Published online Dec 18, 2023. doi: 10.5500/wjt.v13.i6.331
Peer-review started: August 16, 2023
First decision: September 14, 2023
Revised: October 19, 2023
Accepted: November 3, 2023
Article in press: November 3, 2023
Published online: December 18, 2023
Processing time: 123 Days and 17.4 Hours
The increasing kidney retransplantation rate has created a parallel field of research, including the risk factors and outcomes of this advanced form of renal replacement therapy. The presentation of experiences from different kidney transplantation centers may help enrich the literature on kidney retransplantation, as a specific topic in the field of kidney transplantation.
To identify the risk factors affecting primary graft function and graft survival rates after second kidney transplantation (SKT).
The records of SKT cases performed between January 1977 and December 2014 at a European tertiary-level kidney transplantation center were retrospectively reviewed and analyzed. Beside the descriptive characteristics, the survivals of patients and both the first and second grafts were described using Kaplan-Meier curves. In addition, Kaplan-Meier analyses were also used to estimate the survival probabilities at 1, 3, 5, and 10 post-operative years, as well as at the longest follow-up duration available. Moreover, bivariate associations between various predictors and the categorical outcomes were assessed, using the suitable biostatistical tests, according to the predictor type.
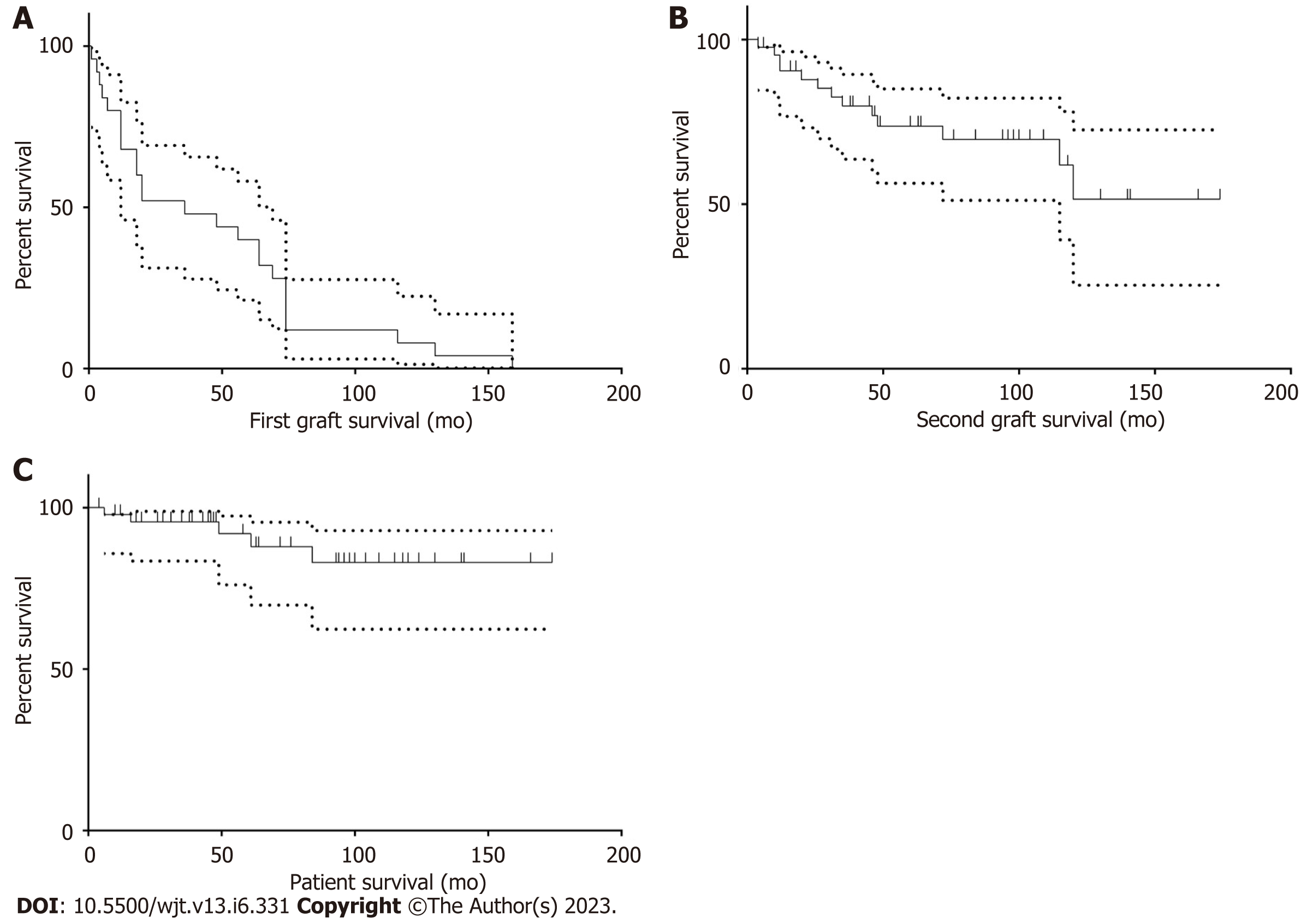
Out of 1861 cases of kidney transplantation, only 48 cases with SKT were eligible for studying, including 33 men and 15 women with a mean age of 42.1 ± 13 years. The primary non-function (PNF) graft occurred in five patients (10.4%). In bivariate analyses, a high body mass index (P = 0.009) and first graft loss due to acute rejection (P = 0.025) were the only significant predictors of PNF graft. The second graft survival was reduced by delayed graft function in the first (P = 0.008) and second (P < 0.001) grafts. However, the effect of acute rejection within the first year after the first transplant did not reach the threshold of significance (P = 0.053). The mean follow-up period was 59.8 ± 48.6 mo. Censored graft/patient survival rates at 1, 3, 5 and 10 years were 90.5%/97.9%, 79.9%/95.6%, 73.7%/91.9%, and 51.6%/83.0%, respectively.
Non-immediate recovery modes of the first and second graft functions were significantly associated with unfavorable second graft survival rates. Patient and graft survival rates of SKT were similar to those of the first kidney transplantation.
Core Tip: Second kidney transplantation (SKT) is a viable option for patients with failed first kidney transplantation (FKT). Although the first primary nonfunction graft is a common contributor to SKT, it is also a potential outcome among a major proportion of those populations. Also, it is a significant risk factor for graft survival among those patients with functioning SKTs. Hence, the non-immediate recovery of the first graft function and delayed graft function in the second graft are significantly associated with unfavorable second graft survival rates. Inspite of this wide spectrum of risk factors, patient and graft survival rates in SKT seemed to be similar to those of FKT. SKT should be recommended for patients with failed FKT.
- Citation: Khalil M, Gadelkareem RA, Abdallah MA, Sayed MAB, Elanany FG, Fornara P, Mohammed N. Predictors of graft function and survival in second kidney transplantation: A single center experience. World J Transplant 2023; 13(6): 331-343
- URL: https://www.wjgnet.com/2220-3230/full/v13/i6/331.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i6.331
Kidney transplantation is the optimal treatment of end-stage renal disease (ESRD), because it provides better outcomes in survival rates, quality of life, and economic saving[1,2]. However, the expected survival of renal allografts is relatively lower than the patients’ survival. This discrepancy between the patient and graft survival rates resulted in a progressively increasing number of patients who may need kidney retransplantation (KRT)[3-5]. Rates of KRT represent more than 15% of patients on the waiting lists[2,3,5], where the second kidney transplantation (SKT) is the most frequent form[5,6]. The numbers of KRT being still relatively far less than that of the first kidney transplantation (FKT) has resulted in persistent debates about the risk factors that may affect KRT and its controversial survival benefits. The magnitude of the reported outcomes of KRT has been shown to be either inferior or acceptable relative to those of FKT[5,7,8]. Beside the potential exposure to the same risk factors of FKT, recipients of KRT are prone to additional factors that may evolve from the repeated process such as sensitization and technical difficulties[5,6,9].
The unresolved debates about the risk factors and survival rates represented our rationale to present the current single center experience of SKT and explore the predictors for the graft function and survival of SKT.
The electronic and manual records of the cases of KRT which were performed between January 1977 and December 2014 at Urology Department, Martin-Luther University, Halle (Saale), Germany were reviewed for the characteristics of the FKT and SKT processes. The effects of these variables on the primary graft function and the survival of both graft and patient were evaluated in SKT.
The target population was the adult patients who received SKT. Exclusion criteria were blood grouping or human leucocytic antigen (HLA) incompatible transplants; immunosuppression protocols other than basiliximab or anti-thymoglobulin for induction, and steroid, tacrolimus or cyclosporine, and mycophenolate mofetil for maintenance; missing data; and SKT within the year just before data collection.
The authors confirm that all the experimental protocols of this study were approved by the Ethical Committee (Institutional Review Board; IRB) of the Faculty of Medicine, Assiut University, Egypt and Martin-Luther University, Germany (IRB approval number: 17200548/2015).
The statistical methods were implemented using IBM® SPSS® Statistics 23 and GraphPad Prism® 6. Two-tailed P values < 0.05 were considered significant.
After excluding primary non-function grafts, the survivals of both the first and second grafts were described using Kaplan-Meier curves. The same method was also used to describe patient survival after the second transplantation for the whole study sample. Moreover, regarding the graft and the patient survivals after SKT, Kaplan-Meier analyses were also used to estimate the survival probabilities at 1, 3, 5, and 10 post-operative years, as well as at the longest follow-up duration available.
Bivariate associations between various predictors and the categorical outcomes were assessed according to the predictor type. For quantitative predictors, the independent-samples t test was used when all outcome groups were normally distributed. Otherwise, the independent-samples Mann-Whitney U test and the Kruskal-Wallis test were used for binary and multinomial outcomes, respectively. For categorical predictors, Fisher’s exact test was used.
As regards the second graft survival, associations with categorical predictors were evaluated by Kaplan-Meier curves for the strata of each predictor; the similarity between these curves for each predictor was tested by the log-rank test. On the other hand, associations with quantitative predictors were evaluated by Cox regression, where testing of the proportional hazards assumption was done by correlating ranked survival times with Schoenfeld residuals.
Between January 1977 and December 2014, a total of 1861 kidney transplants were done, of whom 176 cases had SKT. Only 48 cases were eligible for the current study. Characteristics of patients, donors, FKT, and SKT are summarized in Table 1. Twenty-three cases (47.9%) had primary non-function (PNF) first graft, while only five cases (10.4%) had PNF second graft. Patients with PNF grafts were excluded from the graft survival analyses. The median survival time for the first graft was 36 mo, while it was undefined for the graft and the patient after SKT (Figure 1). Survival probabilities of the graft and the patient after the SKT are shown in Table 2. The follow-up period ranged from 12 to 174 mo.
| Variable | Value1 | |
| Recipient age at SKT (yr) | 47.5 (41.3-56; 24-70) | |
| Recipient sex | Male | 33 (68.8) |
| Female | 15 (31.3) | |
| Recipient BMI (kg/m2) at SKT | 24.7 (22.13-26.95; 19-33.5) | |
| Causes of ESRD | Glomerulonephritis | 16 (33.3) |
| DM | 1 (2.1) | |
| Hypertension | 4 (8.3) | |
| PCKD | 4 (8.3) | |
| Others | 23 (47.9) | |
| Overall duration of dialysis (mo.) | 95 (76-121.8; 29-244) | |
| Start of first graft function | PNF | 23 (47.9) |
| DGF | 8 (16.7) | |
| Immediate | 17 (35.4) | |
| GFR one year after FKT (mL/min/1.73 m2) | 0 (0-29.3; 0-78.8) | |
| Attacks of acute rejection in first year after FKT2 | 0 (0-1; 0-6) | |
| First graft loss due to rejection | 3 (6.3) | |
| First graft nephrectomy | 37 (77.1) | |
| SKT donor type | Living | 3 (6.3) |
| Deceased | 45 (93.8) | |
| SKT donor age (yr) | 50 (36.3-60.8; 16-74) | |
| Recipient age minus donor age (yr) at SKT | 0 (-10-7; -39-34) | |
| SKT donor BMI (kg/m2) | 25 (23-27; 19-37.9) | |
| Recipient BMI minus donor BMI (kg/m2) at SKT | -0.45 (-3.8-3.15; -16.7-9.6) | |
| SKT PRA level | 0-30% | 35 (72.9) |
| 31-80% | 10 (20.8) | |
| > 80% | 3 (6.3) | |
| SKT HLA mismatches | 2 (1.3-3.8; 0-6) | |
| SKT laterality relative to FKT | Ipsilateral | 1 (2.1) |
| Contralateral | 47 (97.9) | |
| Number of renal arteries at SKT | Single | 43 (89.6) |
| Double | 5 (10.4) | |
| SKT operative time (min) | 140 (113-170; 82-236) | |
| SKT ischemia time (min) | 708 (531-897; 74-1319) | |
| SKT operative revision | 24 (50) | |
| SKT vascular complications | 8 (16.7) | |
| Start of second graft function | PNF | 5 (10.4) |
| DGF | 10 (20.8) | |
| Immediate | 33 (68.8) | |
| Attacks of acute rejection in first year after SKT | 0 (0-1; 0-3) | |
| GFR one year after SKT (mL/min/1.73 m2) | 36 (22.8-52.8; 0-82.4) | |
| Follow-up time (months) | Second graft survival | Patient survival after second kidney transplantation | ||||
| Survival probability (%) | Upper 95% confidence limit | Lower 95% confidence limit | Survival probability (%) | Upper 95% confidence limit | Lower 95% confidence limit | |
| 12 | 90.53 | +5.81 | -13.84 | 97.87 | +1.83 | -12.04 |
| 36 | 79.88 | +9.55 | -16.22 | 95.60 | +3.29 | -12.10 |
| 60 | 73.71 | +11.31 | -17.34 | 91.92 | +5.51 | -15.82 |
| 120 | 51.57 | +21.01 | -26.12 | 83.04 | +9.90 | -20.65 |
| 174 (study max.) | 51.57 | +21.01 | -26.12 | 83.04 | +9.90 | -20.65 |
PNF graft occurred in five patients (10.4%). In bivariate analyses, a high body mass index (BMI) of the recipient was the only significant quantitative predictor of PNF graft (P = 0.009) (Tables 3 and 4). Also, first graft loss due to acute rejection was the only significant categorical predictor of PNF graft (P = 0.025) (Table 5).
| Primary non-function (n = 5) | Primary function (n = 43) | P value1 | |||
| Mean | SE | Mean | SE | ||
| Recipient age (yr) | 47.8 | 5.4 | 47.9 | 1.8 | 0.98 |
| Donor age (yr) | 49.6 | 7.5 | 48.0 | 2.2 | 0.82 |
| Recipient BMI (kg/m2) | 28.04 | 0.83 | 24.20 | 0.47 | 0.009 |
| Total ischemia time (min) | 655 | 98 | 711 | 48 | 0.70 |
| Operative time (min) | 150 | 20 | 142 | 6 | 0.66 |
| Primary non-function (n = 5) | Primary function (n = 43) | P value | |||
| Median | Mean rank | Median | Mean rank | ||
| Duration of first graft function (mo) | 0 | 17.6 | 4 | 25.3 | 0.26 |
| Total duration of dialysis before second transplantation (including before first transplantation) (mo) | 93 | 19.3 | 96 | 25.1 | 0.39 |
| Variables | Primary non-function (n = 5) | Primary function (n = 43)1 | Odds ratio2 | P value | |
| DM as a cause of ESRD | No | 5 | 42 | 0 | 1 |
| Yes | 0 | 1 | |||
| First graft function | No | 3 | 20 | 0.84 | |
| Delayed | 1 | 7 | |||
| Instant | 1 | 16 | |||
| Acute rejection in first year after first transplantation | No | 3 | 24 | 0.94 | 1 |
| Yes | 2 | 17 | |||
| First graft loss by acute rejection | No | 3 | 42 | 28 | 0.025 |
| Yes | 2 | 1 | |||
| First graft nephrectomy | No | 0 | 11 | Not assessed3 | 0.58 |
| Yes | 5 | 32 | |||
| Living donor | No | 5 | 40 | 0 | 1 |
| Yes | 0 | 3 | |||
| PRA grouping | 0% to 30% | 3 | 32 | 0.33 | |
| 31% to 80% | 1 | 9 | |||
| Over 80% | 1 | 2 | |||
| Number of HLA mismatches | 0 | 1 | 6 | 0.51 | |
| 1 to 3 | 2 | 27 | |||
| 4 to 6 | 2 | 10 | |||
| Over one artery | No | 5 | 38 | 0 | 1 |
| Yes | 0 | 5 | |||
| Vascular complications | No | 3 | 37 | 4.1 | 0.19 |
| Yes | 2 | 6 |
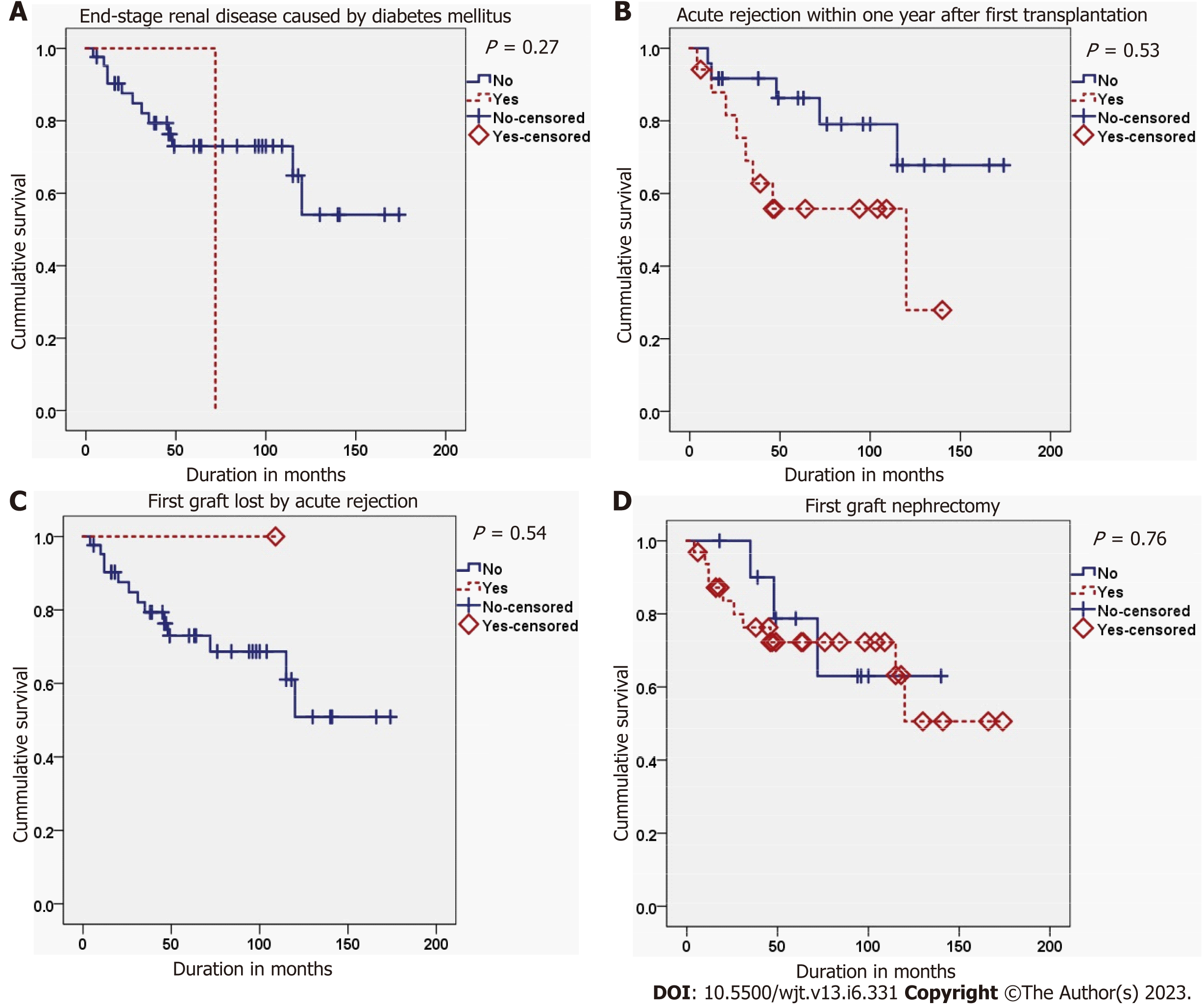
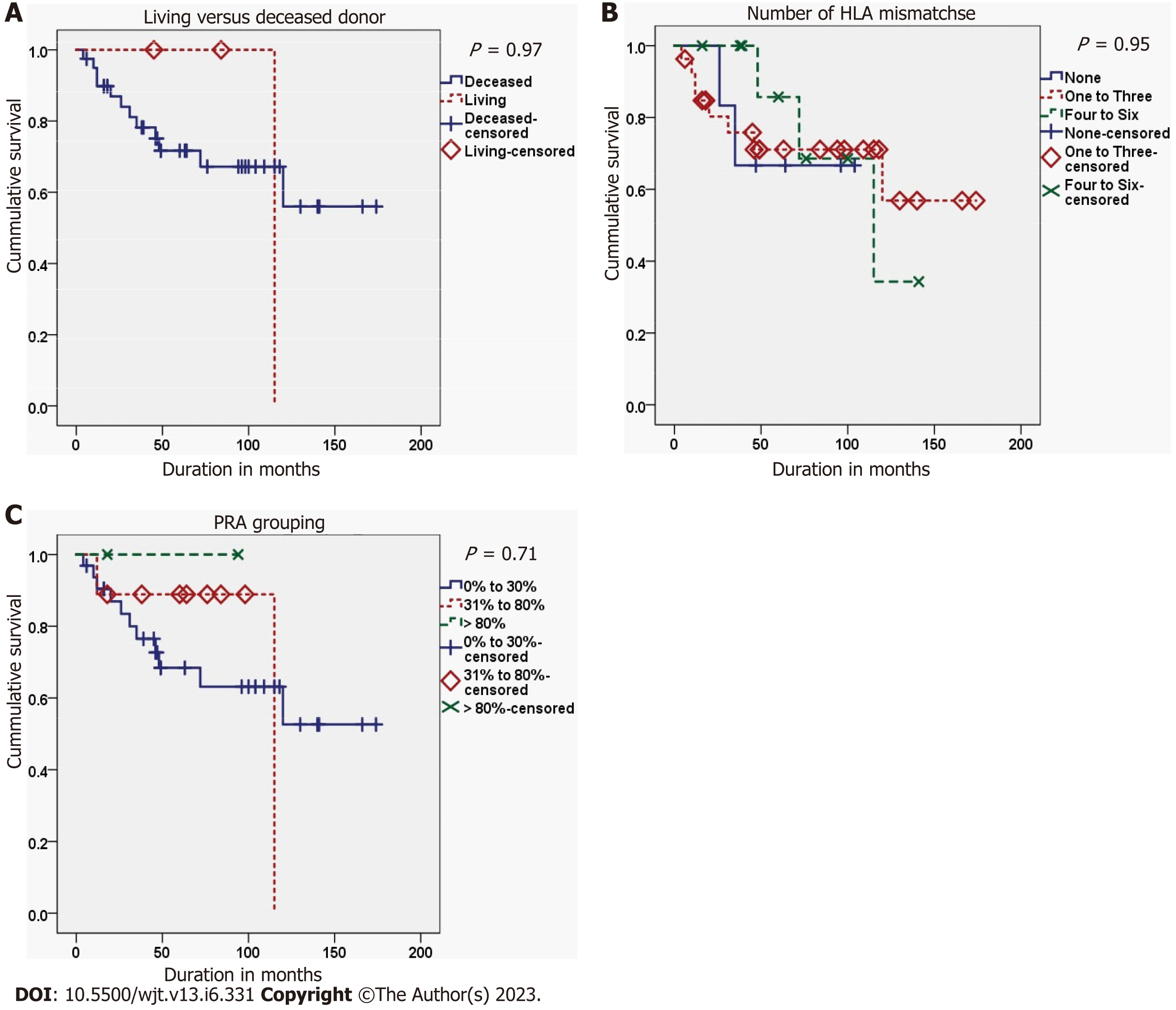
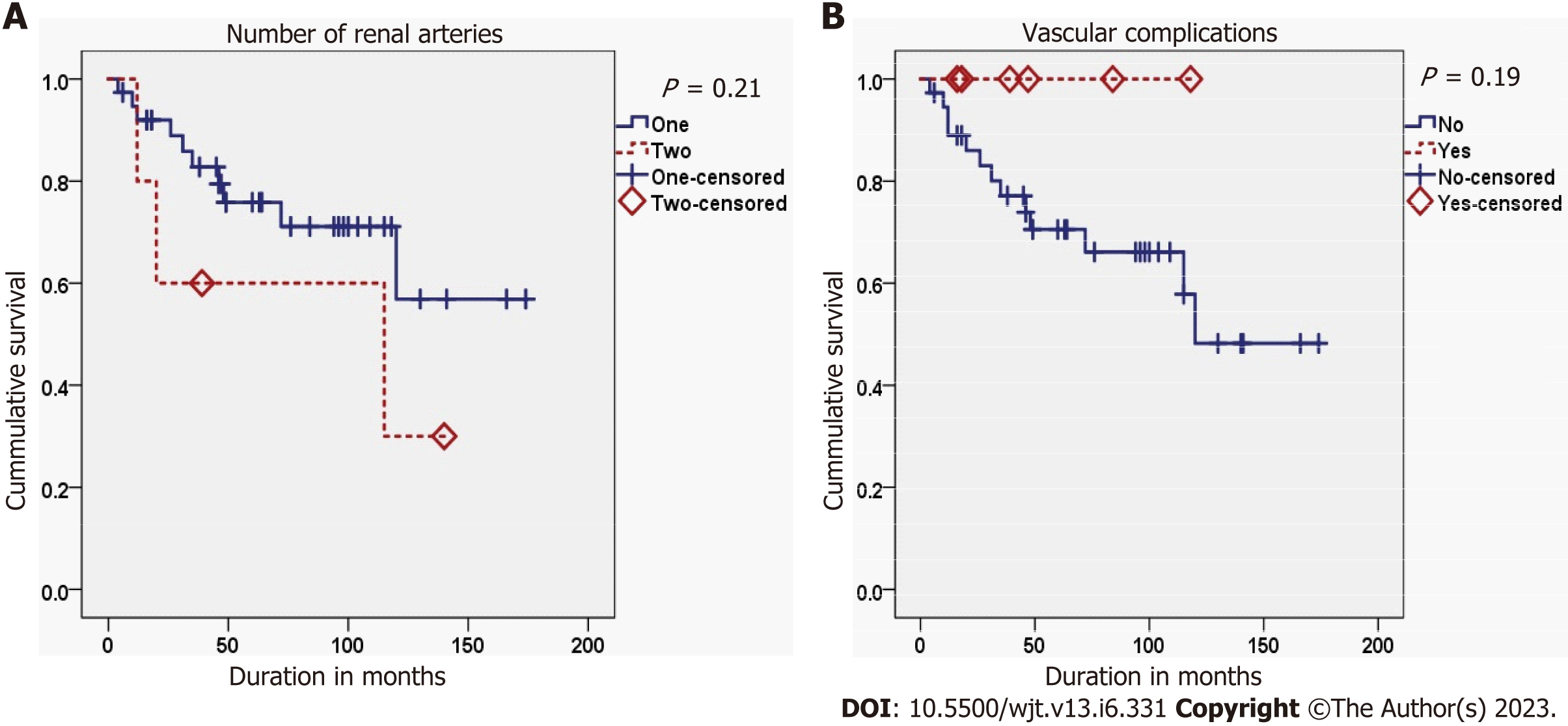
The second graft survival was best in cases with a PNF first graft, while it was worst in cases with a delayed graft function (DGF) of the first graft (P = 0.008). Also, the second graft survival was better in cases with an immediate second graft function than in those with a delayed second graft function (P < 0.001) (Figure 2). Finally, the occurrence of acute rejection within the first year after the FKT decreased the survival of the second graft, but didn’t reach the threshold of significance (P = 0.053) (Tables 6 and 7; Figures 3-5).
| Variables | Events (n = 13) | Censored (n = 30)1 | Log-rank statistic | P value | |
| DM as a cause of ESRD | No | 12 | 30 | 1.218 | 0.270 |
| Yes | 1 | 0 | |||
| First graft function | No | 2 | 18 | 9.684 | 0.008 |
| Delayed | 4 | 3 | |||
| Instant | 7 | 9 | |||
| Acute rejection in first year after first transplantation | No | 5 | 19 | 3.757 | 0.053 |
| Yes | 8 | 9 | |||
| First graft loss by acute rejection | No | 13 | 29 | 0.369 | 0.543 |
| Yes | 0 | 1 | |||
| First graft nephrectomy | No | 3 | 8 | 0.097 | 0.756 |
| Yes | 10 | 22 | |||
| Living donor | No | 12 | 28 | 0.002 | 0.965 |
| Yes | 1 | 2 | |||
| PRA grouping | 0% to 30% | 11 | 21 | 0.693 | 0.707 |
| 31% to 80% | 2 | 7 | |||
| Over 80% | 0 | 2 | |||
| Number of HLA mismatches | 0 | 2 | 4 | 0.106 | 0.948 |
| 1 to 3 | 8 | 19 | |||
| 4 to 6 | 3 | 7 | |||
| Over one artery | No | 10 | 28 | 1.584 | 0.208 |
| Yes | 3 | 2 | |||
| Vascular complications | No | 13 | 24 | 1.723 | 0.189 |
| Yes | 0 | 6 | |||
| Delayed second graft function | No | 7 | 26 | 12.238 | 0.0005 |
| Yes | 6 | 4 |
| Variables | HR | 95%CI for HR | P value | P value for PH testing1 | |
| Lower bound | Upper bound | ||||
| Recipient age (yr) | 0.976 | 0.930 | 1.023 | 0.306 | 0.074 |
| Recipient BMI (kg/m2) | 0.980 | 0.810 | 1.185 | 0.833 | 0.787 |
| Duration of first graft function (mo) | 1.007 | 0.994 | 1.020 | 0.307 | 0.059 |
| Total duration of dialysis before second transplantation (including before first transplantation) (mo) | 1.006 | 0.995 | 1.017 | 0.295 | 0.061 |
| Donor age (yr) | 1.016 | 0.979 | 1.055 | 0.396 | 0.852 |
| Recipient age minus donor age (yr) | 0.972 | 0.937 | 1.009 | 0.140 | 0.306 |
| Recipient BMI minus donor BMI (kg/m2) | 0.984 | 0.893 | 1.085 | 0.751 | 0.410 |
| Total ischemia time (min) | 1.001 | 0.999 | 1.003 | 0.284 | 0.579 |
| Operative time (min) | 0.995 | 0.979 | 1.010 | 0.497 | 0.363 |
No significant associations were found between panel reactive antibodies (PRA) categories at SKT on one hand and first graft nephrectomy (P = 0.784), the duration before first graft nephrectomy (P = 0.497), or acute rejection of the second graft in the first year after SKT (P = 0.223) on the other hand. Also, no significant association was found between the number of second graft arteries and the vascular complications of SKT (P = 0.382).
Graft loss is always a potential outcome after variable periods of FKT[3,8-10]. This outcome created an imperative need for KRT[11]. Nowadays, there is a progressive rise in the numbers of patients receiving this line of treatment. KRT entails more risk factors for unfavorable outcomes than FKT[6,12]. Also, there are substantial controversies about the differences between FKT and SKT regarding patient and graft survival rates[7]. The current study targeted the potential risk factors affecting the second graft function in a large-volume kidney transplantation center.
In our study, the mean patient age at SKT was similar to that reported in other studies[5,13]. Also, our results resembled other studies regarding the gender distribution at SKT[5,13,14]. Causes of ESRD before kidney transplantation are not the same among the different world regions. Diabetic and hypertensive nephropathies represent the main causes in the United States. However, in the current series, glomerulonephritis was the leading cause, as in other countries[5,13].
It has been reported that occurrence of certain clinical outcomes after FKT is significantly associated with more likelihood of the same outcomes after KRT which increases the chances of graft loss[13]. In general, graft loss can be classified into three major categories: PNF grafts, patient death with a functioning graft, and loss of a previously functioning graft due to different medical and surgical causes[15,16].
PNF graft is defined as the permanent absence of functions of the transplanted kidney starting immediately after transplantation. It accounts for 0.6%-8% of all renal graft loss and it is significantly associated with poor patient survival[15,17]. In our series, a slightly higher rate was observed in SKT (10.4%), while the rate was much higher in FKT (47.9%). The major cause of PNF grafts has been reported to be venous or arterial thrombosis occurring within 1-2 d after transplantation[15]. In our series, although the odds of PNF in cases with vascular complications was 4.1 times higher than in cases without these complications, the result was statistically insignificant probably due to the small sample. However, high recipients’ BMI and first graft loss due to acute rejection were significantly associated with the occurrence of PNF after SKT. This might be attributable to the same mechanisms that decrease the second graft survival[15]. To our knowledge, it seems that these factors have not yet been studied relative to PNF graft after SKT.
The third category of kidney transplantation loss outcomes is the loss of the graft which functioned for a certain period before being permanently non-functioning. The risk factors of this outcome are multiple and have different tributaries. Regarding the elements of kidney transplantation process (recipient, donor, and process) and the previously proposed categorizations in the literature[5,14,18], the potential predictors or risk factors that affect the outcome of SKT could be classified into five classes: recipient-related, donor-related, FKT process-related, SKT process-related, and common factors.
The recipient-related risk factors include patient’s age, sex, BMI, race, the cause of ESRD, and the associated comorbidities like diabetes mellitus and hypertension[5,13,19,20]. The second class risk factors are the donor-related factors either in FKT and SKT processes such as donor type (living or deceased), age, sex, and relatedness[5,13,14,21]. In the current series, the studied group of these factors showed no significant effects on SKT graft survival. We examined the effect of two further potential recipient-related variables; the differences between recipients’ and donors’ age and BMIs. Although they have been studied previously for their effect on FKT graft survival[22,23], they haven’t been tested upon KRT survival so far. However, no significant association with the second graft survival could be found. It may be better demonstrated in larger studies.
The third class includes the factors from FKT process such as duration of FKT graft function and estimated glomerular filtration rate at one year after FKT[13,21,24-26]. The fourth class of risk factors includes factors that affect only SKT process such as sensitization due to previous transplantation represented by PRA level, first graft nephrectomy, and serum creatinine at one year after SKT[5,21,25].
The fifth class consists of the common variables between FKT and SKT processes and they represent the major proportion of risk factors. They involve all the phases of the process; factors in the preoperative phase such as number of HLA mismatches[4,5,18], and duration of dialysis[13,27]; factors in the operative and perioperative phases such as ischemia time, DGF[20,28], mode of recovery of graft function[13,27], and surgical complications[14]; factors in the postoperative phase such as acute rejection[13,27]; and factors involving the whole phases such as immunosuppressive regimens[5,12,29], and volume of transplantation center[18]. The reported incidence of DGF among KRTs ranged from 26.7%-39%[5,7,20]. In our study, the non-immediate mode of recovery of first graft function and DGF of second graft were the only significant predictors for low second graft survival. It has been reported that occurrence of acute rejection during their first year post FKT is significantly associated with occurrence of acute rejection during KRT[13,21]. The current results showed that the incidence of acute rejection in FKT approached the threshold of significance in affection of the graft survival of SKT. This insignificant association could be attributed to the small sample size. The significant association of the mode of recovery of FKTs and the nearly significant association of the incidence of acute rejection among FKTs with the SKT graft survival, without the same effect on the SKT, could be attributed to the more stringent immunosuppression protocols and precise donor selection. This may improve the SKT graft function recovery and decrease the incidence of acute rejections. Thus, it may improve the short-term results to some extent but, it doesn’t exterminate the inherent high risk of those patients[9,30].
With controversy, rates of graft and patient survivals of KRTs have been reported as inferior[3,14] or insignificantly different from those of FKT[4,5,21]. In the current study, the long-term graft survival rates were similar to FKT. This outcome is similar to the other studies[4,21].
This study was conducted in a large-volume kidney transplantation center and extracted from a relatively large reviewed number of kidney transplantations. Also, new potential predictors including the differences in age and BMI between the recipients and donors were studied for their effect on graft survival.
Limitations of the current study were the relatively small sample size that didn’t allow for adequate powerful statistical tests such as the multivariate analysis and lack of reporting of some complications as post-transplant neoplastic diseases and infections. Specifically, there were some missing data, such as the levels of the donor specific antibodies against the HLA alleles of the first graft and the pathological evaluation of the donors. In addition, the retrospective studying has its mere limitations of difficult implementation of comparison and randomization.
SKT is a viable option for patients with failed FKT. Demographics and clinical characteristics of the patients accessing SKT are not significantly different from those of FKT. There are multiple potential factors that may originate from the different components and phases of SKT and could affect the survival outcomes. Although the first PNF graft is a common contributor to SKT, it is also a potential outcome among a major proportion of those populations. Also, it is a significant risk factor for graft survival among those patients with functioning SKTs. So, the non-immediate recovery of the first graft function and DGF in the second graft are significantly associated with unfavorable second graft survival rates. Inspite of this wide spectrum of risk factors, patient and graft survival rates in SKT seemed to be similar to those of FKT.
The increasing kidney retransplantation rate has created a parallel field of research, including the risk factors and outcomes of this advanced form of renal replacement therapy. The presentation of experiences from different kidney transplantation (KT) centers may help enrich the literature on kidney retransplantation, as a specific topic in the field of KT.
Despite the potential high risks of repeated KT, increase of the rate of second KT (SKT) seems to be a modifiable variable and may provide better outcomes than return to dialysis in patients with failed first KT.
To identify the risk factors affecting primary graft function and graft survival rates after SKT.
The records of SKT cases performed between January 1977 and December 2014 at a European tertiary-level kidney transplantation center were retrospectively reviewed and analyzed. Beside the descriptive characteristics, the survivals of patients and both the first and second grafts were described using Kaplan-Meier curves. In addition, Kaplan-Meier analyses were also used to estimate the survival probabilities at 1, 3, 5, and 10 post-operative years, as well as at the longest follow-up duration available. Moreover, bivariate associations between various predictors and the categorical outcomes were assessed, using the suitable biostatistical tests, according to the predictor type. However, associations with quantitative predictors were evaluated by Cox regression.
Out of 1861 cases of kidney transplantation, only 48 cases with SKT were eligible for studying, including 33 men and 15 women with a mean age of 42.1 ± 13 years. The primary non-function (PNF) graft occurred in five patients (10.4%). In bivariate analyses, a high body mass index (P = 0.009) and first graft loss due to acute rejection (P = 0.025) were the only significant predictors of PNF graft. The second graft survival was reduced by delayed graft function in the first (P = 0.008) and second (P < 0.001) grafts. However, the effect of acute rejection within the first year after the first transplant did not reach the threshold of significance (P = 0.053). The mean follow-up period was 59.8 ± 48.6 mo. Censored graft/patient survival rates at 1, 3, 5 and 10 years were 90.5%/97.9%, 79.9%/95.6%, 73.7%/91.9%, and 51.6%/83.0%, respectively.
Non-immediate recovery modes of the first and second graft functions were significantly associated with unfavorable second graft survival rates. Patient and graft survival rates of SKT were similar to those of the first KT.
Repeated kidney transplantation may provide better outcomes in patients with failed previous grafts. However, this approach may be associated with higher risks than the first time due to the surgical difficulties and immunological sensitization. Controlling of these risk factors can enhance the outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Egyptian Urological Association.
Specialty type: Transplantation
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gong N, China S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1045] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 2. | United States Renal Data System. 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2021. (Accessed March 10, 2022). Available from: www.usrds.org. |
| 3. | Magee JC, Barr ML, Basadonna GP, Johnson MR, Mahadevan S, McBride MA, Schaubel DE, Leichtman AB. Repeat organ transplantation in the United States, 1996-2005. Am J Transplant. 2007;7:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Barocci S, Valente U, Fontana I, Tagliamacco A, Santori G, Mossa M, Ferrari E, Trovatello G, Centore C, Lorenzi S, Rolla D, Nocera A. Long-term outcome on kidney retransplantation: a review of 100 cases from a single center. Transplant Proc. 2009;41:1156-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ingsathit A, Kantachuvesiri S, Rattanasiri S, Avihingsanon Y, Premasathian N, Pongskul C, Jittikanont S, Lumpaopong A, Sumethkul V. Long-term outcome of kidney retransplantation in comparison with first kidney transplantation: a report from the Thai Transplantation Registry. Transplant Proc. 2013;45:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Kienzl-Wagner K, Mark W, Maglione M, Brandacher G, Öllinger R, Margreiter R, Pratschke J, Bösmüller C. Single-center experience with third and fourth kidney transplants. Transpl Int. 2011;24:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Khalil AK, Slaven JE, Mujtaba MA, Yaqub MS, Mishler DP, Taber TE, Sharfuddin AA. Re-transplants compared to primary kidney transplants recipients: a mate kidney paired analysis of the OPTN/UNOS database. Clin Transplant. 2016;30:566-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Fallahzadeh MK, Birdwell KA. Waitlist Mortality for Second Kidney Transplants. Clin J Am Soc Nephrol. 2022;17:6-7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Mouquet C, Benalia H, Chartier-Kastler E, Sylla C, Coriat P, Bitker MO, Richard F. [Renal retransplantation in adults. Comparative prognostic study]. Prog Urol. 1999;9:239-243. [PubMed] |
| 10. | Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 11. | Barba Abad J, Robles García JE, Saiz Sansi A, Tolosa Eizaguirre E, Romero Vargas L, Algarra Navarro R, Rosell Costa D, Zudaire Bergera JJ, Berián Polo JM, Pascual Piedrola JI. Impact of renal retransplantation on graft and recipient survival. Arch Esp Urol. 2011;64:363-370. [PubMed] |
| 12. | Santos AH Jr, Casey MJ, Womer KL. Analysis of Risk Factors for Kidney Retransplant Outcomes Associated with Common Induction Regimens: A Study of over Twelve-Thousand Cases in the United States. J Transplant. 2017;2017:8132672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Heaphy EL, Poggio ED, Flechner SM, Goldfarb DA, Askar M, Fatica R, Srinivas TR, Schold JD. Risk factors for retransplant kidney recipients: relisting and outcomes from patients' primary transplant. Am J Transplant. 2014;14:1356-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Ahmed K, Ahmad N, Khan MS, Koffman G, Calder F, Taylor J, Mamode N. Influence of number of retransplants on renal graft outcome. Transplant Proc. 2008;40:1349-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 631] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 16. | Yu MY, Kim YC, Lee JP, Lee H, Kim YS. Death with graft function after kidney transplantation: a single-center experience. Clin Exp Nephrol. 2018;22:710-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Parasuraman R, L Zhang P, Samarapungavan D, Pothugunta K, Reddy G, Rocher L, Dumler F, Raofi V, Cohn S, Koffron A. Primary nonfunction of renal allograft secondary to acute oxalate nephropathy. Case Rep Transplant. 2011;2011:876906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Gjertson DW. A multi-factor analysis of kidney regraft outcomes. Clin Transpl. 2002;335-349. [PubMed] |
| 19. | Pour-Reza-Gholi F, Nafar M, Saeedinia A, Farrokhi F, Firouzan A, Simforoosh N, Basiri A, Einollahi B. Kidney retransplantation in comparison with first kidney transplantation. Transplant Proc. 2005;37:2962-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Arnol M, Prather JC, Mittalhenkle A, Barry JM, Norman DJ. Long-term kidney regraft survival from deceased donors: risk factors and outcomes in a single center. Transplantation. 2008;86:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | El-Agroudy AE, Wafa EW, Bakr MA, Donia AF, Ismail AM, Shokeir AA, Shehab El-Dein AB, Ghoneim MA. Living-donor kidney retransplantation: risk factors and outcome. BJU Int. 2004;94:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kostakis ID, Moris DN, Barlas A, Bokos I, Darema M, Theodoropoulou E, Karaolanis G, Kostakis A, Boletis I, Zavos G. Impact of donor and recipient age difference on long-term allograft survival after living donor renal transplantation: analysis of 478 cases. Clin Transplant. 2013;27:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Wang HH, Lin KJ, Liu KL, Chu SH, Hsieh CY, Chiang YJ. Size does matter-donor-to-recipient body mass index difference may affect renal graft outcome. Transplant Proc. 2012;44:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Almond PS, Matas AJ, Gillingham K, Troppmann C, Payne W, Dunn D, Sutherland D, Najarian JS. Risk factors for second renal allografts immunosuppressed with cyclosporine. Transplantation. 1991;52:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Arndorfer JA, Meier-Kriesche HU, Ojo AO, Gruber SA, Cibrik DM, Lake KD, Kaplan B, Leichtman AB. Time to first graft loss as a risk factor for second renal allograft loss. Transplant Proc. 2001;33:1188-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Abouljoud MS, Deierhoi MH, Hudson SL, Diethelm AG. Risk factors affecting second renal transplant outcome, with special reference to primary allograft nephrectomy. Transplantation. 1995;60:138-144. [PubMed] |
| 27. | Johnston O, Rose CL, Gill JS. Risks and benefits of preemptive second kidney transplantation. Transplantation. 2013;95:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Thompson JS, Thacker LR 2nd, Krishnan G. Human leukocyte antigens DR and AB and kidney retransplantation. Transplantation. 2003;75:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Schold J, Poggio E, Goldfarb D, Kayler L, Flechner S. Clinical outcomes associated with induction regimens among retransplant kidney recipients in the United States. Transplantation. 2015;99:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Zhu L, Fu C, Lin K, Wang Z, Guo H, Chen S, Lin Z, Chen Z, Chen G. Patterns of Early Rejection in Renal Retransplantation: A Single-Center Experience. J Immunol Res. 2016;2016:2697860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |