Published online Mar 18, 2023. doi: 10.5500/wjt.v13.i3.96
Peer-review started: December 2, 2022
First decision: December 13, 2022
Revised: December 22, 2022
Accepted: March 6, 2023
Article in press: March 6, 2023
Published online: March 18, 2023
Processing time: 104 Days and 18.2 Hours
Children with acute liver failure (ALF) who meet the criteria are eligible for super-urgent transplantation, whereas children with end-stage chronic liver disease (ESCLD) are usually transplanted electively. Pediatric liver trans
To determine if there is a difference in post-operative complications and survival outcomes between ALF and ESCLD in PLT.
This was a retrospective observational study of all primary PLTs performed at a single center between 2000 and 2019. ALF and ESCLD groups were compared for pretransplant recipient, donor and operative parameters, and post-operative outcomes including graft and patient survival.
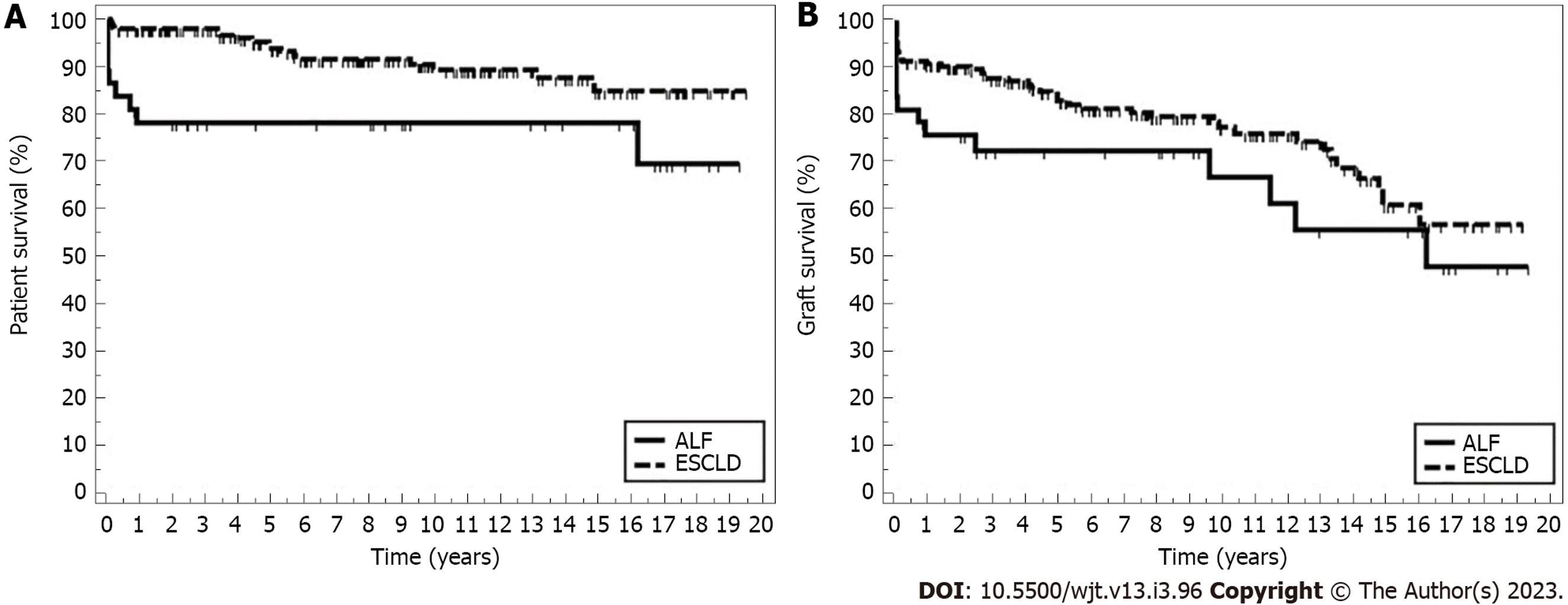
Over a 20-year study period, 232 primary PLTs were performed at our center; 195 were transplanted for ESCLD and 37 were transplanted for ALF. The ALF recipients were significantly older (median 8 years vs 5.4 years; P = 0.031) and heavier (31 kg vs 21 kg; P = 0.011). Living donor grafts were used more in the ESCLD group (34 vs 0; P = 0.006). There was no difference between the two groups concerning vascular complications and rejection, but there were more bile leaks in the ESCLD group. Post-transplant patient survival was significantly higher in the ESCLD group: 1-, 5-, and 10-year survival rates were 97.9%, 93.9%, and 89.4%, respectively, compared to 78.3%, 78.3%, and 78.3% in the ALF group (P = 0.007). However, there was no difference in 1-, 5-, and 10-year graft survival between the ESCLD and ALF groups (90.7%, 82.9%, 77.3% vs 75.6%, 72.4%, and 66.9%; P = 0.119).
Patient survival is inferior in ALF compared to ESCLD recipients; the main reason is death in the 1st year post-PLT in ALF group. Once the ALF children overcome the 1st year after transplant, their survival stabilizes, and they have good long-term outcomes.
Core Tip: To the best of our knowledge, this is the first study to compare the complications and survival outcomes in acute liver failure (ALF) and end-stage chronic liver disease (ESCLD) children post-pediatric liver transplantation (PLT). This study not only showed that survival in the ALF group was significantly inferior post-PLT but also showed a different pattern of survival where ALF survival was mostly affected in the 1st year post-transplant and then stabilized, whereas ESCLD survival declined steadily over time.
- Citation: Alnagar AM, Hakeem AR, Daradka K, Kyrana E, Methga M, Palaniswamy K, Rajwal S, Mulla J, O'meara M, Upasani V, Vijayanand D, Prasad R, Attia MS. Long-term outcomes of pediatric liver transplantation in acute liver failure vs end-stage chronic liver disease: A retrospective observational study . World J Transplant 2023; 13(3): 96-106
- URL: https://www.wjgnet.com/2220-3230/full/v13/i3/96.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i3.96
Liver disease is a leading cause of morbidity and mortality in children. The spectrum of liver path
The diagnosis of ALF in children can be challenging, as hepatic encephalopathy in this age group is usually difficult to define, particularly in its early stages and sometimes it may not be clinically evident until the ALF becomes advanced[2-4]. Also in some cases, accurate diagnosis of the etiology of ALF may not be possible, primarily in candidates who present with unrecognized metabolic diseases shortly after birth or those who clinically deteriorate over a short period not allowing enough room for full bio
Managing children suffering from ALF is a dynamic process, with the decision to list for PLT made in an emergent manner, when the probability of spontaneous recovery is low but also before any irreversible neurological or respiratory sequalae take place[2]. At times, the window from presentation to PLT may span from only few hours up to a small number of days, posing significant challenges for the clinical team[5]. Optimum clinical and logistical management is essential as successful PLT in this special group has a dramatic effect on their survival[4-6]. Hence at the national level, the graft allocation system gives ALF children the highest priority being labeled as “super-urgent.”
In comparison, the clinical and logistic dynamics are completely different in the case of children with ESCLD because PLT is usually performed on an elective basis, as candidates are usually in less critical clinical condition and the transplant team has enough time for evaluation and planning, aiming for optimum timing and potentially improved outcomes.
Both groups were discussed among other categories in publications describing the experience of PLT centers, but to the best of our knowledge, no previous studies have directly compared the outcomes of PLT in ALF and ESCLD settings. This study was conducted to determine if there is a difference in post-PLT complications between the ALF and ESCLD groups, and to describe any variance in survival between the two cohorts. The importance of this comparison is to provide insights for transplant centers and organ allocation systems dealing with these two divergent groups to make the best use of limited resources including a limited graft pool and to anticipate differences in behavior between candidates in each group to tailor their clinical care accordingly. This comparison also opens the door for future research to overcome obstacles and improve PLT outcomes, especially in the ALF group where the underlying cause of the liver failure remains unknown in a considerable number of children.
This was a retrospective cross-sectional observational study of the long-term outcomes of PLT performed at Leeds Teaching Hospitals NHS Trust between 2000 and 2019. Hospital documents and electronic records were used to retrieve donor and recipient data. Ethical approval was not required for this retrospective analysis of already collected data, and the study was registered as a service improvement project within the hospital clinical governance department, with no patient identifiable information stored while collecting and analyzing the data for this project.
The inclusion criteria were all PLT recipients of the first liver transplant in our center ≤ 18 years with either ALF or ESCLD as the recorded indication for PLT. Exclusion criteria were retransplants, primary PLTs for liver tumors, or metabolic disorders without underlying liver disease. Retransplants were excluded as they represent a heterogenous group with well-reported inferior outcomes compared to primary transplants. Children with liver tumors are unique with a well-defined transplant indication, and their disease may be complicated by the burden of chemotherapy before undergoing a liver transplant, although they tend to be systemically well with no effects of acute or chronic liver disease. So, we think they should be ideally studied separately. PLT for metabolic diseases usually has excellent patient and graft survival than PLT for other indications. In addition to this, genotypic and phenotypic diversity in metabolic disorders complicate the possibility of forecasting long-term outcomes in this group of children; hence, they were excluded from the study.
For the objective of this article, ALF was specified to match the Pediatric Acute Liver Failure Study Group[6] definition, as biochemical proof of liver injury, excluding records of recognized chronic liver illness, international normalized ratio > 1.5 if the patient had encephalopathy or > 2.0 if the patient did not have encephalopathy, and coagulopathy not rectified by vitamin K use.
ESCLD was specified as enduring hepatic inflammation identified by biochemical investigations and clinical examination, spanning more than 6 mo causing cirrhosis or permanent liver injury[7].
Data were collected through a retrospective case note review. We recorded 24 peritransplant parameters for this study, which were grouped into four classes: Pretransplant recipient as well as donor parameters, operative parameters, and post-transplant recipient observations. Pretransplant recipient variables were sex, age, weight, liver failure category (ALF and ESCLD), fundamental liver illness etiology, time on the transplant waiting list, and patient location when graft became available (an indirect marker of recipient sickness instantly pretransplant). Donor parameters were sex, age, weight, type of graft (living and deceased), and type of graft (whole or variant graft such as split or reduced). The intraoperative parameters studied were warm ischemia time and cold ischemia time. The post-operative outcomes studied were the incidence of vascular and biliary complications, post-transplant pediatric intensive care unit (PICU) and overall hospital stay, the incidence of biopsy-proven acute/chronic rejection, retransplantation, causes of graft and patient loss, and 1-, 5-, and 10-year graft as well as patient survival.
The primary outcome of this article was to determine if there is a difference in patient and graft survival in PLT recipients transplanted for ALF or ESCLD. The secondary outcome was to compare the incidence of vascular, biliary complications, and biopsy-proven rejection in PLT for both groups.
Data were evaluated using IBM SPSS software version 20.0. (IBM Corp., Armonk, NY, United States). Kolmogorov-Smirnov was employed to validate the normality of variable allocation. Assessments for categorical variables were conducted using the χ2 test (Fisher’s exact adjustment). Student’s t-test was applied to assess two normally distributed quantitative variables between two cohorts, whereas the Mann-Whitney test was applied to assess non-normally distributed quantitative data. Missing data were taken into account through statistical evaluation. The Kaplan-Meier survival curve was employed to examine the graft and patient survival. The significance of the recorded outcomes was determined at the 5% level.
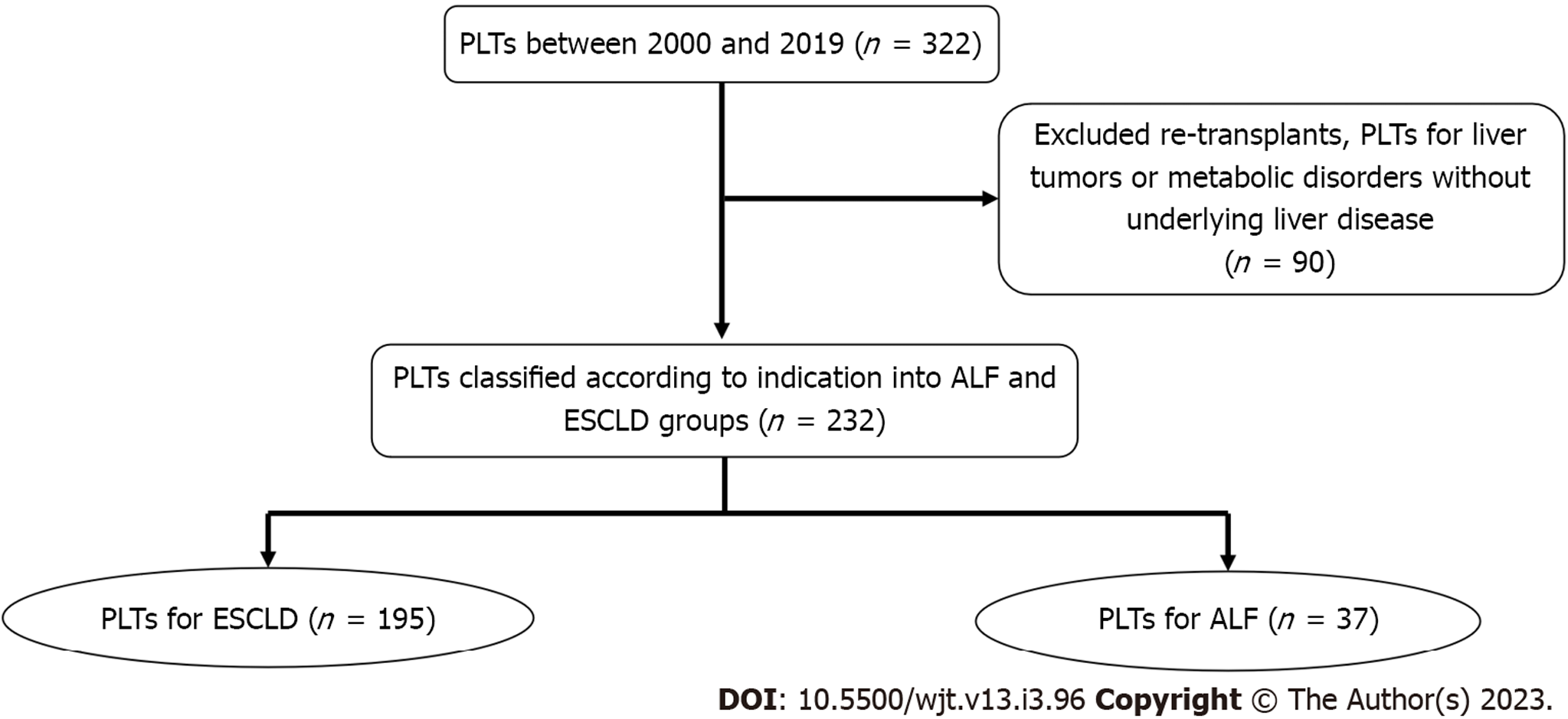
Between November 2000 and August 2019, 322 PLTs were performed in our center. We excluded retransplants, transplants for children with liver tumors, or metabolic disorders without underlying liver disease (90 PLTs) from the final analysis. The remaining 232 PLTs were classified into 195 PLTs due to ESCLD and 37 PLTs as emergency management of ALF (Figure 1). The median follow-up for the ALF group was 8.3 years (1–19.3 years), while the median follow-up for the ESCLD group was 8.1 years (1–19.6 years).
During the study period, 232 children presented to our institute with ALF. Unfortunately, 58 (25.0%) of them did not survive to undergo transplantation, 37 (15.9%) underwent transplantation, and 137 (59.1%) recovered without transplantation.
Both groups were homogenous in terms of recipients’ sex (P = 0.312), whereas recipients’ age and weight were significantly higher in the ALF group. Further analysis of the ALF group showed that 6 patients were transplanted at below 1 year of age, 10 patients were transplanted at 1 year to 4 years of age, 3 patients were transplanted at above 4 years to 10 years of age, and 18 patients were older than 10 years of age at the time of transplant. Biliary atresia and progressive familial intrahepatic cholestasis were the most common causes of liver failure in the ESCLD group, whereas seronegative hepatitis and autoimmune hepatitis were the most common causes in the ALF group (Table 1). There was a significant difference in waiting time on the transplant list between both groups; the median waiting time for the ALF group was 3 d (1–41 d), whereas the median waiting time for ESCLD patients was 60.5 d (1-560 d; P < 0.001). The location of the recipient when the liver graft became available also showed a significant difference as the home locality was greater in the ESCLD group, whereas in the ALF group, hospital, as well as PICU locality (with or without invasive ventilation), were significantly higher (Table 1).
| Demographics | ALF (n = 37), % | ESCLD (n = 195), % | P value |
| Donor demographics | |||
| Sex | |||
| Male | 45.7 | 46.9 | 0.902 |
| Female | 54.3 | 53.1 | |
| Weight, kg | 66.2 (8–90) | 65.8 (10–98) | 0.912 |
| Age, yr | 35.2 (0.9–65) | 29 (1–66) | 0.039 |
| Type of liver graft | |||
| Whole liver | 14 (37.8) | 38 (19.7) | 0.016 |
| Variant graft including split, reduced, living donor | 23 (62.2) | 155 (80.3) | |
| Recipient demographics | |||
| Sex | |||
| Male | 16 (43.2) | 102 (52.3) | 0.312 |
| Female | 21 (56.8) | 93 (47.7) | |
| Age at transplant, yr | 8 (0.1–16.7) | 5.4 (0.3–17.2) | 0.031 |
| Weight, kg | 31 (2.7–66.5) | 21 (4.7–89) | 0.011 |
| Etiology of liver disease | |||
| Alagille's syndrome | 0 (0) | 12 (6.2) | 0.222 |
| Alpha-1-AT deficiency | 0 (0) | 15 (7.7) | 0.138 |
| Autoimmune | 4 (10.8) | 13 (6.7) | 0.487 |
| Biliary atresia | 0 (0) | 92 (47.2) | < 0.001 |
| Biliary cirrhosis | 0 (0) | 2 (1.0) | 1.000 |
| CF liver disease | 0 (0) | 13 (6.7) | 0.232 |
| Drug induced | 2 (5.4) | 0 (0) | 0.025 |
| Hepatitis A | 2 (5.4) | 0 (0) | 0.025 |
| HSV | 2 (5.4) | 0 (0) | 0.025 |
| Seronegative hepatitis | 17 (45.9) | 0 (0) | < 0.001 |
| PFIC | 0 (0) | 29 (14.9) | 0.006 |
| Post-liver resection | 2 (5.4) | 0 (0) | 0.025 |
| PSC | 0 (0) | 8 (4.1) | 0.361 |
| Others | 8 (21.6) | 11 (5.6) | 0.004 |
| Location of the recipient when the graft is available | |||
| Home | 0 (0) | 137 (71.4) | < 0.001 |
| Hospital | 15 (40.5) | 47 (24.5) | 0.044 |
| PICU not ventilated | 7 (18.9) | 5 (2.6) | 0.001 |
| PICU ventilated | 15 (40.5) | 3 (1.6) | < 0.001 |
There was no statistically significant difference in terms of donor sex or weight; however, donors in the ALF group were significantly older than those in the ESCLD group (Table 1). Concerning the graft resource, living donors were used more commonly in the ESCLD cohort (34 donors) than in the ALF cohort, which did not receive any graft from living donors (P = 0.006).
Technical variant (reduced and split) grafts were used significantly more in the ESCLD group (Table 1). There was no difference in cold ischemia time between the two groups, but warm ischemia time was significantly longer in the ALF group (median 55 min, 32–81 min) than in the ESCLD group (median 45.5 min, 29–81 min; P = 0.004).
Vascular complications occurrence: There was no distinction among the groups regarding vascular complications. Five patients in the ALF group and forty-one patients in the ESCLD group had at least one post-transplant vascular event (Table 2). Some patients in the ESCLD group had more than one vascular complication; two recipients had both hepatic artery thrombosis and portal vein thrombosis (PVT), two recipients developed both hepatic artery stenosis and portal vein stenosis (PVS), and one patient had PVS then PVT (Table 2).
| Outcome | ALF among n = 37, n (%) | ESCLD among n = 195, n (%) | P value |
| Vascular complications | |||
| AV malformation post-liver biopsy | 0 (0) | 1 (0.5) | 1.000 |
| Retroperitoneal hematoma, femoral vein bypass cannula | 0 (0) | 1 (0.5) | 1.000 |
| HAS | 2 (5.4) | 13 (6.7) | 1.000 |
| PVS | 0 (0) | 14 (7.2) | 0.134 |
| HAT | 1 (2.7) | 10 (5.1) | 1.000 |
| PVT | 1 (2.7) | 7 (3.6) | 1.000 |
| HVS | 1 (2.7) | 0 (0) | 0.159 |
| Biliary complications | |||
| CHD sludge | 0 (0) | 2 (1) | 1.000 |
| Biliary stricture | 3 (8.1) | 21 (10.8) | 0.775 |
| Bile leak | 0 (0) | 23 (11.8) | 0.031 |
| Cause of graft loss | n = 5 | n = 27 | |
| HAT | 0 (0) | 9 (33.3) | 0.288 |
| PNF | 2 (40) | 5 (18.5) | 0.296 |
| Chronic rejection | 3 (60) | 6 (22.2) | 0.121 |
| Biliary tract complications | 0 (0) | 7 (25.9) | 0.560 |
| Cause of death | n = 9 | n = 17 | |
| Unknown | 0 (0) | 5 (29.4) | 0.129 |
| Cardiopulmonary | 3 (33.3) | 1 (5.9) | 0.104 |
| Cerebral oedema | 1 (11.1) | 0 (0) | 0.346 |
| Fungal infection | 1 (11.1) | 0 (0) | 0.346 |
| Gastrointestinal | 0 (0) | 1 (5.9) | 1.000 |
| Intracranial hemorrhage | 1 (11.1) | 0 (0) | 0.346 |
| Liver failure | 1 (11.1) | 4 (23.5) | 0.628 |
| Recurrence of disease | 0 (0) | 1 (5.9) | 1.000 |
| Sepsis | 2 (22.2) | 5 (29.4) | 1.000 |
Incidence of biliary complications: Bile leak was significantly higher in the ESCLD group, whereas biliary stricture and common hepatic duct sludge showed no significant difference between the two groups. It was also of note that three of the ESCLD recipients developed both bile leakage and biliary stricture (Table 2).
Incidence of rejection: There was no difference in biopsy-proven acute or chronic rejection between the two groups. Seventeen (45.9%) ALF patients had one or more episodes of rejection and sixty-two patients (31.8%) in the ESCLD group experienced rejection (P = 0.096).
PICU stay post-PLT was longer in the ALF group by a median of half a day (2.5 vs 2 d), but this difference was not statistically significant (P = 0.112). In keeping with their sick status before the transplant, the ALF patients had a longer median hospital stay (29 vs 21 d) compared to ESCLD patients (P = 0.013).
There was no difference among the studied groups in terms of the need for retransplantation; 5 (13.5%) in the ALF group and 27 (13.8%) in the ESCLD group (P = 0.957).
During the follow-up period, 17 (8.7%) of the ESCLD recipients died while in the ALF group, and 9 (24.3%) recipients died (P = 0.011) (Figure 2A and Table 3). Sepsis and liver failure were the two most common causes of death in PLT recipients, but there was no statistically significant difference between the two groups (Table 2). Analysis of ALF group survival in conjunction with the age of transplant (less than 1 year, 1-4 years, 4-10 years, more than 10 years) showed 5-year patient survival of 50%, 90%, 100%, and 77.8%, respectively.
| Survival outcome | Mean | %1 yr | %5 yr | %10 yr | %End of study | Log rank | |
| χ2 | P value | ||||||
| Patient survival | |||||||
| ALF | 14.86 | 78.3 | 78.3 | 78.3 | 69.6 | 7.370 | 0.007 |
| ESCLD | 17.71 | 97.9 | 93.9 | 89.4 | 85.0 | ||
| Graft survival | |||||||
| ALF | 12.46 | 75.6 | 72.4 | 66.9 | 47.8 | 2.426 | 0.119 |
| ESCLD | 14.46 | 90.7 | 82.9 | 77.3 | 56.8 | ||
Median graft survival was longer in the ESCLD group (2412 d) compared to the ALF group (2292 d), but this difference was not statistically significant (P = 0.587) (Figure 2B and Table 3). Despite some causes of graft failure being more frequent in one group than the other, there was no statistically significant difference (Table 2).
In the United Kingdom, children with ALF or ESCLD who are candidates for PLT are referred to one of the three centers in the country for evaluation, including our center. When the indication for PLT is established, patients are offered transplantation if there is more than 50% survival likelihood at 5 years after PLT with an acceptable quality of life. But the dynamics of PLT are completely divergent between ALF and ESCLD settings, leading to separate selection criteria for candidates needing emergency transplantation compared to elective procedure.
The studied cohorts have diverse etiology with distinct differences in short-term prognosis. Similarly, graft allocation procedures are unique for elective and emergency transplantation, showing that those cohorts have a distinct probability of mortality without PLT. In our study, the percentage of PLTs performed as emergency management for ALF was 11.5%, consistent with studies from other centers[8-10], whereas some other authors have reported higher rates of PLT in ALF patients that may reach up to 35%[11,12]; this difference reflects the absence of a reliable prognostic model for ALF patients that can accurately predict ALF candidates who would not survive without PLT.
Identifying ALF patients who would be saved by successful PLT and differentiating them from those who may recover spontaneously or those who will eventually die with or without PLT cannot be guided by currently available data[13-16]. Using isolated biomarkers such as ammonia, actin-free Gc-globulin, and lactate is not reliable and cannot be applied to all ALF patients without being sufficiently studied in the pediatric population[17-19]. Prognostic scoring models applying clinical and laboratory variables such as King’s college criteria and liver injury unit score cannot be confidently used to anticipate ALF patient death[19-21].
In terms of the etiology of liver failure, biliary atresia was the most common indication for PLT in ESCLD patients in our study (47.2%), consistent with the literature[1,8,11,22,23]. On the other hand, the etiology of the underlying liver disease could not be identified in 45.9% of our ALF recipients. Most of the literature concerned with PLT in ALF patients has shown the same observation[3,12,24,25]. Failure to identify the etiology of ALF is probably multifactorial. First, most of the data concerned with ALF in children and infants are retrieved from case reports, exploration of adult data, individual practice, and retrospective studies of single centers[2]. Second, the rapid progression of liver failure to transplant or death does not give enough room for the extensive work-up required[4]. The inability to identify the cause of ALF is always stressful for the treating physicians as it may affect the outcome of this group of patients because of its effects on prognosis, therapy, and prevention.
Until 2008, we were using molecular adsorbent recirculating system as bridging therapy for ALF patients, but we did not find it helpful in terms of changing outcomes for patients. We use plas
The location of the recipient when the graft became available showed a significant difference between the two groups. ALF patients are mostly located in the hospital ward or PICU while ESCLD patients are usually admitted to our center from home. This is explained by the fact that ALF patients are usually sicker in the immediate pretransplant period; thus, they are usually hospitalized or even admitted to the PICU if multiple organ support is needed, while ESCLD patients are mostly less critical and thus usually followed up in outpatient clinics and admitted to the transplant center only when the graft becomes available. This was also reflected in the post-transplant hospital stay that was significantly longer in ALF patients who are expected to require a longer time to recover before discharge.
In contrast to the ESCLD group, ALF candidates in our study did not receive a living donor graft; this is possibly linked to a brief window that does not provide sufficient time for meticulous assessment of possible living donors. Living donation intended for ALF candidates has consistently been a matter of discussion[26]. Various studies have expressed concerns about the ultra-short period utilized for radiological and clinical assessment of the living donor along with the emotional element in PLT that might affect the outcomes[27]. Other reports claim that PLT using living donor grafts has a possibly better outcome due to a briefer waiting period as the sick child does not have to wait for the liver graft allocation system to receive a suitable graft in addition to the presumed better-quality graft due to limited cold ischemia time as shown by the inferior primary non-function incidence in grafts from living donors[28].
Technical variant grafts were used significantly higher in the ESCLD group; this is explained by the significantly lower age at transplant of this group compared to the ALF group, so smaller grafts were needed to match recipient size. This can also explain higher rates of bile leakage in the ESCLD group from the cut surface of reduced or split grafts.
ALF recipients in our study have a significantly lower survival rate than ESCLD recipients, which has been reported in multiple studies[11,22], while only one study to the best of our knowledge has reported similar patient survival in both groups[25]. This can be explained by multiple factors. First, the more critical condition of ALF patients in the pretransplant period that was reflected in our study by significantly higher PICU location of these patients when the graft becomes available and was linked in some reports to low post-transplant survival[11]. Second is the scarcity of suitable grafts for the pediatric population[29], which prolongs the waiting time of ALF patients and may put pressure on the transplant centers to accept marginal grafts, as survival of this critical group is largely dependent on receiving a PLT within a short window of opportunity[28]. The third is the fact that the etiology is unknown in most cases of ALF, and this will surely affect post-transplant disease management and progression.
Interestingly, we noticed that the recipient’s survival in the ALF group is most affected in the 1st-year post-transplant and remained almost stable after that, while survival in the ESCLD group continued to decline gradually over the years. This observation is probably related to how unwell the ALF recipients were at the time of transplant (a considerable proportion was in intensive care), but after the 1st year, survival stabilizes. Whereas in ESCLD, the 1st-year outcomes are better, probably because most of these patients were stable and admitted for transplant from home. However, after that, there seems to be a steady decrease in their survival, possibly because of the effects of disease chronicity where ESCLD itself or its underlying etiology like cystic fibrosis or alpha-1-antitrypsin deficiency would have affected other systems such as the lungs and kidneys and this effect would reflect on patient survival over the years.
There were some limitations in this study. The larger study cohort, longer follow-up duration, single-center population with uniform pretransplant and post-transplant protocol, and careful retrieval of data allowed us to overcome the limitation of the retrospective nature of the study. More importantly, no such studies are comparing ALF with ESCLD in the paediatric transplant literature.
This was a retrospective study that compared the long-term outcomes of PLT in ALF and ESCLD settings. Survival of PLT recipients was significantly higher in the ESCLD group due to multiple factors such as the critical general condition of ALF patients in the peritransplant time, scarcity of suitable grafts for pediatric recipients, and obscure etiology of ALF in most of the cases. The rate of complications did not show a significant difference apart from higher rates of bile leak in the ESCLD group.
Settings of pediatric liver transplantation (PLT) in end-stage chronic liver disease (ESCLD) and acute liver failure (ALF) are divergent. ALF recipients are transplanted within a narrow window of opportunity, whereas ESCLD recipients are usually transplanted electively.
Outcomes of PLT in ALF and ESCLD were previously described by different centers but to the best of our knowledge, they were not compared to establish if there is a difference in post-PLT survival and complication rates between these two groups.
To determine if there is a difference in post-operative complications and survival outcomes between the ALF and ESCLD in PLT.
This was a retrospective observational study of all primary PLTs performed at a single center between 2000 and 2019. ALF and ESCLD groups were compared for the pretransplant recipient, donor and operative parameters, and post-operative outcomes including graft and patient survival.
During the 20-year study period, 232 primary PLTs were performed at our center; 195 were transplanted for ESCLD and 37 were transplanted for ALF. The ALF recipients were significantly older (median 8 years vs 5.4 years; P = 0.031) and heavier (31 vs 21 kg; P = 0.011). Living donor grafts were used more in the ESCLD group (34 vs 0; P = 0.006). There was no difference between the two groups concerning vascular complications and rejection, but there were more bile leaks in the ESCLD group. Post-transplant patient survival was considerably superior in the ESCLD group: 1-, 5-, and 10-year survival rates were 97.9%, 93.9%, and 89.4% correspondingly compared to 78.3%, 78.3%, and 78.3% in the ALF group (P = 0.007). However, there was no difference in 1-, 5-, and 10-year graft survival rates between the ESCLD and ALF groups - 90.7%, 82.9%, and 77.3% vs 75.6%, 72.4%, and 66.9% (P = 0.119).
Post-PLT survival in ALF patients is inferior to ESCLD patients. This may be due to several factors including uncertainty of the underlying pathology in most ALF patients and the more critical clinical status of ALF candidates in the immediate pre-transplant period. Survival post-PLT in the ALF group was adversely affected in the 1st year and then stabilized, while post-PLT survival in the ESCLD group showed a gradual decline over the study period.
Future research should address the dilemma of identifying the underlying pathology in a considerable portion of ALF candidates and should also try to overcome liver graft shortage by identifying methods to widen the graft pool.
This article would not have been achievable without the passion and commitment of clinicians, transplant coordinators, and nurses. The authors would like to thank Mark Stringer, Ernest Hidalgo, Suzanne Davison, and Patricia McClean, for their unique impact on manuscript writing and patient support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Oley MH, Indonesia; Patel MV, India S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Uribe M, Buckel E, Ferrario M, Godoy J, González G, Hunter B, Ceresa S, Cavallieri S, Berwart F, Herzog C, Santander MT, Calabrán L. Pediatric liver transplantation: ten years of experience in a multicentric program in Chile. Transplant Proc. 2005;37:3375-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Rivera-Penera T, Moreno J, Skaff C, McDiarmid S, Vargas J, Ament ME. Delayed encephalopathy in fulminant hepatic failure in the pediatric population and the role of liver transplantation. J Pediatr Gastroenterol Nutr. 1997;24:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Squires RH Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, Dhawan A, Rosenthal P, Rodriguez-Baez N, Murray KF, Horslen S, Martin MG, Lopez MJ, Soriano H, McGuire BM, Jonas MM, Yazigi N, Shepherd RW, Schwarz K, Lobritto S, Thomas DW, Lavine JE, Karpen S, Ng V, Kelly D, Simonds N, Hynan LS. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 568] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 4. | Sundaram SS, Alonso EM, Narkewicz MR, Zhang S, Squires RH; Pediatric Acute Liver Failure Study Group. Characterization and outcomes of young infants with acute liver failure. J Pediatr. 2011;159:813-818.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Bhatia V, Lodha R. Intensive care management of children with acute liver failure. Indian J Pediatr. 2010;77:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Ng VL, Li R, Loomes KM, Leonis MA, Rudnick DA, Belle SH, Squires RH; Pediatric Acute Liver Failure Study Group (PALFSG). Outcomes of Children With and Without Hepatic Encephalopathy From the Pediatric Acute Liver Failure Study Group. J Pediatr Gastroenterol Nutr. 2016;63:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Mews C, Sinatra F. Chronic liver disease in children. Pediatr Rev. 1993;14:436-444. [PubMed] |
| 8. | Broering DC, Kim JS, Mueller T, Fischer L, Ganschow R, Bicak T, Mueller L, Hillert C, Wilms C, Hinrichs B, Helmke K, Pothmann W, Burdelski M, Rogiers X. One hundred thirty-two consecutive pediatric liver transplants without hospital mortality: lessons learned and outlook for the future. Ann Surg. 2004;240:1002-12; discussion 1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Venick RS, Farmer DG, Soto JR, Vargas J, Yersiz H, Kaldas FM, Agopian VG, Hiatt JR, McDiarmid SV, Busuttil RW. One Thousand Pediatric Liver Transplants During Thirty Years: Lessons Learned. J Am Coll Surg. 2018;226:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15 Suppl 2:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Guariso G, Visonà Dalla Pozza L, Manea S, Salmaso L, Lodde V, Facchin P; SIGENP Group of Liver Transplantation. Italian experience of pediatric liver transplantation. Pediatr Transplant. 2007;11:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Farmer DG, Venick RS, McDiarmid SV, Duffy JP, Kattan O, Hong JC, Vargas J, Yersiz H, Busuttil RW. Fulminant hepatic failure in children: superior and durable outcomes with liver transplantation over 25 years at a single center. Ann Surg. 2009;250:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Squires JE, Rudnick DA, Hardison RM, Horslen S, Ng VL, Alonso EM, Belle SH, Squires RH. Liver Transplant Listing in Pediatric Acute Liver Failure: Practices and Participant Characteristics. Hepatology. 2018;68:2338-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Bhaduri BR, Mieli-Vergani G. Fulminant hepatic failure: pediatric aspects. Semin Liver Dis. 1996;16:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, Stribling R, Crippin JS, Flamm S, Somberg KA, Rosen H, McCashland TM, Hay JE, Lee WM. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 254] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM; U. S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1562] [Cited by in RCA: 1459] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 17. | Bagchi A, Kumar S, Ray PC, Das BC, Gumma PK, Kar P. Predictive value of serum actin-free Gc-globulin for complications and outcome in acute liver failure. J Viral Hepat. 2015;22:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Schiødt FV, Bangert K, Shakil AO, McCashland T, Murray N, Hay JE, Lee WM; Acute Liver Failure Study Group. Predictive value of actin-free Gc-globulin in acute liver failure. Liver Transpl. 2007;13:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Cholongitas EB, Betrossian A, Leandro G, Shaw S, Patch D, Burroughs AK. King's criteria, APACHE II, and SOFA scores in acute liver failure. Hepatology. 2006;43:881; author reply 882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Cholongitas E, Theocharidou E, Vasianopoulou P, Betrosian A, Shaw S, Patch D, O'Beirne J, Agarwal B, Burroughs AK. Comparison of the sequential organ failure assessment score with the King's College Hospital criteria and the model for end-stage liver disease score for the prognosis of acetaminophen-induced acute liver failure. Liver Transpl. 2012;18:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Lu BR, Zhang S, Narkewicz MR, Belle SH, Squires RH, Sokol RJ; Pediatric Acute Liver Failure Study Group. Evaluation of the liver injury unit scoring system to predict survival in a multinational study of pediatric acute liver failure. J Pediatr. 2013;162:1010-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Martin SR, Atkison P, Anand R, Lindblad AS; SPLIT Research Group. Studies of Pediatric Liver Transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr Transplant. 2004;8:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Feng S, Si M, Taranto SE, McBride MA, Mudge C, Stritzel S, Roberts JP, Rosenthal P. Trends over a decade of pediatric liver transplantation in the United States. Liver Transpl. 2006;12:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Baliga P, Alvarez S, Lindblad A, Zeng L; Studies of Pediatric Liver Transplantation Research Group. Posttransplant survival in pediatric fulminant hepatic failure: the SPLIT experience. Liver Transpl. 2004;10:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Heffron TG, Pillen T, Smallwood G, Rodriguez J, Sekar S, Henry S, Vos M, Casper K, Gupta NA, Fasola CG, Romero R. Pediatric liver transplantation for acute liver failure at a single center: a 10-yr experience. Pediatr Transplant. 2010;14:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Éboli L, Tannuri AC, Gibelli N, Silva T, Braga P, Tannuri U. Comparison of the Results of Living Donor Liver Transplantation Due to Acute Liver Failure and Biliary Atresia in a Quaternary Center. Transplant Proc. 2017;49:832-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Cariús LP, Pacheco-Moreira LF, Balbi E, Leal CR, Gonzalez AC, Agoglia LV, Araújo C, Enne M, Martinho JM. Living donor liver transplantation for acute liver failure: a single center experience. Transplant Proc. 2009;41:895-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Szymczak M, Kaliciński P, Kowalewski G, Broniszczak D, Markiewicz-Kijewska M, Ismail H, Stefanowicz M, Kowalski A, Teisseyre J, Jankowska I, Patkowski W. Acute liver failure in children-Is living donor liver transplantation justified? PLoS One. 2018;13:e0193327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Wu YM, Ho MC, Hu RH, Ko WJ, Yang PM, Lai MY, Lee PH. Liver transplantation for acute hepatic failure. Transplant Proc. 2004;36:2226-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |