabolic syndrome which included IFG showed a high RR of 11, which was much higher than the RR of 5 in individuals for whom IFG was excluded in analysis[175].
The development of metabolic syndrome has been studied in kidney donors. An analysis of 2018 Living kidney donors, when matched with control non-donors, found that the living kidney donors showed a lower absolute prevalence for all metabolic risk factors, except for those who were either overweight or obese[176]. However, in another study, more donors developed new onset metabolic syndrome compared to the control group[177]. Martín-Alemañy et al[178] reported that living kidney donors had a high frequency of cardiometabolic risk factors and metabolic syndrome at the time of donation, which significantly increased over time[178]. In fact, metabolic syndrome was found to be a major barrier to kidney donation in one of the studies[179]. Therefore, one should carefully evaluate potential donors with PD and metabolic syndrome as they may be at risk of developing DM and cardiovascular complications.
Family history is one of the recognized risk factors for the development of type 2 diabetes. Familial predisposition is usually due to a combination of environmental and behavioral risk factors with genetic propensity due to various genes[180,181]. The prevalence of diabetes among individuals who have a first-degree relative with diabetes was 14.3% and it was significantly higher than individuals without a family history (3.2%)[180]. The authors classified family history risk categories of diabetes as high (at least two generations have first degree relative with diabetes), moderate (one generation of first-degree relatives with diabetes) and average (no first-degree relatives with diabetes). The prevalence rates of diabetes were 32.7% in a high-risk family, 20.1% in a moderate risk family and 8.4% in an average risk family[182]. Therefore, family history risk categories of diabetes have a significant and graded association with the prevalence of diabetes. In the EPIC-InterAct study, the authors investigated the association between a family history of diabetes among different family members and the incidence of type 2 diabetes and also studied the extent of genetic, anthropometric and lifestyle risk factors in familial predisposition. The study found that lifestyle, anthropometric and genetic risk factors contributed only minimally, with most of the risk being attributed to positive family history[183]. The Health Examinees-Gem study was done in Korea and aimed to find associations between a family history of diabetes with adherence to regular exercise, healthy diet and body composition, and clusters of healthy behaviors. The participants of the study were found to be strictly adherent to exercise and healthy diet but were found to not have a normal body composition[184]. Therefore, prediabetic kidney donors should always be evaluated with respect to their detailed family history of DM or PD. Figure 2 shows potential risk factors of the development of diabetes in a prediabetic kidney donor.
The Amsterdam Forum on the Care of the Living Kidney Donor (2006)[185] recommends to exclude individuals with a history of diabetes or fasting blood glucose ≥ 126 mg/dL (7.0 mmol/L) on at least two occasions[or 2-h glucose with oral glucose tolerance test ≥ 200 mg/dL (11.1 mmol/L)], but do not have any recommendations for PD[185].
Caring for Australian and New Zealanders with Kidney Impairment (CARI) guidelines[186] recommend checking fasting blood sugar twice in all kidney donors. Those with sugar ≥ 7 mmol/L on both occasions are considered diabetic and this is considered to be an absolute contraindication. The guidelines used the criteria of IFG as 6.1-6.9 mmol/L. Any donor with at least one occasion of IFG should have a 2 h oral glucose tolerance test. Those with normal fasting sugars were allowed to donate. Patients at high risk for the development of type 2 DM were advised to have an oral glucose tolerance test. The characteristics of high risk for developing type 2 diabetes mentioned in CARI guidelines included family history, age > 45 years, being an Aboriginal or Torres Strait Islander and obesity. If the 2-h glucose of an oral glucose tolerance test result is ≥ 11.1 mmol/L then the patient is considered diabetic and this is an absolute contra-indication to a living kidney donation. Donors with IGT and a blood sugar between 7.8-11.0 mmol/L are considered not fit to donate. Donors with glucose tolerance < 7.8 mmol/L are normal and considered to not be a contraindication to donation. Furthermore, a past history of gestational diabetes was considered as contraindication to donation.
The American Society of Transplantation (AST)[187] states that the risk of DM in donors with PD is higher than that for a healthy donor. PD also increases the future risk of diabetic kidney disease. United Network of Organ Sharing excludes donors with diabetes from donation whilst AST recommend potential donors with PD to do lifestyle modifications. The AST recommends changes in diet, to do more exercise and to lose weight to achieve euglycemia and reduce the risk for future DM[187].
The British Transplantation Society (BTS) and United Kingdom Renal Association published their guidelines in 2018[188]. All potential living kidney donors must have a fasting plasma glucose done. A fasting plasma glucose concentration between 6.1-6.9 mmol/L is suggestive of IFG and an oral glucose tolerance test should be undertaken in these donors. These guidelines also recommend an oral glucose tolerance test in prospective donors with an increased risk of type 2 diabetes such as a family history of diabetes, history of gestational diabetes, ethnicity or obesity. If an oral glucose tolerance test shows persistent IFG or IGT, then careful assessment should be clinically done using the diabetes risk calculator[189]. Unlike other guidelines, these guidelines do not exclude the diabetic completely. Diabetics can be taken as donors provided there is no target organ damage and cardiovascular risk factors such as obesity, hypertension or hyperlipidemia are optimally managed. Furthermore, thorough assessment should be done to ascertain the lifetime risk of cardiovascular and progressive CKD in the presence of a single kidney.
The Kidney Disease: Improving Global Outcomes (KDIGO) published its guidelines for the care of live kidney donors in 2019. The guidelines suggest to take a history of DM, gestational diabetes, and family history of diabetes. Blood sugar status should be assessed by checking fasting blood glucose and/or HbA1c before donation. The guidelines also recommend doing a two-hour glucose tolerance testing or HbA1c testing for donor candidates with elevated fasting blood glucose, history of gestational diabetes, or family history of diabetes in a first-degree relative. Decisions regarding donors with PD or DM should be taken on an individual basis, keeping in view their future risk. Furthermore, KDIGO guidelines recommend that donors with PD and DM should be explained that their condition may progress and could result in end organ damage[190].
European Best Practice Guidelines, published in 2015, recommended that DM is a contra-indication to donation, other than in exceptional circumstances (1D), and that IGT is not an absolute contra-indication to donation (2C)[191].
Looking at these guidelines, there is variability in recommendation for donations in prediabetics and there is a need to build a uniform consensus among the transplant community across the globe.
Various diabetes risk scores and risk calculators have been reported in the literature. The AST guidelines have mentioned the diabetes risk calculator provides accurate and individualized risk for future development of diabetes[187-192]. The Renal Association and BTS also recommend a diabetic risk calculator[188,189]. The University of Minnesota developed an apparatus that predicted risk of hypertension, type 2 diabetes, and reduced e GFR using data of living kidney donor program from 1963 through 2017 with a median follow up of 22.8 years. It requires donor age, sex, race, smoking status, estimated GFR, serum creatinine, (capillary or serum) glucose, BMI, systolic blood pressure, diastolic blood pressure, family history of hypertension and dyslipidemia. It also took into consideration the relationship to the recipient and whether the recipient has type 1 or type 2 DM. Unfortunately, prediction for hypertension and diabetes may not be valid for non-white donors[193].
There are various risks score models and risk calculators available. Prominent risk assessment tools include the Australian 5-year type 2 Diabetes Risk Assessment (AUSDRISK)[194], the Diabetes United Kingdom 10-year Know your Risk[195], The Finnish Diabetes Risk Score (FINDRISC)[196], and the ADA type 2 Diabetes Risk Test[197]. Age, sex, family history of diabetes, BMI and history of hypertension are included in all the country-specific calculators. The ADA calculator does not include ethnicity but takes gestational diabetes into consideration. The AUSDRISK and United Kingdom calculator, on the other hand, take ethnicity but not gestational diabetes into consideration. The AUSDRISK also includes smoking, fruit and vegetable intake and personal history of elevated glucose level. Waist circumference is included in both the AUSDRISK and United Kingdom calculators. Physical activity is included in the AUSDRISK and ADA calculators. The FINDRISC diabetes calculator includes gender, weight, height, age, waist circumference, and physical activity for more than 30 min, vegetable and fruits intake, use of blood pressure medications, high glucose level in past, and family history of diabetes in two generations. A systematic review done in 2011 identified 43 risk models for the prediction of the risk of DM[198]. This systematic review found poor methods including pre-screening univariate variables, the categorization of continuous risk predictors and the poor handling of missing data which could jeopardize model development. The other problem found was universal validation. Most risk scores show overall good results in predicting DM in populations for whom they were developed. However, the performance of these risk scores is more heterogeneous and generally weaker in external populations[199]. Unfortunately, most of these risk detection models have not been validated in kidney donors. It may be reasonable to use a well validated local risk calculator or risk score for all prediabetic kidney donors in that particular area to provide more accurate and individualized risk for the future development of diabetes.
There were about 88751 patients on the waiting list for a kidney until September 2023, as per Organ Procurement & Transplant Network data. Only 20445 of the patients were transplanted until September 2023[200]. About 15824 kidneys were obtained from deceased donors and another 4621 were from living donors. This reflects that approximately only a quarter of the patients on the waiting list could get a kidney. Because of a global organ shortage and unmet needs for kidneys, many centers accept increasingly complex live donors including prediabetics. The lack of evidence for long-term outcomes for pre-diabetic kidney donors for the future risk of development of diabetes, development of CKD and other complications of PD have contributed to the conundrum of using complex donors. As discussed, a couple of studies with short term duration (ranging from 88 months to 10.4 years) in kidney donors having PD did not find an increased risk of CKD[14,33,34]. After progression of PD to DM, approximately another 19 years are needed for progression of microalbuminuria to macroalbuminuria and then to development of ESRD[37]. Keeping these facts in mind, to know the real sequelae of PD in a kidney donor, we need long term studies of at least 19 years to effectively follow up.
Post donation, the prediabetic kidney donors are left with only one kidney. Most of the evidence regarding PD and its complications are derived from studies done in the general population[20,39,41]. Development of diabetes and CKD are not the only worries. Other complications of PD including cardiovascular disease[7,72,73], stroke[76,81-84], neuropathies[85-88,90,122], retinopathy[91-95], dementia[96-101,103-105], depression[106-116], cancers[117-119], non-alcoholic fatty liver disease[124-128] and increased all-cause mortality[46,131,132] are well established in the general population. Therefore, it is the responsibility of the transplant team that there should be no maleficence and every effort should be taken to follow the ethical principle “first do no harm”[201]. Every effort should be made to avoid any subtle form of coercion from the family in case of live related kidney donation. A well-informed consent form showing detailed risk vs benefits and alternative options other than a transplant should be available for both the donor and recipient to protect both of them equally. Unfortunately, the guidelines from various societies and organizations are variable, leading to further confusion[185-187,190,191]. We feel that, while evaluating a potential prediabetic kidney donor, one has to look at overall risk of development of diabetes. Donors with IFG should undergo a glucose tolerance test and, if IGT is detected, then great care should be taken to further evaluate these donors. The combination of IFG and IGT poses a great risk of developing renal dysfunction[20] and peripheral neuropathy[88]. Similarly, two hours IGT has been a strong predictor of stroke and cardiovascular events[76,83]. Therefore, prediabetic kidney donors with IFG and IGT should be considered as high risk and may not be suitable candidates. Those with isolated IFG with normal glucose tolerance should be further evaluated. If they have no risk factors (age, ethnicity, smoking, obesity, gestational diabetes and metabolic syndrome) they may represent a low-risk case. IFG along with a single or combination of risk factors such as age, family history, ethnicity, smoking, obesity, gestational diabetes and metabolic syndrome may contribute to the status of a high-risk donor. A well designed and validated local risk score or calculator may be used in these cases. Those with high risk should be excluded and those with low risk may be accepted provided they are willing to undergo long term lifestyle modification and accept the risk.
We suggest the following recommendations:
There is a need for greater consensus amongst regional societies to call for unified position statements regarding kidney donation in donors with PD, through international transplant societies like The Transplantation Society or International Society of Nephrology.
Kidney donor with PD and abnormal IFG along with IGT are considered high risk even in the absence of other risk factors. If appropriate lifestyle modification fails a reversion to euglycemia, they then should not donate.
Kidney donors with isolated IFG with normal IGT should be evaluated for other risk factors such as age, family history, ethnicity, smoking, obesity, gestational diabetes and metabolic syndrome. The presence of any risk factors in kidney donors, along with IFG, make them high risk. Appropriate lifestyle modifications are recommended to achieve euglycemia and possible donation in the future.
A locally well designed and validated risk score or risk calculator may be helpful in identifying high risk donors and should be used to identify high risk donors.
All kidney donors with PD should be advised about modifiable risk factors such as smoking, weight loss and correction of any component of a metabolic syndrome, if present.
Comprehensive risk explanations should be done. The donor should be aware of possible development of diabetes and various complications of PD, including CKD and cardiovascular events. Both the donors and recipient should know about alternative therapies such as hemodialysis and peritoneal dialysis. Donors with a poor track record or history and who are unable to lose weight or quit smoking should be excluded through collaboration with donor advocacy or social workers.
In case if donation is made, there is a need for an enhanced medical follow up of a kidney donor who has history of PD. They should have greater access to clinics, health club memberships, a dietician and medications. Lifestyle modification should be re-enforced through continuous education.
There is a need for long-term well designed prospective studies in kidney donors with PD to know the long-term risk of diabetes and the various complications associated with PD.
It will be interesting to assess the efficacy of sodium-glucose cotransporter-2, glucagon like peptide 1, mineralocorticoid receptor antagonist and renin angiotensin inhibitors in kidney donors with PD and the effect of this on long term renal and cardiovascular outcomes.
Most of the knowledge regarding PD and the risk of DM and other complications is derived from studies done in the general population. Unfortunately, there is limited work done in kidney donors with PD. Most of these studies are retrospective in nature, with a small sample size and a shorter follow up. As a result, this is one of the limitations of our review.
The global prevalence of PD is high. PD increases the risk of DM, CKD, cardiovascular events, stroke, neuropathy, retinopathy, dementia, depression, cancer, non-alcoholic fatty liver disease and increases all-cause mortality. Increasing age, obesity, smoking, certain ethnicities, gestational diabetes, metabolic syndrome and a family history of diabetes make it riskier for prediabetics to donate. There is limited research on the impact of PD in kidney donors and there is a need for prospective long term follow up studies. The combination of IFG and IGT has greater association with CKD, cardiovas
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Batta A, India; Phillips R, United Kingdom S-Editor: Li L L-Editor: A P-Editor: Zhao S
| 1. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2129] [Article Influence: 425.8] [Reference Citation Analysis (0)] |
| 2. | Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ, Magliano DJ, Selvin E. Global Prevalence of Prediabetes. Diabetes Care. 2023;46:1388-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 228] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 3. | Bullard KM, Saydah SH, Imperatore G, Cowie CC, Gregg EW, Geiss LS, Cheng YJ, Rolka DB, Williams DE, Caspersen CJ. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999-2010. Diabetes Care. 2013;36:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G; 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 2168] [Article Influence: 180.7] [Reference Citation Analysis (0)] |
| 5. | Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of Type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990-2000. Diabet Med. 2007;24:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B; American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 968] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 7. | Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM, Wong TY, McNeil J, Shaw JE. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 8. | Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 637] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 9. | Jeon HJ, Bae HJ, Ham YR, Choi DE, Na KR, Ahn MS, Lee KW. Outcomes of end-stage renal disease patients on the waiting list for deceased donor kidney transplantation: A single-center study. Kidney Res Clin Pract. 2019;38:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 881] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 11. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3856] [Article Influence: 148.3] [Reference Citation Analysis (1)] |
| 12. | Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 705] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 13. | Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Øyen O, Reisæter A, Pfeffer P, Jenssen T, Leivestad T, Line PD, Øvrehus M, Dale DO, Pihlstrøm H, Holme I, Dekker FW, Holdaas H. Long-term risks for kidney donors. Kidney Int. 2014;86:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 574] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 14. | Chandran S, Masharani U, Webber AB, Wojciechowski DM. Prediabetic living kidney donors have preserved kidney function at 10 years after donation. Transplantation. 2014;97:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 1017] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | Brands MW, Bell TD, Rodriquez NA, Polavarapu P, Panteleyev D. Chronic glucose infusion causes sustained increases in tubular sodium reabsorption and renal blood flow in dogs. Am J Physiol Regul Integr Comp Physiol. 2009;296:R265-R271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Palatini P, Mormino P, Dorigatti F, Santonastaso M, Mos L, De Toni R, Winnicki M, Dal Follo M, Biasion T, Garavelli G, Pessina AC; HARVEST Study Group. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. 2006;70:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Pruijm M, Wuerzner G, Maillard M, Bovet P, Renaud C, Bochud M, Burnier M. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol Dial Transplant. 2010;25:2225-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Melsom T, Mathisen UD, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I, Eriksen BO. Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care. 2011;34:1546-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Markus MRP, Ittermann T, Baumeister SE, Huth C, Thorand B, Herder C, Roden M, Siewert-Markus U, Rathmann W, Koenig W, Dörr M, Völzke H, Schipf S, Meisinger C. Prediabetes is associated with microalbuminuria, reduced kidney function and chronic kidney disease in the general population: The KORA (Cooperative Health Research in the Augsburg Region) F4-Study. Nutr Metab Cardiovasc Dis. 2018;28:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Franciosi M, Pellegrini F, Sacco M, De Berardis G, Rossi MC, Strippoli GF, Belfiglio M, Tognoni G, Valentini M, Nicolucci A; IGLOO (Impaired Glucose tolerance, and Long-term Outcomes Observational Study) Study Group. Identifying patients at risk for microalbuminuria via interaction of the components of the metabolic syndrome: a cross-sectional analytic study. Clin J Am Soc Nephrol. 2007;2:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Tapp RJ, Shaw JE, Zimmet PZ, Balkau B, Chadban SJ, Tonkin AM, Welborn TA, Atkins RC. Albuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am J Kidney Dis. 2004;44:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Sarafidis PA, Bakris GL. Microalbuminuria and chronic kidney disease as risk factors for cardiovascular disease. Nephrol Dial Transplant. 2006;21:2366-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Mac-Moune Lai F, Szeto CC, Choi PC, Ho KK, Tang NL, Chow KM, Li PK, To KF. Isolate diffuse thickening of glomerular capillary basement membrane: a renal lesion in prediabetes? Mod Pathol. 2004;17:1506-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Bangstad HJ, Osterby R, Dahl-Jørgensen K, Berg KJ, Hartmann A, Nyberg G, Frahm Bjørn S, Hanssen KF. Early glomerulopathy is present in young, type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1993;36:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Bangstad HJ, Østerby R, Rudberg S, Hartmann A, Brabrand K, Hanssen KF. Kidney function and glomerulopathy over 8 years in young patients with Type I (insulin-dependent) diabetes mellitus and microalbuminuria. Diabetologia. 2002;45:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Garg AX, Muirhead N, Knoll G, Yang RC, Prasad GV, Thiessen-Philbrook H, Rosas-Arellano MP, Housawi A, Boudville N; Donor Nephrectomy Outcomes Research (DONOR) Network. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Gourishankar S, Courtney M, Jhangri GS, Cembrowski G, Pannu N. Serum cystatin C performs similarly to traditional markers of kidney function in the evaluation of donor kidney function prior to and following unilateral nephrectomy. Nephrol Dial Transplant. 2008;23:3004-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Anjum S, Muzaale AD, Massie AB, Bae S, Luo X, Grams ME, Lentine KL, Garg AX, Segev DL. Patterns of End-Stage Renal Disease Caused by Diabetes, Hypertension, and Glomerulonephritis in Live Kidney Donors. Am J Transplant. 2016;16:3540-3547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11:1650-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Steiner RW, Ix JH, Rifkin DE, Gert B. Estimating risks of de novo kidney diseases after living kidney donation. Am J Transplant. 2014;14:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Fehrman-Ekholm I, Nordén G, Lennerling A, Rizell M, Mjörnstedt L, Wramner L, Olausson M. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82:1646-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Okamoto M, Suzuki T, Fujiki M, Nobori S, Ushigome H, Sakamoto S, Yoshimura N. The consequences for live kidney donors with preexisting glucose intolerance without diabetic complication: analysis at a single Japanese center. Transplantation. 2010;89:1391-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Hebert SA, Murad DN, Nguyen DT, Graviss EA, Adrogue HE, Matas AJ, Ibrahim HN. Outcomes of Kidney Donors With Impaired Fasting Glucose. Transplantation. 2022;106:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1935] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 36. | Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5:49-56. [PubMed] |
| 37. | Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1141] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 38. | Fox CS, Larson MG, Leip EP, Meigs JB, Wilson PW, Levy D. Glycemic status and development of kidney disease: the Framingham Heart Study. Diabetes Care. 2005;28:2436-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Selvin E, Ning Y, Steffes MW, Bash LD, Klein R, Wong TY, Astor BC, Sharrett AR, Brancati FL, Coresh J. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes. 2011;60:298-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Plantinga LC, Crews DC, Coresh J, Miller ER 3rd, Saran R, Yee J, Hedgeman E, Pavkov M, Eberhardt MS, Williams DE, Powe NR; CDC CKD Surveillance Team. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 41. | Redon J, Morales-Olivas F, Galgo A, Brito MA, Mediavilla J, Marín R, Rodríguez P, Tranche S, Lozano JV, Filozof C; MAGAL Group. Urinary albumin excretion and glomerular filtration rate across the spectrum of glucose abnormalities in essential hypertension. J Am Soc Nephrol. 2006;17:S236-S245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Schöttker B, Brenner H, Koenig W, Müller H, Rothenbacher D. Prognostic association of HbA1c and fasting plasma glucose with reduced kidney function in subjects with and without diabetes mellitus. Results from a population-based cohort study from Germany. Prev Med. 2013;57:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Bigotte Vieira M, Neves JS, Leitão L, Baptista RB, Magriço R, Viegas Dias C, Oliveira A, Carvalho D, Mc Causland FR. Impaired Fasting Glucose and Chronic Kidney Disease, Albuminuria, or Worsening Kidney Function: A Secondary Analysis of SPRINT. J Clin Endocrinol Metab. 2019;104:4024-4032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Furukawa M, Onoue T, Kato K, Wada T, Shinohara Y, Kinoshita F, Goto M, Arima H, Tsushita K. Prediabetes is associated with proteinuria development but not with glomerular filtration rate decline: A longitudinal observational study. Diabet Med. 2021;38:e14607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016;33:1615-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 46. | Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W, Roden M, Herder C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65:275-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 47. | Gottwald-Hostalek U, Gwilt M. Vascular complications in prediabetes and type 2 diabetes: a continuous process arising from a common pathology. Curr Med Res Opin. 2022;38:1841-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3393] [Article Influence: 484.7] [Reference Citation Analysis (0)] |
| 49. | World Health Organization; International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF Consultation. 2006. [cited 22 August 2023]. Available from: https://apps.who.int/iris/handle/10665/43588. |
| 50. | Genuth S. Classification and diagnosis of diabetes mellitus. Med Clin North Am. 1982;66:1191-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 52. | Lee CMY, Colagiuri S, Woodward M, Gregg EW, Adams R, Azizi F, Gabriel R, Gill TK, Gonzalez C, Hodge A, Jacobs Jr DR Jr, Joseph JJ, Khalili D, Magliano DJ, Mehlig K, Milne R, Mishra G, Mongraw-Chaffin M, Pasco JA, Sakurai M, Schreiner PJ, Selvin E, Shaw JE, Wittert G, Yatsuya H, Huxley RR. Comparing different definitions of prediabetes with subsequent risk of diabetes: an individual participant data meta-analysis involving 76 513 individuals and 8208 cases of incident diabetes. BMJ Open Diabetes Res Care. 2019;7:e000794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Fehrman-Ekholm I, Dunér F, Brink B, Tydén G, Elinder CG. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 2001;72:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 54. | Ramcharan T, Matas AJ. Long-term (20-37 years) follow-up of living kidney donors. Am J Transplant. 2002;2:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Iglesias-Márquez RA, Calderón S, Santiago-Delpín EA, Rivé-Mora E, González-Caraballo Z, Morales-Otero L. The health of living kidney donors 20 years after donation. Transplant Proc. 2001;33:2041-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Eberhard OK, Kliem V, Offner G, Oldhafer K, Fangmann J, Pichlmay R, Koch KM, Brunkhorst R. Assessment of long-term risks for living related kidney donors by 24-h blood pressure monitoring and testing for microalbuminuria. Clin Transplant. 1997;11:415-419. [PubMed] |
| 57. | Hakim RM, Goldszer RC, Brenner BM. Hypertension and proteinuria: long-term sequelae of uninephrectomy in humans. Kidney Int. 1984;25:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 236] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Miller IJ, Suthanthiran M, Riggio RR, Williams JJ, Riehle RA, Vaughan ED, Stubenbord WT, Mouradian J, Cheigh JS, Stenzel KH. Impact of renal donation. Long-term clinical and biochemical follow-up of living donors in a single center. Am J Med. 1985;79:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Talseth T, Fauchald P, Skrede S, Djøseland O, Berg KJ, Stenstrøm J, Heilo A, Brodwall EK, Flatmark A. Long-term blood pressure and renal function in kidney donors. Kidney Int. 1986;29:1072-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Undurraga A, Roessler E, Arcos O, González F, Espinoza O, Herrera S, Ayala A, Reynolds E, Espinoza M, Hidalgo F. Long-term follow-up of renal donors. Transplant Proc. 1998;30:2283-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Watnick TJ, Jenkins RR, Rackoff P, Baumgarten A, Bia MJ. Microalbuminuria and hypertension in long-term renal donors. Transplantation. 1988;45:59-65. [PubMed] |
| 62. | Williams SL, Oler J, Jorkasky DK. Long-term renal function in kidney donors: a comparison of donors and their siblings. Ann Intern Med. 1986;105:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Haugen AJ, Hallan S, Langberg NE, Dahle DO, Pihlstrøm H, Birkeland KI, Reisaeter A, Midtvedt K, Hartmann A, Holdaas H, Mjøen G. Increased long-term risk for hypertension in kidney donors - a retrospective cohort study. Transpl Int. 2020;33:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Holscher CM, Haugen CE, Jackson KR, Garonzik Wang JM, Waldram MM, Bae S, Locke JE, Reed RD, Lentine KL, Gupta G, Weir MR, Friedewald JJ, Verbesey J, Cooper M, Segev DL, Massie AB. Self-Reported Incident Hypertension and Long-Term Kidney Function in Living Kidney Donors Compared with Healthy Nondonors. Clin J Am Soc Nephrol. 2019;14:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC, Rosas-Arellano MP, Housawi A, Garg AX; Donor Nephrectomy Outcomes Research (DONOR) Network. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 66. | Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1358] [Cited by in RCA: 1269] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 67. | Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, Atkins R, Badawi A, Ballew SH, Banerjee A, Barregård L, Barrett-Connor E, Basu S, Bello AK, Bensenor I, Bergstrom J, Bikbov B, Blosser C, Brenner H, Carrero JJ, Chadban S, Cirillo M, Cortinovis M, Courville K, Dandona L, Dandona R, Estep K, Fernandes J, Fischer F, Fox C, Gansevoort RT, Gona PN, Gutierrez OM, Hamidi S, Hanson SW, Himmelfarb J, Jassal SK, Jee SH, Jha V, Jimenez-Corona A, Jonas JB, Kengne AP, Khader Y, Khang YH, Kim YJ, Klein B, Klein R, Kokubo Y, Kolte D, Lee K, Levey AS, Li Y, Lotufo P, El Razek HMA, Mendoza W, Metoki H, Mok Y, Muraki I, Muntner PM, Noda H, Ohkubo T, Ortiz A, Perico N, Polkinghorne K, Al-Radaddi R, Remuzzi G, Roth G, Rothenbacher D, Satoh M, Saum KU, Sawhney M, Schöttker B, Shankar A, Shlipak M, Silva DAS, Toyoshima H, Ukwaja K, Umesawa M, Vollset SE, Warnock DG, Werdecker A, Yamagishi K, Yano Y, Yonemoto N, Zaki MES, Naghavi M, Forouzanfar MH, Murray CJL, Coresh J, Vos T; Global Burden of Disease 2013 GFR Collaborators; CKD Prognosis Consortium; Global Burden of Disease Genitourinary Expert Group. Global Cardiovascular and Renal Outcomes of Reduced GFR. J Am Soc Nephrol. 2017;28:2167-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 68. | Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1728] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 69. | Haugen AJ, Hallan S, Langberg NE, Dahle DO, Pihlstrøm H, Birkeland KI, Reisæter AV, Midtvedt K, Hartmann A, Holdaas H, Mjøen G. Increased risk of ischaemic heart disease after kidney donation. Nephrol Dial Transplant. 2022;37:928-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Garg AX, Meirambayeva A, Huang A, Kim J, Prasad GV, Knoll G, Boudville N, Lok C, McFarlane P, Karpinski M, Storsley L, Klarenbach S, Lam N, Thomas SM, Dipchand C, Reese P, Doshi M, Gibney E, Taub K, Young A; Donor Nephrectomy Outcomes Research Network. Cardiovascular disease in kidney donors: matched cohort study. BMJ. 2012;344:e1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Garg AX, Prasad GV, Thiessen-Philbrook HR, Ping L, Melo M, Gibney EM, Knoll G, Karpinski M, Parikh CR, Gill J, Storsley L, Vlasschaert M, Mamdani M; Donor Nephrectomy Outcomes Research (DONOR) Network. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation. 2008;86:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 72. | Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Coronary-heart-disease risk and impaired glucose tolerance. The Whitehall study. Lancet. 1980;1:1373-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 481] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 73. | Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 820] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 74. | Færch K, Vistisen D, Johansen NB, Jørgensen ME. Cardiovascular risk stratification and management in pre-diabetes. Curr Diab Rep. 2014;14:493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Brannick B, Dagogo-Jack S. Prediabetes and Cardiovascular Disease: Pathophysiology and Interventions for Prevention and Risk Reduction. Endocrinol Metab Clin North Am. 2018;47:33-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 76. | DECODE Study Group; the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1050] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 77. | Ning F, Tuomilehto J, Pyörälä K, Onat A, Söderberg S, Qiao Q; DECODE Study Group. Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care. 2010;33:2211-2216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Qiao Q, Pyörälä K, Pyörälä M, Nissinen A, Lindström J, Tilvis R, Tuomilehto J. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J. 2002;23:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | DECODA Study Group; International Diabetes Epidemiology Group. Cardiovascular risk profile assessment in glucose-intolerant Asian individuals--an evaluation of the World Health Organization two-step strategy: the DECODA Study (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia). Diabet Med. 2002;19:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 80. | Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 677] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 81. | Fonville S, Zandbergen AA, Koudstaal PJ, den Hertog HM. Prediabetes in patients with stroke or transient ischemic attack: prevalence, risk and clinical management. Cerebrovasc Dis. 2014;37:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Vermeer SE, Sandee W, Algra A, Koudstaal PJ, Kappelle LJ, Dippel DW; Dutch TIA Trial Study Group. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke. 2006;37:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Qureshi AI, Giles WH, Croft JB. Impaired glucose tolerance and the likelihood of nonfatal stroke and myocardial infarction: the Third National Health and Nutrition Examination Survey. Stroke. 1998;29:1329-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;344:e3564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 85. | Pfannkuche A, Alhajjar A, Ming A, Walter I, Piehler C, Mertens PR. Prevalence and risk factors of diabetic peripheral neuropathy in a diabetics cohort: Register initiative "diabetes and nerves". Endocr Metab Sci. 2020;1:100053. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 86. | Katon JG, Reiber GE, Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999-2004. Diabetes Care. 2013;36:1604-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Bongaerts BW, Rathmann W, Kowall B, Herder C, Stöckl D, Meisinger C, Ziegler D. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35:1891-1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 89. | Lee CC, Perkins BA, Kayaniyil S, Harris SB, Retnakaran R, Gerstein HC, Zinman B, Hanley AJ. Peripheral Neuropathy and Nerve Dysfunction in Individuals at High Risk for Type 2 Diabetes: The PROMISE Cohort. Diabetes Care. 2015;38:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 90. | Kirthi V, Perumbalath A, Brown E, Nevitt S, Petropoulos IN, Burgess J, Roylance R, Cuthbertson DJ, Jackson TL, Malik RA, Alam U. Prevalence of peripheral neuropathy in pre-diabetes: a systematic review. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 91. | Nagi DK, Pettitt DJ, Bennett PH, Klein R, Knowler WC. Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Diabet Med. 1997;14:449-456. [PubMed] [DOI] [Full Text] |
| 92. | Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 93. | Kirthi V, Nderitu P, Alam U, Evans JR, Nevitt S, Malik RA, Hopkins D, Jackson TL. The prevalence of retinopathy in prediabetes: A systematic review. Surv Ophthalmol. 2022;67:1332-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Lott ME, Slocomb JE, Shivkumar V, Smith B, Quillen D, Gabbay RA, Gardner TW, Bettermann K. Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Acta Ophthalmol. 2013;91:e462-e469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003;121:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 96. | D'Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol. 1996;13:227-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 337] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 97. | Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Tang J, Pei Y, Zhou G. When aging-onset diabetes is coming across with Alzheimer disease: comparable pathogenesis and therapy. Exp Gerontol. 2013;48:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Stolk RP, Breteler MM, Ott A, Pols HA, Lamberts SW, Grobbee DE, Hofman A. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care. 1997;20:792-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 100. | Stolk RP, Pols HA, Lamberts SW, de Jong PT, Hofman A, Grobbee DE. Diabetes mellitus, impaired glucose tolerance, and hyperinsulinemia in an elderly population. The Rotterdam Study. Am J Epidemiol. 1997;145:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: A population-based cohort study. Alzheimers Dement. 2019;15:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 102. | Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 357] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 103. | van Agtmaal MJM, Houben AJHM, de Wit V, Henry RMA, Schaper NC, Dagnelie PC, van der Kallen CJ, Koster A, Sep SJ, Kroon AA, Jansen JFA, Hofman PA, Backes WH, Schram MT, Stehouwer CDA. Prediabetes Is Associated With Structural Brain Abnormalities: The Maastricht Study. Diabetes Care. 2018;41:2535-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 104. | Casagrande SS, Lee C, Stoeckel LE, Menke A, Cowie CC. Cognitive function among older adults with diabetes and prediabetes, NHANES 2011-2014. Diabetes Res Clin Pract. 2021;178:108939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 105. | Dybjer E, Nilsson PM, Engström G, Helmer C, Nägga K. Pre-diabetes and diabetes are independently associated with adverse cognitive test results: a cross-sectional, population-based study. BMC Endocr Disord. 2018;18:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | Topaloğlu US, Erol K. Fatigue, anxiety and depression in patients with prediabetes: a controlled cross-sectional study. Diabetol Int. 2022;13:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 107. | Burns RJ, Deschênes SS, Schmitz N. Associations Between Depressive Symptoms and Indices of Obesity in Adults With Prediabetes and Normal Blood Glucose Levels: Results From the Emotional Health and Wellbeing Study. Can J Diabetes. 2018;42:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, Pothos EN, Kahn CR. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A. 2015;112:3463-3468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 109. | Chen S, Zhang Q, Dai G, Hu J, Zhu C, Su L, Wu X. Association of depression with pre-diabetes, undiagnosed diabetes, and previously diagnosed diabetes: a meta-analysis. Endocrine. 2016;53:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 110. | Nouwen A, Nefs G, Caramlau I, Connock M, Winkley K, Lloyd CE, Peyrot M, Pouwer F; European Depression in Diabetes Research Consortium. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care. 2011;34:752-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 111. | Deschênes SS, Burns RJ, Graham E, Schmitz N. Prediabetes, depressive and anxiety symptoms, and risk of type 2 diabetes: A community-based cohort study. J Psychosom Res. 2016;89:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 112. | Graham E, Au B, Schmitz N. Depressive symptoms, prediabetes, and incident diabetes in older English adults. Int J Geriatr Psychiatry. 2017;32:1450-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Jiang L, Atasoy S, Johar H, Herder C, Peters A, Kruse J, Ladwig KH. Anxiety boosts progression of prediabetes to type 2 diabetes: findings from the prospective Cooperative Health Research in the Region of Augsburg F4 and FF4 studies. Diabet Med. 2020;37:1737-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 114. | Bhurtyal N, Paudel K, Shah S, Paudel S, Kafle MP, Shah DS. Anxiety and depression among living kidney donors in tertiary care hospital of low resource country setting Nepal. Ann Med Surg (Lond). 2022;80:104119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 115. | Holscher CM, Leanza J, Thomas AG, Waldram MM, Haugen CE, Jackson KR, Bae S, Massie AB, Segev DL. Anxiety, depression, and regret of donation in living kidney donors. BMC Nephrol. 2018;19:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Wirken L, van Middendorp H, Hooghof CW, Sanders JF, Dam RE, van der Pant KAMI, Wierdsma JM, Wellink H, van Duijnhoven EM, Hoitsma AJ, Hilbrands LB, Evers AWM. Psychosocial consequences of living kidney donation: a prospective multicentre study on health-related quality of life, donor-recipient relationships and regret. Nephrol Dial Transplant. 2019;34:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 117. | Lambe M, Wigertz A, Garmo H, Walldius G, Jungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control. 2011;22:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 118. | Yun JE, Jo I, Park J, Kim MT, Ryu HG, Odongua N, Kim E, Jee SH. Cigarette smoking, elevated fasting serum glucose, and risk of pancreatic cancer in Korean men. Int J Cancer. 2006;119:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Zhou XH, Qiao Q, Zethelius B, Pyörälä K, Söderberg S, Pajak A, Stehouwer CD, Heine RJ, Jousilahti P, Ruotolo G, Nilsson PM, Calori G, Tuomilehto J; DECODE Study Group. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53:1867-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 120. | Ke J, Lin T, Liu X, Wu K, Ruan X, Ding Y, Liu W, Qiu H, Tan X, Wang X, Chen X, Li Z, Cao G. Glucose Intolerance and Cancer Risk: A Community-Based Prospective Cohort Study in Shanghai, China. Front Oncol. 2021;11:726672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, Huang Y. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57:2261-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 122. | Lee SC, Chan JC. Evidence for DNA damage as a biological link between diabetes and cancer. Chin Med J (Engl). 2015;128:1543-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 123. | Engels EA, Fraser GE, Kasiske BL, Snyder JJ, Utt J, Lynch CF, Li J, Pawlish KS, Brown S, Yu KJ, Pfeiffer RM. Cancer risk in living kidney donors. Am J Transplant. 2022;22:2006-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 124. | Vesa CM, Behl T, Nemeth S, Bratu OG, Diaconu CC, Moleriu RD, Negrut N, Zaha DC, Bustea C, Radu FI, Bungau S. Prediction of NAFLD occurrence in prediabetes patients. Exp Ther Med. 2020;20:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Le P, Chaitoff A, Rothberg MB, Alkhouri N, McCullough A. Trends in Prevalence of Nonalcoholic Fatty Liver Disease in US Adults with Prediabetes. J Gen Intern Med. 2019;34:2336-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Pappachan JM, Babu S, Krishnan B, Ravindran NC. Non-alcoholic Fatty Liver Disease: A Clinical Update. J Clin Transl Hepatol. 2017;5:384-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 127. | Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 128. | Hossain IA, Akter S, Bhuiyan FR, Shah MR, Rahman MK, Ali L. Subclinical inflammation in relation to insulin resistance in prediabetic subjects with nonalcoholic fatty liver disease. BMC Res Notes. 2016;9:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Cen J, Han Y, Liu Y, Hu H. Evaluated Glomerular Filtration Rate Is Associated With Non-alcoholic Fatty Liver Disease: A 5-Year Longitudinal Cohort Study in Chinese Non-obese People. Front Nutr. 2022;9:916704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 130. | Katchman H, Zelber-Sagi S, Baruch R, Berman G, Schwartz IF, Schwartz D, Nakache R, Goykhman Y, Katz P, Shibolet O, Shashar M, Grupper A. Progression and new onset of nonalcoholic fatty liver disease in living kidney donors compared to healthy controls. Clin Transplant. 2018;32:e13240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 131. | Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, Yang Y, Hu Y, Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 409] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 132. | Islam Z, Akter S, Inoue Y, Hu H, Kuwahara K, Nakagawa T, Honda T, Yamamoto S, Okazaki H, Miyamoto T, Ogasawara T, Sasaki N, Uehara A, Yamamoto M, Kochi T, Eguchi M, Shirasaka T, Shimizu M, Nagahama S, Hori A, Imai T, Nishihara A, Tomita K, Sone T, Konishi M, Kabe I, Mizoue T, Dohi S; Japan Epidemiology Collaboration on Occupational Health Study Group. Prediabetes, Diabetes, and the Risk of All-Cause and Cause-Specific Mortality in a Japanese Working Population: Japan Epidemiology Collaboration on Occupational Health Study. Diabetes Care. 2021;44:757-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 133. | Cao Z, Li W, Wen CP, Li S, Chen C, Jia Q, Zhang W, Tu H, Wu X. Risk of Death Associated With Reversion From Prediabetes to Normoglycemia and the Role of Modifiable Risk Factors. JAMA Netw Open. 2023;6:e234989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 134. | Kowall B, Lehnich AT, Strucksberg KH, Führer D, Erbel R, Jankovic N, Moebus S, Jöckel KH, Stang A. Associations among sleep disturbances, nocturnal sleep duration, daytime napping, and incident prediabetes and type 2 diabetes: the Heinz Nixdorf Recall Study. Sleep Med. 2016;21:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 135. | Wang HB, Yan WH, Dou JT, Lu ZH, Wang BA, Mu YM. Association between Self-reported Snoring and Prediabetes among Adults Aged 40 Years and Older without Diabetes. Chin Med J (Engl). 2017;130:791-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Kim NH, Cho NH, Yun CH, Lee SK, Yoon DW, Cho HJ, Ahn JH, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Shin C. Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care. 2013;36:3909-3915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Gagnon C, Magliano DJ, Ebeling PR, Dunstan DW, Zimmet PZ, Shaw JE, Daly RM. Association between hyperglycaemia and fracture risk in non-diabetic middle-aged and older Australians: a national, population-based prospective study (AusDiab). Osteoporos Int. 2010;21:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 138. | Zaccardi F, Rocca B, Pitocco D, Tanese L, Rizzi A, Ghirlanda G. Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: a meta-analysis. Diabetes Metab Res Rev. 2015;31:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 139. | Yan Z, Cai M, Han X, Chen Q, Lu H. The Interaction Between Age and Risk Factors for Diabetes and Prediabetes: A Community-Based Cross-Sectional Study. Diabetes Metab Syndr Obes. 2023;16:85-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 140. | Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, Zhang X, Li C, Huang Z, Sun X, Wang L, Zhou M, Wu J, Wang Y. Prevalence and Treatment of Diabetes in China, 2013-2018. JAMA. 2021;326:2498-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 558] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 141. | Suastika K, Dwipayana P, Semadi MS, Kuswardhani RAT. Age is an Important Risk Factor for Type 2 Diabetes Mellitus and Cardiovascular Diseases. In: Chackrewarthy S. Glucose Tolerance. London: InTech, 2012. [DOI] [Full Text] |
| 142. | Rooney MR, Rawlings AM, Pankow JS, Echouffo Tcheugui JB, Coresh J, Sharrett AR, Selvin E. Risk of Progression to Diabetes Among Older Adults With Prediabetes. JAMA Intern Med. 2021;181:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 143. | Shang Y, Marseglia A, Fratiglioni L, Welmer AK, Wang R, Wang HX, Xu W. Natural history of prediabetes in older adults from a population-based longitudinal study. J Intern Med. 2019;286:326-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 144. | Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG; American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Am J Clin Nutr. 2004;80:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 145. | Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 797] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 146. | Miao Z, Alvarez M, Ko A, Bhagat Y, Rahmani E, Jew B, Heinonen S, Muñoz-Hernandez LL, Herrera-Hernandez M, Aguilar-Salinas C, Tusie-Luna T, Mohlke KL, Laakso M, Pietiläinen KH, Halperin E, Pajukanta P. The causal effect of obesity on prediabetes and insulin resistance reveals the important role of adipose tissue in insulin resistance. PLoS Genet. 2020;16:e1009018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 147. | Bugeja A, Harris S, Ernst J, Burns KD, Knoll G, Clark EG. Changes in Body Weight Before and After Kidney Donation. Can J Kidney Health Dis. 2019;6:2054358119847203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 148. | Praga M, Hernández E, Herrero JC, Morales E, Revilla Y, Díaz-González R, Rodicio JL. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000;58:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 226] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 149. | Nogueira JM, Weir MR, Jacobs S, Haririan A, Breault D, Klassen D, Evans D, Bartlett ST, Cooper M. A study of renal outcomes in African American living kidney donors. Transplantation. 2009;88:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 150. | Bergman BC, Perreault L, Hunerdosse D, Kerege A, Playdon M, Samek AM, Eckel RH. Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes. 2012;61:3156-3166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 151. | Houston TK, Person SD, Pletcher MJ, Liu K, Iribarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ. 2006;332:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 152. | Aeschbacher S, Schoen T, Clair C, Schillinger P, Schönenberger S, Risch M, Risch L, Conen D. Association of smoking and nicotine dependence with pre-diabetes in young and healthy adults. Swiss Med Wkly. 2014;144:w14019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 153. | Centers for Disease Control and Prevention. Smoking and Diabetes. May 5, 2022. [Cited 5 January 2024]. Available From: https://www.cdc.gov/tobacco/campaign/tips/diseases/diabetes.html. |
| 154. | Rimm EB, Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Hennekens CH, Speizer FE. Cigarette smoking and the risk of diabetes in women. Am J Public Health. 1993;83:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 155. | Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 361] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 156. | Śliwińska-Mossoń M, Milnerowicz H. The impact of smoking on the development of diabetes and its complications. Diab Vasc Dis Res. 2017;14:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 157. | Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res. 2017;184:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 158. | Heldt J, Torrey R, Han D, Baron P, Tenggardjaja C, McLarty J, Lindler T, Baldwin DD. Donor Smoking Negatively Affects Donor and Recipient Renal Function following Living Donor Nephrectomy. Adv Urol. 2011;2011:929263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 159. | Khalil MAM, Tan J, Khamis S, Khalil MA, Azmat R, Ullah AR. Cigarette Smoking and Its Hazards in Kidney Transplantation. Adv Med. 2017;2017:6213814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 160. | Parker ED, Lin J, Mahoney T, Ume N, Yang G, Gabbay RA, ElSayed NA, Bannuru RR. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care. 2024;47:26-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 247] [Article Influence: 247.0] [Reference Citation Analysis (0)] |
| 161. | NHS Health and Social Care Information Centre, Public Health Statistics. Health Survey for England - 2004: Health of ethnic minorities, Headline results. In: Part of Health Survey for England, Country, Regions, Strategic Health Authorities. England: NHS, 2006. |
| 162. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 8961] [Article Influence: 426.7] [Reference Citation Analysis (1)] |
| 163. | Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 1014] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 164. | Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2141] [Cited by in RCA: 2333] [Article Influence: 145.8] [Reference Citation Analysis (1)] |
| 165. | Herath H, Herath R, Wickremasinghe R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women-A community based retrospective cohort study. PLoS One. 2017;12:e0179647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 166. | Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1541] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 167. | Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009;181:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 168. | Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 169. | Bellos I, Pergialiotis V. Risk of pregnancy complications in living kidney donors: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2022;270:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 170. | Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3603] [Cited by in RCA: 3666] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 171. | Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care. 2002;25:1790-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 309] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 172. | Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1034] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 173. | Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 454] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 174. | Salminen M, Kuoppamäki M, Vahlberg T, Räihä I, Irjala K, Kivelä SL. Metabolic syndrome defined by modified International Diabetes Federation criteria and type 2 diabetes mellitus risk: a 9-year follow-up among the aged in Finland. Diab Vasc Dis Res. 2013;10:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 175. | Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1298] [Cited by in RCA: 1367] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 176. | Kang E, Park J, Kim HJ, Park S, Park M, Kim Y, Kim K, Park SM, Chae DW, Chin HJ, Lee JP, Lee S, Kim SW, Cho JH, Han M, Kim YC, Kim YS, Choi I, Lee H. Metabolic risks in living kidney donors in South Korea. Kidney Res Clin Pract. 2021;40:645-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 177. | Grupper A, Angel Y, Baruch A, Schwartz IF, Schwartz D, Nakache R, Goykhman Y, Katz P, Nachmany I, Lubezky N, Weinstein T, Shashar M, Ben-Bassat OK, Berliner S, Rogowski O, Zeltser D, Shapira I, Shenhar-Tsarfaty S. Long term metabolic and renal outcomes of kidney donors compared to controls with excellent kidney function. BMC Nephrol. 2019;20:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 178. | Martín-Alemañy G, Pérez-Navarro M, Rosas-Herrera A, Hinojosa-Heredia H, Fuentes-Méndez L, Valdez-Ortiz R. Changes in cardiometabolic risk factors and metabolic syndrome over time in living kidney donors: a retrospective cohort study. Nutr Hosp. 2021;38:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 179. | López Y López LR, Martínez González J, Bahena Méndez J, Espinoza-Peralta D, Campos Nolasco NP, Curiel Hernández RE, Sebastián Díaz MA, Wasung de Lay M, Vázquez Dávila RA, Carmona-Escamilla MA. Metabolic Syndrome, a Real Barrier for Living Kidney Donor Transplant. Transplant Proc. 2020;52:1072-1076. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 180. | Annis AM, Caulder MS, Cook ML, Duquette D. Family history, diabetes, and other demographic and risk factors among participants of the National Health and Nutrition Examination Survey 1999-2002. Prev Chronic Dis. 2005;2:A19. [PubMed] |
| 181. | Scheuner MT, Wang SJ, Raffel LJ, Larabell SK, Rotter JI. Family history: a comprehensive genetic risk assessment method for the chronic conditions of adulthood. Am J Med Genet. 1997;71:315-324. [PubMed] [DOI] [Full Text] |
| 182. | Zhang J, Yang Z, Xiao J, Xing X, Lu J, Weng J, Jia W, Ji L, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Lin L, Wang N, Yang W; China National Diabetes and Metabolic Disorders Study Group. Association between family history risk categories and prevalence of diabetes in Chinese population. PLoS One. 2015;10:e0117044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 183. | InterAct Consortium; Scott RA, Langenberg C, Sharp SJ, Franks PW, Rolandsson O, Drogan D, van der Schouw YT, Ekelund U, Kerrison ND, Ardanaz E, Arriola L, Balkau B, Barricarte A, Barroso I, Bendinelli B, Beulens JW, Boeing H, de Lauzon-Guillain B, Deloukas P, Fagherazzi G, Gonzalez C, Griffin SJ, Groop LC, Halkjaer J, Huerta JM, Kaaks R, Khaw KT, Krogh V, Nilsson PM, Norat T, Overvad K, Panico S, Rodriguez-Suarez L, Romaguera D, Romieu I, Sacerdote C, Sánchez MJ, Spijkerman AM, Teucher B, Tjonneland A, Tumino R, van der A DL, Wark PA, McCarthy MI, Riboli E, Wareham NJ. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia. 2013;56:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 184. | Choi J, Choi JY, Lee SA, Lee KM, Shin A, Oh J, Park J, Song M, Yang JJ, Lee JK, Kang D. Association between family history of diabetes and clusters of adherence to healthy behaviors: cross-sectional results from the Health Examinees-Gem (HEXA-G) study. BMJ Open. 2019;9:e025477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 185. | Delmonico F; Council of the Transplantation Society. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79:S53-S66. [PubMed] |
| 186. | Kanellis J; CARI. The CARI guidelines. Justification for living donor kidney transplantation. Nephrology (Carlton). 2010;15:S72-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 187. | American Society of Transplantation. Chapter 05: Kidney donation for people with pre-diabetes. In: Living Kidney donation: Medical Toolkit. 2023. [cited 3 January 2024]. Available from: https://www.myast.org/cdn/farfuture/Kot4K2avIGZ1L6mY3_PU5uEtV8t34Mu60f8sN2YymFI/mtime:1516737263/sites/default/files/pdf/Ch%205_AST-18-LiveDonorToolKit-Medical-Chapter5-D3.pdf. |
| 188. | Andrews PA, Burnapp L. British Transplantation Society / Renal Association UK Guidelines for Living Donor Kidney Transplantation 2018: Summary of Updated Guidance. Transplantation. 2018;102:e307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 189. | Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ. 2009;338:b880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 190. | Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberú J, Bakr MA, Gallon L, Garvey CA, Guleria S, Li PK, Segev DL, Taler SJ, Tanabe K, Wright L, Zeier MG, Cheung M, Garg AX. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101:S1-S109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 191. | Abramowicz D, Cochat P, Claas FH, Heemann U, Pascual J, Dudley C, Harden P, Hourmant M, Maggiore U, Salvadori M, Spasovski G, Squifflet JP, Steiger J, Torres A, Viklicky O, Zeier M, Vanholder R, Van Biesen W, Nagler E. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant. 2015;30:1790-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 192. | Heikes KE, Eddy DM, Arondekar B, Schlessinger L. Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 193. | Palzer EF, Vempati S, Helgeson ES, Matas AJ. Long-Term Living Kidney Donor Risk: A Web-Based Calculator. J Am Soc Nephrol. 2020;31:2968-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 194. | Chen L, Magliano DJ, Balkau B, Colagiuri S, Zimmet PZ, Tonkin AM, Mitchell P, Phillips PJ, Shaw JE. AUSDRISK: an Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust. 2010;192:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 195. | Organization UKD. Diabetes risk factors. UK: UK DO; 23 SEPTEMBER, 2023.. |
| 196. | Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1233] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 197. | American Diabetes Association. Tools and calculators: Take the Type 2 Risk Test: American Diabetes Association. 2023. [cited 3 January 2024]. Available from: Available from: https://diabetes.org/diabetes-risk-test. |
| 198. | Collins GS, Mallett S, Omar O, Yu LM. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med. 2011;9:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 349] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 199. | Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev. 2011;33:46-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 200. | OPTN Transplant data [Internet]. optn. 2021. [cited 3 January 2024]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. |
| 201. | Beauchamp TL, Childress JF. Principles of biomedical ethics. 5th ed. NY: Oxford University Press; 2001. |