Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.89702
Peer-review started: November 9, 2023
First decision: November 29, 2023
Revised: December 13, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: March 18, 2024
Processing time: 126 Days and 16.9 Hours
Prolonged donor hepatectomy time may be implicated in early and late complications of liver transplantation.
To evaluate the impact of donor hepatectomy time on outcomes of liver transplant recipients, mainly early allograft dysfunction.
This multicenter retrospective study included brain-dead donors and adult liver graft recipients. Donor-recipient matching was obtained through a crossover list. Clinical and laboratory data were recorded for both donors and recipients. Donor hepatectomy, cold ischemia, and warm ischemia times were recorded. Primary outcome was early allograft dysfunction. Secondary outcomes included need for retransplantation, length of intensive care unit and hospital stay, and patient and graft survival at 12 months.
From January 2019 to December 2021, a total of 243 patients underwent a liver transplant from a brain-dead donor. Of these, 57 (25%) developed early allograft dysfunction. The median donor hepatectomy time was 29 (23–40) min. Patients with early allograft dysfunction had a median hepatectomy time of 25 (22–38) min, whereas those without it had a median time of 30 (24–40) min (P = 0.126).
Donor hepatectomy time was not associated with early allograft dysfunction, graft survival, or patient survival following liver transplantation.
Core Tip: This study aims to evaluate the impact of donor hepatectomy time on outcomes of liver transplant recipients. This is a multicenter retrospective study that included brain-dead donors and adult liver graft recipients. A total of 243 patients underwent liver transplantation form brain-dead donors. The median duration of donor hepatectomy was 29 (23–40) min. Patients with early allograft dysfunction had a median hepatectomy time of 25 (22-38) min, while those without had a median time of 30 (24–40) min (P = 0.126). Duration of donor hepatectomy was not associated with early allograft dysfunction, graft survival, or patient survival following liver transplantation.
- Citation: Custodio G, Massutti AM, Caramori A, Pereira TG, Dalazen A, Scheidt G, Thomazini L, Leitão CB, Rech TH. Association of donor hepatectomy time with liver transplantation outcomes: A multicenter retrospective study. World J Transplant 2024; 14(1): 89702
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/89702.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.89702
The main source of livers for transplantation is brain-dead donors[1]. During liver harvesting and storage processes, the organs are exposed to numerous cellular insults[2]. As a result, transplantation becomes a race against time. In order to mitigate the negative effects of ischemia, efforts have focused on organ preservation by reducing cold ischemia time and implementing different organ perfusion techniques[3,4].
However, a novel concept has emerged regarding the development of early allograft dysfunction: Donor hepatectomy time, also referred to as donor warm ischemia time[5,6]. Hepatectomy time is defined as the interval from aortic cross-clamping to placing the liver at low temperatures. Despite the brief duration of donor warm ischemia (minutes) in contrast to the long duration of cold ischemia (hours), in the warm phase the organs are maintained at relatively high temperatures and at high metabolic demands[5,7].
Despite the significant role of donor hepatectomy time in graft outcomes, it has received insufficient attention[6,8]. Recently, Gilbo et al[5] demonstrated an association between longer hepatectomy times and early surgical complications[5]. They showed that a 10-min increase in donor hepatectomy time produced a similar effect of 1-h increase in cold ischemia time. Similarly, Adelmann et al[8] demonstrated that hepatectomy time was independently associated with early allograft dysfunction[8].
To address the shortage of organs and improve liver transplantation outcomes, it is crucial to continuously explore opportunities to enhance donor, graft, and recipient care. One such method involves reducing the duration of ischemic phases, which has been demonstrated to be of great importance. Therefore, our study aimed to evaluate the impact of donor hepatectomy time on outcomes of liver transplant recipients.
This is a multicenter retrospective study. The study was approved by the reference Ethics Committee at the Universidade Federal Rio Grande do Sul (PROPESQ UFRGS, project No. 5.526.176), Brazil. The study adheres to the guidelines set forth by the Helsinki Declaration, as well as to local standards and Brazilian legislation[9]. The Ethics Committee did not require informed consent due to the retrospective design and the anonymization of donors and recipients prior to analysis.
This study included brain-dead donors from 19 regional centers in the state of Santa Catarina, Brazil, and adult liver transplant recipients from brain-dead donors at Hospital Santa Isabel, a general hospital in the city of Blumenau, state of Santa Catarina, Brazil, from January 2019 to December 2021. In order to be eligible, patients had to be over 18 years of age and have received a liver transplant in the Liver Transplantation Center at Hospital Santa Isabel. Exclusion criteria were retransplantation, grafts from living-related donors, split liver grafts, and intraoperative death.
Donor-recipient matching was obtained through a crossover list provided by the regional organ distribution center of the state of Santa Catarina. Clinical and laboratory data were recorded for both donors and recipients, and the donor risk index (DRI) was calculated to assess organ quality[10]. The DRI considers 8 donor characteristics, namely age, height, ethnicity, cause of death, donation after circulatory death, donor hospital location, split liver graft, and cold ischemia time. The DRI assesses the risk of graft loss in comparison to an ideal donor[10,11]. A DRI score ≥ 1.4 predicts graft failure[11]. Model for end-stage liver disease (MELD) scores were calculated for recipients. The MELD score is a prospectively developed and validated scoring system for assessing the severity of chronic liver disease that uses patients’ laboratory values for serum bilirubin, serum creatinine, and the international normalized ratio (INR) for prothrombin time to predict 3-month survival[12].
Donor hepatectomy time as well as cold and warm ischemia times were analyzed. Donor hepatectomy time, also known as donor warm ischemia time, is the interval from the start of aortic cold flush in the donor to the completion of donor hepatectomy, during which the liver is transferred to ice-cold preservation solution on the back table[7]. Cold ischemia time refers to the interval from the start of cold flush (both aortic and portal) in the donor to the moment the liver is removed from ice storage and placed in the recipient abdomen for implantation[7]. Warm ischemia time in the recipient is the interval between the removal of the liver from the cold solution and organ reperfusion in the recipient[5,7].
The criteria for early allograft dysfunction were defined as the presence of any of the following postoperative laboratory findings: (1) Serum bilirubin > 10 mg/dL on day 7 after transplant; (2) INR > 1.6 on day 7 after transplant; and (3) Alanine or aspartate aminotransferase levels > 2000 IU/L within the first 7 d after transplant[13]. Graft survival was defined as the time from liver transplantation to either retransplantation or death from any cause[14]. Patient survival was defined as the time from transplantation to death from any cause. Graft and patient survival were evaluated at 12 mo. Patients were followed up until their last visit to the Liver Transplantation Center at Hospital Santa Isabel.
Primary outcome was early allograft dysfunction. Secondary outcomes included need for retransplantation, length of intensive care unit (ICU) and hospital stay, and patient and graft survival at 12 months.
Livers were procured regionally at 19 centers in the state of Santa Catarina, Brazil. The procedure involved isolating the liver and extracting it after dissection of the biliary duct, portal vein, and hepatic artery, along with en-bloc resection of the celiac trunk and aortic patch. The liver was then flushed and cooled through both the abdominal aorta and portal vein and immersed in ice-cold preservation solution (Institute George Lopez 1 solution). Skilled senior staff members performed all liver transplants, with most recipients receiving an inferior vena cava-sparing piggyback anastomosis, although some required replacement of the inferior vena cava. The portal vein was reconstructed in a standard end-to-end fashion. An end-to-end hepatic artery anastomosis was performed, with multiple anastomoses performed in cases of abnormal donor or recipient hepatic artery anatomy. Sequential portal and arterial reperfusion were employed. A standard triple immunosuppression regimen consisting of a calcineurin inhibitor, steroids, and an antimetabolite was administered to all patients[15].
Categorical variables were expressed as percentages. Continuous data were presented as mean (SD) if normally dis
Between January 2019 and December 2021, a total of 243 patients underwent a liver transplant from a brain-dead donor. Table 1 presents the main baseline characteristics of donors, recipients, and surgical procedures. The donors were predominantly male (n = 150, 62%), with a mean age of 41 (SD, 14) years. Stroke was the leading cause of brain death (n = 118, 48.6%), followed by traumatic brain injury (n = 96, 39.5%) and anoxic encephalopathy (n = 19, 7.8%). The median DRI was 1.3 (1.1–1.6). The recipients were mostly male (n = 175, 72%), with a mean age of 56 (SD, 11) years and a body mass index (BMI) of 27.8 (SD, 4.8) kg/m2. The primary indications for liver transplantation were viral hepatitis (n = 78, 32%), alcoholic liver disease (n = 63, 26%), and non-alcoholic fatty liver disease (n = 29, 12%).
| Donor characteristics | Values |
| Demographics | |
| Age (yr) | 41 ± 14 |
| Men | 150 (62) |
| BMI (kg/m2) | 25.5 ± 3.5 |
| Cause of death | |
| Stroke | 118 (48.6) |
| Traumatic brain injury | 96 (39.5) |
| Anoxic encephalopathy | 19 (7.8) |
| Others | 10 (4.1) |
| Organ Procurement | |
| Regional | 215 (88.5) |
| Local | 28 (11.5) |
| Disease severity | |
| Time on MV before donation (d) | 4 (3-7) |
| Presence of sepsis | 125 (51.4) |
| Need for vasopressors | 201 (82.7) |
| Cardiac arrest | 48 (19.8) |
| Biochemical measurements | |
| ALT (U/L) | 29 (19-62) |
| AST (U/L) | 40 (24-70) |
| Bilirubin (mg/dL) | 0.5 (0.3-0.8) |
| Creatinine (mg/dL) | 1 (0.7-1.4) |
| Sodium (mEq/L) | 148 ± 10 |
| Platelets (10³/mm³) | 158 (106-212) |
| Blood glucose (mg/dL) | 243 ± 91 |
| Recipients’ characteristics | Values |
| Demographics | |
| Age (yr) | 56 ± 11 |
| Men | 175 (72) |
| BMI (kg/m2) | 27.8 ± 4.8 |
| Blood group | |
| O | 89 (36.6) |
| A | 108 (44.5) |
| B | 34 (14) |
| AB | 11 (4.5) |
| Indications for liver transplantation | |
| Viral hepatitis | 78 (32) |
| Alcoholic liver disease | 63 (26) |
| Non-alcoholic steatohepatitis | 29 (12) |
| Cryptogenic | 23 (9.5) |
| Others | 50 (20.5) |
| Disease severity | |
| MELD score | 20 ± 8 |
| Presence of HCC | 92 (38) |
| Previous abdominal surgery | 88 (36.2) |
| Previous decompensation | 153 (63) |
| Biochemical measurements | |
| ALT (U/L) | 611 (375-1041) |
| AST (U/L) | 1055 (580-1829) |
| Bilirubin (mg/dL) | 4 (2.3-6.2) |
| Creatinine (mg/dL) | 0.9 (0.7-1.2) |
| Platelets (10³/mm³) | 105 (67-142) |
| INR | 2.1 (1.7-2.7) |
| Albumin (g/dL) | 2.6 (2.3-2.9) |
| Surgical procedures | |
| Cold ischemia time (min) | 405 (329-492) |
| Warm ischemia time (min) | 34 (30-37) |
| Donor hepatectomy time (min) | 29 (23-40) |
| Need for thrombectomy | 33 (13.6) |
| Need for arterial reconstruction | 31 (12.8) |
Donor hepatectomy time ranged from 15 to 93 min, with a median of 29 (23–40) min. There was a difference in hepa
Early allograft dysfunction was observed in 57 patients (25%). The median donor hepatectomy time had no impact on the development of early allograft dysfunction. Patients with early allograft dysfunction had a median donor hepatectomy time of 25 (22–38) min, whereas those without it had a median time of 30 (24–40) min (P = 0.126) (Table 2). Similarly, other surgical times were not associated with early allograft dysfunction (Table 2).
| All patients (n = 228) | With EAD (n = 57) | Without EAD (n = 171) | P value | |
| Donors’ characteristics | ||||
| Age (yr) | 41 ± 14 | 43 ± 14 | 40 ± 14 | 0.186 |
| BMI (kg/m2) | 25.5 ± 3.6 | 26 ± 4.1 | 25.3 ± 3.5 | 0.286 |
| Need for vasopressors | 187 (82) | 44 (77.2) | 143 (8.6) | 0.273 |
| Time on MV before donation (d) | 4 (3-7) | 5 (4-11) | 4 (3-7) | 0.001 |
| Cardiac arrest | 41 (18) | 14 (24.6) | 27 (15.8) | 0.135 |
| DRI score | 1.3 (1.1-1.6) | 1.3 (1.1-1.5) | 1.4 (1.1-1.7) | 0.224 |
| Recipients’ characteristics | ||||
| Age (yr) | 56 ± 11 | 53 ± 13 | 58 ± 10 | 0.021 |
| BMI (kg/m2) | 27.7 ± 4.8 | 28.9 ± 5.9 | 27.4 ± 4.1 | 0.112 |
| Indication for transplantation | 0.079 | |||
| Alcoholic liver disease | 62 (27.2) | 13 (22.8) | 49 (28.7) | |
| Viral hepatitis | 74 (32.4) | 16 (28.1) | 58 (33.9) | |
| Non-alcoholic steatohepatitis | 26 (11.4) | 8 (14) | 18 (10.5) | |
| Cryptogenic | 21 (9.2) | 4 (7.0) | 17 (9.9) | |
| Others | 45 (19.7) | 16 (28.1) | 29 (17) | |
| MELD score | 19 (14-24) | 20 (13-25) | 18 (12-23) | 0.047 |
| Biochemistry at ICU admission | ||||
| Albumin (g/dL) | 2.6 (2.3-2.9) | 2.5 (2.3-1.7) | 2.7 (2.3-2.9) | 0.314 |
| Creatinine (mg/dL) | 0.9 (0.7-1.2) | 0.9 (0.7-1.3) | 0.8 (0.7-1.1) | 0.009 |
| Platelets (10³/mm³) | 105 (67-142) | 104 (74-143) | 108 (82-157) | 0.057 |
| AST (U/L) | 1055 (580-1829) | 1370 (739-3174) | 1003 (561-1434) | < 0.001 |
| ALT (U/L) | 611 (375-1041) | 799 (435-1583) | 488 (289-826) | < 0.001 |
| INR | 2.1 (1.7-2.7) | 2.7 (1.9-3.7) | 2.1 (1.7-2.7) | < 0.001 |
| Bilirubin (mg/dL) | 4 (2.3-6.2) | 6.5 (4.1-8.8) | 3.7 (2.5-5.3) | 0.077 |
| Surgical procedures | ||||
| Donor hepatectomy time (min) | 29 (23-40) | 30 (23-39) | 0.126 | |
| Cold ischemia time (min) | 405 (329-492) | 388 (311-495) | 407 (334-483) | 0.291 |
| Warm ischemia time (min) | 34 (30-37) | 35 (30-39) | 34 (3037) | 0.079 |
When each of the 3 criteria for early allograft dysfunction was analyzed separately, no significant correlation was found between donor hepatectomy time and postoperative markers of liver graft function on ICU admission, day 1, or day 7 (Table 3).
| Hepatectomy time | r | P value |
| Graft function markers | ||
| At admission | ||
| AST (IU/L) | -0.017 | 0.797 |
| ALT (IU/L) | 0.005 | 0.943 |
| INR | 0.033 | 0.617 |
| Bilirubin (mg/dL) | 0.069 | 0.287 |
| At day 1 | ||
| AST (IU/L) | -0.083 | 0.213 |
| ALT (IU/L) | 0.041 | 0.541 |
| INR | -0.051 | 0.449 |
| Bilirubin (mg/dL) | 0.054 | 0.419 |
| At day 7 | ||
| AST (IU/L) | -0.026 | 0.717 |
| ALT (IU/L) | 0.068 | 0.336 |
| INR | -0.055 | 0.443 |
| Bilirubin (mg/dL) | 0.087 | 0.234 |
Donor hepatectomy time did not differ significantly between survivors and non-survivors [29 (24–38) vs 26 (21–42) min, P = 0.787], patients with and without graft survival at 12 months [29 (24–38) vs 27 (21–45) min, P = 0.893], or patients requiring and not requiring retransplantation [30 (24–42) vs 29 (24–40) min, P = 0.951].
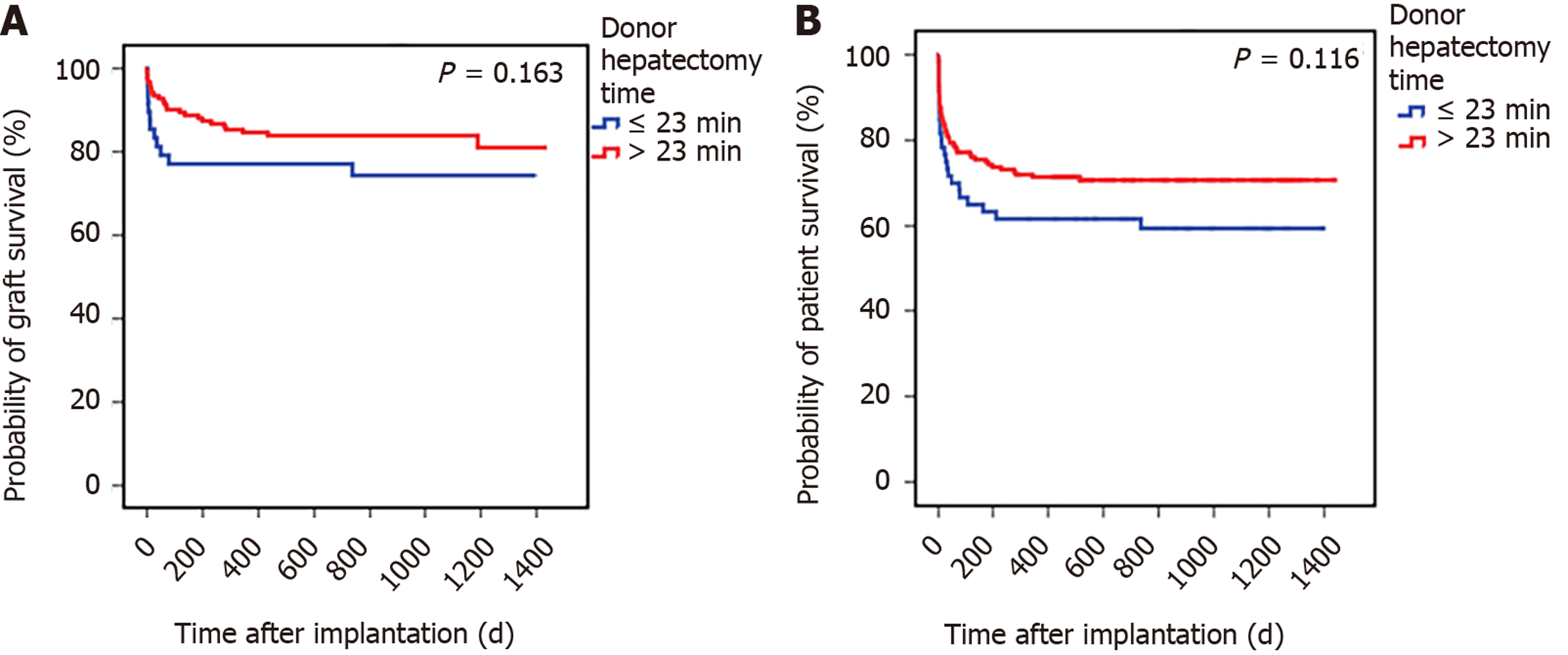
To better understand the impact of donor hepatectomy time, we categorized patients based on the discriminative power of hepatectomy time to predict the outcome determined by the ROC curve, which was set at 23 min. The effects of hepatectomy time below and above this cutoff are detailed in Table 4. Figure 1 illustrates the survival analysis for grafts (Figure 1A) and for patients (Figure 1B) according to hepatectomy times below and above the cutoff value (23 min).
| Outcomes | All patients (n = 243) | Patients with hepatectomy time < 23 min (n = 61) | Patients with hepatectomy time ≥ 23 min (n = 182) | P value |
| Early allograft dysfunction1 | 57 (25) | 19 (33.9) | 38 (22.1) | 0.076 |
| Need for retransplantation | 13 (5.3) | 4 (6.6) | 9 (4.9) | 0.628 |
| Graft survival2 | 166 (81.8) | 37 (75.5) | 129 (83.8) | 0.192 |
| Patient survival | 167 (68.7) | 37 (60.7) | 130 (71.4) | 0.116 |
| LOS, hospital (d) | 10 (8-14) | 10 (7-16) | 10 (8-13) | 0.790 |
| LOS, ICU (d) | 4 (3-6) | 4 (3-6.5) | 4 (3-5) | 0.417 |
Arterial anatomy type was not associated with donor hepatectomy time. The median procedure duration was 29 (23–38) min for donors with standard arterial anatomy and 28 (24–41) min for donors with unusual arterial anatomy (P = 0.688).
Donors with hepatectomy time < 23 min were receiving vasopressors in a similar number to those with hepatectomy time > 23 min [n = 55 (90.2%) vs n = 146 (80.2%), respectively, P = 0.075]. Likewise, donors who had hepatectomy times either above or below the cutoff (23 min) required similar doses of preoperative vasopressors. The dose administered was 0.12 (0.04–0.22) mcg/kg/min for donors above the cutoff and 0.13 (0.05–0.26) mcg/kg/min for donors below the cutoff (P = 0.507).
In this multicenter retrospective study involving liver recipients from brain-dead donors, we did not find any evidence of an association between donor hepatectomy time and the development of early allograft dysfunction. Furthermore, our findings indicate that longer hepatectomy times did not affect either graft or patient survival.
Previous literature reports donor hepatectomy time ranging from 32 to 51 min, with a median of 40 min[5,16]. Two single-center retrospective studies investigated whether donor hepatectomy and implantation time increased the incidence of early allograft dysfunction, but their results were inconclusive[5,8]. Adelmann et al[8] suggested that prolonged donor hepatectomy time increased the risk of early allograft dysfunction, but no adjustment was made for confounders, such as cold ischemia time[8]. Conversely, Gilbo et al[5] showed that the risk of developing early allograft dysfunction was not influenced by donor hepatectomy time but rather by implantation time, which had a linear effect on the development of early allograft dysfunction, increasing the risk by 15% for every 10-min increase in time[5]. Our findings align with these results, as we showed that donor hepatectomy time was not associated with an increased risk of early allograft dysfunction. It is reasonable to conceive that hepatectomy times in our province are sufficiently short (11 min below the median time reported in the literature) to allow for reduced risk of early allograft dysfunction or other clinical outcomes.
Although consensus on the optimal donor hepatectomy time remains inconclusive, studies have suggested that minimizing ischemia times[7,17], especially cold ischemia time[18,19], is associated with better outcomes and fewer early surgical complications, including non-anastomotic biliary strictures[5,20]. However, the impact of donor hepatectomy time, which is relatively brief compared to other ischemia times, on clinical outcomes has received limited attention. In this study, we showed that donor hepatectomy time was not associated with graft or patient survival, need for retransplantation, or length of ICU or hospital stay. Probably, other donor, recipient, and surgical procedure characteristics, such as previous comorbidities[21], age[22], underlying disease[19], and bleeding volume[23,24], are better determinants of these outcomes than hepatectomy time itself. For instance, liver grafts recovered from donors after cardiac death undergo distinct ischemic insults during procurement, exhibiting differences in nature and severity of injury. Using the Euro
Unstable patients and those with unusual arterial anatomy may have prolonged hepatectomy times. In our study, the presence of unusual arterial anatomy or vasopressor dose had no significant impact on donor hepatectomy time, although this result should be considered exploratory.
Our study is one of the few studies that have been specifically designed to investigate the association between donor hepatectomy time and the development of early allograft dysfunction. Nevertheless, given the multicenter nature of the study, it is essential to acknowledge some limitations. First, although this study represents the largest dataset to test this hypothesis, it is still underpowered. Based on the 5-min difference that we found in median hepatectomy time between patients with and without early allograft dysfunction, our results have a power of 71%. However, it is highly unlikely that an increment in sample size would change results, as a very short hepatectomy time was observed overall. Second, since donor hepatectomy time is not considered crucial, surgeons may have provided less accurate information in this regard, but data were collected from patients’ medical records. Third, the retrospective nature of the study resulted in some missing information, including 15 patients without the primary outcome. Fourth, unfortunately we do not have data on the impact of donor hepatectomy time after cardiac death, as well described[26], because this type of donation is not currently available in Brazil.
In conclusion, donor hepatectomy time was not associated with early allograft dysfunction, graft survival, or patient survival following liver transplantation. While there is a need for policies and interventions to enhance post-transplant outcomes, it appears that the current donor hepatectomy time is already sufficiently short to further mitigate risks. We suggest that future research efforts should focus on exploring alternative strategies other than further reducing donor hepatectomy time.
To address the shortage of organs and improve liver transplantation outcomes, it is crucial to explore opportunities to enhance donor, graft, and recipient care. One such method involves reducing the duration of ischemic phases, which has been demonstrated to be of great importance.
There is a need for policies and interventions to improve post-transplant results, it appears that the donor's hepatectomy time may be a factor contributing to this improvement.
This study aimed to evaluate the impact of donor hepatectomy timing on outcomes in liver transplant recipients, particularly early allograft dysfunction. We know that transplantation is a race against time, and better understanding the importance of these times is essential for a more accurate strategy.
This is a multicenter retrospective study. The study included brain-dead donors from 19 regional centers in the state of Santa Catarina, Brazil, and adult liver transplant recipients from brain-dead donors at Hospital Santa Isabel, a general hospital in the city of Blumenau, state of Santa Catarina, Brazil, from January 2019 to December 2021. The discriminative power of donor hepatectomy time to predict the outcome was determined by analyzing receiver operating characteristic curves, and patients were divided into two groups: Below and above the cutoff.
In this multicenter retrospective study involving liver recipients from brain-dead donors, we did not find any evidence of an association between donor hepatectomy time and the development of early allograft dysfunction. Furthermore, our findings indicate that longer hepatectomy times did not affect either graft or patient survival. We believe that the exceptionally short median donor hepatectomy time of < 29 min in our study, along with the absence of prolonged warm ischemia typical of donors after cardiac death, explains the lack of association between donor hepatectomy time and outcomes in our cohort of brain-dead donors.
Donor hepatectomy times did not affect either graft or patient survival. The new methods that this study proposed was to evaluate hepatectomy time in centers where this time is already reduced in relation to other centers already studied.
While there is a need for policies and interventions to enhance post-transplant outcomes, it appears that the current donor hepatectomy time is already sufficiently short to further mitigate risks. We suggest that future research efforts should focus on exploring alternative strategies other than further reducing donor hepatectomy times.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsoulfas G, Greece S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Noguchi H, Iwanaga Y, Okitsu T, Nagata H, Yonekawa Y, Matsumoto S. Evaluation of islet transplantation from non-heart beating donors. Am J Transplant. 2006;6:2476-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Banker A, Bhatt N, Rao PS, Agrawal P, Shah M, Nayak M, Mohanka R. A Review of Machine Perfusion Strategies in Liver Transplantation. J Clin Exp Hepatol. 2023;13:335-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Tchilikidi KY. Liver graft preservation methods during cold ischemia phase and normothermic machine perfusion. World J Gastrointest Surg. 2019;11:126-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 5. | Gilbo N, Fieuws S, Meurisse N, Nevens F, van der Merwe S, Laleman W, Verslype C, Cassiman D, van Malenstein H, Roskams T, Sainz-Barriga M, Pirenne J, Jochmans I, Monbaliu D. Donor Hepatectomy and Implantation Time Are Associated With Early Complications After Liver Transplantation: A Single-center Retrospective Study. Transplantation. 2021;105:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Jochmans I, Fieuws S, Tieken I, Samuel U, Pirenne J. The Impact of Hepatectomy Time of the Liver Graft on Post-transplant Outcome: A Eurotransplant Cohort Study. Ann Surg. 2019;269:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Heylen L, Pirenne J, Naesens M, Sprangers B, Jochmans I. "Time is tissue"-A minireview on the importance of donor nephrectomy, donor hepatectomy, and implantation times in kidney and liver transplantation. Am J Transplant. 2021;21:2653-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Adelmann D, Roll GR, Kothari R, Syed S, Burdine LJ, Tavakol M, Niemann CU. The Impact of Deceased Donor Liver Extraction Time on Early Allograft Function in Adult Liver Transplant Recipients. Transplantation. 2018;102:e466-e471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 18260] [Article Influence: 1521.7] [Reference Citation Analysis (0)] |
| 10. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1487] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 11. | Lozanovski VJ, Probst P, Arefidoust A, Ramouz A, Aminizadeh E, Nikdad M, Khajeh E, Ghamarnejad O, Shafiei S, Ali-Hasan-Al-Saegh S, Seide SE, Kalkum E, Nickkholgh A, Czigany Z, Lurje G, Mieth M, Mehrabi A. Prognostic role of the Donor Risk Index, the Eurotransplant Donor Risk Index, and the Balance of Risk score on graft loss after liver transplantation. Transpl Int. 2021;34:778-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Inaba K, Barmparas G, Resnick S, Browder T, Chan LS, Lam L, Talving P, Demetriades D. The Model for End-Stage Liver Disease score: an independent prognostic factor of mortality in injured cirrhotic patients. Arch Surg. 2011;146:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 874] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 14. | Duan X, Yan L, Shen Y, Zhang M, Bai X, Liang T. Outcomes of liver transplantation using moderately steatotic liver from donation after cardiac death (DCD). Ann Transl Med. 2020;8:1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Best LM, Leung J, Freeman SC, Sutton AJ, Cooper NJ, Milne EJ, Cowlin M, Payne A, Walshaw D, Thorburn D, Pavlov CS, Davidson BR, Tsochatzis E, Williams NR, Gurusamy KS. Induction immunosuppression in adults undergoing liver transplantation: a network meta-analysis. Cochrane Database Syst Rev. 2020;1:CD013203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Jochmans I, van Rosmalen M, Pirenne J, Samuel U. Adult Liver Allocation in Eurotransplant. Transplantation. 2017;101:1542-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Al-Kurd A, Kitajima T, Delvecchio K, Tayseer Shamaa M, Ivanics T, Yeddula S, Yoshida A, Rizzari M, Collins K, Abouljoud M, Nagai S. Short recipient warm ischemia time improves outcomes in deceased donor liver transplantation. Transpl Int. 2021;34:1422-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Figiel W, Smoter P, Krasnodębski M, Rykowski P, Morawski M, Grąt M, Patkowski W, Zieniewicz K. Predictors of Long-Term Outcomes After Liver Transplantation Depending on the Length of Cold Ischemia Time. Transplant Proc. 2022;54:1025-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Lozanovski VJ, Döhler B, Weiss KH, Mehrabi A, Süsal C. The Differential Influence of Cold Ischemia Time on Outcome After Liver Transplantation for Different Indications-Who Is at Risk? A Collaborative Transplant Study Report. Front Immunol. 2020;11:892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | van Leeuwen OB, van Reeven M, van der Helm D, IJzermans JNM, de Meijer VE, van den Berg AP, Darwish Murad S, van Hoek B, Alwayn IPJ, Porte RJ, Polak WG. Donor hepatectomy time influences ischemia-reperfusion injury of the biliary tree in donation after circulatory death liver transplantation. Surgery. 2020;168:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Lazzeri C, Bonizzoli M, Ghinolfi D, De Simone P, Pezzati D, Rreka E, Bombardi M, Migliaccio ML, Peris A. Comorbidities and Age in Brain-Dead Donors and Liver Transplantation: A 15-Year Retrospective Investigation. Exp Clin Transplant. 2020;18:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol. 2019;70:745-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 23. | Tan L, Wei X, Yue J, Yang Y, Zhang W, Zhu T. Impact of Perioperative Massive Transfusion on Long Term Outcomes of Liver Transplantation: a Retrospective Cohort Study. Int J Med Sci. 2021;18:3780-3787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Rana A, Petrowsky H, Hong JC, Agopian VG, Kaldas FM, Farmer D, Yersiz H, Hiatt JR, Busuttil RW. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg. 2013;216:902-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Bekki Y, Kozato A, Kusakabe J, Tajima T, Fujiki M, Gallo A, Melcher ML, Bonham CA, Sasaki K. Impact of the donor hepatectomy time on short-term outcomes in liver transplantation using donation after circulatory death: A review of the US national registry. Clin Transplant. 2022;36:e14778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Meier RPH, Kelly Y, Yamaguchi S, Braun HJ, Lunow-Luke T, Adelmann D, Niemann C, Maluf DG, Dietch ZC, Stock PG, Kang SM, Feng S, Posselt AM, Gardner JM, Syed SM, Hirose R, Freise CE, Ascher NL, Roberts JP, Roll GR. Advantages and Limitations of Clinical Scores for Donation After Circulatory Death Liver Transplantation. Front Surg. 2021;8:808733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |