Published online Dec 18, 2023. doi: 10.5500/wjt.v13.i6.368
Peer-review started: August 30, 2023
First decision: September 29, 2023
Revised: October 16, 2023
Accepted: October 26, 2023
Article in press: October 26, 2023
Published online: December 18, 2023
Processing time: 109 Days and 20.3 Hours
Tacrolimus extended-release tablets have been Food and Drug Administration-approved for use in the de novo kidney transplant population. Dosing requi
To obtain target trough concentrations of extended-release tacrolimus in de novo kidney transplant recipients according to CYP3A5 genotype.
Single-arm, prospective, single-center, open-label, observational study (ClinicalTrials.gov: NCT037
Mean time to therapeutic tacrolimus trough concentration was longer in CYP3A5 intermediate and extensive metabolizers vs CYP3A5 non-expressers (6 d vs 13.5 d vs 4.5 d; P = 0.025). Mean tacrolimus doses and weight-based doses to achieve therapeutic concentration were higher in CYP3A5 intermediate and extensive metabolizers vs CYP3A5 non-expressers (16 mg vs 16 mg vs 12 mg; P = 0.010) (0.20 mg/kg vs 0.19 mg/kg vs 0.13 mg/kg; P = 0.018). CYP3A5 extensive metabolizers experienced lower mean tacrolimus trough concentrations throughout the study period compared to CYP3A5 intermediate metabolizers and non-expressers (7.98 ng/mL vs 9.18 ng/mL vs 10.78 ng/mL; P = 0 0.008). No differences were identified with regards to kidney graft function at 30-d post-transplant. Serious adverse events were reported for 13 (36%) patients.
Expression of CYP3A5 leads to higher starting doses and incremental dosage titration of extended-release tacro
Core Tip: In this single-arm, prospective, observational study we study once-daily, extended release tacrolimus dosing. Here we find that the expression of the cyctrochrome P450 enzyme, CYP3A5, is an important clinical factor to determine optimal dosage requirements after kidney transplantation. In kidney transplant recipients who express CYP3A5 activity, higher doses of extended-release tacrolimus are required to attain therapeutic trough concentrations. Delays in achieving therapeutic trough concentrations has been linked to increase rates of acute rejection which highlights the importance of this research in identifying dosing considerations for extended-release tacrolimus in the de novo kidney transplant setting.
- Citation: Diamond A, Karhadkar S, Chavin K, Constantinescu S, Lau KN, Perez-Leal O, Mohrien K, Sifontis N, Di Carlo A. Dosing strategies for de novo once-daily extended release tacrolimus in kidney transplant recipients based on CYP3A5 genotype. World J Transplant 2023; 13(6): 368-378
- URL: https://www.wjgnet.com/2220-3230/full/v13/i6/368.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i6.368
Outcomes after kidney transplantation have been significantly improved with advances in immunosuppressive therapies. Tacrolimus is currently marketed in various formulations that have proven to be highly effective in preventing acute rejection after kidney transplantation[1-3]. Compared to immediate release tacrolimus, once daily extended-release formulations have demonstrated similar efficacy and safety in the de novo kidney transplant setting leading to increased utilization[1,2,4,5]. Life cycle pharma tacrolimus (LCPT) (Envarsus®; Veloxis Pharmaceuticals) was designed to enhance the bioavailability of tacrolimus[6]. In published studies, utilization of LCPT has been shown to provide rapid achievement of target trough concentrations following kidney transplantation[1,2,7]. In addition, a once daily LCPT dosing regimen results in lower peak concentrations with equivalent overall exposure compared to immediate-release and other extended-release tacrolimus formulations[3]. Similar efficacy and safety profiles have been demonstrated when comparing LCPT to other available tacrolimus formulations[1-3,7,8].
The use of LCPT has been Food and Drug Administration (FDA)-approved for the prophylaxis of organ rejection in de novo kidney transplant patients and in kidney transplant patients converted from tacrolimus immediate-release formulations[6]. The recommended FDA-approved dosing for de novo kidney transplant recipients is 0.14 mg/kg/d, however various starting doses have been evaluated in clinical trials[1,2,6]. Some kidney transplant recipients are known to metabolize tacrolimus at a higher or lower rate due to the presence of genetic polymorphisms that affect its metabolism[9]. The metabolism of LCPT occurs primarily within the cytochrome P450 (CYP) system, of which approximately 55 different genes have been identified in the human genome[10]. A multitude of CYP enzymes exist, including CYP3A5 which is known to be an integral component of tacrolimus metabolism. In addition, genetic variation affecting CYP3A5 function is known to impact overall tacrolimus exposure as well as dosing requirements to attain therapeutic concentrations[11,12]. The most common genetic variants (CYP3A5*3 and CYP3A5*6) of CYP3A5 in the general population produce non-functional versions of the enzyme[13,14]. On the other hand, the presence of at least one CYP3A5*1 allele would confer activity to CYP3A5 (commonly known as an expresser) which has been shown to lead to higher dosage requirements of tacrolimus to attain therapeutic concentrations[9]. Previous data has illustrated that CYP3A5 expressers can require up to 2-fold higher tacrolimus doses to achieve similar trough concentrations compared to CYP3A5 non-expressers[15]. CYP3A5 genetic variation may also lead to delays in time to achievement of target trough concentrations, which has been linked to higher rates of acute rejection. Furthermore, knowledge of CYP3A5 genetic variants in transplant patients may lead to prevention of subtherapeutic and supratherapeutic concentrations in the early post-transplant period potentially lowering the risks of acute rejection and drug toxicities[16,17].
The primary objective of this study was to identify the time to therapeutic trough concentration of de novo once-daily LCPT in kidney transplant recipients according to CYP3A5 expresser status. Secondary objectives include the description of the distribution of common CYP3A5 variants in our population and to identify the dose required (total and weight-based) to obtain target trough concentrations according to expresser status.
We conducted a single-arm, prospective, open-label, single-center, observational study (ClinicalTrials.gov: NCT03
All patients received LCPT tablets orally once daily at a starting dose of 0.13 mg/kg/day based on actual body weight. If a patient weighed more than 120% of their ideal body weight, an adjusted body weight was calculated for initial drug dosing[18]. All doses were rounded to the nearest 1 mg increment and adjusted to maintain a tacrolimus trough concentration of 8-10 ng/mL for the first 30 d after kidney transplant. No dose adjustments were performed during the first 48 h after the initial dose or subsequent dose adjustments to allow steady state concentrations to be achieved. All patients received additional immunosuppression with antithymocyte globulin (Thymoglobulin®; Sanofi Pharmaceuticals) or basiliximab (Simulect®; Novartis Pharmaceuticals) induction and mycophenolate sodium 720 mg by mouth every 12 h. Antithymocyte globulin dosing ranged between 4-6 mg/kg based on immunologic risk and was dosed by actual body weight unless the patient was greater than 120% of their ideal body weight, for which an adjusted body weight was utilized. Adjustments to mycophenolate sodium dosing was at the discretion of the treating physician, based on adverse effects, lab abnormalities, and other clinical considerations. All patients received daily pulse-dose methylprednisolone for 5 d according to institutional protocol. Prednisone maintenance immunosuppression was utilized in some recipients based on immunologic risk and the presence of an automimmune kidney disease at the time of kidney transplant. Patients requiring prednisone received a maintenance dose of prednisone 5-10 mg by mouth daily.
Two buccal swab samples were collected from each patient using DNA/RNA Shield™ collection tubes (Zymo Research Corporation). The samples were stored frozen at -20 °C until obtaining samples from all patients included in the study. The DNA extraction was performed with NucleoMag® DNA Swab extraction kit (Takara Bio Inc.) following the manufacturer's recommendations. A Tecan Spark Plate Reader was used to determine the DNA concentration using the NanoQuant Plate™. The DNA concentration was normalized at 5 ng/L for all the samples with molecular biology grade water for real-time PCR analysis.
We processed all the patient's DNA samples on the same day for DNA genotyping. We performed DNA single nuc
The primary efficacy endpoint was the time to therapeutic tacrolimus trough concentration during the first 30 d after kidney transplantation. Therapeutic tacrolimus trough concentration was defined as tacrolimus trough concentration 8 ng/mL. Secondary efficacy endpoints included the tacrolimus dose and weight-based tacrolimus dose required to achieve an initial therapeutic trough concentration. Safety outcomes measured included incidence of hyperkalemia (serum potassium > 5.5 mEq/L) and incidence of tremor. Tremor was assessed utilizing the quality of life in essential tremor (QUEST) questionnaire and was completed at 30 d post-kidney transplant[20]. Incidence of serious adverse events (SAEs) and drug discontinuation due to adverse events (AEs) were also reported.
Descriptive statistics were used to characterize the baseline demographics of the entire cohort (intent to treat population). Continuous parametric data are presented as mean ± SD while continuous non-parametric data are presented as median (25%-75% interquartile range). Analysis of outcomes according to CYP3A5 expresser status within the modified intent-to-treat (ITT) population were completed using the Kruskal-Wallis or ANOVA test for continuous data and the chi-squared test for categorical data. Tests were corrected for multiple comparisons as necessary utilizing the Bonferroni method. All tests were two-tailed, and P < 0.05 was used to represent statistical significance. Time to therapeutic tacrolimus trough concentration was analyzed using a Kaplan-Meier time-to-event analysis. All analyses were performed using SPSS, version 26 for windows (Armonk, NY; IBM Inc.).
A total of 36 patients (ITT population) were enrolled and 35 patients completed the entire 30-d treatment period. One patient withdrew prior to the end of the study time period due to neurologic toxicity and tremors. Patients who were able to complete genotype testing were included in the final analysis [n = 34; modified ITT (mITT) population]. All 34 patients were included in the mITT analysis and patients were stratified based on CYP3A5 phenotype. Of the 34 total patients, 15 (44.1%) were found to be non-expressers of CYP3A5, while 13 (38.2%) and 6 (17.6%) were found to be intermediate and extensive metabolizers, respectively. The population was predominantly black (66.7%), male (55.6%), and recipients of a deceased donor kidney transplant (69.4%) with a mean age of 55.5 years (Table 1). Baseline characteristics were similar between groups except for a higher percentage of black patients (92.3% vs 83.3% vs 46.7%; P = 0.026) in the CYP3A5 intermediate and extensive metabolizer groups compared to CYP3A5 non-expressers (Table 2).
| All patients (n = 36) | |
| Age (yr), mean ± SD | 55.5 ± 13.7 |
| Male, n (%) | 20 (55.6) |
| Race, n (%) | |
| White | 9 (25.0) |
| Black | 24 (66.7) |
| Hispanic | 2 (5.6) |
| Transplant type, n (%) | |
| Deceased donor | 25 (69.4) |
| Living donor | 11 (30.6) |
| Hypertension, n (%) | 28 (77.8) |
| Diabetes mellitus, n (%) | 9 (25.0) |
| Focal segmental glomerulosclerosis, n (%) | 1 (2.8) |
| Polycystic kidney disease, n (%) | 1 (2.8) |
| HIV-associated nephropathy, n (%) | 1 (2.8) |
| Lupus nephritis, n (%) | 1 (2.8) |
| Transplant number, n (%) | |
| One | 35 (97.2) |
| Two | 1 (2.8) |
| cPRA (%), median (IQR) | 0 (0-10.0) |
| Actual body weight (kg), mean ± SD | 87.4 ± 18.4 |
| Dosing weight, n (%) | |
| Actual | 16 (44.4) |
| Adjusted | 20 (55.6) |
| BMI (kg/m2), mean ± SD | 30.0 ± 5.5 |
| Non-expresser (n = 15) | Intermediate metabolizer (n = 13) | Extensive metabolizer (n = 6) | P value | |
| Age (yr), mean ± SD | 53.8 ± 12.6 | 49.0 ± 14.0 | 58.3 ± 14.1 | 0.354 |
| Male, n (%) | 9 (60.0) | 8 (61.5) | 2 (33.3) | 0.470 |
| Race, n (%) | 0.026 | |||
| White | 7 (46.7) | 1 (7.7) | 0 | |
| Black | 7 (46.7) | 12 (92.3) | 5 (83.3) | |
| Hispanic | 1 (6.7) | 0 | 0 | |
| Transplant type, n (%) | 0.550 | |||
| Deceased donor | 12 (80.0) | 8 (61.5) | 4 (66.7) | |
| Living donor | 3 (20.0) | 5 (38.5) | 2 (33.3) | |
| Hypertension, n (%) | 12 (80.0) | 9 (69.2) | 6 (100.0) | 0.304 |
| Diabetes mellitus, n (%) | 2 (13.3) | 4 (30.8) | 2 (33.3) | 0.457 |
| Focal segmental glomerulosclerosis, n (%) | 1 (6.7) | 0 | 0 | 0.521 |
| Polycystic kidney disease, n (%) | 0 | 1 (7.7) | 0 | 0.435 |
| HIV-associated nephropathy, n (%) | 1 (6.7) | 0 | 0 | 0.521 |
| Lupus nephritis, n (%) | 0 | 1 (7.7) | 0 | 0.435 |
| Transplant number, n (%) | 0.435 | |||
| One | 15 (100.0) | 12 (92.3) | 6 (100.0) | |
| Two | 0 | 1 (7.7) | 0 | |
| cPRA (%), median (IQR) | 0 (0-20.8) | 10.0 (0-10.0) | 0 (0-20.8) | 0.732 |
| Actual body weight (kg), mean ± SD | 85.5 ± 16.8 | 91.8 ± 22.2 | 77.4 ± 10.5 | 0.286 |
| Dosing weight, n (%) | 0.987 | |||
| Actual | 7 (53.3) | 6 (53.8) | 3 (50.0) | |
| Adjusted | 8 (46.7) | 7 (46.2) | 3 (50.0) | |
| BMI (kg/m2), mean ± SD | 29.68 ± 5.0 | 30.68 ± 6.9 | 28.88 ± 4.5 | 0.805 |
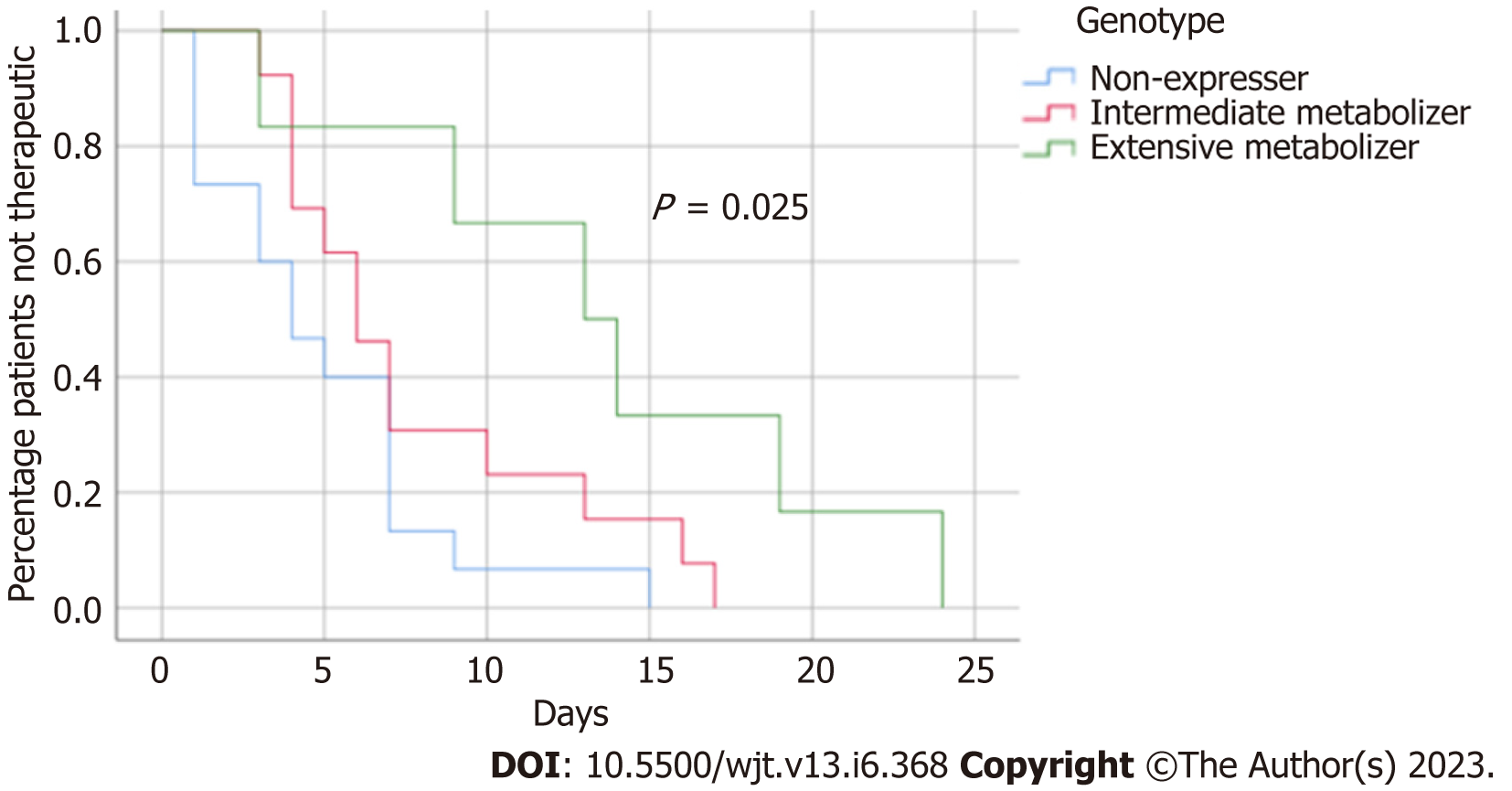
Mean time to therapeutic tacrolimus trough concentration was longer in CYP3A5 intermediate and extensive metabolizers compared to CYP3A5 non-expressers (P = 0.025). A Kaplan Meier analysis demonstrated that the highest incidence of patients not achieving therapeutic tacrolimus trough concentration by 7 d post-transplant were CYP3A5 extensive metabolizers followed by CYP3A5 intermediate metabolizers. Only 13.3% of CYP3A5 non-expressers failed to achieve a therapeutic tacrolimus trough by 7 d post-transplant compared to approximately 30.8% of CYP3A5 intermediate metabolizers and 83.3% of CYP3A5 extensive metabolizers (Figure 1). Mean tacrolimus doses to achieve therapeutic concentration were higher in CYP3A5 intermediate and extensive metabolizers compared to CYP3A5 non-expressers (16 mg vs 16 mg vs 12 mg; P = 0.010). Mean weight-based tacrolimus doses to achieve therapeutic tacrolimus trough concentrations were also higher in CYP3A5 intermediate and extensive metabolizers compared to CYP3A5 non-expressers (0.20 mg/kg vs 0.19 mg/kg vs 0.13 mg/kg; P = 0.018) (Table 3).
| Non-expressers (n = 15) | Intermediate metabolizer (n = 13) | Extensive metabolizer (n = 6) | P value | |
| Time (d) to therapeutic tacrolimus concentration, median (IQR) | 4.5 (1.0-7.0) | 6.0 (4.0-11.5) | 13.5 (7.5-20.25) | 0.025 |
| Tacrolimus dose (mg) at therapeutic concentration, median (IQR) | 12 (10-14) | 16 (13-20) | 16 (11-20.5) | 0.010 |
| Weight-based tacrolimus dose (mg/kg) at therapeutic concentration, median (IQR) | 0.13 (0.12-0.165) | 0.20 (0.125-0.25) | 0.19 (0.138-0.265) | 0.018 |
| Tacrolimus dose (mg), median (IQR) | 9.6 (9.2-10.1) | 12.5 (10.6-14.5) | 13.8 (10.4-14.4) | 0.011 |
| Weight-based tacrolimus dose (mg/kg), median (IQR) | 0.128 (0.102-0.142) | 0.136 (0.108-0.169) | 0.176 (0.128-0.217) | 0.074 |
| Tacrolimus trough concentration (ng/mL), mean ± SD | 10.78 ± 2.1 | 9.18 ± 1.6 | 7.98 ± 1.3 | 0.008 |
| Weight-based tacrolimus dose at day 30 (mg/kg), mean ± SD | 0.103 ± 0.429 | 0.154 ± 0.620 | 0.167 ± 0.590 | 0.022 |
| Potassium (mEq/L), mean ± SD | 4.29 ± 0.36 | 4.68 ± 0.35 | 4.35 ± 0.59 | 0.041 |
| Serum creatinine (mg/dL) at day 30, median (IQR) | 1.76 (1.29-2.62) | 1.75 (1.27-2.65) | 1.94 (1.2-3.0) | 0.906 |
| eGFR (mL/min/1.73 m2) at day 30, median (IQR) | 40.0 (27.0-58.0) | 46.0 (30.0-58.5) | 31.5 (25.0-56.3) | 0.701 |
Mean daily tacrolimus dose, daily weight-based tacrolimus dose, and tacrolimus trough concentrations throughout the 30-d study period were compared amongst the three groups. A higher mean daily tacrolimus dose was seen in CYP3A5 intermediate and extensive metabolizers compared to poor metabolizers (12.5 mg vs 13.8 mg vs 9.6 mg; P = 0.011). While not statistically significant, a higher daily weight-based tacrolimus dose was seen in CYP3A5 intermediate and extensive metabolizers compared to CYP3A5 non-expressers (0.136 mg/kg vs 0.176 mg/kg vs 0.128 mg/kg; P = 0.074). CYP3A5 extensive metabolizers experienced lower mean tacrolimus trough concentrations throughout the study period compared to CYP3A5 intermediate metabolizers and non-expressers (7.98 ng/mL vs 9.18 ng/mL vs 10.78 ng/mL; P = 0.008). No statistically significant differences in kidney graft function at 30-d post-transplant were observed between CYP3A5 extensive metabolizers, intermediate metabolizers, and non-expressers measured by mean serum creatinine (1.94 mg/dL vs 1.76 mg/dL vs 1.76 mg/dL; P = 0.906) and mean estimated glomerular filtration rate (31.5 mL/min/1.73 m2 vs 46 mL/min/1.73 m2 vs 40 mL/min/1.73 m2; P = 0.701) (Table 3).
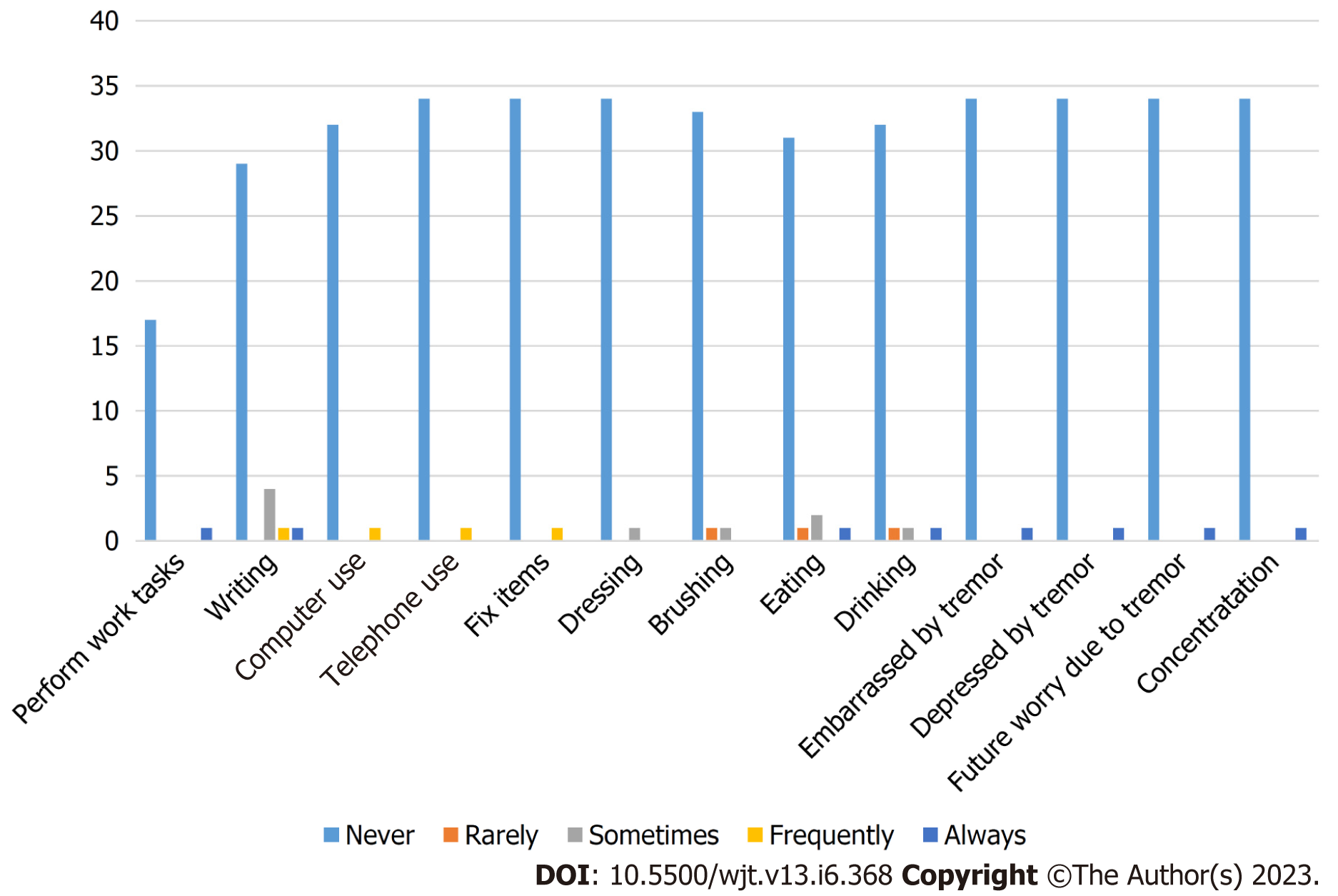
Safety endpoints were evaluated as part of the ITT analysis. SAEs were reported for 13 (36%) patients with 1 SAE (2.8%) attributed to study drug. The one patient who experienced a SAE attributed to study drug resulted in neurotoxicity which led to study drug discontinuation. Assessment of tremor using the QUEST questionnaire revealed that the majority of patients experienced no significant impact of tremor on their quality of life. A further description of patient responses to the QUEST questionnaire are summarized in Figure 2. A total of 11 (31%) patients enrolled experienced at least one potassium value above 5.5 mEq/L. Mean potassium values did differ throughout the 30-d study period between extensive metabolizer, intermediate metabolizer, and non-expresser groups, but were not clinically significant (4.35 vs 4.68 vs 4.29; P = 0.041).
To our knowledge, this is the first prospective observational study to provide outcomes data for the de novo dosing of extended-release tacrolimus, LCPT, in a predominant CYP3A5*1 expresser kidney transplant population. This research evaluates a lower initial LCPT dose of 0.13 mg/kg/d compared with the FDA-approved initial LCPT dosing of 0.14 mg/kg/d. A starting LCPT dose of 0.17 mg/kg/d was commonly evaluated in other de novo kidney transplant populations[1,2]. Genetic polymorphisms have been shown in numerous studies to directly affect dosage requirements of extended-release tacrolimus preparations, including LCPT[8,12]. In addition, several other patient specific factors may affect tacrolimus absorption including age, ethnicity, body weight, hepatic function, drug-drug interactions, and oral intake[15,21]. The incorporation of a CYP3A5 genotype testing variable provides a clearer understanding of LCPT dosage requi
Delayed time to therapeutic tacrolimus trough concentrations results in higher rates of acute cellular rejection[15-17]. The expression of at least one CYP3A5*1 allele is associated with a delayed time to achieve initial therapeutic tacrolimus trough concentration as well as a decreased time within therapeutic tacrolimus trough concentration range after kidney transplantation[12,15]. The significant impact of CYP3A5 activity on tacrolimus metabolism warrants investigation into dosing of once daily tacrolimus formulations in CYP3A5 expressers. Participants in this study who expressed at least one CYP3A5*1 allele or two CYP3A5*1 alleles had significant increases in LCPT dosing requirements compared to those who did not express any CYP3A5*1 alleles. Higher dosing requirements in CYP3A5 expressers noted in this study leads to the consideration of a need for higher initial LCPT de novo dosing in this population to avoid delays in attainment of therapeutic tacrolimus trough concentrations.
Since LCPT dosing has not been evaluated in prior studies based on CYP3A5 genotype, it is worthwhile to compare the results of our study to a different once daily tacrolimus formulation (Advagraf®, Astagraf®; Astellas Pharmaceuticals). De Meyer et al[12] showed that CYP3A5 expressers, both intermediate and extensive metabolizers, required higher tacrolimus extended-release doses by day 30 compared to CYP3A5 non-expressers (CYP3A5 intermediate metabolizer: 0.30 mg/kg/d vs CYP3A5 extensive metabolizer: 0.46 mg/kg/d vs CYP3A5 non-expresser: 0.15 mg/kg/d; P < 0.001). We identified a similar trend as De Meyer et al[12] in which CYP3A5 expressers regardless of intermediate or extensive metabolizer phenotype, require higher LCPT dosing than CYP3A5 non-expressers. However, lower doses of LCPT appear to be required for CYP3A5 expressers in our study compared to other available once daily tacrolimus formulations (Astagraf®) when comparing dosing at day 30 post-kidney transplant (CYP3A5 intermediate metabolizer: 0.17 mg/kg/d vs 0.30 mg/kg/d; CYP3A5 extensive metabolizer: 0.16 mg/kg/d vs 0.46 mg/kg/d). One limitation to this comparison is the goal tacrolimus trough concentration, since in De Meyer et al[12] it was 8-12 ng/mL at day 30 compared to our study goal of 8-10 ng/mL, although median trough concentrations were similar at day 30 for both studies.
Guidelines from the Clinical Pharmacogenetics Implementation Consortium for CYP3A5 Genotype and Tacrolimus Dosing provide clinical recommendations for dosing based on CYP3A5 genotype[19]. These guidelines provide clinical evidence for dose adjustments required for immediate-release tacrolimus dosing, but do not discuss the implications for dose adjustments for tacrolimus extended-release formulations, such as LCPT. Recommendations for all CYP3A5 expressers is to provide initial tacrolimus dosing of 1.5-2 times the recommended starting dose and to not exceed a starting dose of 0.3 mg/kg/d. CYP3A5 expressers in our study required approximately 1.5 times the recommended FDA-approved 0.14 mg/kg/d starting dose for LCPT to achieve therapeutic tacrolimus trough concentrations[6]. The majority of CYP3A5 expressers in this study required an approximate 20% reduction in the mean LCPT dose at day 30 from the time of attainment of therapeutic tacrolimus trough concentrations. The delayed time to achieve therapeutic tacrolimus trough concentration for CYP3A5 expressers compared to CYP3A5 non-expressers leads to CYP3A5 expressers requiring higher initial starting doses of LCPT. However, patients may require dose reduction over time to maintain therapeutic tacrolimus concentrations. Patients in this study identified as CYP3A5 intermediate metabolizers required a mean of 0.2 mg/kg/d to achieve therapeutic tacrolimus concentration vs 0.19 mg/kg/d for CYP3A5 extensive metabolizers. Given the similarity in doses required to achieve therapeutic tacrolimus concentrations between these two groups, a similar dosing strategy for all CYP3A5 expressers could be utilized. Two large sample size studies evaluated clinical outcomes associated with de novo use of LCPT in kidney transplant recipients. Budde et al[1] evaluated the incidence of biopsy-proven acute rejection, graft failure, patient survival, and AEs at 12 mo while Rostaing et al[2] evaluated similar outcomes at both 12 and 24 mo after kidney transplantation. Both of these studies evaluated an initial starting dose of 0.17 mg/kg/d, which is the maximum dose of LCPT evaluated in clinical studies with at least 12 mo efficacy and safety outcomes. CYP3A5 genotype was not performed for these studies and our study provides additional data regarding initial de novo LCPT dosing based on CYP3A5 genotype. Based on these findings, we suggest that CYP3A5 expressers may require higher initial starting doses of approximately 0.2 mg/kg/d. In order to avoid delays in attaining therapeutic tacrolimus trough concentrations, 0.2 mg/kg/d may be used as an initial starting LCPT dose for CYP3A5 expressers barring no other clinical barriers to higher starting doses.
This study has several limitations to the interpretation and generalizability of its findings. The single-center design of this study reflects the clinical approach of one institution which may not be applicable to all kidney transplant recipients. CYP3A5 genotype may not be the only genetic consideration when determining an individual’s genetic predisposition to the metabolism of tacrolimus. Genetic differences in CYP3A4 activity and other SNPs within the CYP system could play a role in determining the metabolic rate of tacrolimus which is not captured in our study[12]. Our study evaluated dose requirements and other clinical outcomes through the first 30 d after kidney transplantation. This follow-up period only provides information on short-term LCPT dosing outcomes and future studies with long-term follow-up periods should be performed. This study is also limited by its open-label design and relatively smaller number of patients enrolled. In addition, the single-arm design of this study did not allow for a comparator arm and future randomized controlled studies should be performed to further evaluate dosing requirements of LCPT in the de novo kidney transplant population.
Expression of CYP3A5 metabolic activity is an important clinical factor needed to determine optimal LCPT dosage requirements in the de novo kidney transplant recipient. It is expected that CYP3A5 expressers would require a higher initial starting dose as well as higher incremental dosage titration to achieve therapeutic tacrolimus trough concentrations in a reasonable timeframe. Prospective identification of CYP3A5 genotype may lead to optimized dosing of LCPT in the de novo kidney transplant setting. Future, randomized, larger-scale studies should be conducted to determine the optimal de novo dosing of LCPT after kidney transplantation.
Tacrolimus has been extensively studied and shown to require significant dose adjustments in CYP3A5 expressers compared to non-expressers. Data regarding the impact of CYP35 expresser status on the dosing of tacrolimus extended-release tablets has not been published and is important to understand given the vastly different pharmacokinetic profile of this tacrolimus formulation. There is an increased use of tacrolimus extended-release tablets in the de novo setting warranting further investigation into this clinical question.
The main concerns when initiating tacrolimus in the de novo kidney transplant setting is to achieve therapeutic tacrolimus trough concentrations in a reasonable timeframe while also avoiding drug toxicity. The rationale behind this research is to identify dosing strategies that should be considered when initiating tacrolimus extended-release immediately after kidney transplant. Particular, research evaluating dosing strategies in patients known to have higher tacrolimus dose requirements (i.e. CYP3A5 expressers) will provide data for transplant centers to make educated clinical decisions surrounding dosing and dosing adjustments for tacrolimus extended-release tablet formulations.
The main objectives of this research was to identify the time to therapeutic tacrolimus trough concentration as well as the dose required to obtain that trough concentration. These objectives were realized as well as the differences in dosing requirements amongst CYP3A5 expressers compared to non-expressers. The significance of these objectives warrant further investigation towards linking clinical outcomes such as acute rejection and graft function outside of the first month after transplant in patients receiving tacrolimus extended-release tablets in the de novo kidney transplant setting.
Patients (n = 36) were consented to receive tacrolimus extended-release tablets at a dose of 0.13 mg/kg/d at the time of kidney transplantation. Dosing was adjusted to maintain therapeutic trough concentrations of 8-10 ng/mL which assisted in identifying the primary objective of time to therapeutic concentration. In addition, all patients that consented to CYP3A5 genotype testing (n = 34) were included in additional data analysis to describe dosing requirements for tacrolimus extended-release tablets in patients that were CYP3A5 expressers compared to CYP3A5 non-expressers. These methods allowed the authors to describe initial dosing requirements as well as the impact of CYP3A5 metabolism on tacrolimus extended-release dosing and attainment of target trough concentrations.
This research demonstrated that kidney transplant recipients who are expressers of CYP3A5 exhibited higher dose requirements for tacrolimus extended-release tablets and also experienced delays in attaining therapeutic trough concentrations compared to CYP3A5 non-expressers. These findings are pertinent to the field of solid organ transplant since transplant centers that utilize tacrolimus extended-release tablets in the de novo setting should be aware of the higher dosing needs in this patient population. In addition, transplant recipients suspected to or known to be CYP3A5 expressers may require more aggressive dose titration to achieve and maintain target tacrolimus trough concentrations. Future research in this area should focus on clinical outcomes beyond our study period of 30 d to determine the impact on acute rejection and kidney graft function.
Overall, this study provides additional clinical information regarding the dosing requirements of tacrolimus extended-release tablets in the de novo kidney transplant setting. To our knowledge, this is the first prospective observational study to provide outcomes data for the de novo dosing of tacrolimus extended-release tablets. The findings from this research validate that the impact of CYP3A5 expression has a clinical impact on the pharmacokinetic profile of tacrolimus extended-release tablets similar to findings published with tacrolimus immediate-release. New approaches to dosing and dose titration for tacrolimus extended-release tablets have been proposed by this research in the de novo kidney transplant setting and can be used as a guide when making clinical decisions in this patient population.
Future research should aim to randomize patients to various doses of tacrolimus extended-release tablets to offer a more advanced comparison of different initial dosing strategies. Conducting CYP3A5 genotype analyses prior to study drug initiation would be beneficial in future studies in order to further assess the impact of pharmacokinetic variations in metabolism on tacrolimus extended-release tablets.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira GSA, Brazil S-Editor: Lin C L-Editor: A P-Editor: Zhang YL
| 1. | Budde K, Bunnapradist S, Grinyo JM, Ciechanowski K, Denny JE, Silva HT, Rostaing L; Envarsus study group. Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of Phase III, double-blind, randomized trial. Am J Transplant. 2014;14:2796-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Rostaing L, Bunnapradist S, Grinyó JM, Ciechanowski K, Denny JE, Silva HT Jr, Budde K; Envarsus Study Group. Novel Once-Daily Extended-Release Tacrolimus Versus Twice-Daily Tacrolimus in De Novo Kidney Transplant Recipients: Two-Year Results of Phase 3, Double-Blind, Randomized Trial. Am J Kidney Dis. 2016;67:648-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am J Transplant. 2017;17:432-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Andrés A, Delgado-Arranz M, Morales E, Dipalma T, Polanco N, Gutierrez-Solis E, Morales JM, Praga M, Gutierrez E, Gonzalez E. Extended-release tacrolimus therapy in de novo kidney transplant recipients: single-center experience. Transplant Proc. 2010;42:3034-3037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Fanous H, Zheng R, Campbell C, Huang M, Nash MM, Rapi L, Zaltzman JS, Prasad GV. A comparison of the extended-release and standard-release formulations of tacrolimus in de novo kidney transplant recipients: a 12-month outcome study. Clin Kidney J. 2013;6:45-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Philosophe B, Leca N, West-Thielke PM, Horwedel T, Culkin-Gemmell C, Kistler K, Stevens DR. Evaluation of Flexible Tacrolimus Drug Concentration Monitoring Approach in Patients Receiving Extended-Release Once-Daily Tacrolimus Tablets. J Clin Pharmacol. 2018;58:891-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96:191-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Trofe-Clark J, Brennan DC, West-Thielke P, Milone MC, Lim MA, Neubauer R, Nigro V, Bloom RD. Results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients. Am J Kidney Dis. 2018;71:315-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Khan AR, Raza A, Firasat S, Abid A. CYP3A5 gene polymorphisms and their impact on dosage and trough concentration of tacrolimus among kidney transplant patients: a systematic review and meta-analysis. Pharmacogenomics J. 2020;20:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 703] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 11. | Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1595] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 12. | De Meyer M, Haufroid V, Kanaan N, Darius T, Buemi A, De Pauw L, Eddour DC, Wallemacq P, Mourad M. Pharmacogenetic-based strategy using de novo tacrolimus once daily after kidney transplantation: prospective pilot study. Pharmacogenomics. 2016;17:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR. Genetic variability in CYP3A5 and its possible consequences. Pharmacogenomics. 2004;5:243-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Suarez-Kurtz G, Vargens DD, Santoro AB, Hutz MH, de Moraes ME, Pena SD, Ribeiro-dos-Santos Â, Romano-Silva MA, Struchiner CJ. Global pharmacogenomics: distribution of CYP3A5 polymorphisms and phenotypes in the Brazilian population. PLoS One. 2014;9:e83472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 675] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 16. | Richards KR, Hager D, Muth B, Astor BC, Kaufman D, Djamali A. Tacrolimus trough level at discharge predicts acute rejection in moderately sensitized renal transplant recipients. Transplantation. 2014;97:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Tripathi M, Gohel K, Hegde U, Gang S, Rajapurkar M. Correlation of Trough Tacrolimus Level with Early Acute Rejections in Renal Allograft Recipients- A Prospective Study. Int Gyn & Women’s Health. 2018;1:38-49.. [DOI] [Full Text] |
| 18. | Jasiak-Panek NM, Wenzler E, Patel S, Thielke JJ, Progar K, Brandt S, Huang YJ, Benedetti E, West-Thielke PM. A randomized, open-label pharmacokinetic trial of tacrolimus extended-release dosing in obese de novo kidney transplant recipients. Clin Transplant. 2019;33:e13640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ, Leeder JS, van Schaik R, Thummel KE, Klein TE, Caudle KE, MacPhee IA. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 2015;98:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 531] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 20. | Langone A, Steinberg SM, Gedaly R, Chan LK, Shah T, Sethi KD, Nigro V, Morgan JC; STRATO Investigators. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP-TacrO (STRATO): an open-label, multicenter, prospective phase 3b study. Clin Transplant. 2015;29:796-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Kim IW, Noh H, Ji E, Han N, Hong SH, Ha J, Burckart GJ, Oh JM. Identification of factors affecting tacrolimus level and 5-year clinical outcome in kidney transplant patients. Basic Clin Pharmacol Toxicol. 2012;111:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |