Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.88891
Peer-review started: October 13, 2023
First decision: November 2, 2023
Revised: November 8, 2023
Accepted: December 11, 2023
Article in press: December 11, 2023
Published online: March 18, 2024
Processing time: 154 Days and 5.8 Hours
Liver transplantation (LT) is a life-saving intervention for patients with end-stage liver disease. However, the equitable allocation of scarce donor organs remains a formidable challenge. Prognostic tools are pivotal in identifying the most suitable transplant candidates. Traditionally, scoring systems like the model for end-stage liver disease have been instrumental in this process. Nevertheless, the landscape of prognostication is undergoing a transformation with the integration of machine learning (ML) and artificial intelligence models.
To assess the utility of ML models in prognostication for LT, comparing their per
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, we conducted a thorough and standardized literature search using the PubMed/MEDLINE database. Our search imposed no restrictions on publication year, age, or gender. Exclusion criteria encompassed non-English stu
Our search yielded a total of 64 articles, with 23 meeting the inclusion criteria. Among the selected studies, 60.8% originated from the United States and China combined. Only one pediatric study met the criteria. Notably, 91% of the studies were published within the past five years. ML models consistently demonstrated satisfactory to excellent area under the receiver operating characteristic curve values (ranging from 0.6 to 1) across all studies, surpassing the performance of traditional scoring systems. Random forest exhibited superior predictive capa
This study underscores the potential of ML models in guiding decisions related to allograft allocation and LT, marking a significant evolution in the field of prognostication.
Core Tip: This systematic review highlights the promising role of machine learning (ML) models in improving prognostication for liver transplantation (LT). ML models consistently outperformed traditional scoring systems, demonstrating excellent predictive capabilities for various post-transplant complications, including mortality, sepsis, and acute kidney injury. The findings underscore the potential of ML in enhancing decision-making related to organ allocation and LT, representing a substantial advancement in prognostication methods.
- Citation: Chongo G, Soldera J. Use of machine learning models for the prognostication of liver transplantation: A systematic review. World J Transplant 2024; 14(1): 88891
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/88891.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.88891
Liver transplantation (LT) has long been a transformative intervention for individuals afflicted with acute and chronic-end-stage liver ailments. In addition to restoring patients' health, LT can enhance their overall well-being and potentially extend their lifespan by up to 15 years[1]. This treatment approach is firmly established as a last resort when alternative methods and therapies have proven ineffective. According to the Scientific Registry of Transplant Recipients in the United States, the survival rates for patients after deceased donor LT are commendable, standing at approximately 90% at one year and 77% at five years post-LT[2]. Nevertheless, the field of LT confronts a range of challenges, encompassing can
The persistent scarcity of donor organs has emerged as a critical and ongoing concern. While living donation has bolstered liver transplant numbers in some regions, in others, the field has stagnated. Consequently, there has been a concerted effort over the past decade to augment the pool of deceased donors. This endeavor has led to increased utilization of liver allografts obtained after cardiac death (DCD), as well as those from marginal and extended donor criteria[3]. Despite these improvements, a notable number of DCD livers remain unused due to suboptimal allograft function and unacceptable donor parameters. This predicament has given rise to the concept of mechanical perfusion for solid organ transplantation, aiming to expand the available organ pool, particularly for liver allografts, further under
A recent study emphasized the multifaceted challenges inherent to LT. In 2017, the United States recorded a waiting list of 14360 candidates eagerly awaiting LT[5]. Furthermore, the study reported an average hospital expenditure exceeding $490000 per patient associated with LT in 2011[5]. Evidently, there is an escalating demand for a more efficient system of liver organ allocation to optimize outcomes within a society grappling with diminishing liver organ donations and escalating expenditures linked to the care of end-stage liver disease patients.
The allocation of liver allografts to patients in need has relied on various scoring tools. Initially, Child-Turcotte-Pugh (CTP) score served this purpose, but the Model for End-stage Liver Disease (MELD) has now become the preferred score for organ allocation. Additionally, several other scoring systems, such as survival outcomes following LT (SOFT), balance of risk (BAR), donor risk index (DRI), age, bilirubin, international normalized ratio (INR), and creatinine (ABIC), chronic liver failure (CLIF)-Consortium Organ Failure scoreC OFs (CLIF-C OFs), CLIF-Consortium score for Acute on Chronic Liver Failure (CLIF-C ACLFs), and CLIF-Sequential Organ Failure Assessment score (SOFA), have been employed in this context.
The CTP score, initially validated for predicting postoperative mortality in cirrhotic patients, incorporates clinical and biochemical data, including serum albumin, serum bilirubin, INR or prothrombin time, ascites, and encephalopathy, to assess the prognosis of end-stage liver disease. The total Child-Pugh (CP) score is calculated by assigning points to each variable, with a maximum score of 15 points (Supplementary Table 1). CP class A corresponds to a score of 5-6 points, with a 10% mortality rate. CP class B corresponds to a score of 7-9 points, with a 30% mortality rate, while CP class C repre
However, the use of CTP for liver transplant allocation had significant limitations. It relied on subjective assessments of ascites and encephalopathy, lacked an evaluation of renal function, and had a limited scoring range, making it challenging to differentiate patients based on disease severity. This limitation was evident when patients with different INR and bilirubin levels were assigned the same CTP score, potentially leading to misleading prioritization[9]. Other drawbacks of the CTP score include the empirical selection of variables and the interdependence of some variables, such as coagulation and albumin, which could result in an imbalance in their influence within the score.
The CTP score's arbitrary cutoffs for quantitative variables lack evidence of optimality in defining hepatic changes and mortality risk, hindering its reliability in predicting prognosis in liver cirrhosis and post-LT[10]. Conversely, MELD score, originally designed for predicting survival after trans-jugular intrahepatic Porto-systemic shunt procedures, has been extended to assess prognosis in liver cirrhosis and serves as a tool for liver organ allocation[11]. MELD score's has a good reliability in predicting 1-year and 5-year survival across diverse liver diseases, including alcoholic cirrhosis and hepatitis[12]. Additionally, MELD score has prognostic value in conditions like spontaneous bacterial peritonitis, variceal bleeding, and hepatorenal syndrome (HRS)[13]. In cases of variceal bleeding, the MELD score's predictive ability was comparable to the CTP score. Concerning HRS, a high MELD score (> 20) has been linked to a median survival of just 1 mo for type 1 HRS, while type 2 HRS patients' survival correlated with their MELD score, with a median survival of 3 mo for MELD > 20 and 11 mo for MELD < 20[14]. To enhance its predictive power, the MELD score has evolved into multiple versions, including MELD sodium (MELD NA) and Delta MELD (D-MELD).
MELD NA, developed due to the observation of dilutional hyponatremia in cirrhotic patients, stems from systemic arterial vasodilation-induced antidiuretic hormone release, which was linked to portal hypertension severity[15]. Hypon
The D-MELD was introduced to address the limitation of a single MELD score at a specific time. While it is useful in predicting survival in cirrhotic patients awaiting transplantation, conflicting evidence exists. The potential bias in frequent laboratory testing for acutely worsening patients also complicates its use[20,21]. In summary, all versions of the MELD score have limitations, including susceptibility to therapeutic interventions, empirical variable selection, limited predictive ability for post-transplant mortality, and the need for on-site computation[10].
To improve the prediction of post-liver transplant mortality, various prediction tools have been explored, including the DRI, eurotransplant-donor risk Index (ET-DRI), SOFT, pre-allocation SOFT (p-SOFT), BAR, ABIC, CLIF C OFs, CLIF-C ACLFs, and the CLIF-SOFA. The DRI, predating the MELD score, was initially considered as an independent predictor of allograft failure across different MELD categories. However, numerous studies have revealed its limited association with outcomes[22]. The DRI's limitations include its validation in the pre-MELD era, the absence of recipient-related risk factors as the fact that is impractical for predicting morbidity and graft failure due to its poor predictive ability, inclusion of irrelevant factors (e.g., ethnicity), and omission of relevant factors[23].
The ET-DRI replaces ethnicity and height risk factors with parameters like the latest gamma-glutamyl transferase and rescue offer in the Eurotransplant context. Although it has been shown to be potentially useful for liver allocation, studies have consistently shown its limited predictive ability for early post-transplant outcomes[22-26]. Overall, the ET-DRI is consistently considered an unreliable tool for predicting morbidity and mortality after LT.
Various prediction tools have been explored to enhance post-liver transplant prognostication. The SOFT score (Supplementary Table 2) has been tested for predicting 90-d post-transplant mortality[22,27]. A derivative of SOFT, the p-SOFT score (Supplementary Table 3), exhibited promising predictive accuracy[22]. However, the complexity of these scores, which involve multiple subjective and semi-quantitative variables, hampers their prompt clinical assessment and decision-making. Furthermore, their predictive ability for major morbidity at 3 mo appears limited[22,28].
The BAR score (Supplementary Table 4) offers promise by evaluating both recipient and donor factors for severe complications and 90-d mortality[22,28]. This tool has shown robustness in various patient populations, including pediatric, adolescent, and living donor liver transplant recipients[29,30]. However, in specific patient subgroups, BAR's accuracy in assessing short-term outcomes, including major complications, 90-d mortality, and ICU and hospital stay length, may be suboptimal[22].
The ABIC score (Supplementary material) aim to predict outcomes in patients with alcoholic hepatitis. While it has shown potential, its validation has been inconsistent, and it may not be widely applicable. Additionally, it primarily assesses the risk of wait-time mortality, making it unsuitable for post-liver transplant mortality assessment[31,32].
The CLIF-SOFA score (Supplementary Table 5), a modified version of the SOFA, is tailored for end-stage liver disease patients. This adaptation replaces platelet count and Glasgow coma scale with INR and hepatic encephalopathy, respectively. Additionally, it incorporates terlipressin and renal replacement therapy into cardiovascular and renal parameters, respectively, and includes SpO2/FiO2 as an alternative respiratory parameter for patients without an arterial line[33].
In a study published in 2014, the CLIF-SOFA score proved to be a significant predictor of 1-year post-LT mortality, surpassing the SOFA score in discriminatory power on several post-transplant days[34]. CLIF-SOFA score exhibited greater numerical differences between 1-year survivor and non-survivor groups, especially post-LT. Furthermore, CLIF-SOFA score trends reflected patients' responses to therapeutic strategies, with a CLIF-SOFA score > 8 on post-transplant day 7 indicating delayed recovery from multiple organ dysfunction, associated with higher acute rejection rates and poorer 1-year survival rates.
The CLIF-C OFs, a simplified version of CLIF-SOFA, uses a 3-point range per organ system and performs similarly to CLIF-SOFA, outperforming SOFA[35]. This score has proven to be an excellent prognostic tool for short-term outcomes in LT. Another variation, the CLIF-C ACLFs (Supplementary material), designed for acute-on-chronic liver failure (ACLF) patients, includes the CLIF-SOFA score, age, and white-cell count. Jalan et al[35] demonstrated the superiority of the CLIF-ACLF score in terms of performance compared to CLIF-SOFA and CLIF-C OFs scores. However, inferior per
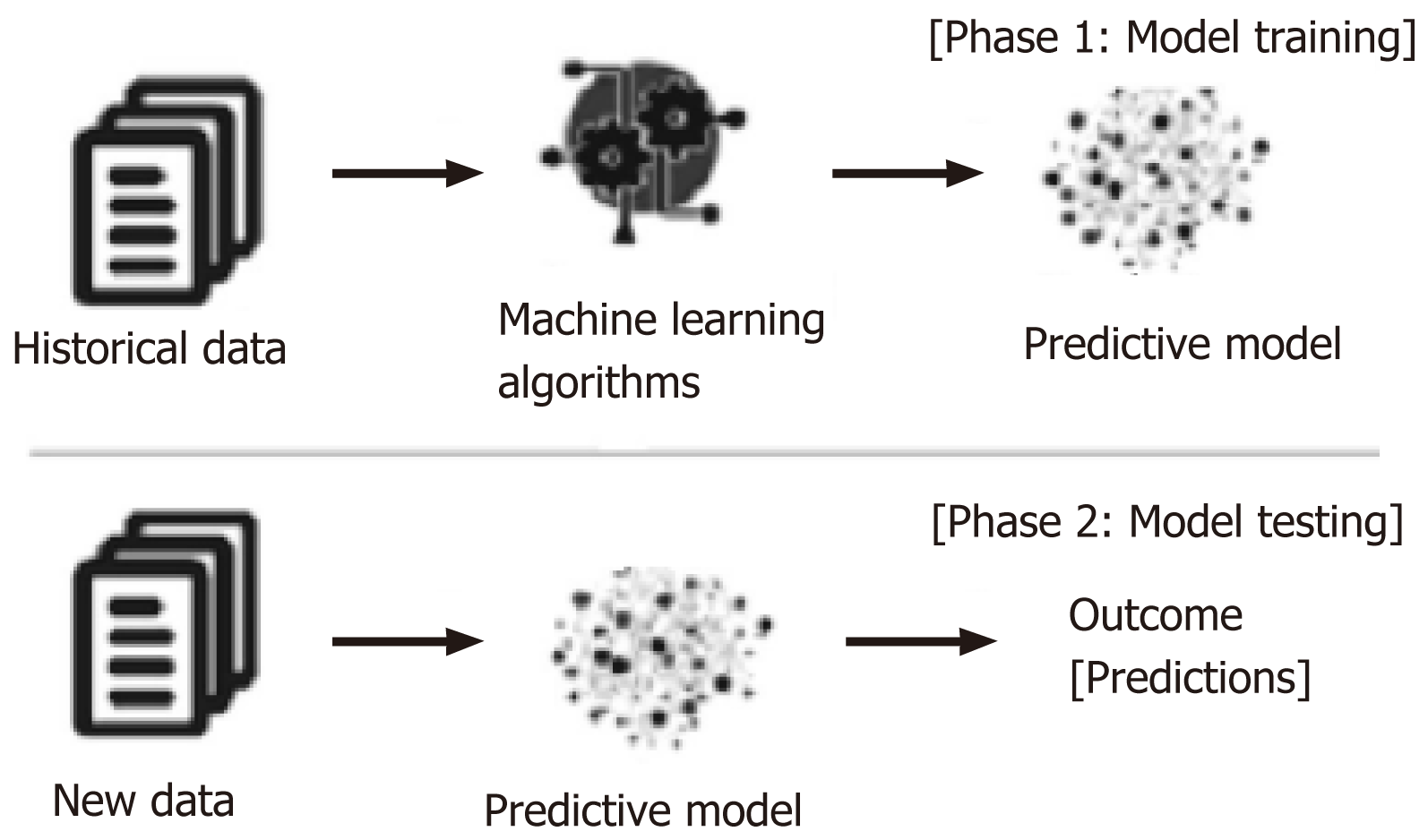
In response to the limitations of existing prognostic scores, there is a growing interest in harnessing machine learning (ML) models and algorithms to enhance the prediction of outcomes in LT. ML models serve as a bridge between organ allocation and achieving optimal results, capitalizing on the increasing use of artificial intelligence (AI) in medicine over the past decade (Figure 1). ML algorithms, as illustrated in Figure 2, rely on various types of input data, including structured, semi-structured, and unstructured data. Structured data, characterized by well-defined formats and adherence to specific data models, is organized in a tabular fashion and includes information like names, dates, and addresses. Semi-structured data, found in NoSQL databases, JSON documents, HTML, and XML, possesses organizational properties that enable analysis. On the other hand, unstructured data, comprising text and multimedia materials from sources like emails, sensor data, and web pages, lacks predefined formats, making it more challenging to process and analyze. To extract valuable insights from data for building intelligent applications in specific problem domains, various ML techniques are applied based on their learning capabilities[44]. Mohammed et al[45] categorized ML algorithms into four main groups: Supervised, unsupervised, semi-supervised, and reinforcement learning (Supple-mentary Table 6). Supervised learning involves mapping input to output based on labeled training data, typically used for tasks like classification and regression. Unsupervised learning, on the other hand, analyzes unlabeled datasets without human intervention and is employed for tasks such as clustering and dimensionality reduction, focusing on extracting generative features and identifying meaningful trends.
In the realm of ML, several techniques are employed to enhance predictive models for various applications, including LT prognostication. One such technique is semi-supervised learning, which effectively leverages both labeled and unlabeled data to achieve improved prediction outcomes, especially when labeled data is limited. This approach plays a crucial role in bridging the gap between supervised and unsupervised learning methods, finding utility in domains such as machine translation, data labeling, and text classification[46].
Reinforcement learning, on the other hand, offers a distinct approach by focusing on environment-driven algorithms that enable software agents and machines to autonomously evaluate optimal behavior within specific contexts. This methodology relies on the concept of rewards and penalties, aiming to utilize insights gained from interactions with the environment to maximize rewards or minimize risks. While reinforcement learning possesses significant potential in training AI models, it is better suited for complex scenarios rather than straightforward problems[47].
Within the realm of classification algorithms, several notable methods find application in health-related domains. Logistic regression (LR) stands as a commonly used technique, relying on logistic functions to estimate probabilities. While LR can excel in linearly separable datasets, it may suffer from overfitting in high-dimensional scenarios. Regularization techniques like L1 and L2 regularization are often employed to mitigate this issue[46].
Support vector machine (SVM) is another prominent classification method with applications in health data. SVM operates in high-dimensional spaces by constructing hyperplanes that maximize the margin between data points in different classes. The choice of kernel functions, such as polynomial, linear, radial basis function, and sigmoid, sig
Random forest (RF) offers a distinct ensemble classification technique, widely used in ML and data science applications. RF employs parallel ensembling, training multiple decision tree classifiers on different data subsets and combining their outcomes through averaging or majority voting. This approach effectively addresses overfitting concerns and enhances prediction accuracy, making it suitable for both continuous and categorical data in classification and regression problems[40].
Additionally, Adaptive Boosting (AdaBoost) serves as a valuable classification algorithm in the realm of health data. It adopts a sequential ensembling approach to improve the performance of weak classifiers by learning from their errors. By combining multiple underperforming classifiers, AdaBoost creates a robust classifier with high accuracy, boosting the performance of decision trees, base estimators, and binary classification tasks. However, it's essential to note that AdaBoost can be susceptible to overfitting and sensitivity to noisy data and outliers[48].
These various ML techniques have been instrumental in addressing complex problems in health-related domains, including LT prognostication. However, they also come with their own set of challenges, such as overfitting and interpretability issues. Therefore, periodic reviews are crucial to evaluate their performance and reliability compared to traditional scoring methods. This study aims to conduct a systematic review of observational studies, assessing the effectiveness of ML models in LT prognostication and comparing their performance with established scoring systems.
Extreme gradient boosting (XGBoost) stands out as a prominent classifier, belonging to the ensemble learning algorithm family, akin to RF. XGBoost represents a specific variant of gradient boosting that intricately considers detailed approximations when determining the optimal model. It effectively addresses overfitting concerns by minimizing the loss function and employing advanced regularization techniques, including L1 and L2 regularization. These regularization methods are implemented through the computation of second-order gradients of the loss function, resulting in enhanced model generalization and performance[48].
In the domain of ML, artificial neural networks (ANN) and deep learning techniques hold significant sway. Deep learning, a subset of ANN-based approaches, encompasses representation learning and comprises multiple layers, including input, hidden, and output layers. These layers collaboratively facilitate learning from data, giving rise to a computational architecture that excels, particularly when dealing with large datasets. Notable deep learning algorithms encompass multilayer perceptron, long short-term memory recurrent neural network, convolutional neural network, and ConvNet, among others[49].
ML demonstrates versatility by not only addressing diagnostic challenges but also serving as a valuable tool in prog
Within the context of LT, ML models have garnered increasing attention, underscoring the need for periodic assessments of their reliability and performance compared to conventional scoring systems. To this end, this study endeavors to conduct a systematic review of observational studies. The objective is to comprehensively evaluate the evidence concerning the deployment of ML models for prognostication in LT. This evaluation encompasses an assessment of their performance and reliability, juxtaposed with the array of traditional scoring systems currently avai
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines to ensure a standardized approach[52].
A comprehensive literature search was conducted using the PubMed/MEDLINE search engine by one researcher. The search strategy included the following terms: ("ML" OR "AI") AND ("LT" OR "Allograft liver") AND ("Prognosis" OR "Mortality" OR "Prognostication"). A reference manager tool, Zotero, was utilized for sorting and managing references.
All observational studies discussing ML models and prognosis of LT, regardless of the year of publication, age, or sex, were included. Studies written in English were considered. Additionally, studies examining ML models and the risk of post-transplant complications were included, as these complications often contribute to transplant failure or mortality. Exclusion criteria encompassed non-English papers, review articles, case reports, conference articles, studies with missing data, or studies with evident methodological flaws.
The systematic search was conducted by one reviewer, who screened the potential studies based on their titles and abstracts. Full-text versions of eligible studies were obtained and thoroughly analyzed for content and methodology.
A summary of the included studies was created, providing a narrative overview of each paper's objectives, methods, results, and conclusions. After reviewing the full papers, data on various elements was extracted including; study type, population studied and year of study, purpose of the study, setting of the study, its methods and results, conclusion, limitations and strengths of the study as well as a summary of the study. Additionally, if reported by the studies, a com
By systematically extracting relevant information from the selected studies, a comprehensive understanding of the role of AI in LT prognosis was obtained. The data synthesis process involved organizing and presenting the findings in a coherent manner, allowing for a comprehensive evaluation of the current literature in this field.
This approach enabled to examine the various methodologies employed in the studies, identify key trends, and evaluate the potential benefits and limitations of using ML models for prognostication in LT. The synthesized data from the included studies will contribute to providing valuable insights into the current state of research on the role of AI in predicting outcomes in LT.
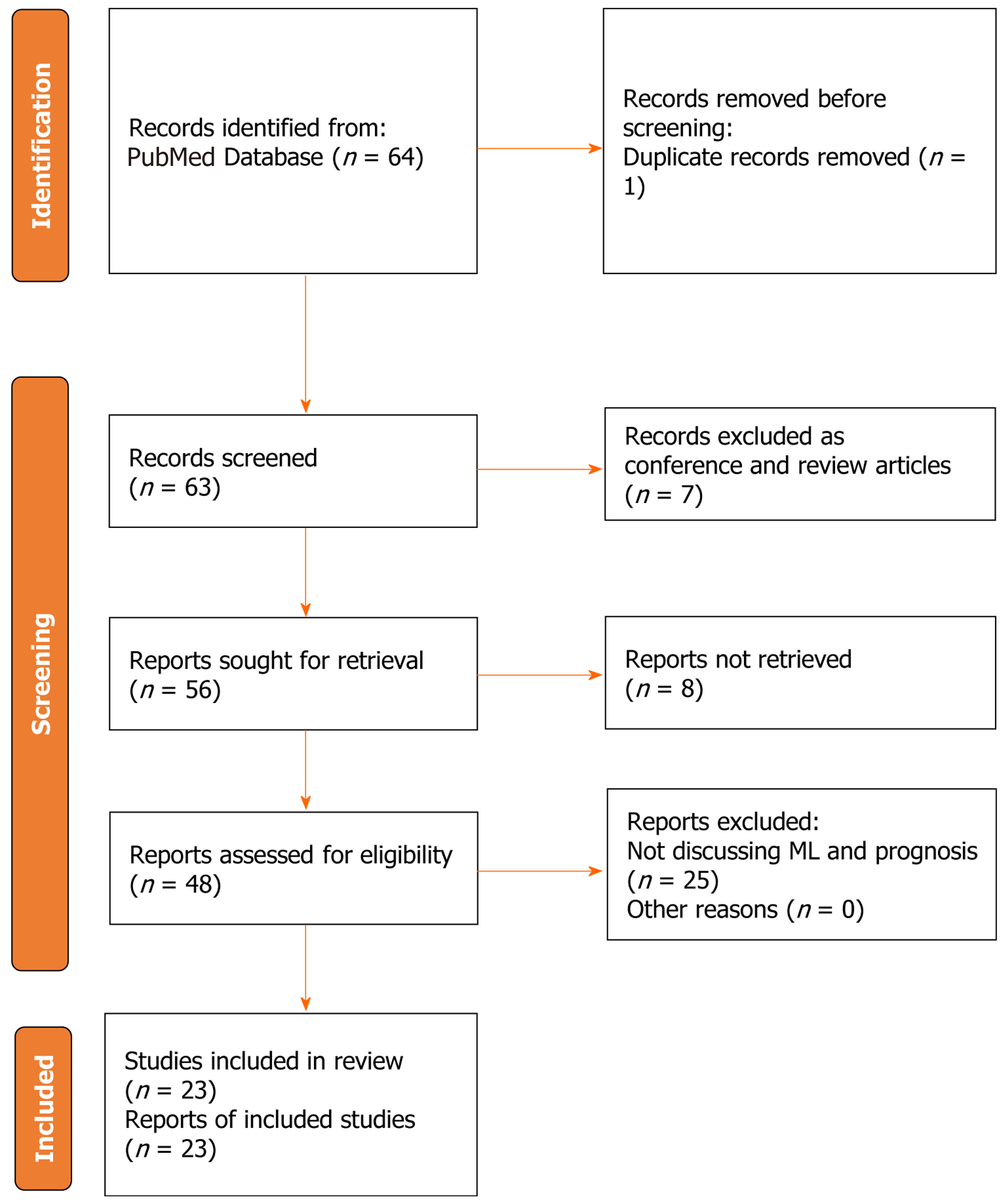
Using the predetermined search strategy, a total of 64 references were initially identified. Among these, 7 references were excluded as they were conference articles or review papers. Additionally, 1 duplicate article was removed, and 8 articles were excluded as they were abstracts only and could not be accessed for full-text reading. Subsequently, a thorough evaluation of the remaining 48 articles was conducted through full-text reading and content analysis. Following the comprehensive assessment, 23 studies met the inclusion criteria and were included in the final analysis. The selection process and reasons for exclusion of certain studies are visually represented in Figure 3, which depicts the flowchart illustrating the search strategy employed. Table 1, summarizes the findings of every study included[53-74].
| Ref. | Context | Aim | Methods | Results | Conclusion |
| Briceño et al[53], 2014 | A Spanish study using a two-fold ANN model which included, the positive survival and the negative loss models were implored to predict 3 mo graft survival post LT | To test the accuracy of ANN inpredicting post-transplant outcomes and compare with other conventional models | Sixty-four donor and recipient variables from a set of 1003 LT from a multicenter study including 11 Spanish centers were included. For each D-R pair, common statistics (simple and multiple regression models) and ANN formulae for two non-complementary probability-models of 3-months graft-survival and -loss were calculated: a positive-survival (NN-CCR) and a negative-loss (NN-MS) model. The NN models were obtained by using the Neural Net Evolutionary Programming (NNEP) algorithm. Additionally, receiver-operating curves (ROC) were performed to validate ANN against other scores | Optimal results for NN-CCR and NN-MS models were obtained, with the best performance in predicting the probability of graft-survival (90.79%) and -loss (71.42%) for each D-R pair, significantly improving results from multiple regressions. ROC curves for 3- months graft-survival and –loss predictions were significantly more accurate for ANN than for other scores in both NN-CCR (AUROC-ANN = 0.80 vs –MELD = 0.50; -D-MELD = 0.54; -P- 5 SOFT = 0.54; -SOFT = 0.55; –BAR = 0.67 and -DRI = 0.42) and NN-MS (AUROC-ANN = 0.82 vs – MELD = 0.41; -D-MELD = 0.47; -P-SOFT = 0.43; -SOFT = 0.57, -BAR = 0.61 and -DRI = 0.48) | ANN maybe considered a powerful decision-making technology for this dataset, optimizing the principles of justice, efficiency and equity. This may be a useful tool for predicting 3-months outcome and a potential research area for future D-R matching models |
| Ershoff et al[54], 2020 | An American study in which DNN was trained on pre transplant data and compared with the BAR and SOFT scores in predicting 90-d mortality post LT | The primary aim of the study was to classify recipients with 90-d post-liver transplant mortality using DNNs | In this study, we trained a DNN to predict 90-d post -transplant mortality using preoperative variables and compared the performance to that of the Survival Outcomes Following Liver Transplantation (SOFT) and Balance of Risk (BAR) scores, using United Network of Organ Sharing data on adult patients who received a deceased donor liver transplant between 2005 and 2015 (n = 57544). The DNN was trained using 202 features, and the best DNN’s architecture consisted of 5 hidden layers with 110 neurons each | The area under the receiver operating characteristics curve (AUC) of the best DNN model was 0.703 (95%CI: 0.682-0.726) as compared to 0.655 (95%CI: 0.633-0.678) and 0.688 (95%CI: 0.667-0.711) for the BAR score and SOFT score, respectively | Despite the complexity of DNN, it did not achieve a significantly higher discriminative performance than the SOFT score. Future risk models will likely benefit from the inclusion of other data sources, including high-resolution clinical features for which DNNs are particularly apt to outperform conventional statistical methods |
| Lau et al[55], 2015 | An Australian study proposing an algorithm made from 15 donor, recipient and transplant factors selected by ML predicting mortality within 30 days after LT | To evaluate the utility of machine-learning algorithms, such as random forests and artificial neural networks, to predict outcome based on donor and recipient variables which are known before organ allocation | Liver transplant data from the Austin Hospital, Melbourne, Australia, from 2010 to 2013 has been included in the study. The top 15 donor, recipient, and transplant factors influencing the outcome of graft failure within 30 days were selected using a machine learning methodology. An algorithm predicting the outcome of interest was developed using those factors | Donor risk index predicts the outcome with an area under the receiver operating characteristic curve (AUC-ROC) value of 0.680 (95%CI: 0.669-0.690). The combination of the factors used in donor risk index with the model for end-stage liver disease score yields an AUC-ROC of 0.764 (95%CI: 0.756-0.771), whereas survival outcomes after liver transplantation (LT) score obtains an AUC-ROC of 0.638 (95%CI: 0.632-0.645). The top 15 donor and recipient characteristics within random forests results in an AUC-ROC of 0.818 (95%CI: 0.812-0.824) | This study confirms that machine-learning algorithms based on donor and recipient variables which are known before organ allocation can be utilized to predict transplant outcomes |
| Liu et al[56], 2020 | A Chinese study using ML to predict 30 d survival after LT | To use data-driven technique to develop a predictive model using ML to predict postoperative survival within 30 days for the patients who have undergone LT | We use random forest (RF) to select important features, including clinically used features and new features discovered from physiological measurement values. Moreover, we propose a new imputation method to deal with the problem of missing values and the results show that it outperforms the other alternatives. In the predictive model, we use patients’ blood test data within 1–9 d before surgery to construct the model to predict postoperative patients’ survival | The experimental results on a real data set indicate that RF outperforms the other alternatives. The experimental results on the temporal validation set show that our proposed model achieves AUC of 0.771 and specificity of 0.815 | ML can detect the high risk patients in early phase after LT, and discover important factors that are essential in LT |
| Yang et al[57], 2022 | A Chinese study in which conventional Scoring systems were compared with ML models in predicting 90 day survival in ACLF patients following LT | To compare the predictive value of conventional models and ML models for predicting 90-d post-transplant survival of ACLF patients based on preoperative variables | Preoperative data of 132 ACLF patients receiving LT at our center were investigated retrospectively. Cox regression was performed to determine the risk factors for short-term survival among ACLF patients following LT. Five conventional score systems (the MELD score, ABIC, CLIF-C OFs, CLIF-SOFAs and CLIF-C ACLFs) in forecasting short term survival were estimated through the ROC. Four machine-learning (ML) models, including support vector machine (SVM), logistic regression (LR), multi-layer perceptron (MLP) and random forest (RF), were also established for short-term survival prediction | Cox regression analysis demonstrated that creatinine (Cr) and international normalized ratio (INR) were the two independent predictors for short-term survival among ACLF patients following LT. The ROC curves showed that the AUC ML models was much larger than that of conventional models in predicting short term survival. Among conventional models the model for end stage liver disease (MELD) score had the highest AUC (0.704), while among ML models the RF model yielded the largest AUC (0.940). (AUROC) of MELDs (AUROC: 0.704) was higher than those of ABIC (AUROC: 0.607), CLIF-C OFs (AUROC: 0.606), CLIF-C ACLFs (AUROC: 0.653), and CLIF-SOFAs (AUROC: 0.633) for prediction of the 90-d outcome in ACLF patients following LT | Compared with the traditional methods, the ML models showed good performance in the prediction of short-term prognosis among ACLF patients following LT and the RF model perform the best |
| Andres et al[58], 2018 | A United States study using ML to construct a prediction tool called PSSP using SRTR data to predict survival following LT for PSC and compared with cox regression in survival analysis | To develop ML models to predict individual survival after LT for Primary Sclerosing Cholangitis (PSC) | We applied a software tool, PSSP, to adult patients in the Scientific Registry of Transplant Recipients (n = 2769) who received a LT for PSC between 2002 and 2013; this produced a model for predicting individual survival distributions for novel patients. We also developed an appropriate evaluation measure, D-calibration, to validate this model | The learned PSSP model showed an excellent D-calibration (P = 1.0), and passed the single-time calibration test (Hosmer-Lemeshow P value of over 0.05) at 0.25, 1, 5 and 10 yr. In contrast, the model based on traditional Cox regression showed worse calibration on long-term survival and failed at 10 yr (Hosmer-Lemeshow P value = 0.027). The overall KM survival curve at 0.25, 1, 3, 5 and 10-yr showed survival probabilities of: 95.6%, 93%, 87.6%, 84.1% and 72% | Our empiricalresults show that the individual survival distributions produced by these models are well calibrated, which means they can be used for this screening task of deciding whether a candidateshould be added to the LT waiting list as they can help predict the survival of a possible recipient (or of a donor/recipient pair) |
| Kong et al[59], 2020 | A Chinese study in which Logistic regression and artificial neural network(ANN) analysis were used to determine the preoperative independent risk factors and protective factors for the survival or death of patients90 days after surgery | To develop a simple ML model for quick prediction of the short-term survival ofpatients after LT in the event that the donor's information is not available in advance | A total of 1495 adult patients underwent LT in the present study. Three-quarters of recipients were randomly selected into the test set (n = 1121), while the remaining 25% formed the validation set (n = 374). Univariate and multivariate analysis and machine-learning techniques were applied to evaluate possible influencing factors. To further simplify the model, a weighted-scoring system was designed considering each influencing factor and its importance in an ANN | In the test set, multivariate analysis identified creatinine, age, and total bilirubin as independent risk factors, while albumin was an independent protective factor. Logistic regression analysis showed the C-statistic to be 0.650, while ANN indicated this to be 0.698. We simplified the model to obtain the final scoring model, for which the C-statistic was 0.636, and defined four risk grades. The 90-d mortality rates corresponding to the four risk levels were 6.2%, 11.8%, 24.0%, and 34.9%, respectively. In the validation set, the C-statistic value of the original model was 0.668 and that of the simplified model was 0.647 | We demonstrated that the postoperative 90-d mortality followingadult LT can be predicted using a scoring system based on recipients' preoperative characteristics |
| Bertsimas et al[60], 2019 | An American study using Optimized prediction of mortality (OPOM) utilizing machine-learning optimal classification tree models trained to predict a candidate’s 3-months waitlist mortality or removal using the standard transplant analysis andresearch (STAR) dataset | To utilize a state-of-the-art machine-learning method-termed optimal classification trees (OCTs)-to generatea more accurate prediction of a liver candidate’s 3-months wait-list mortality or removal | An OPOM was developed (http://www.opom.online) utilizing machine-learning optimal classification tree models trained to predict a candidate’s 3-months waitlist mortality or removal utilizing the STAR dataset. The Liver Simulated Allocation Model (LSAM) was then used to compare OPOM to MELD-based allocation. Out-of-sample area under the curve (AUC) was also calculated for candidate groups of increasing disease severity | OPOM considerably outperformed both MELD variants when predicting the 3-months probability of dying or becoming unsuitable for transplant for all patients (0.859 vs 0.841 for MELD-Na, and 0.823for Match MELD) and across all exception statuses. In addition, analysis of out-of-sample AUC for OPOM, Match MELD and MELD-Na, for subpopulations of patients with increasing dis-ease severity, revealed a notable decline in predictive power for Match MELD and MELD-Na as disease severity increased, whereas OPOM’s predictive power was maintained. The largest divergence in predictive power between OPOM and MELD was at the higher disease severity brackets, with OPOM outperforming Match MELD by up to 16% | OPOM more accurately and objectively prioritizes candidates for LT based on disease severity, allowing for more equitable allocation of livers with a resultant sig- nificant number of additional lives saved every year. These data demonstrate the potential of machine learning technology to help guide clinical practice, and potentially guide national policy |
| He et al[61], 2021 | An American study using image omics and multi-network based deep learning model that converts expertise in LT, full-slide image digitization, and deep machine learning, and integrates multimodality data of quantitative image features with relevant clinical data to identify pre-clinical and biological markers for predicting good post-transplant outcomes, regardless of size | To develop a convergent artificial intelligence (AI) model that combines transient clinical data with quantitative histologic and radiomic features for more objective risk assessment of LT for HCC patient | Patients who received a LT for HCC between 2008-2019 were eligible for inclusion in the analysis. All patients with post-LT recurrence were included, and those without recurrence were randomly selected for inclusion in the deep learning model. Pre- and post-transplant magnetic resonance imaging (MRI) scans and reports were compressed using Caps Net networks and natural language processing, respectively, as input for a multiple feature radial basis function network. We applied a histological image analysis algorithm to detect pathologic areas of interest from explant tissue of patients who recurred. The multilayer perceptron was designed as a feed forward, supervised neural network topology, with the final assessment of recurrence risk. We used AUC and F-1 score to assess the predictability of different network combinations | A total of 109 patients were included (87 in the training group, 22 in the testing group), of which 20 were positive for cancer recurrence. Seven models (AUC; F-1 score) were generated, including clinical features only (0.55; 0.52), MRI only (0.64; 0.61), pathological images only (0.64; 0.61), MRI plus pathology (0.68; 0.65), MRI plus clinical (0.78, 0.75), pathology plus clinical (0.77; 0.73), and a combination of clinical, MRI, and pathology features (0.87; 0.84). The final combined model showed 80% recall and 89% precision. The total accuracy of the implemented model was 82% | We validated that the deep learning model combining clinical features and multi scale histopathologic and radiomic image features can be used to discover risk factors for recurrence beyond tumor size and biomarker analysis |
| Pinto-Marques et al[62], 2022 | A Portuguese study in which the ML model, Hepato-Predict was constructed on retrospective LT data for HCC based on the assessment of a gene expression signature plus clinical variables | To propose a new decision algorithm combining biomarkers measured in a tumor biopsy with clinical variables, to predict recurrence after LT | A literature systematic review singled out candidate biomarkers whose RNA levels were assessed by quantitative PCR in tumor tissue from 138 HCC patients submitted to LT (> 5 yr follow up, 32% beyond Milan criteria). The resulting 4 gene signature was combined with clinical variables to develop a decision algorithm using machine learning approaches. The method was named HepatoPredict | HepatoPredict identifies 99% disease-free patients (> 5 yr) including many outside clinical criteria (16%-24%). Has increased positive predictive value (88.5%-94.4%) without any loss of long-term overall survival or recurrence rates for patients deemed eligible by HepatoPredict; those deemed ineligible display marked reduction of survival and increased recurrence in the short and long term | HepatoPredict outperforms conventional clinical-pathologic selection criteria (Milan, UCSF), providing superior prognostic information |
| Lai et al[63], 2023 | A Taiwanese study in which the ML model ResNet-18 was trained on FDG-PET-CT images to predict outcomes in HCC patients undergoing LT | To evaluate the performance of deep learning from 18F-FDG PET-CT images to predict overall survival in HCC patients before LT | We retrospectively included 304 patients with HCC who underwent 18F-FDG PET/CT before LT between January 2010 and December 2016. The hepatic areas of 273 of the patients were segmented by software, while the other 31 were delineated manually. We analyzed the predictive value of the deep learning model from both FDG PET/CT images and CT images alone | The results of the developed prognostic model were obtained by combining FDG PET-CT images and combining FDG CT images (0.807 AUC vs 0.743 AUC). The model based on FDG PET-CT images achieved somewhat better sensitivity than the model based on CT images alone (0.571 SEN vs 0.432 SEN) | Our retrospective study indicated that an automated 3D ResNet-18 convolutional neural network with FDG-PET-CT has promise for predicting clinical outcomes in patientswith HCC undergoing LDLT and that Automatic liver segmentation from 18F-FDG PET-CT images is feasible and can be utilized to train deep-learning models |
| Kazemi et al[64], 2019 | Iranian study aimed at modelling patient survival after LT using machine-learning methods to investigate influential factors and compare the performance of these methods with a classic statistic method, cox regression | To Identify effective factors for patient survival after LT using ML techniques | Our study included 902 adults who received livers from deceased donors from March 2011 to March 2014 at the Shiraz Organ Transplant Center (Shiraz, Iran). In a 3-step feature selection method, effective features of 6-month survival were extracted by: (1) F statistics, Pearson chi-square, and likelihood ratio chi-square; (2) 5 machine earning techniques. To evaluate the performance of the machine-learning techniques, Cox regression was applied to the data set. Evaluations were based on the area under the receiver operating characteristic curve and sensitivity of models; and (3) We also constructed a model using all factors identified in the previous step | The model predicted survival based on 26 identified effective factors. In the following order, graft failure, Aspergillus infection, acute renal failure and vascular complications after transplant, as well as graft failure diagnosis interval, previous diabetes mellitus, Model for End-Stage Liver Disease score, donor inotropic support, units of packed cell received, and previous recipient dialysis, were found to be predictive factors in patient survival. The area under the receiver operating characteristic curve and model sensitivity were 0.90 and 0.81, respectively | Data mining analyses can help identify effective features of patient survival after livertransplant and build models with equal or higher performance than Cox regression. The order ofinfluential factors identified with the machine learning model was close to clinical experiments |
| Nitski et al[65], 2021 | An American study that examined retrospective data of transplant recipients from the SRTR and UHN to assess the role of deep learning algorithms to predict complications resulting in death after liver transplant over multiple time frames in comparison with logistic regression | To assess the ability of deep learning algorithms of longitudinal data from two prospective cohorts to predict complications resulting in death after LT over multiple timeframes, compared with logistic regression models | In this machine learning analysis, model development was done on a set of 42 146 liver transplant recipients [mean age 48.6 yr (SD 17.3); 17 196 (40.8%) women] from the Scientific Registry of Transplant Recipients (SRTR) in the United States. Transferability of the model was further evaluated by fine-tuning on a dataset from the UHN in Canada [n = 3269; mean age 52.5 yr (11.1); 1079 (33.0%) women]. The primary outcome was cause of death, as recorded in the databases, due to cardiovascular causes, infection, graft failure, or cancer, within 1 yr and 5 yr of each follow-up examination after transplantation. We compared the performance of four deep learning models against logistic regression, assessing performance using the AUROC | In both datasets, deep learning models outperformed logistic regression, with the Transformer model achieving the highest AUROCs in both datasets (P < 0.0001). The AUROC for the Transformer model across all outcomes in the SRTR dataset was 0.804 (99%CI: 0.795-0.854) for 1-yr predictions and 0.733 (0.729-0.769) for 5-yr predictions. In the UHN dataset, the AUROC for the top-performing deep learning model was 0.807 (0.795-0.842) for 1-yr predictions and 0.722 (0.705–0.764) for 5-yr predictions. AUROCs ranged from 0.695 (0.680–0.713) for prediction of death from infection within 5 yr to 0.859 (0.847-0.871) for prediction of death by graft failure within 1 yr | Deep learning algorithms can incorporate longitudinal information to continuously predict long-term outcomes after LT, outperforming logistic regression models |
| Ivanics et al[66], 2022 | A multinational study of ML models assessing their 90-d predictive value post LT across United States, Canada and | To evaluate the feasibility of developing MLA-based models to predict 90-d post-LT mortality using 3 large nationaltransplant registries and to evaluate the external validity of the models across countries | We used data from 3 national registries and developed machine learning algorithm (MLA)–based models to predict 90-d post-LT mortality within and across countries. Predictive performance and external validity of each model were assessed. Prospectively collected data of adult patients (aged ≥ 18 yr) who underwent primary LTs between January 2008 and December 2018 from the Canadian Organ Replacement Registry (Canada), National Health Service Blood and Transplantation (United Kingdom), and United Network for Organ Sharing (United States) were used to develop MLA models to predict 90-d post-LT mortality. Models were developed using each registry individually (based on variables inherent to the individual databases) and using all 3 registries combined (variables in common between the registries [harmonized]). The model performance was evaluated using AUROC curve. The number of patients included was as follows: Canada, n = 1214; the United Kingdom, n = 5287; and the United States, n = 59558 | The best performing MLA-based model was ridge regression across both individual registries and harmonized data sets. Model performance diminished from individualized to the harmonized registries, especially in Canada (individualized ridge: AUROC, 0.74; range, 0.73-0.74; harmonized: AUROC, 0.68; range, 0.50-0.73) and US (individualized ridge: AUROC, 0.71; range, 0.70-0.71; harmonized: AUROC, 0.66; range, 0.66-0.66) data sets. External model performance across countries was poor overall | External model performance across countries was poor overall. MLA-based models yield a fair discriminatory potential when used within individual databases. However, the external validity of these models is poor when applied across countries |
| Cheong et al[67], 2021 | A Korean study assessing the role of pre LT hyperlactatemia in early mortality post LT | To study important variables for pre-LT hyperlactatemia and examine the impact of preoperative hyperlactatemia on 30 and 90 d mortality after LT | A total of 2002 patients from LT registry between January 2008 and February 2019 were analyzed. Six organ failures (liver, kidney, brain, coagulation, circulation, and lung) were defined by criteria of EASL-CLIF ACLF Consortium. Variable importance of pre-operative hyperlactatemia was examined by machine learning using random survival forest (RSF). Kaplan-Meier Survival curve analysis was performed to assess 90-d mortality | Median lactate level was 1.9 mmol/L (interquartile range: 1.4, 2.4 mmol/L) and 107 (5.3%) patients showed > 4.0 mmol/L. RSF analysis revealed that the four most important variables for hyperlactatemia were MELD score, circulatory failure, hemoglobin, and respiratory failure. The 30-d and 90-d mortality rates were 2.7% and 5.1%, whereas patients with lactate > 4.0 mmol/L showed increased rate of 15.0% and 19.6%, respectively | Pre-LT lactate > 4.0 mmol/L was associated with increased early post-LT mortality. Our results suggest that future study of correcting modifiable risk factors may play a role in preventing hyperlactatemia and lowering early mortality after LT |
| Kulkarni et al[68], 2021 | An American study using Random Forest approach to identify key predictors of outcomes in pediatric candidates less than 2 yr of age undergoing LT | To identify key predictors of LT outcomes in Pediatric candidates less than 2 yr of age using random forest approach | SRTR database was queried for children < 2 yr listed for initial LT during 2002-17 (n = 4973). Subjects were divided into three outcome groups; bad (death or removal for too sick to transplant), good (spontaneous improvement) and transplant. Demographic, clinical, listing history and laboratory variables at the time of listing (baseline variables), and changes in variables between listing and prior to outcome (trajectory variables) were analyzed using random forest analysis | 81.5% candidates underwent LT, 12.3% had bad outcome. RF model including both baseline and trajectory variables improved prediction compared to model using baseline variables alone. RF analyses identified change in serum creatinine and listing status as the most predictive variables. 80% of subjects listed with a PELD score at time of listing and outcome underwent LT, while 70% of subjects in both bad and good outcome groups were listed with either Status 1 (A or B) prior to an outcome, regardless of initial listing status. Increase in creatinine on LT waitlist was predictive of bad outcome. Longer time spent on WL was predictive of good outcome. Subjects with biliary atresia, liver tumors and metabolic disease had LT rate > 85%; while > 20% of subjects with acute liver failure had a bad outcome | Change in creatinine, listing status, need for RRT, time spent on LT waitlist and diagnoses were the most predictive variables |
| Molinari et al[69], 2019 | An American study using ML techniques to identify predictors of short and long term mortality post cadaveric LT | To develop a scoring system using ML that could stratify patients by their risk of death after LT based only on preoperative variables. Secondary aims were to assess whether the model could also predict 1- and 5-yr patient survival | The study population was represented by 30458 adults who underwent LT in the United States between January 2002 and June 2013. Machine learning techniques identified recipient age, Model for End-Stage Liver Disease score, body mass index, diabetes, and dialysis before LT as the strongest predictors for 90-d postoperative mortality. A weighted scoring system (minimum of 0 to a maximum of 6 points) was subsequently developed | Recipients with 0, 1, 2, 3, 4, 5, and 6 points had an observed 90-d mortality of 6.0%, 8.7%, 10.4%, 11.9%, 15.7%, 16.0%, and 19.7%, respectively (P ≤ 0.001). One-year mortality was 9.8%, 13.4%, 15.8%, 17.2%, 23.0%, 25.2%, and 35.8% (P ≤ 0.001) and five-year survival was 78%, 73%, 72%, 71%, 65%, 59%, and 48%, respectively (P = 0.001). The mean 90-d mortality for the cohort was 9%. The area under the curve of the model was 0.952 for the discrimination of patients with 90-day mortality risk ≥ 10% | Short- and long-term outcomes of patients undergoing cadaveric LT can be predicted using a scoring system based on recipients’ preoperative characteristics |
| Cooper et al[70], 2022 | A United States study predicting the risk of GVHD among patients undergoing OLT using ML models | To develop ML algorithms for predicting the risk of GVHD among patients undergoing OLT | To develop a predictive model, we retrospectively evaluated the clinical features of 1938 donor-recipient pairs at the time they underwent OLT at our center; 19 (1.0%) of these recipients developed GVHD. This population was divided into training (70%) and test (30%) sets. A total of 7 machine-learning classification algorithms were built based on the training data set to identify patients at high risk for GVHD | The C5.0, heterogeneous ensemble, and generalized gradient boosting machine (GGBM) algorithms predicted that 21% to 28% of the recipients in the test data set were at high risk for developing GVHD, with an AUROC of 0.83 to 0.86. The 7 algorithms were then evaluated in a validation data set of 75 more recent donor-recipient pairs who underwent OLT at our center; 2 of these recipients developed GVHD. The logistic regression, heterogeneous ensemble, and GGBM algorithms predicted that 9% to 11% of the validation recipients were at high risk for developing GVHD, with an AUROC of 0.93 to 0.96 that included the 2 recipients who developed GVHD | we show that a machine-learning approach can predict which recipients are at high risk for developing GVHD after OLT based on factors known or measurable at the time of transplantation |
| He et al[71], 2021 | A Chinese study comparing the predicting power of ML models and logistic regression for AKI among patients undergoing DCDLT | To compare the performance of ML algorithms to that of a logistic regression model for predicting AKI after LT using preoperative and intraoperative data | A total of 493 patients with donation after cardiac death LT (DCDLT) were enrolled. AKI was defined according to the clinical practice guidelines of kidney disease: improving global outcomes (KDIGO). The clinical data of patients with AKI (AKI group) and without AKI (non-AKI group) were compared. With logistic regression analysis as a conventional model, four predictive machine learning models were developed using the following algorithms: Random forest, support vector machine, classical decision tree, and conditional inference tree. The predictive power of these models was then evaluated using the AUC | The incidence of AKI was 35.7% (176/493) during the follow-up period. Compared with the non AKI group, the AKI group showed a remarkably lower survival rate (P < 0.001). The random forest model demonstrated the highest prediction accuracy of 0.79 with AUC of 0.850 (95%CI: 0.794-0.905), which was significantly higher than the AUCs of the other machine learning algorithms and logistic regression models (P < 0.001) | The random forest model based on machine learning algorithms for predicting AKI occurring after DCDLT demonstrated stronger predictive power than other models in our study |
| Chen et al[72], 2023 | A Chinese study predicting the risk of sepsis within 7 days post LT | Our study aimed to develop and validate a predictive model for postoperative sepsis within 7 days in LT recipients using ML technology | Data of 786 patients who received LT from January 2015 to January 2020 was retrospectively extracted from the big data platform of Third Affiliated Hospital of Sun Yat-sen University. Seven ML models were developed to predict postoperative sepsis. The AUC, sensitivity, specificity, accuracy, and f1-score were evaluated as the model performances. The model with the best performance was validated in an independent dataset involving 118 adult LT cases from February 2020 to April 2021. The postoperative sepsis-associated outcomes were also explored in the study | After excluding 109 patients according to the exclusion criteria, 677 patients who underwent LT were finally included in the analysis. Among them, 216 (31.9%) were diagnosed with sepsis after LT, which were related to more perioperative complications, increased postoperative hospital stay and mortality after LT (all P < 0.05). Our results revealed that a larger volume of red blood cell infusion, ascitic removal, blood loss and gastric drainage, less volume of crystalloid infusion and urine, longer anesthesia time, higher level of preoperative TBIL were the top 8 important variables contributing to the prediction of post-LT sepsis. The RF model showed the best overall performance to predict sepsis after LT among the seven ML models developed in the study, with an AUC of 0.731, an accuracy of 71.6%, the sensitivity of 62.1%, and specificity of 76.1% in the internal validation set, and a comparable AUC of 0.755 in the external validation set | The random forest classifier model showed the best overall performance to predict sepsis after LT |
| Lee et al[73], 2018 | A Korean study comparing the predicting power for AKI post LT of ML models and logistic regression | To compare the performance of machine learning approaches with that of logistic regression analysis to predict AKI after LT | We reviewed 1211 patients and preoperative and intraoperative anesthesia and surgery-related variables were obtained. The primary outcome was postoperative AKI defined by acute kidney injury network criteria. The following machine learning techniques were used: decision tree, random forest, gradient boosting machine, support vector machine, naïve Bayes, multilayer perceptron, and deep belief networks. These techniques were compared with logistic regression analysis regarding the AUROC | AKI developed in 365 patients (30.1%). The performance in terms of AUROC was best in gradient boosting machine among all analyses to predict AKI of all stages (0.90, 95%CI: 0.86-0.93) or stage 2 or 3 AKI. The AUROC of logistic regression analysis was 0.61 (95%CI: 0.56-0.66). Decision tree and random forest techniques showed moderate performance (AUROC 0.86 and 0.85, respectively) | In our comparison of seven machine learning approaches with logistic regression analysis, the gradient boosting machine showed the best performance with the highest AUROC |
| Bredt et al[74], 2022 | A Brazilian study investigating risk factors of AKI post DDLT using ML and Logistic regression | To identify the risk factors of AKI after deceased-donor LT (DDLT) and compare the prediction performance of ANN with that of LR for this complication | Adult patients with no evidence of end-stage kidney dysfunction (KD) who underwent the first DDLT according to model for end-stage liver disease (MELD) score allocation system were evaluated. AKI was defined according to the International Club of Ascites criteria, and potential predictors of postoperative AKI were identified by LR. The prediction performance of both ANN and LR was tested | The incidence of AKI was 60.6% (n = 88/145) and the following predictors were identified by LR: MELD score > 25 (OR = 1.999), preoperative kidney dysfunction (OR = 1.279), extended criteria donors (OR = 1.191), intraoperative arterial hypotension (OR = 1.935), intraoperative massive blood transfusion (MBT) (OR = 1.830), and postoperative serum lactate (SL) (OR = 2.001). The area under the receiver-operating characteristic curve was best for ANN (0.81, 95%CI: 0.75-0.83) than for LR (0.71, 95%CI: 0.67-0.76). The root-mean-square error and mean absolute error in the ANN model were 0.47 and 0.38, respectively | The severity of liver disease, pre-existing kidney dysfunction, marginal grafts, hemodynamic instability, MBT, and SL are predictors of postoperative AKI, and ANN has better prediction performance than LR in this scenario |
The majority of the included studies were considered to be of good quality, despite being observational in nature and not appraised using any specific quality assessment tool. Many of these studies incorporated validation sets in their analyses, which contributes to the robustness of their findings.
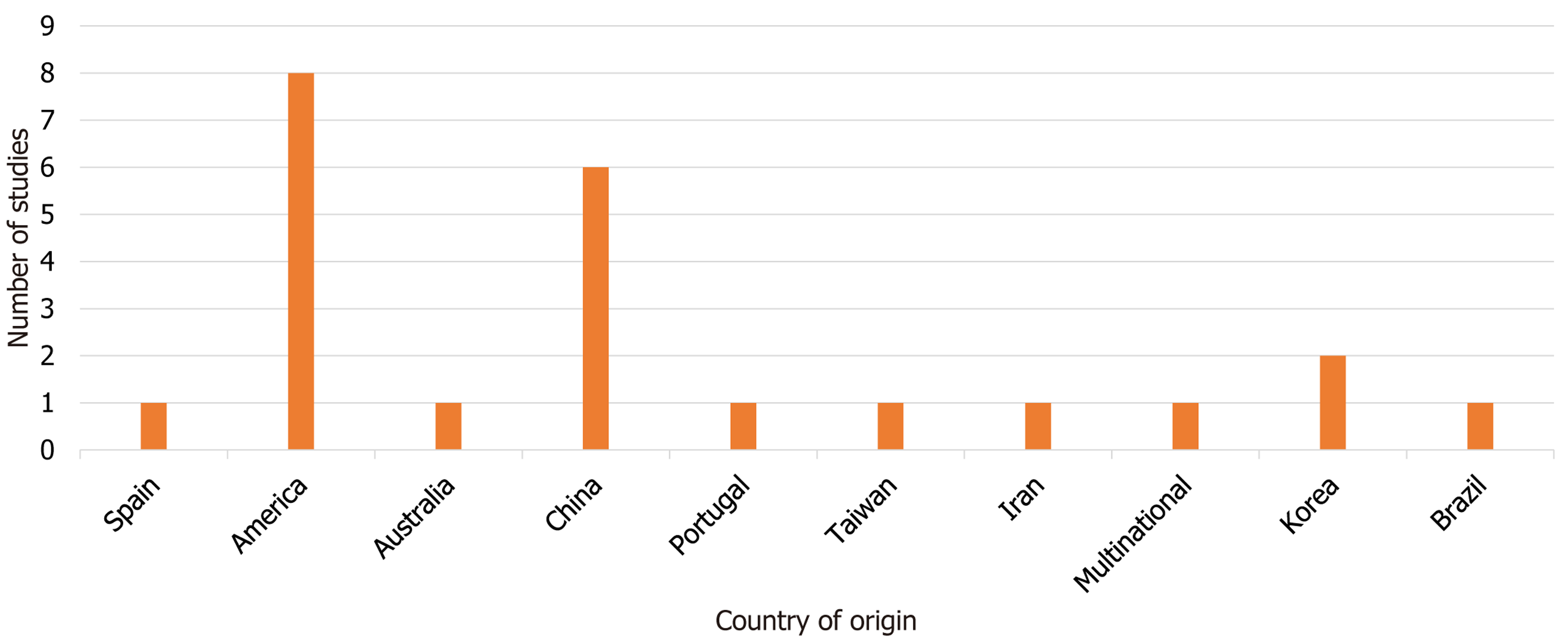
Study outcomes: The studies assessed in this systematic review covered a range of transplantation reasons, including ACLF from various causes, primary sclerosing cholangitis (PSC), and hepatocellular carcinoma (HCC). Among the 23 studies analyzed, the highest number (8 studies, accounting for 34.8%) were conducted in America, followed by 6 studies (26%) from China. Additionally, 2 studies (8.7%) were from Korea, while the remaining studies originated from Spain, Australia, Portugal, Taiwan, Iran, and Brazil, each contributing 1 study (4.3%). Furthermore, there was one multinational study involving participants from the United States, Canada, and the United Kingdom, which represented 4.3% of the total sample as depicted by Figure 4.
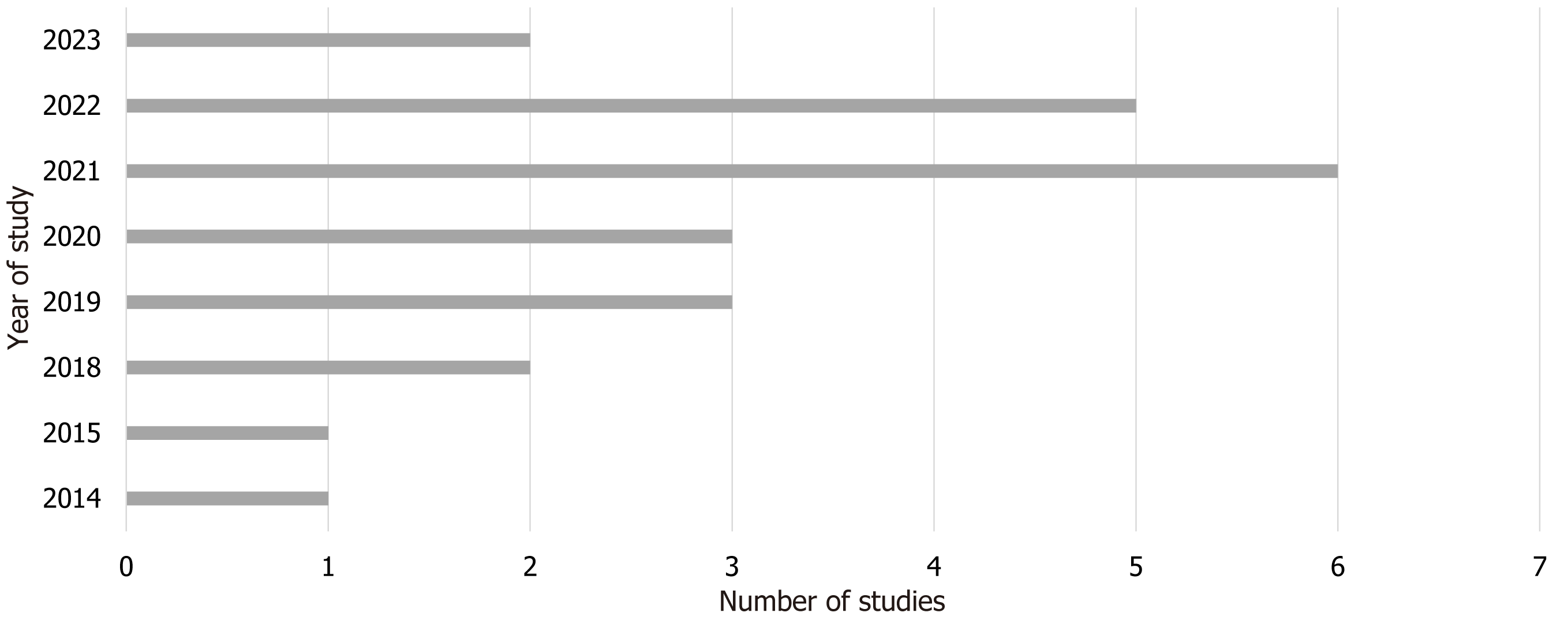
The studies analyzed in this review spanned from 2014 to 2023. Notably, the highest proportion of studies (26%, 6 studies) were published in 2021, followed by 5 studies (21.7%) from 2022. Studies from 2019, 2020, 2018, and 2023 accounted for 13% (3 studies) each, while 2014 and 2015 each contributed 1 study (4.3%) as shown in Figure 5. Regarding the age of participants, one study involved individuals under 18 years old, while the remaining 22 studies focused on adults aged 18.
The primary outcomes of interest in the included studies were mortality and the emergence of complications post liver transplant. Most of the studies reported the receiver operating characteristic (ROC) curve and used the area under the ROC curve (AUROC) as a measure of predictive performance. AUROC values were categorized as excellent (0.9-1), very good (0.8-0.9), good (0.7-0.8), satisfactory (0.6-0.7), and unsatisfactory (0.5-0.6) based on previous classification[75].
Across all the studies, ML algorithms and models were developed using pre-transplant donor and/or recipient variables. Short-term mortality predictions were typically up to 90 d, while long-term predictions extended up to 5 years. Analysis of AUROC demonstrated that ML models consistently yielded satisfactory to excellent results in predicting short and long-term mortality or the risk of complications post liver transplant.
Furthermore, the AUROC analysis revealed that ML models outperformed traditional models and scoring systems, including commonly used models such as MELD, D-MELD, SOFT, P-SOFT, BAR, DRI score, ABIC, CLIF-C OFs, CLIF-C ACLFs, and CLIF SOFA. Additionally, ML models showed superiority over models based on Cox and LR. Detailed comparisons and findings are presented in Table 1.
Sub-analysis: In terms of predicting 90-d mortality, the RF model demonstrated the highest area under the curve (AUC) value of 0.940 compared to other ML models. Additionally, among the six studies identified in the literature search that discussed the prediction of complications post liver transplant using ML models, an analysis of the AUC values indicated that the 'gradient boosting machine' model performed better than other ML models in predicting the risk of graft-versus-host disease (GVHD), pneumonia, and acute kidney injury (AKI). On the other hand, the RF model showed better performance in predicting the risk of sepsis and AKI post liver transplant. Detailed results and comparisons are provided in Table 1.
This sub-analysis highlights the specific performance of ML models in predicting 90-d mortality and the risk of complications following LT. The RF model exhibited superior predictive capability for mortality within the 90-d timeframe.
Furthermore, when examining the prediction of post-transplant complications, the 'gradient boosting machine' model demonstrated better performance in predicting GVHD, pneumonia, and AKI, while the RF model showed greater effectiveness in predicting the risk of sepsis and AKI. These findings emphasize the potential of ML techniques in enhancing prognostic accuracy and tailoring clinical management strategies in LT.
The review highlights a limited number of studies, just 64, that have explored the application of ML models in the context of LT. This scarcity of research, despite an unrestricted search, indicates a historical lack of emphasis on the potential of ML models in the realm of prognosis and transplant decision-making. Factors contributing to this limited attention include lingering perceptions of ML models as associated with science fiction and concerns regarding potential errors and patient harm. However, it's noteworthy that ML models have advanced in sophistication and have implemented strategies to address challenges like overfitting. Their effectiveness is contingent upon access to substantial datasets for continuous learning and refinement[76].
In recent years, there has been a notable surge in research at the intersection of ML and LT, particularly within the last five years. Among the 23 studies reviewed, a substantial majority (91%) were conducted between 2018 and 2023, sig
Crucially, ML methods employed for the allocation of orthotopic liver transplants, whether from living donors, deceased donors, or cadaveric sources, should be rooted in population-specific parameters pertaining to the recipient. This individualized approach is essential to ensure post-transplant longevity and minimize the risk of complications. The utilization of ML models that take into account an individual's unique population parameters or variables to assess the risk of mortality prior to transplantation holds the potential to prevent unnecessary mortality and morbidity associated with high-risk transplantations[78].
Concerning the underlying reasons for transplantation, factors such as ACLF, PSC, and HCC have been prominent considerations. Existing studies have demonstrated the pivotal role of LT as a life-saving intervention for ACLF patients[79]. ACLF can manifest at any stage of chronic liver disease, leading to a rapid deterioration in liver function and a high mortality rate within a short timeframe[80], as it is noticeable a high mortality rate for non-transplanted ACLF patients within 28 and 90 d[81,82].
LT is a critical treatment option for various liver-related conditions, including ACLF, PSC, and HCC. However, the efficacy of LT in ACLF patients remains debated, with conflicting findings suggesting no significant survival advantage over non-transplanted patients[83]. ML models have the potential to improve the assessment of short-term mortality risk in ACLF patients post-transplantation, thereby aiding in the allocation of liver allografts and potentially enhancing outcomes[79]. It is imperative to expand the scope of research on ML models in LT to encompass diverse patient populations, thereby increasing the external validity of these models. Customizing ML algorithms to specific transplant registries and incorporating population-specific parameters can enhance the accuracy and effectiveness of prognosis and decision-making in LT.
PSC is a chronic liver disease characterized by progressive bile duct inflammation, cholestasis, and fibrosis. LT is the primary treatment for end-stage PSC, yielding generally favorable outcomes, although complications like cholangiocarcinoma, recurrent disease, worsening of inflammatory bowel disease, and an elevated risk of colonic cancer pose challenges[84]. Cholangiocarcinoma develops in 8%-18% of long-standing PSC patients[85], and PSC recurrence post-transplantation is observed in some cases[86]. Increased dysplasia and colon cancer risk are also associated with colitis patients having coexisting PSC[87,88]. Consequently, accurate evaluation and allocation of liver allografts in PSC patients are critical, with ML algorithms incorporating pertinent variables from PSC patients facilitating informed and precise decision-making[86-89].
HCC is a common indication for LT, ranking fifth among the most prevalent malignancies and being the third leading cause of cancer-related mortality worldwide[90-92]. LT offers a promising therapeutic option for long-term survival in HCC cases by addressing both advanced liver disease and HCC itself[93,94]. However, the risk of HCC recurrence post-transplantation underscores the necessity for careful patient selection. HCC recurrence occurs most frequently among liver transplant recipients compared to other liver diseases, estimated at 8%-20%[95]. Guidelines recommend active post-transplant surveillance for HCC patients, such as regular liver imaging tests within the first postoperative year and subsequent monitoring to detect lung metastases[96]. Tumor recurrence in HCC patients after transplantation is often attributed to advanced tumor burden and unclear tumor biology[97].
The Milan criteria, comprising specific size and number requirements for liver lesions along with the absence of vascular invasion or extra-hepatic metastases, were established to guide LTs for HCC[98]. Transplantations adhering to these criteria have demonstrated comparable survival outcomes to those performed for cirrhosis. However, criticism of the Milan criteria centers on their strictness in terms of lesion size and number, with some studies suggesting successful transplantation outcomes for HCC patients beyond these criteria. Additionally, the Milan criteria do not account for tumor biology, potentially limiting their applicability[99].
Down-staging, a strategy involving loco-regional therapy to reduce tumor burden and bring lesions outside the transplant criteria within the criteria, has shown promise in achieving favorable long-term outcomes for HCC patients beyond the Milan criteria. Nevertheless, tumor recurrence remains a concern, occurring in 8%-20% of transplanted HCC patients, typically within 2 years post-transplantation, with a median survival of 1 year following recurrence diagnosis[100].
To address the risk of tumor recurrence, various prognostic scores have been developed, such as the Risk Estimation of Tumor REcurrence After Transplant (RETREAT) score. This score considers three factors associated with post-transplant HCC recurrence: explant liver tumor burden, microvascular invasion evidence, and alpha-fetoprotein levels at the time of transplant. The RETREAT score ranges from 0 to 8, with higher scores indicating an elevated risk of recurrence. A score of 0 corresponds to a 1% recurrence rate at 1 year and a 2.9% recurrence rate at 5 years. Conversely, RETREAT scores of 5 or higher are associated with 1- and 5-year HCC recurrence rates of 39.3% and 75.2%, respectively[101]. Deep learning models can be used for diagnosis of HCC[102,103].
The RETREAT score, while valuable for post-transplant management, has limitations as it relies on factors that assess explant tissue biology and anatomy. This restricts its utility to assessing transplant failure risk after transplantation. ML models, utilizing pre-transplant data in HCC patients, can effectively allocate liver allografts before transplantation, thereby enhancing long-term survival prospects[101].
Although ML is gaining traction in various medical disciplines, this review reveals a dearth of pediatric studies among the 23 studies discussing ML and LT. This shortage reflects the limited interest in applying ML in pediatric patients, aligning with trends in other pediatric disciplines where ML adoption has been low. Consequently, there's a clear need for more research on ML in pediatric LT to assess its impact in this domain[104]. Furthermore, the high mortality rate in pediatric acute liver failure underscores the importance of robust criteria, including ML models, to inform decision-making in this patient group[105].
Evaluating ML model performance involves various metrics like accuracy, precision, confusion matrix, recall, speci
The utilization of ML algorithms in LT prognostication is a significant advancement. These models are primarily based on pre-transplant donor and recipient data, allowing for accurate predictions before transplantation. Considering that crucial decisions regarding LT must be made pre-procedure, ML models hold promise in addressing the complex challenge of allocating allografts to the most suitable recipients[101].
Numerous studies reviewed consistently indicate that ML models provide satisfactory to excellent predictions for both short- and long-term mortality or complication risks[106]. Additionally, emerging evidence suggests that AI can surpass traditional tools in predicting cardiac events post LT[107] and mortality related to esophageal variceal bleeding[108,109]. Accurate predictions of short- and long-term complications following LT are crucial, as they inform the need for addi
Long-term survivors face increased risks of comorbidities like metabolic syndrome, renal dysfunction, cardiovascular disease, and extrahepatic malignancies, necessitating multidisciplinary management strategies to prevent medical complications and their associated cost implications[111,112]. Metabolic syndrome, in particular, is prevalent among liver transplant recipients and is associated with chronic liver disease progression and increased cardiovascular risk[110]. Sustained transient post-transplant diabetes significantly elevates the long-term risk of major adverse cardiac events and mortality[113]. Therefore, precise prognostication of patients at risk of long-term complications is essential, and AI algorithms offer promise in enhancing risk assessment and improving patient outcomes.
Furthermore, ML models consistently outperform traditional scoring systems, including MELD, D-MELD, SOFT, p-SOFT, BAR, DRI score, ABIC, CLIF-C OFs, CLIF-C ACLFs, and CLIF SOFA, as well as models based on Cox and LR. This finding is particularly significant given the limitations of traditional scoring systems in predicting post-transplant outcomes[101]. The incorporation of ML algorithms in organ allocation can enhance efficiency by preventing unnecessary transplantations and allocating allografts to patients with a higher likelihood of success. This optimization helps manage the associated costs of transplant failure and complications, especially considering the limited availability of donor organs. Regarding short and long-term mortality prediction (90-d), the RF model consistently exhibits the highest AUC[114,115].
ML models provide numerous advantages, such as managing large datasets, objectivity, and assisting in cases with similar probabilities. In LT, ANNs and RF classifiers are the commonly used AI models. ANNs excel at identifying complex patterns beyond human capability and can yield near-perfect predictions, reaching up to 95% accuracy in 3-months graft survival. However, ANNs lack transparency regarding the variables they consider. In contrast, RF models offer better confidence in utilizing marginal organs, resulting in improved post-transplantation outcomes[114].
RF models exhibit superiority when predicting the risk of sepsis and AKI. Although overall survival post-LT has improved, post-transplantation infections remain a significant challenge, contributing to morbidity and mortality. Studies reveal that 35%-55% of liver transplant recipients experience infection-related complications, including bacterial, fungal, and multidrug-resistant infections. Most of these infections occur within the first six months after transplantation and are responsible for a significant portion of early post-transplant deaths[116-119].
AKI and chronic renal dysfunction are common complications following LT. Contributing factors include long-term exposure to immunosuppressive medications like calcineurin inhibitors, preoperative kidney dysfunction, perioperative AKI/hypertension, diabetes mellitus (DM), and atherosclerosis pre- and/or post-transplantation. Long-term data indicates that kidney failure, defined as a glomerular filtration rate of 29 mL/min/1.73 m² or less or the development of end-stage renal disease, occurs in 18% at 5 years and 25% at 10 years post-transplantation[120]. Factors significantly asso
The use of ML models in predicting the risk of sepsis and AKI is vital to enhance post-liver transplant outcomes. Post-transplant infections and AKI are associated with increased healthcare costs, prolonged hospital stays, and adverse effects on both allograft and patient survival[116,119]. Also, ML models have been used for the diagnosis of appendicitis and heart disease[123,124]. Employing ML models for predicting and managing these complications holds the potential to yield improved patient outcomes, reduced healthcare expenditures, and an overall better quality of life.
Despite the demonstrated superiority of ML models in the review, certain limitations must be acknowledged. Many studies relied on retrospective designs, which can introduce biases and impact result generalizability. Prospective studies with larger sample sizes and more diverse populations are necessary to validate ML model performance across different contexts and patient groups.
Another limitation stems from the lack of standardization and consistency in data collection and reporting of LT-related variables across various centers and studies. Data collection disparities can result in inconsistencies and hinder accurate comparisons of different ML models. Efforts should be made to standardize data collection practices in LT research to enhance the reliability and general applicability of ML models.
The underrepresentation of pediatric LT in the reviewed studies underscores a research gap. Pediatric patients have unique considerations and challenges in LT, and developing ML models tailored to this population could significantly enhance their outcomes.
Ethical considerations are paramount when implementing ML models in clinical decision-making. These models must be transparent, explainable, and accountable to ensure that clinicians and patients comprehend the rationale behind predictions, enabling informed decisions. Furthermore, addressing the black box dilemma of AI models for prognostication is imperative, as ensuring transparency and interpretability in these models is essential to uphold ethical standards in healthcare decision-making.
This study reveals a significant surge in interest in the application of ML for liver transplant prognostication, with the majority of the studies emerging within the past five years. Notably, the United States and China stand out as the frontrunners in this field. This research also emphasizes that the performance of ML models exhibits variability when applied across different countries, underscoring limited external validity. Consequently, ML algorithms tailored to each country's unique transplant registry data demonstrate greater reliability.
Furthermore, the study highlights the superior predictive accuracy of ML models built on pre-transplant data in comparison to established scoring systems like MELD, irrespective of the underlying cause of hepatic failure, including HCC. Additionally, the study suggests that when selecting an ML model for predicting the risk of sepsis and AKI post-LT, the RF model may be the most suitable choice.
Overall, the use of ML models in LT has the potential to optimize organ allocation, improve patient outcomes, and reduce healthcare costs. However, more prospective studies with larger and diverse populations are needed to validate ML model performance and standardize data collection practices in LT research. Additionally, the inclusion of pediatric patients in ML research is crucial to address their unique needs. With continued research and advancements in ML techniques, ML models are poised to play an increasingly pivotal role in LT in the coming years.
Liver transplantation (LT) is a life-saving procedure for individuals with end-stage liver disease, offering not only health restoration but also a potential 15-year extension of life. However, the equitable allocation of donor organs remains a challenge due to donor scarcity. While the survival rates post-transplant are commendable, the shortage of donor organs persists, pushing the field towards utilizing less conventional donors. An efficient system of liver organ allocation is essential as there's a growing demand, leading to escalating healthcare costs. Traditional scoring systems like Child-Turcotte-Pugh and model for end-stage liver disease (MELD) have been employed for organ allocation, but they have limitations, such as empirical variable selection and limited predictive ability.
The primary challenge in LT is optimizing organ allocation. The scarcity of donor organs necessitates accurate prognostication for organ allocation and transplant success. While traditional scoring systems have been useful, they are not without limitations. Therefore, there's a need to explore more reliable and predictive methods. In this context, machine learning (ML) models present a promising avenue. ML algorithms can analyze various data types, from structured to unstructured, and offer a new dimension in predictive accuracy. Their ability to handle complex datasets and discover intricate patterns makes them suitable for enhancing prog
The primary objectives of this study are to comprehensively assess the effectiveness of ML models in LT prognostication and to compare their performance and reliability with traditional scoring systems. This evaluation involves a systematic review of observational studies to determine the real-world utility of ML models in predicting transplant outcomes. Realizing these objectives is crucial for advancing the field of LT and ensuring that patients receive the most suitable organs, ultimately improving survival rates and healthcare resource allocation. Moreover, the study aims to bridge the gap between ML and traditional scoring systems, shedding light on the potential of ML models to revolutionize prognostication in LT.
This systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines and conducted a comprehensive literature search on PubMed/MEDLINE using specific terms related to ML, artificial intelligence (AI), LT, and prognosis. It included all relevant observational studies without restrictions on publication year, age, or gender, focusing on ML models for LT prognosis and post-transplant complications. Exclusion criteria covered non-English papers, review articles, case reports, conference papers, studies with missing data, or methodological flaws. A single reviewer screened and analyzed eligible studies, summarizing their objectives, methods, results, and con
In this systematic review, an initial pool of 64 references was identified and refined through a selection process. After excluding conference articles, review papers, and duplicates, 23 studies were included for analysis. These studies spanned from 2014 to 2023 and covered various transplantation reasons, with the majority conducted in the United States (34.8%), followed by China (26%). The primary outcomes assessed were mortality and post-transplant complications, with ML models consistently outperforming traditional models and scoring systems. The receiver operating characteristic curve analysis demonstrated ML models' excellent predictive performance for both short-term and long-term outcomes. Notably, the Random forest (RF) model excelled in predicting 90-d mortality, while the 'gradient boosting machine' model showed proficiency in forecasting complications like graft-versus-host disease, pneumonia, and acute kidney injury (AKI). The RF model was particularly adept at predicting sepsis and AKI. These findings highlight the potential of ML to enhance prognostic accuracy and inform clinical management in LT.
This study underscores the growing interest in applying ML to liver transplant prognostication, with a surge in research within the last five years. Notably, the United States and China have been leaders in this field. The research emphasizes the need for customized ML algorithms, adapted to each country's unique transplant registry data, to enhance the reli
The future of research in this field should focus on conducting more prospective studies with larger and diverse patient populations to validate the performance of ML models and enhance their generalizability. Standardizing data collection practices in LT research is crucial to ensure consistency and facilitate accurate comparisons of different ML models. Furthermore, there is a pressing need to include pediatric patients in ML research to address their unique requirements and challenges in LT. Ethical considerations should remain paramount, with a focus on ensuring transparency, explainability, and accountability in ML models to uphold ethical standards in healthcare decision-making. Continued advancements in ML techniques and the expansion of research efforts are expected to play an increasingly pivotal role in LT, offering the potential to further enhance patient care and clinical decision-making in the coming years.
We extend our appreciation to the Faculty of Life Sciences and Education at the University of South Wales for the Gastroenterology MSc program and their invaluable support in our work. We sincerely acknowledge the efforts of the University of South Wales and commend them for their commitment to providing life-long learning opportunities and advanced life skills to Healthcare professionals.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Federação Brasileira De Gastroenterologia; Sociedade Brasileira de Hepatologia.
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mijwil MM, Iraq S-Editor: Qu XL L-Editor: A P-Editor: Zhang YL
| 1. | Dababneh Y, Mousa OY. Liver Transplantation. 2023 Apr 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 2. | Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17 Suppl 1:174-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 3. | Jadlowiec CC, Taner T. Liver transplantation: Current status and challenges. World J Gastroenterol. 2016;22:4438-4445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 238] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 4. | Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med. 1968;278:608-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Siddiqui NA, Ullah N, Shaikh JR, Bhandari S, Ullah U, Khan SF, Khan OQ, Mohammed Abdul MK. Worse Outcomes Associated With Liver Transplants: An Increasing Trend. Cureus. 2021;13:e17534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Mansour A, Watson W, Shayani V, Pickleman J. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122:730-5; discussion 735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 250] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Rashed E, Soldera J. CLIF-SOFA and CLIF-C scores for the prognostication of acute-on-chronic liver failure and acute decompensation of cirrhosis: A systematic review. World J Hepatol. 2022;14:2025-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 8. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5738] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 9. | Tsoris A, Marlar CA. Use Of The Child Pugh Score In Liver Disease. 2023 Mar 13. In: StatPearls [Internet]. StatPearls Publishing;. 2023 Jan. [PubMed] |
| 10. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 427] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 11. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 12. | Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, Lucey MR. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol. 2004;40:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 282] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, Pandya P, Sitaraman S, Shen J. Model for end-stage liver disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology. 2002;35:1282-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Albillos A, Colombato LA, Groszmann RJ. Vasodilatation and sodium retention in prehepatic portal hypertension. Gastroenterology. 1992;102:931-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis. 2000;32:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, Kamath P, Klintmalm G. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 549] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 19. | Londoño MC, Cárdenas A, Guevara M, Quintó L, de Las Heras D, Navasa M, Rimola A, Garcia-Valdecasas JC, Arroyo V, Ginès P. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56:1283-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, Burroughs AK. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Bambha K, Kim WR, Kremers WK, Therneau TM, Kamath PS, Wiesner R, Rosen CB, Thostenson J, Benson JT, Dickson ER. Predicting survival among patients listed for liver transplantation: an assessment of serial MELD measurements. Am J Transplant. 2004;4:1798-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Boecker J, Czigany Z, Bednarsch J, Amygdalos I, Meister F, Santana DAM, Liu WJ, Strnad P, Neumann UP, Lurje G. Potential value and limitations of different clinical scoring systems in the assessment of short- and long-term outcome following orthotopic liver transplantation. PLoS One. 2019;14:e0214221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Mataya L, Aronsohn A, Thistlethwaite JR Jr, Friedman Ross L. Decision making in liver transplantation--limited application of the liver donor risk index. Liver Transpl. 2014;20:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, Porte RJ, Rogiers X, Ringers J; European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC). The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 25. | Schoening W, Helbig M, Buescher N, Andreou A, Schmitz V, Bahra M, Puhl G, Pascher A, Pratschke J, Seehofer D. Eurotransplant donor-risk-index and recipient factors: influence on long-term outcome after liver transplantation - A large single-center experience. Clin Transplant. 2016;30:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Reichert B, Kaltenborn A, Goldis A, Schrem H. Prognostic limitations of the Eurotransplant-Donor Risk Index in liver transplantation. J Negat Results Biomed. 2013;12:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS Jr, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 28. | Schlegel A, Linecker M, Kron P, Györi G, De Oliveira ML, Müllhaupt B, Clavien PA, Dutkowski P. Risk Assessment in High- and Low-MELD Liver Transplantation. Am J Transplant. 2017;17:1050-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, Geier A, Clavien PA. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745-53; discussion 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 30. | Ma Y, Wang Q, Yang J, Yan L. Comparison of Different Scoring Systems Based on Both Donor and Recipient Characteristics for Predicting Outcome after Living Donor Liver Transplantation. PLoS One. 2015;10:e0136604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R, Arroyo V, Caballería J, Ginès P, Bataller R. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Papastergiou V, Tsochatzis EA, Pieri G, Thalassinos E, Dhar A, Bruno S, Karatapanis S, Luong TV, O'Beirne J, Patch D, Thorburn D, Burroughs AK. Nine scoring models for short-term mortality in alcoholic hepatitis: cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther. 2014;39:721-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2172] [Article Influence: 181.0] [Reference Citation Analysis (5)] |
| 34. | Pan HC, Jenq CC, Tsai MH, Fan PC, Chang CH, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC. Scoring systems for 6-month mortality in critically ill cirrhotic patients: a prospective analysis of chronic liver failure - sequential organ failure assessment score (CLIF-SOFA). Aliment Pharmacol Ther. 2014;40:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 735] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 36. | Grochot RM, Luz LB, Garcia R, Balbinot RA, Balbinot SS, Soldera J. CLIF-SOFA is superior to other liver-specific scores for predicting mortality in acute-on-chronic liver failure and decompensated cirrhosis. Austin J Gastroenterol. 2019;6:1105. |
| 37. | Motola-Kuba M, Escobedo-Arzate A, Tellez-Avila F, Altamirano J, Aguilar-Olivos N, González-Angulo A, Zamarripa-Dorsey F, Uribe M, Chávez-Tapia NC. Validation of prognostic scores for clinical outcomes in cirrhotic patients with acute variceal bleeding. Ann Hepatol. 2016;15:895-901. [PubMed] [DOI] [Full Text] |
| 38. | Jacques ROC, Massignan LS, Winkler MS, Balbinot RS, Balbinot RA, Balbinot SS, Soldera J. Liver-specific scores as predictors of mortality in spontaneous bacterial peritonitis. GastroHep. 2020;2:224-31. [DOI] [Full Text] |
| 39. | Terres AZ, Balbinot RS, Muscope ALF, Longen LM, Schena B, Cini BT, Rost Jr. GL, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Predicting mortality for Hepatorenal Syndrome with liver-specific scores. GastroHep. 2:336-343. [DOI] [Full Text] |
| 40. | Jacques ROC, Massignan LDS, Winkler MS, Balbinot RS, Balbinot SS, Soldera J. Acute-On-Chronic Liver Failure Is Independently Associated With Lower Survival In Patients With Spontaneous Bacterial Peritonitis. Arq Gastroenterol. 2021;58:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Rost GL Jr, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Acute-on-chronic liver failure is independently associated with higher mortality for cirrhotic patients with acute esophageal variceal hemorrhage: Retrospective cohort study. World J Clin Cases. 2023;11:4003-4018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 42. | Grochot RM, Luz LB, Garcia R, Balbinot RA, Balbinot SS, Soldera J. Acute–on–chronic liver failure data from a teaching hospital in Brazil. A Historical Cohort. International Journal of Scientific Research. 2020;9:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 43. | Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Luis Rost G Jr, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Evidence-based protocol for diagnosis and treatment of hepatorenal syndrome is independently associated with lower mortality. Gastroenterol Hepatol. 2022;45:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Sarker IH. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput Sci. 2021;2:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 751] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
| 45. | Mohammed M, Khan MB, Bashier, EB. Machine learning: algorithms and applic-ations. Google scholar. 2016;. [DOI] [Full Text] |
| 46. | Han J, Kamber M, Pei J. Data Mining: Concepts and Techniques, 3rd edn Morgan Kaufmann. Waltham, Massachusetts. 2011. Available from: https://linkinghub.elsevier.com/retrieve/pii/C20090618195. |
| 47. | Kaelbling LP, Littman ML, Moore AW. Reinforcement Learning: A Survey. J Artif Intell Res. 1996;4:237-285. |
| 48. | Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J. Scikit-learn: Machine learning in Python. J Mach Learn Res. 2011;12:2825-30. |
| 49. | Xin Y, Kong L, Liu Z, Chen Y, Li Y, Zhu H, Gao M, Hou H, Wang C. Machine learning and deep learning methods for cybersecurity. Ieee access. 2018;6:35365-81 Available from: https://ieeexplore.ieee.org/abstract/document/8359287/. |
| 50. | Fatima M, Pasha M. Survey of machine learning algorithms for disease diagnostic. J Intell Learn Syst Appl. 2017;9:1-6. |
| 51. | McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2911] [Cited by in RCA: 2730] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 52. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40624] [Article Influence: 10156.0] [Reference Citation Analysis (2)] |
| 53. | Briceño J, Cruz-Ramírez M, Prieto M, Navasa M, Ortiz de Urbina J, Orti R, Gómez-Bravo MÁ, Otero A, Varo E, Tomé S, Clemente G, Bañares R, Bárcena R, Cuervas-Mons V, Solórzano G, Vinaixa C, Rubín A, Colmenero J, Valdivieso A, Ciria R, Hervás-Martínez C, de la Mata M. Use of artificial intelligence as an innovative donor-recipient matching model for liver transplantation: results from a multicenter Spanish study. J Hepatol. 2014;61:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 54. | Ershoff BD, Lee CK, Wray CL, Agopian VG, Urban G, Baldi P, Cannesson M. Training and Validation of Deep Neural Networks for the Prediction of 90-Day Post-Liver Transplant Mortality Using UNOS Registry Data. Transplant Proc. 2020;52:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Lau L, Kankanige Y, Rubinstein B, Jones R, Christophi C, Muralidharan V, Bailey J. Machine-Learning Algorithms Predict Graft Failure After Liver Transplantation. Transplantation. 2017;101:e125-e132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 56. | Liu CL, Soong RS, Lee WC, Jiang GW, Lin YC. Predicting Short-term Survival after Liver Transplantation using Machine Learning. Sci Rep. 2020;10:5654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Yang M, Peng B, Zhuang Q, Li J, Liu H, Cheng K, Ming Y. Models to predict the short-term survival of acute-on-chronic liver failure patients following liver transplantation. BMC Gastroenterol. 2022;22:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Andres A, Montano-Loza A, Greiner R, Uhlich M, Jin P, Hoehn B, Bigam D, Shapiro JAM, Kneteman NM. A novel learning algorithm to predict individual survival after liver transplantation for primary sclerosing cholangitis. PLoS One. 2018;13:e0193523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Kong L, Lv T, Jiang L, Yang J. A simple four-factor preoperative recipient scoring model for prediction of 90-day mortality after adult liver Transplantation:A retrospective cohort study. Int J Surg. 2020;81:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Bertsimas D, Kung J, Trichakis N, Wang Y, Hirose R, Vagefi PA. Development and validation of an optimized prediction of mortality for candidates awaiting liver transplantation. Am J Transplant. 2019;19:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 61. | He T, Fong JN, Moore LW, Ezeana CF, Victor D, Divatia M, Vasquez M, Ghobrial RM, Wong STC. An imageomics and multi-network based deep learning model for risk assessment of liver transplantation for hepatocellular cancer. Comput Med Imaging Graph. 2021;89:101894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Pinto-Marques H, Cardoso J, Silva S, Neto JL, Gonçalves-Reis M, Proença D, Mesquita M, Manso A, Carapeta S, Sobral M, Figueiredo A, Rodrigues C, Milheiro A, Carvalho A, Perdigoto R, Barroso E, Pereira-Leal JB. A Gene Expression Signature to Select Hepatocellular Carcinoma Patients for Liver Transplantation. Ann Surg. 2022;276:868-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Lai YC, Wu KC, Chang CJ, Chen YJ, Wang KP, Jeng LB, Kao CH. Predicting Overall Survival with Deep Learning from 18F-FDG PET-CT Images in Patients with Hepatocellular Carcinoma before Liver Transplantation. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 64. | Kazemi A, Kazemi K, Sami A, Sharifian R. Identifying Factors That Affect Patient Survival After Orthotopic Liver Transplant Using Machine-Learning Techniques. Exp Clin Transplant. 2019;17:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Nitski O, Azhie A, Qazi-Arisar FA, Wang X, Ma S, Lilly L, Watt KD, Levitsky J, Asrani SK, Lee DS, Rubin BB, Bhat M, Wang B. Long-term mortality risk stratification of liver transplant recipients: real-time application of deep learning algorithms on longitudinal data. Lancet Digit Health. 2021;3:e295-e305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 66. | Ivanics T, So D, Claasen MPAW, Wallace D, Patel MS, Gravely A, Choi WJ, Shwaartz C, Walker K, Erdman L, Sapisochin G. Machine learning-based mortality prediction models using national liver transplantation registries are feasible but have limited utility across countries. Am J Transplant. 2023;23:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 67. | Cheong Y, Lee S, Lee DK, Kim KS, Sang BH, Hwang GS. Preoperative hyperlactatemia and early mortality after liver transplantation: selection of important variables using random forest survival analysis. Anesth Pain Med (Seoul). 2021;16:353-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Kulkarni S, Chi L, Goss C, Lian Q, Nadler M, Stoll J, Doyle M, Turmelle Y, Khan A. Random forest analysis identifies change in serum creatinine and listing status as the most predictive variables of an outcome for young children on liver transplant waitlist. Pediatr Transplant. 2021;25:e13932. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Molinari M, Ayloo S, Tsung A, Jorgensen D, Tevar A, Rahman SH, Jonassaint N. Prediction of Perioperative Mortality of Cadaveric Liver Transplant Recipients During Their Evaluations. Transplantation. 2019;103:e297-e307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Cooper JP, Perkins JD, Warner PR, Shingina A, Biggins SW, Abkowitz JL, Reyes JD. Acute Graft-Versus-Host Disease After Orthotopic Liver Transplantation: Predicting This Rare Complication Using Machine Learning. Liver Transpl. 2022;28:407-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | He ZL, Zhou JB, Liu ZK, Dong SY, Zhang YT, Shen T, Zheng SS, Xu X. Application of machine learning models for predicting acute kidney injury following donation after cardiac death liver transplantation. Hepatobiliary Pancreat Dis Int. 2021;20:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Chen C, Yang D, Gao S, Zhang Y, Chen L, Wang B, Mo Z, Yang Y, Hei Z, Zhou S. Development and performance assessment of novel machine learning models to predict pneumonia after liver transplantation. Respir Res. 2021;22:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Lee HC, Yoon SB, Yang SM, Kim WH, Ryu HG, Jung CW, Suh KS, Lee KH. Prediction of Acute Kidney Injury after Liver Transplantation: Machine Learning Approaches vs. Logistic Regression Model. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 74. | Bredt LC, Peres LAB, Risso M, Barros LCAL. Risk factors and prediction of acute kidney injury after liver transplantation: Logistic regression and artificial neural network approaches. World J Hepatol. 2022;14:570-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Trifonova OP, Lokhov PG, Archakov AI. [Metabolic profiling of human blood]. Biomed Khim. 2014;60:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Clancey WJ, Shortliffe EH. Readings in medical artificial intelligence: the first decade. Addison-Wesley Longman Publishing Co., Inc. 1984. Available from: https://dL.acm.org/doi/book/10.5555/1124. |
| 77. | Zippel C, Bohnet-Joschko S. Rise of Clinical Studies in the Field of Machine Learning: A Review of Data Registered in ClinicalTrials.gov. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Zanetto A, Shalaby S, Gambato M, Germani G, Senzolo M, Bizzaro D, Russo FP, Burra P. New Indications for Liver Transplantation. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Huebener P, Sterneck MR, Bangert K, Drolz A, Lohse AW, Kluge S, Fischer L, Fuhrmann V. Stabilisation of acute-on-chronic liver failure patients before liver transplantation predicts post-transplant survival. Aliment Pharmacol Ther. 2018;47:1502-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 81. | Mahmud N, Kaplan DE, Taddei TH, Goldberg DS. Incidence and Mortality of Acute-on-Chronic Liver Failure Using Two Definitions in Patients with Compensated Cirrhosis. Hepatology. 2019;69:2150-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 82. | Chen Z, Diaz G, Pollicino T, Zhao H, Engle RE, Schuck P, Shen CH, Zamboni F, Long Z, Kabat J, De Battista D, Bock KW, Moore IN, Wollenberg K, Soto C, Govindarajan S, Kwong PD, Kleiner DE, Purcell RH, Farci P. Role of humoral immunity against hepatitis B virus core antigen in the pathogenesis of acute liver failure. Proc Natl Acad Sci U S A. 2018;115:E11369-E11378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 83. | O'Leary JG, Bajaj JS, Tandon P, Biggins SW, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, Lai J, Fallon M, Vargas HE, Thuluvath P, Subramanian R, Thacker LR, Reddy KR. Outcomes After Listing for Liver Transplant in Patients With Acute-on-Chronic Liver Failure: The Multicenter North American Consortium for the Study of End-Stage Liver Disease Experience. Liver Transpl. 2019;25:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Fabia R, Levy MF, Testa G, Obiekwe S, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB. Colon carcinoma in patients undergoing liver transplantation. Am J Surg. 1998;176:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Lerut J, Demetris AJ, Stieber AC, Marsh JW, Gordon RD, Esquivel CO, Iwatsuki S, Starzl TE. Intrahepatic bile duct strictures after human orthotopic liver transplantation. Recurrence of primary sclerosing cholangitis or unusual presentation of allograft rejection? Transpl Int. 1988;1:127-130. [PubMed] |
| 87. | Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, Kowdley KV, Stevens AC, Crispin DA, Emond M, Rubin CE. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 206] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Ballotin VR, Bigarella LG, Riva F, Onzi G, Balbinot RA, Balbinot SS, Soldera J. Primary sclerosing cholangitis and autoimmune hepatitis overlap syndrome associated with inflammatory bowel disease: A case report and systematic review. World J Clin Cases. 2020;8:4075-4093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 91. | Soldera J, Balbinot SS, Balbinot RA, Cavalcanti AG. Diagnostic and Therapeutic Approaches to Hepatocellular Carcinoma: Understanding the Barcelona Clínic Liver Cancer Protocol. Clin Med Insights Gastroenterol. 2016;9:67-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Onzi G, Moretti F, Balbinot SS, Balbinot RA, Soldera J. Hepatocellular carcinoma in non-alcoholic fatty liver disease with and without cirrhosis. Hepatoma Res. 2019;5:7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 93. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3245] [Article Influence: 463.6] [Reference Citation Analysis (1)] |
| 94. | Benten D, Staufer K, Sterneck M. Orthotopic liver transplantation and what to do during follow-up: recommendations for the practitioner. Nat Clin Pract Gastroenterol Hepatol. 2009;6:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 713] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 96. | Greten TF, Malek NP, Schmidt S, Arends J, Bartenstein P, Bechstein W, Bernatik T, Bitzer M, Chavan A, Dollinger M, Domagk D, Drognitz O, Düx M, Farkas S, Folprecht G, Galle P, Geißler M, Gerken G, Habermehl D, Helmberger T, Herfarth K, Hoffmann RT, Holtmann M, Huppert P, Jakobs T, Keller M, Klempnauer J, Kolligs F, Körber J, Lang H, Lehner F, Lordick F, Lubienski A, Manns MP, Mahnken A, Möhler M, Mönch C, Neuhaus P, Niederau C, Ocker M, Otto G, Pereira P, Pott G, Riemer J, Ringe K, Ritterbusch U, Rummeny E, Schirmacher P, Schlitt HJ, Schlottmann K, Schmitz V, Schuler A, Schulze-Bergkamen H, von Schweinitz D, Seehofer D, Sitter H, Straßburg CP, Stroszczynski C, Strobel D, Tannapfel A, Trojan J, van Thiel I, Vogel A, Wacker F, Wedemeyer H, Wege H, Weinmann A, Wittekind C, Wörmann B, Zech CJ. [Diagnosis of and therapy for hepatocellular carcinoma]. Z Gastroenterol. 2013;51:1269-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-8; discussion 228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 470] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 98. | Meyers R, Hiyama E, Czauderna P, Tiao GM. Liver Tumors in Pediatric Patients. Surg Oncol Clin N Am. 2021;30:253-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Rudnick SR, Russo MW. Liver transplantation beyond or downstaging within the Milan criteria for hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2018;12:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 101. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 102. | Ballotin VR, Bigarella LG, Soldera J. Deep learning applied to the imaging diagnosis of hepatocellular carcinoma. Artif Intell Gastrointest Endosc. 2021;2:127-135. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 103. | Soldera J. Artificial Intelligence as a Prognostic Tool for Gastrointestinal Tract Pathologies. Med Vozandes. 2023;34:4-9. |
| 104. | Ashton JJ, Young A, Johnson MJ, Beattie RM. Using machine learning to impact on long-term clinical care: principles, challenges, and practicalities. Pediatr Res. 2023;93:324-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 105. | Grama A, Aldea CO, Burac L, Delean D, Bulata B, Sirbe C, Duca E, Boghitoiu D, Coroleuca A, Pop TL. Etiology and Outcome of Acute Liver Failure in Children-The Experience of a Single Tertiary Care Hospital from Romania. Children (Basel). 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Ferrarese A, Sartori G, Orrù G, Frigo AC, Pelizzaro F, Burra P, Senzolo M. Machine learning in liver transplantation: a tool for some unsolved questions? Transpl Int. 2021;34:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 107. | Soldera J, Corso LL, Rech MM, Ballotin VR, Bigarella LG, Tomé F, Moraes N, Balbinot RS, Rodriguez S, Brandão AB, Hochhegger B. Mo1598 Predicting Post-Liver Transplantation Major Adverse Cardiovascular Events Using A Machine Learning Algorithm. Gastroenterology. 2023;1:S-13. [DOI] [Full Text] |
| 108. | Rech MM, Corso LL, Dal Bó EF, Tomé F, Terres AZ, Balbinot RS, Muscope AL, Eberhardt LZ, Cini BT, Balensiefer JI, Schena B, Longen ML, Rost GL, Balbinot SA, Balbinot RA, Soldera J. Prospective validation of a neural network model for the prediction of 1-year mortality in cirrhotic patients with acute esophageal variceal bleeding. Gastroenterology. 2023;. [DOI] [Full Text] |
| 109. | Soldera J, Tomé F, Corso LL, Rech MM, Ferrazza AD, Terres AZ, Cini BT, Eberhardt LZ, Balensiefer JI, Balbinot RS, Muscope AL. Use of machine learning algorithm to predict re-bleeding and mortality for esophageal variceal bleeding in cirrhotic patients. EMJ Gastroenterol. 2020;9:46-48. |
| 110. | Durand F. How to improve long-term outcome after liver transplantation? Liver Int. 2018;38 Suppl 1:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 111. | Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 112. | Singh S, Watt KD. Long-term medical management of the liver transplant recipient: what the primary care physician needs to know. Mayo Clin Proc. 2012;87:779-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Roccaro GA, Goldberg DS, Hwang WT, Judy R, Thomasson A, Kimmel SE, Forde KA, Lewis JD, Yang YX. Sustained Posttransplantation Diabetes Is Associated With Long-Term Major Cardiovascular Events Following Liver Transplantation. Am J Transplant. 2018;18:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 114. | Briceño J, Ayllón MD, Ciria R. Machine-learning algorithms for predicting results in liver transplantation: the problem of donor-recipient matching. Curr Opin Organ Transplant. 2020;25:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Soldera J, Tomé F, Corso LL, Ballotin VR, Bigarella LG, Balbinot RS, Rodriguez S, Brandão AB, Hochhegger B. 590 Predicting 30 and 365-day mortality after liver transplantation using a machine learning algorithm. Gastroenterology. 2021;160:S-789. [DOI] [Full Text] |
| 116. | Kalpoe JS, Sonnenberg E, Factor SH, del Rio Martin J, Schiano T, Patel G, Huprikar S. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2012;18:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 117. | Rubin RH. The direct and indirect effects of infection in liver transplantation: pathogenesis, impact, and clinical management. Curr Clin Top Infect Dis. 2002;22:125-154. [PubMed] |
| 118. | Kim HK, Park YK, Wang HJ, Kim BW, Shin SY, Lim SK, Choi YH. Epidemiology and clinical features of post-transplant bloodstream infection: an analysis of 222 consecutive liver transplant recipients. Infect Chemother. 2013;45:315-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 119. | Vera A, Contreras F, Guevara F. Incidence and risk factors for infections after liver transplant: single-center experience at the University Hospital Fundación Santa Fe de Bogotá, Colombia. Transpl Infect Dis. 2011;13:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 121. | Patel HK, Patel A, Abouljoud M, Divine G, Moonka DK. Survival after liver transplantation in patients who develop renal insufficiency. Transplant Proc. 2010;42:4167-4170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Saxena V, Lai JC. Kidney Failure and Liver Allocation: Current Practices and Potential Improvements. Adv Chronic Kidney Dis. 2015;22:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Mijwil MM, Aggarwal K. A diagnostic testing for people with appendicitis using machine learning techniques. Multimed Tools Appl. 2022;81:7011-7023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 124. | Salman Shukur B, Mijwil MM. Involving machine learning techniques in heart disease diagnosis: a performance analysis. International Journal of Electrical and Computer Engineering. 2023;13:2177-2185. [DOI] [Full Text] |