Published online Feb 18, 2023. doi: 10.5500/wjt.v13.i2.44
Peer-review started: September 21, 2022
First decision: October 24, 2022
Revised: November 7, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: February 18, 2023
Processing time: 147 Days and 13.4 Hours
As Hepatitis C virus infection (HCV+) rates in kidney donors and transplant recipients rise, direct-acting antivirals (DAA) may affect outcomes.
To analyze the effects of HCV+ in donors, recipients, or both, on deceased-donor (DD) kidney transplantation (KT) outcomes, and the impact of DAAs on those effects.
The Organ Procurement and Transplantation Network data of adult first solitary DD-KT recipients 1994-2019 were allocated into four groups by donor and recipient HCV+ status. We performed patient survival (PS) and death-censored graft survival (DCGS) pairwise comparisons after propensity score matching to assess the effects of HCV+ in donors and/or recipients, stratifying our study by DAA era to evaluate potential effect modification.
Pre-DAA, for HCV+ recipients, receiving an HCV+ kidney was associated with 1.28-fold higher mortality (HR 1.151.281.42) and 1.22-fold higher death-censored graft failure (HR 1.081.221.39) compared to receiving an HCV- kidney and the absolute risk difference was 3.3% (95%CI: 1.8%-4.7%) for PS and 3.1% (95%CI: 1.2%-5%) for DCGS at 3 years. The HCV dual-infection (donor plus recipient) group had worse PS (0.56-fold) and DCGS (0.71-fold) than the dual-uninfected. Donor HCV+ derived worse post-transplant outcomes than recipient HCV+ (PS 0.36-fold, DCGS 0.34-fold). In the DAA era, the risk associated with HCV+ in donors and/or recipients was no longer statistically significant, except for impaired PS in the dual-infected vs dual-uninfected (0.43-fold).
Prior to DAA introduction, donor HCV+ negatively influenced kidney transplant outcomes in all recipients, while recipient infection only relatively impaired outcomes for uninfected donors. These adverse effects disappeared with the introduction of DAA.
Core Tip: In this paper, using data from across 25 years, we demonstrate that the adverse effects of hepatitis C infection in donors and/or recipients on kidney transplant outcomes have disappeared since the introduction of direct-acting antiviral agents.
- Citation: Yuan Q, Hong S, Leya G, Roth E, Tsoulfas G, Williams W, Madsen JC, Elias N. Analysis of the effects of donor and recipient hepatitis C infection on kidney transplant outcomes in the United States. World J Transplant 2023; 13(2): 44-57
- URL: https://www.wjgnet.com/2220-3230/full/v13/i2/44.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i2.44
By improving patients’ quality of life and survival, kidney transplantation (KT) is the optimal treatment for advanced kidney disease, even for Hepatitis C virus infected (HCV+) dialysis patients[1,2]. HCV+ donor kidneys could alleviate transplant organ shortages[3], and most kidney waitlist patients favor accepting an HCV+ kidney over waiting longer for an uninfected (HCV-) kidney[4]. Nonetheless, likely driven by concerns over HCV transmission and transplant outcomes, HCV+ kidneys have traditionally been discarded rather than transplanted into HCV- recipients[5].
Since December 2013, direct-acting antivirals (DAA), including NS3/4A inhibitors (boceprevir, telaprevir, simeprevir, asunaprevir, grazoprevir and paritaprevir), NS5A inhibitors (ombitasvir, ledipasvir, daclatasvir, elbasvir and velpatasvir), NS5B inhibitors (sofosbuvir and dasabuvir)[6], have revolutionized HCV treatment by consistently achieving 95% or better sustained virologic responses[7]. Before the introduction of DAAs, a combination of interferon and ribavirin were the standard scheme for HCV treatment[8]. Concurrently, United States donors who died as a result of drug overdose, many of whom were HCV+, increased from 66 to 1263 between 2000 and 2016. Notably, these donors were young: median age of 31 years[3]. HCV+ kidneys’ superior quality, the increased prevalence of HCV+ in donors and recipients, and DAA treatments, have contributed to soaring numbers of HCV+ donor and/or recipient transplants. In the DAA era, because of the promise of HCV treatment, waitlisted transplant candidates were 2.2 times more likely willing to accept an HCV+ kidney and HCV+ reci
Despite HCV antiviral advancements, the Organ Procurement and Transplantation Network (OPTN) deceased donor (DD) kidneys allocation algorithm uses the Kidney Donor Risk Index (KDRI), for which donor HCV+ status has the largest coefficient amongst dichotomous factors in the calculation[9]. This outdated system overestimates HCV+ kidneys’ risk in the DAA era, depriving candidates of high-quality HCV+ kidneys if they, or their accepting center, decline kidney offers based on KDRI thresholds, thus contributing to HCV+ kidneys’ high discard rate.
We sought to understand the effect of HCV+ in donors and recipients on DD-KT outcomes and discern whether those effects differed among various HCV+ donor and recipient combinations. We hypothesized that donor HCV status could modify HCV effect in recipients, recipient HCV status could modify HCV effect in donors, and those modifications would change favorably following DAA availability. We used national registry data with propensity score matching (PSM) to systematically characterize the effect of HCV+ on KT outcomes both prior to and following the introduction of DAAs.
We used the OPTN Analysis and Research file released in June 2019 based on data collected through March 2019. The content in this paper is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
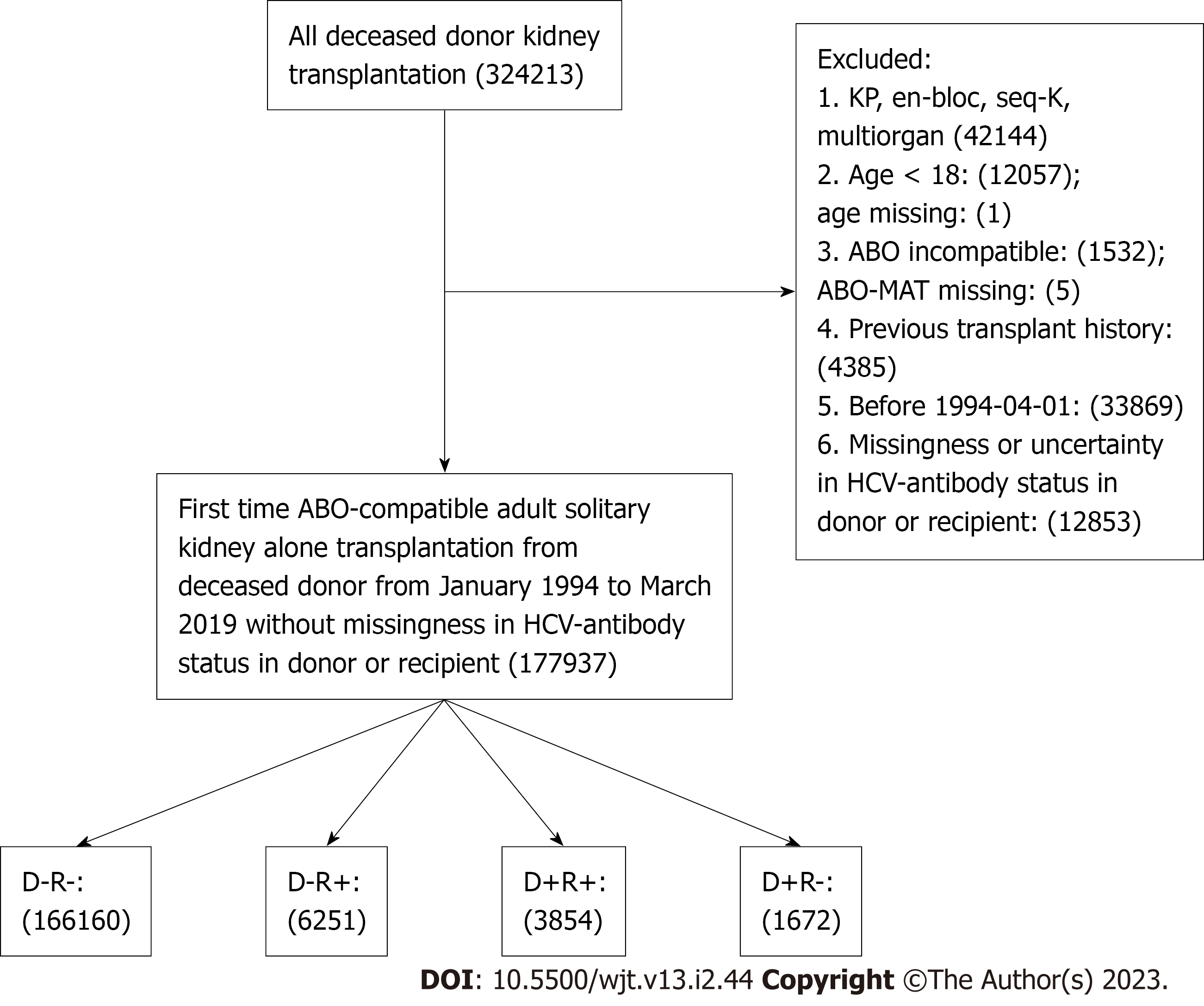
We identified all adult (age ≥ 18) first-time solitary KT recipients from ABO-compatible DD between January 1994 and March 2019 in the United States Patients with missing or uncertain HCV-antibody status in the donor or recipient were excluded. Patients were allocated into four groups according to HCV+ in the donor (D+) or recipient (R+): D-R-, D+R-, D-R+, and D+R+.
The study outcomes were patient survival (PS) and death-censored graft survival (DCGS) following KT. DCGS was defined as time to re-transplantation or dialysis reinstatement, whichever came first. Recipient HCV status is reported but not necessarily confirmed or assessed at transplant. HCV+ status was defined as HCV Ab+ or HCV nucleic acid test (NAT) positive, while HCV- was defined as HCV Ab- without HCV NAT+.
We performed pairwise PS and DCGS comparisons after PSM to assess the effect of donor and recipient HCV+ status on outcomes. Briefly, transplantation of HCV (+) or (-) donors into HCV (-) recipients was used to assess the effect of HCV+ in naïve recipients, as compared to D+R+ vs D-R+ combinations to assess the effect of HCV donor status in HCV infected recipients. D+R- vs D-R- patients addressed HCV donor infection effect in uninfected recipients, while D+R+ vs D-R+ patients addressed the effect in HCV+ recipients. Similarly, D+R+ vs D+R- pairings were compared for the effect of recipient HCV+ on HCV+ donor kidneys’ outcomes, and D-R+ vs D-R- pairings were compared for the effect in HCV- donor kidneys. Finally, D+R+ vs D-R- pairings addressed the effect of HCV+ in both donors and recipients on outcomes, and D+R- vs D-R+ pairings addressed whether HCV+ in donors or recipients alone was more detrimental.
Subject pairs were matched by the probability of positive HCV exposure based on a multivariable logistic regression model with 40 potential predictors from the donor, recipient, and transplant procedure. Supplementary Table 1 shows model variables and missingness. Variables were chosen based on The Scientific Registry of Transplant Recipients risk adjustment models[10]. We used complete-case analysis for categorical variables missing fewer than 1% of values and included a missing indicator in the initial step for those missing more than 1%. For continuous variables, the missing values were imputed with the median, and a missing indicator was also included for those missing percentage > 1% (Supplementary Table 1). The potential outliers of continuous variables were winsorized at 1 and 99 percentiles. By focusing on HCV exposure effect in a sample of subjects that resembles the exposed subjects, we estimated the average treatment effect in the treated. We used the nearest neighbor matching with 1:1 ratio, without replacement, and with a caliper of width equal to 0.2 of the standard deviation (SD) of the logit of the propensity score. We performed balance diagnosis comparing matched groups’ characteristics. An SD greater than 0.1 was considered an imbalance sign, and the propensity score prediction model was refitted ensuring matched groups’ balance (Supplementary Table 1). We further stratified our study by DAA era (before or after December 2013) to evaluate potential effect modification.
Survival rates were presented in Kaplan-Meier curves and analyzed by log-rank tests. Time to outcome was defined as the interval from date-of-transplant to date-of-outcome (death or graft failure) and censored for loss to follow-up or end of study period. Absolute and relative risk differences in mortality and death-censored graft failure (DCGF) were estimated using Austin’s methods[11]. All analyses were performed using RStudio software, version 1.1.456 (R. RStudio, Inc., Boston, MA). A P-value of less than 0.05 identified statistical significance, and all confidence intervals used a 95% threshold.
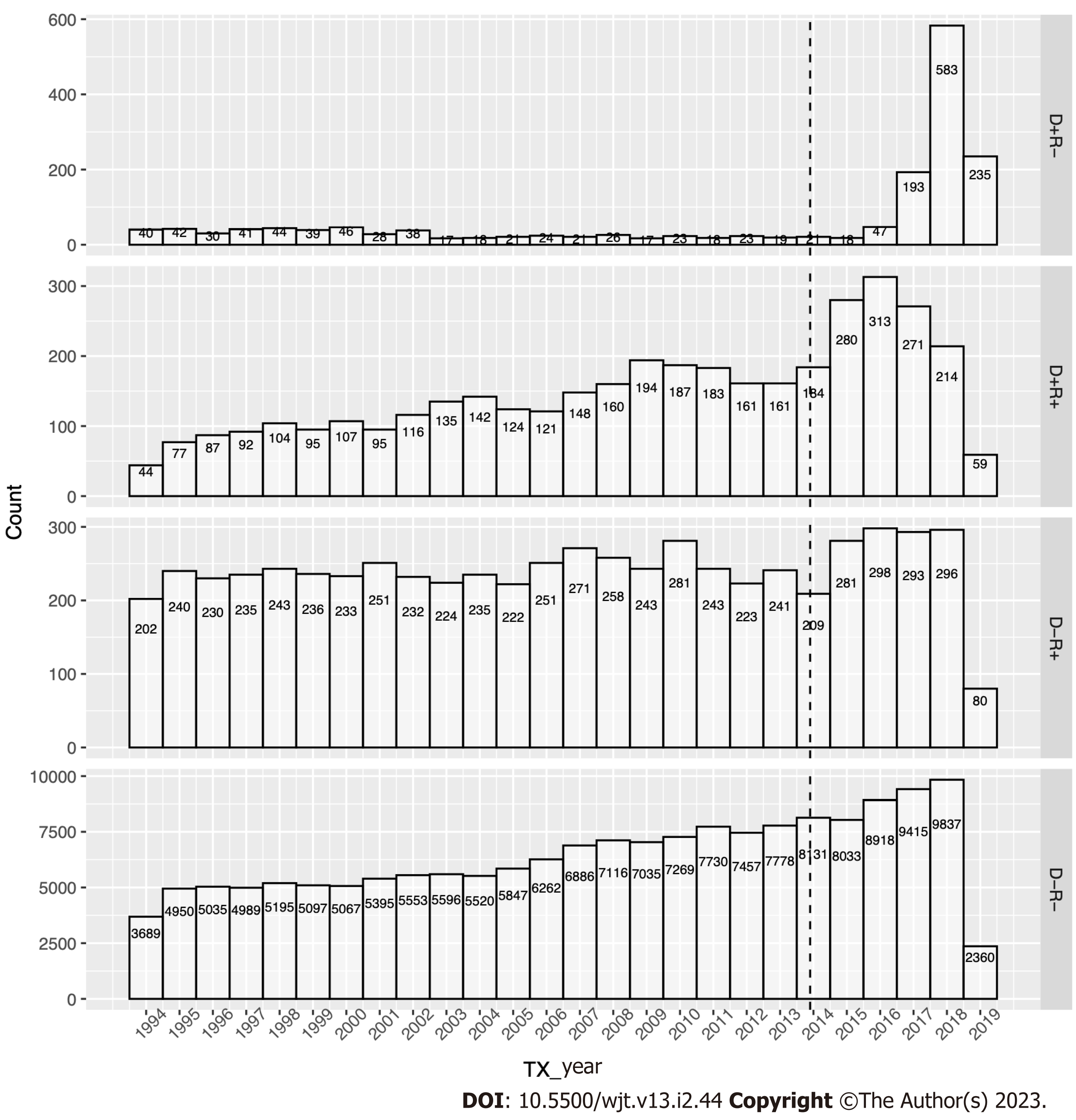
We identified 166,160 D-R-, 6,251 D-R+, 3,854 D+R+, and 1,672 D+R- pairings during the study (Figure 1). D+R+ transplants increased at a similar rate to D-R- transplants in the pre-DAA era, while D+R- and D-R+ transplants remained stable for two decades. However, HCV+ kidney utilization surged in the DAA era, initially with the traditional operating paradigm (D+ to R+), which peaked in 2016 and soon shifted to more robust HCV+ kidneys utilization (D+ to R-) (Figure 2).
Tables 1-3 and Supplementary Table 2 and 3 detail all cohorts’ donor, recipient, and transplant characteristics. The D+R- donors pre-DAA were predominantly male, white or African American, with low body mass index, who succumbed to head trauma, with relatively low serum creatinine, and low rates of donation after circulatory death (DCD), diabetes, and hypertension (Supplementary Table 2A). In contrast, D+R- recipients tended to be older (57 [IQR, 47, 65]) and had less dialysis time. Thirty-seven percent of D+R- and 38% of D+R+ were shared nationally, and D+R- had the longest cold ischemia time (CIT) at 20 h [IQR, 16.0, 26.0]. D+R- and D+R+ cohorts had higher HLA mismatch than D-R- and D-R+. However, the incidence of delayed graft function (DGF) in D+R- was 25.3%, lower than in the D-R+ or D+R+ cohorts and similar to the D-R- cohort (Supplementary Table 2C).
| Characteristics | D-R- | D-R+ | D+R- | D+R+ | P value | |
| n | Pre | 116108 | 4646 | 550 | 2455 | |
| Post | 46099 | 1443 | 1082 | 1303 | ||
| Age (median [IQR]) | Pre | 40.0 [23.0, 51.0] | 40.0 [24.0, 51.0] | 41.0 [34.0, 46.0] | 42.0 [33.0, 49.0] | < 0.001 |
| Post | 40.0 [27.0, 52.0] | 42.0 [29.0, 52.0] | 35.0 [29.0, 44.0] | 32.0 [26.0, 39.0] | < 0.001 | |
| Gender = M (%) | Pre | 69042 (59.5) | 2762 (59.4) | 380 (69.1) | 1574 (64.1) | < 0.001 |
| Post | 28136 (61.0) | 853 (59.1) | 617 (57.0) | 818 (62.8) | 0.012 | |
| BMI (median [IQR]) | Pre | 25.6 [22.4, 29.7] | 25.6 [22.3, 29.4] | 24.8 [22.1, 28.0] | 25.1 [22.3, 28.6] | < 0.001 |
| Post | 27.1 [23.4, 31.9] | 27.5 [23.7, 32.2] | 26.3 [23.3, 30.5] | 25.6 [22.8, 29.4] | < 0.001 | |
| Race (%) | ||||||
| White | Pre | 83490 (71.9) | 3209 (69.1) | 409 (74.4) | 1824 (74.3) | < 0.001 |
| African American | 14085 (12.1) | 698 (15.0) | 91 (16.5) | 341 (13.9) | ||
| Hispanic | 14482 (12.5) | 574 (12.4) | 45 ( 8.2) | 260 (10.6) | ||
| Other | 4051 (3.5) | 165 ( 3.6) | 5 ( 0.9) | 30 (1.2) | ||
| White | Post | 31238 (67.8) | 929 (64.4) | 922 (85.2) | 1103 (84.7) | < 0.001 |
| African American | 6325 (13.7) | 273 (18.9) | 44 (4.1) | 58 (4.5) | ||
| Hispanic | 6421 (13.9) | 187 (13.0) | 94 (8.7) | 116 (8.9) | ||
| Other | 2115 (4.6) | 54 (3.7) | 22 (2.0) | 26 (2.0) | ||
| Cause of death (%) | ||||||
| Anoxia | Pre | 19852 (17.1) | 750 (16.1) | 67 (12.2) | 470 (19.1) | < 0.001 |
| Cerebrovascular/stroke | 44318 (38.2) | 1800 (38.7) | 196 (35.6) | 985 (40.1) | ||
| Head trauma | 48450 (41.7) | 1926 (41.5) | 281 (51.1) | 960 (39.1) | ||
| Other | 3488 (3.0) | 170 (3.7) | 6 (1.1) | 40 (1.6) | ||
| Anoxia | Post | 18056 (39.2) | 574 (39.8) | 779 (72.0) | 882 (67.7) | < 0.001 |
| Cerebrovascular/stroke | 12337 (26.8) | 397 (27.5) | 109 (10.1) | 127 (9.7) | ||
| Head trauma | 14151 (30.7) | 430 (29.8) | 176 (16.3) | 272 (20.9) | ||
| Other | 1555 (3.4) | 42 (2.9) | 18 (1.7) | 22 (1.7) | ||
| DCD = yes (%) | Pre | 10101 (8.7) | 368 (7.9) | 11 (2.0) | 105 (4.3) | < 0.001 |
| Post | 10519 (22.8) | 329 (22.8) | 151 (14.0) | 128 (9.8) | < 0.001 | |
| SCR (median [IQR]) | Pre | 1.0 [0.7, 1.3] | 1.0 [0.7, 1.3] | 0.9 [0.7, 1.2] | 0.9 [0.7, 1.1] | < 0.001 |
| Post | 0.9 [0.7, 1.4] | 1.0 [0.7, 1.4] | 0.9 [0.7, 1.3] | 0.9 [0.7, 1.1] | < 0.001 | |
| History of diabetes = yes (%) | Pre | 6712 (5.8) | 254 (5.5) | 14 (2.5) | 95 (3.9) | < 0.001 |
| Post | 3616 (7.8) | 123 (8.5) | 41 (3.8) | 30 (2.3) | < 0.001 | |
| History of hypertension = yes (%) | Pre | 28967 (24.9) | 1137 (24.5) | 114 (20.7) | 576 (23.5) | 0.038 |
| Post | 13638 (29.6) | 453 (31.4) | 226 (20.9) | 173 (13.3) | < 0.001 | |
| Smoking history = no (%) | Pre | 78484 (67.6) | 3039 (65.4) | 234 (42.5) | 1068 (43.5) | < 0.001 |
| Post | 36564 (79.3) | 1154 (80.0) | 723 (66.8) | 953 (73.1) | < 0.001 | |
| Characteristics | D-R- | D-R+ | D+R- | D+R+ | P value | |
| n | Pre | 116108 | 4646 | 550 | 2455 | |
| Post | 46099 | 1443 | 1082 | 1303 | ||
| Age (median [IQR]) | Pre | 53.0 [42.0, 61.0] | 51.0 [44.0, 58.0] | 57.0 [47.0, 65.0] | 53.0 [47.0, 59.0] | < 0.001 |
| Post | 55.0 [44.0, 64.0] | 59.0 [52.0, 64.0] | 60.0 [52.0, 67.0] | 60.0 [55.5, 65.0] | < 0.001 | |
| Gender = M (%) | Pre | 69448 (59.8) | 3251 (70.0) | 406 (73.8) | 1995 (81.3) | < 0.001 |
| Post | 27135 (58.9) | 994 (68.9) | 739 (68.3) | 1017 (78.1) | < 0.001 | |
| BMI (median [IQR]) | Pre | 26.8 [23.6, 30.9] | 26.1 [23.0, 29.9] | 26.5 [23.6, 29.4] | 26.4 [23.3, 29.8] | < 0.001 |
| Post | 28.6 [24.9, 32.8] | 27.8 [24.4, 31.6] | 29.1 [25.7, 33.3] | 27.8 [24.5, 31.5] | < 0.001 | |
| Race (%) | ||||||
| White | Pre | 56376 (48.6) | 1383 (29.8) | 211 (38.4) | 404 (16.5) | < 0.001 |
| African American | 34683 (29.9) | 2447 (52.7) | 290 (52.7) | 1789 (72.9) | ||
| Hispanic | 15940 (13.7) | 517 (11.1) | 27 (4.9) | 201 (8.2) | ||
| Other | 9109 (7.8) | 299 (6.4) | 22 (4.0) | 61 (2.5) | ||
| White | Post | 16501 (35.8) | 334 (23.1) | 494 (45.7) | 267 (20.5) | < 0.001 |
| African American | 15762 (34.2) | 784 (54.3) | 392 (36.2) | 855 (65.6) | ||
| Hispanic | 9079 (19.7) | 226 (15.7) | 117 (10.8) | 137 (10.5) | ||
| Other | 4757 (10.3) | 99 (6.9) | 79 (7.3) | 44 (3.4) | ||
| Insurance = nonprivate (%) | Pre | 83051 (71.5) | 3733 (80.3) | 418 (76.0) | 1831 (74.6) | < 0.001 |
| Post | 37085 (80.4) | 1265 (87.7) | 831 (76.8) | 993 (76.2) | < 0.001 | |
| Education level (%) | ||||||
| High school | Pre | 44906 (38.7) | 1923 (41.4) | 222 (40.4) | 1195 (48.7) | < 0.001 |
| Technical | 22531 (19.4) | 931 (20.0) | 86 (15.6) | 450 (18.3) | ||
| Post high school degree | 18972 (16.3) | 494 (10.6) | 59 (10.7) | 254 (10.3) | ||
| High school | Post | 19085 (41.4) | 741 (51.4) | 403 (37.2) | 680 (52.2) | < 0.001 |
| Technical | 11577 (25.1) | 369 (25.6) | 280 (25.9) | 318 (24.4) | ||
| Post high school degree | 10687 (23.2) | 230 (15.9) | 331 (30.6) | 214 (16.4) | ||
| ESRD (%) | ||||||
| Diabetes | Pre | 32208 (27.7) | 1193 (25.7) | 178 (32.4) | 783 (31.9) | < 0.001 |
| Hypertension | 30368 (26.2) | 1745 (37.6) | 221 (40.2) | 1108 (45.1) | ||
| Other | 53532 (46.1) | 1708 (36.8) | 151 (27.5) | 564 (23.0) | ||
| Diabetes | Post | 14439 (31.3) | 477 (33.1) | 438 (40.5) | 601 (46.1) | < 0.001 |
| Hypertension | 12513 (27.1) | 543 (37.6) | 270 (25.0) | 444 (34.1) | ||
| Other | 19147 (41.5) | 423 (29.3) | 374 (34.6) | 258 (19.8) | ||
| Dialysis time, day, (median [IQR]) | Pre | 1143 [708, 1691] | 1344 [889, 2164] | 976 [580, 1388] | 1118 [603, 1596] | < 0.001 |
| Post | 1661 [1043, 2373] | 1999 [1364, 2988] | 1257 [637, 1674] | 1065 [593, 1661] | < 0.001 | |
| CPRA (median [IQR]) | Pre | 0.0 [0.0, 2.0] | 0.0 [0.0, 2.0] | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | < 0.001 |
| CPRA (mean [SD]) | Post | 19.3 (32.8) | 20.5 (33.5) | 9.4 (21.9) | 9.3 (21.3) | < 0.001 |
| PVD = yes (%) | Pre | 5374 (4.6) | 210 (4.5) | 26 (4.7) | 111 (4.5) | < 0.001 |
| Post | 4590 (10.0) | 186 (12.9) | 118 (10.9) | 148 (11.4) | 0.001 | |
| Diabetes = yes (%) | Pre | 38827 (33.4) | 1491 (32.1) | 225 (40.9) | 1014 (41.3) | < 0.001 |
| Post | 17213 (37.3) | 583 (40.4) | 521 (48.2) | 714 (54.8) | < 0.001 | |
| Characteristics | D-R- | D-R+ | D+R- | D+R+ | P value | |
| n | Pre | 116108 | 4646 | 550 | 2455 | |
| Post | 46099 | 1443 | 1082 | 1303 | ||
| TX year (median [IQR]) | Pre | 2005 [2000, 2010] | 2004 [1999, 2009] | 2001 [1997, 2007] | 2006 [2001, 2010] | < 0.001 |
| Post | 2016 [2015, 2018] | 2016 [2015, 2018] | 2018 [2017, 2018] | 2016 [2015, 2017] | < 0.001 | |
| Region (%) | ||||||
| 1 | Pre | 4694 (4.0) | 167 (3.6) | 11 (2.0) | 74 (3.0) | < 0.001 |
| 2 | 14123 (12.2) | 661 (14.2) | 112 (20.4) | 823 (33.5) | ||
| 3 | 17507 (15.1) | 704 (15.2) | 64 (11.6) | 235 (9.6) | ||
| 4 | 10816 (9.3) | 414 (8.9) | 29 (5.3) | 136 (5.5) | ||
| 5 | 17680 (15.2) | 655 (14.1) | 63 (11.5) | 260 (10.6) | ||
| 6 | 4356 (3.8) | 156 (3.4) | 6 (1.1) | 7 (0.3) | ||
| 7 | 9360 (8.1) | 403 (8.7) | 54 (9.8) | 137 (5.6) | ||
| 8 | 7738 (6.7) | 252 (5.4) | 23 (4.2) | 55 (2.2) | ||
| 9 | 7150 (6.2) | 355 (7.6) | 29 (5.3) | 222 (9.0) | ||
| 10 | 10186 (8.8) | 457 (9.8) | 104 (18.9) | 192 (7.8) | ||
| 11 | 12498 (10.8) | 422 (9.1) | 55 (10.0) | 314 (12.8) | ||
| 1 | Post | 1601 (3.5) | 62 (4.3) | 35 (3.2) | 65 (5.0) | < 0.001 |
| 2 | 5404 (11.7) | 182 (12.6) | 151 (14.0) | 349 (26.8) | ||
| 3 | 6590 (14.3) | 221 (15.3) | 181 (16.7) | 150 (11.5) | ||
| 4 | 4526 (9.8) | 173 (12.0) | 45 (4.2) | 67 (5.1) | ||
| 5 | 8107 (17.6) | 238 (16.5) | 101 (9.3) | 144 (11.1) | ||
| 6 | 1911 (4.1) | 55 (3.8) | 13 (1.2) | 6 (0.5) | ||
| 7 | 3192 (6.9) | 110 (7.6) | 37 (3.4) | 45 (3.5) | ||
| 8 | 3130 (6.8) | 86 (6.0) | 5 (0.5) | 44 (3.4) | ||
| 9 | 3057 (6.6) | 85 (5.9) | 125 (11.6) | 174 (13.4) | ||
| 10 | 3507 (7.6) | 79 (5.5) | 195 (18.0) | 84 (6.4) | ||
| 11 | 5074 (11.0) | 152 (10.5) | 194 (17.9) | 175 (13.4) | ||
| Shared (%) | ||||||
| Local | Pre | 85197 (73.4) | 3464 (74.6) | 223 (40.5) | 931 (37.9) | < 0.001 |
| Regional | 9559 (8.2) | 377 (8.1) | 124 (22.5) | 591 (24.1) | ||
| National | 21352 (18.4) | 805 (17.3) | 203 (36.9) | 933 (38.0) | ||
| Local | Post | 35095 (76.1) | 1115 (77.3) | 337 (31.1) | 379 (29.1) | < 0.001 |
| Regional | 5349 (11.6) | 157 (10.9) | 345 (31.9) | 348 (26.7) | ||
| National | 5655 (12.3) | 171 (11.9) | 400 (37.0) | 576 (44.2) | ||
| CIT (median [IQR]) | Pre | 18.0 [13.0, 23.1] | 18.0 [13.0, 23.5] | 20.0 [16.0, 26.0] | 19.0 [15.0, 25.0] | < 0.001 |
| Post | 16.7 [11.4, 22.6] | 16.5 [11.0, 22.0] | 18.4 [13.1, 23.8] | 18.0 [12.3, 23.5] | < 0.001 | |
| HLA mismatch = 4-6 (%) | Pre | 76253 (65.7) | 3238 (69.7) | 452 (82.2) | 2151 (87.6) | < 0.001 |
| Post | 35126 (76.2) | 1170 (81.1) | 894 (82.6) | 1154 (88.6) | < 0.001 | |
| DGF = yes (%) | Pre | 28919 (24.9) | 1440 (31.0) | 139 (25.3) | 756 (30.8) | < 0.001 |
| Post | 13468 (29.2) | 496 (34.4) | 222 (20.5) | 284 (21.8) | < 0.001 | |
In the DAA era, HCV+ donors were younger than HCV- donors, with lower rates of diabetes and hypertension. D+R- donors were predominantly white (85.2%) and died primarily of anoxic brain injury (72%) (Supplementary Table 3A). D+R- recipients tended to be white (45.7%), highly educated (30.6% with post high school degree), and less likely to have hypertension as the etiology of renal failure (Supplementary Table 3B). Similar to the pre-DAA transplants, D+R- transplants had the lowest rate of DGF (20.5%) despite the longest CIT (18.4[IQR, 13.1, 23.8]) (Supplementary Table 3C).
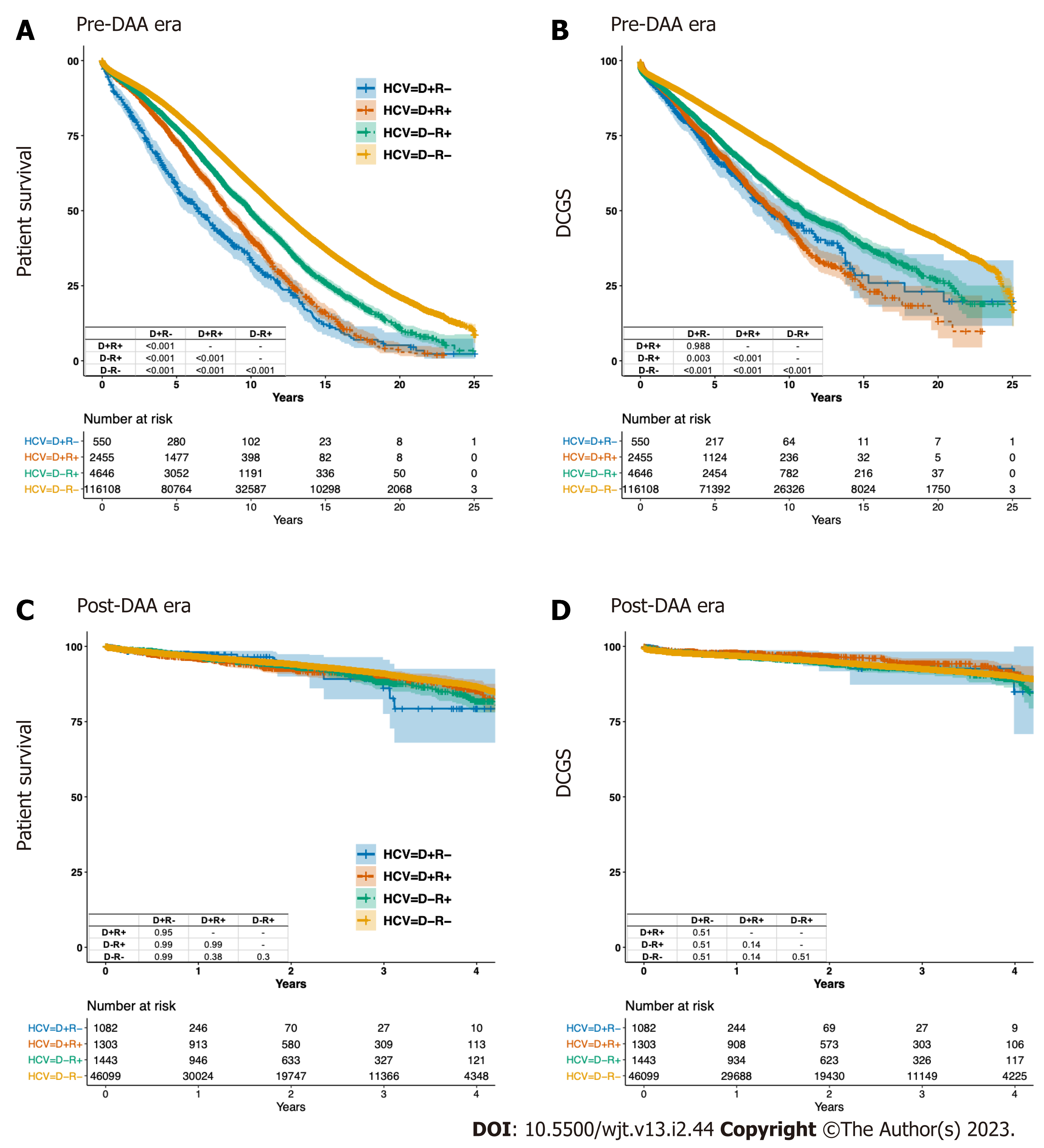
Prior to the DAA era, D-R- patients had the best crude PS and DCGS, while D+R- patients had the worst crude PS and DCGS (Figure 3A and B). The crude 3-year PS was 89.6%, 73.1%, 86.7% and 84.8% for D-R-, D+R-, D-R+ and D+R+, respectively. The crude 3-year DCGS was 88.8%, 80.1%, 84.2% and 82% for D-R-, D+R-, D-R+ and D+R+, respectively (Table 4).
| Cohorts in comparison (%) | D-R+ | D+R+ | D-R- | D+R- | Absolute risk difference | ||
| Crude | Patient survival | Pre | 86.7 (85.8, 87.7) | 84.8 (83.4, 86.3) | 89.6 (89.5, 89.8) | 73.1 (69.4, 76.9) | - |
| Post | 88.1 (85.7, 90.5) | 89.8 (87.7, 92) | 91 (90.6, 91.3) | 86.1 (77.6, 95.6) | - | ||
| Death-censored graft survival | Pre | 84.2 (83.1, 85.3) | 82 (80.4, 83.6) | 88.8 (88.6, 89) | 80.1 (76.6, 83.7) | - | |
| Post | 92.4 (90.6, 94.2) | 94.2 (92.5, 96) | 92.5 (92.2, 92.8) | 92.6 (87.3, 98.3) | - | ||
| D+ vs D- in R+ | Patient survival | Pre | 86.3 (84.4, 88.2) | 84.8 (82.8, 86.8) | - | - | 3.3 (1.8, 4.7) |
| Post | 90.2 (85.5, 95) | 88.7 (84, 93.7) | - | - | 2.7 (-2.9, 8.1) | ||
| Death-censored graft survival | Pre | 83.6 (81.5, 85.8) | 81.6 (79.4, 83.9) | - | - | 3.1 (1.2, 5) | |
| Post | 90.1 (85.5, 94.9) | 92 (87.9, 96.3) | - | - | 0.4 (-5, 6.1) | ||
| D+ vs D- in R- | Patient survival | Pre | - | - | 85.3 (82.3, 88.4) | 73.5 (69.8, 77.4) | 8 (5.2, 10.9) |
| Post | - | - | 89.1 (83.6, 94.9) | 88.6 (81.6, 96.2) | -0.3 (-5.9, 6.1) | ||
| Death-censored graft survival | Pre | - | - | 86.9 (83.9, 89.9) | 80.4 (76.8, 84.1) | 7.4 (4.3, 10.5) | |
| Post | - | - | 92.2 (87.8, 96.8) | 93.8 (88.5, 99.4) | -2.3 (-7.4, 2.1) | ||
| R+ vs R- in D+ | Patient survival | Pre | - | 80.9 (77.3, 84.6) | - | 76.7 (72.8, 80.7) | 0.1 (-2.9, 3.1) |
| Post | - | 88.4 (84.3, 92.8) | - | 85.1 (75.4, 96) | 1.1 (-6.1, 7) | ||
| Death-censored graft survival | Pre | - | 79.4 (75.5, 83.5) | - | 80.6 (76.9, 84.6) | 1.2 (-2.5, 4.9) | |
| Post | - | 93 (89.6, 96.5) | - | 92.3 (86.2, 98.8) | 0.7 (-5, 5.4) | ||
| R+ vs R- in D- | Patient survival | Pre | 86.7 (85.8, 87.7) | - | 88.7 (87.8, 89.6) | - | 2.6 (1.9, 3.2) |
| Post | 88.1 (85.7, 90.5) | - | 89.5 (87.3, 91.8) | - | -0.5 (-3.3, 2.2) | ||
| Death-censored graft survival | Pre | 84.2 (83.1, 85.3) | - | 86.5 (85.5, 87.5) | - | 3.5 (2.6, 4.4) | |
| Post | 92.4 (90.6, 94.2) | - | 92.1 (90.2, 94.1) | - | -0.2 (-2.4, 2) | ||
| D+R+ vs D-R- | Patient survival | Pre | - | 85 (83.4, 86.5) | 89 (87.7, 90.4) | - | 5.3 (4.3, 6.4) |
| Post | - | 88.4 (85.5, 91.4) | 91.8 (89.2, 94.4) | - | 3.3 (0, 6.7) | ||
| Death-censored graft survival | Pre | - | 82.7 (81, 84.4) | 87.8 (86.4, 89.2) | - | 7.1 (5.7, 8.5) | |
| Post | - | 93.3 (90.9, 95.8) | 93.3 (90.9, 95.8) | - | 1.7 (-1.4, 4.5) | ||
| D+R- vs D-R+ | Patient survival | Pre | 85.4 (82.1, 88.8) | - | - | 75.6 (71.6, 79.7) | 5.4 (2.6, 8.6) |
| Post | 88.7 (81.8, 96.3) | - | - | 91.5 (85, 98.6) | 3.2 (-5.9, 11.6) | ||
| Death-censored graft survival | Pre | 84.5 (81.1, 88.1) | - | - | 81.1 (77.3, 85.1) | 4.8 (1.4, 8.2) | |
| Post | 94.4 (89.3, 99.7) | - | - | 93.3 (87.4, 99.5) | 2.5 (-3.6, 10) | ||
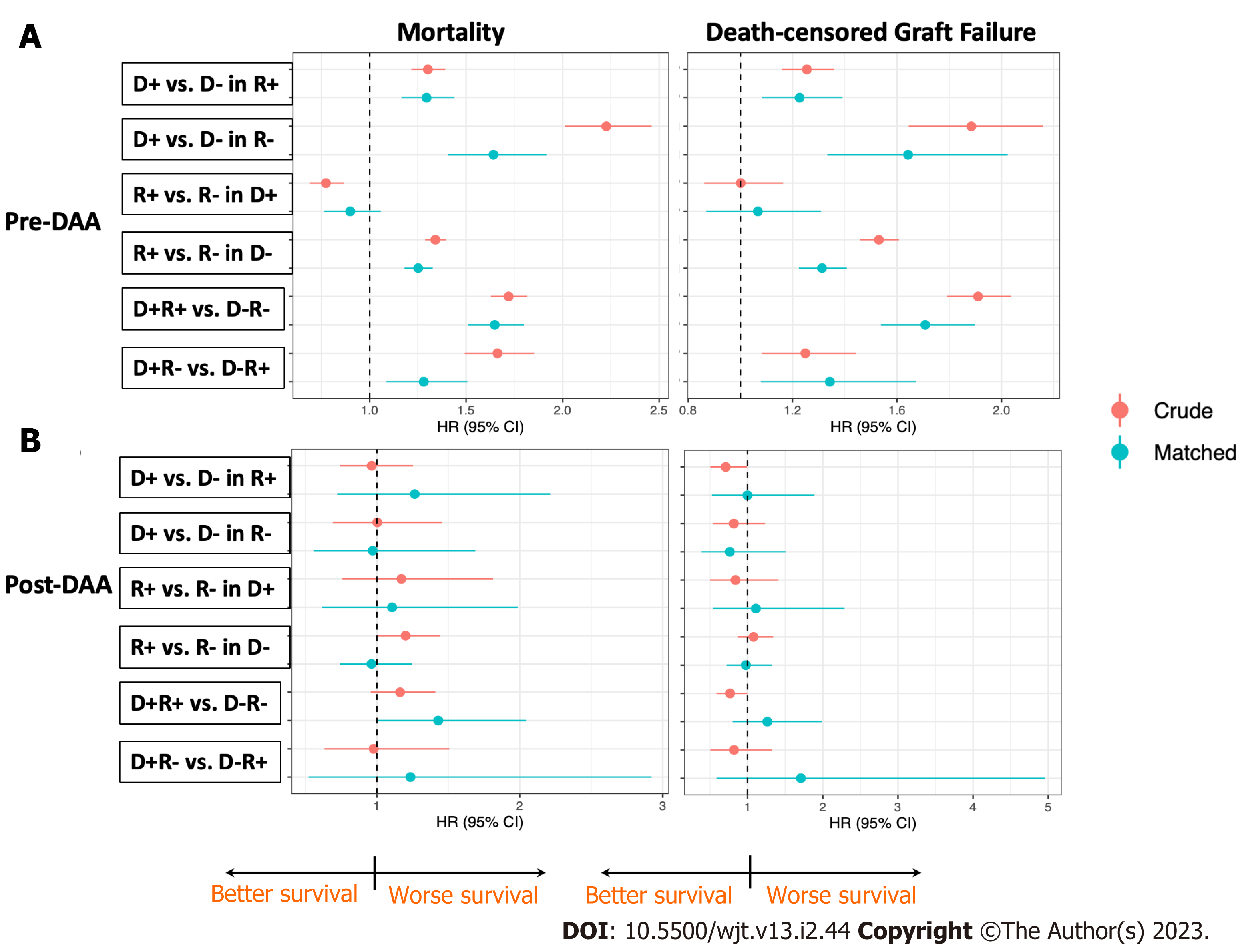
After matching, 1272 pairs of HCV+ and 528 pairs of HCV- recipients were generated. Among the HCV+ recipients, receiving an HCV+ DD kidney was associated with 1.28-fold higher mortality (HR 1.151.281.42) and 1.22-fold higher DCGF (HR 1.081.221.39) compared to receiving an HCV- kidney over the observed period (Figure 4A). The absolute risk difference (aRD) was 3.3% (95%CI: 1.8%, 4.7%) for PS and 3.1% (95%CI: 1.2%, 5%) for DCGS at 3 years (Table 4).
Among HCV- recipients, receiving an HCV+ kidney was associated with 1.55-fold higher mortality (HR 1.331.551.80) and 1.64-fold higher DCGF (HR 1.331.642.02) compared to an HCV- kidney (Figure 4A). The aRD was 8% (95%CI: 5.2%, 10.9%) for PS and 7.4% (95%CI: 4.3%, 10.5%) for DCGS at 3 years (Table 4).
In the DAA era, comparable crude PS and DCGS were observed among all four cohorts (Figure 3C and D). The crude 3-year PS were 91%, 86.1%, 88.1% and 89.8% for D-R-, D+R-, D-R+ and D+R+, respectively. The crude 3-year DCGS were 92.5%, 92.6%, 92.4% and 94.2% for D-R-, D+R-, D-R+ and D+R+, respectively (Table 4). After matching, there were 290 pairs of HCV+ and 791 pairs of HCV- recipients. In contrast with pre-DAA era risks, the risks for PS and DCGS associated with receiving an HCV+ kidney in either HCV+ or HCV- recipients were not statistically significantly different in the DAA era (Figure 4B).
Pre-DAA, HCV+ in recipients of HCV- donor kidneys corelated with significant declines in both crude PS and DCGS. However, HCV+ in recipients of HCV+ donors demonstrated a relative protective effect on mortality by 22% (D+R- vs D+R+, adjusted P < 0.001 for log-Rank test, HR0.690.780.87), despite the DCGS remaining comparable between two groups (D+R- vs D+R+, adjusted P = 0.988 for log-Rank test) (Figure 3A and B).
After matching, we generated 461 pairs of HCV+ and 4646 pairs of HCV- DD. HCV+ in recipients of HCV- donor kidneys was associated with 1.25-fold higher mortality (HR 1.181.251.33) and 1.31-fold higher DCGF (HR 1.221.311.41). The aRD between D-R- and D-R+ was 2.6% (95%CI: 1.9%, 3.2%) for PS and 3.5% (95%CI: 2.6%, 4.4%) for DCGS at 3 years (Table 4). In contrast, HCV+ and HCV- recipients of HCV+ donors demonstrated comparable outcomes (HR 0.8611.18 for mortality, 0.871.011.31 for DCGS) (Figure 4A).
In the DAA era, we generated 508 pairs of HCV+ and 1440 pairs of HCV- recipients after matching. The risk associated with recipient’s HCV+ when receiving either HCV+ or HCV- kidneys was not statistically significantly different in PS or DCGS (Figure 4B).
Pre-DAA, HCV+ in the donor and recipient significantly impaired both PS and DCGS (D-R- vs D+R+, adjusted P < 0.001 for log-Rank test) (Figure 3A and B). There were 2150 pairs of D-R- and D+R+ transplants after matching. HCV+ in donor and recipient was associated with 1.56-fold higher mortality (HR 1.431.561.7) and 1.71-fold higher DCGF (HR 1.541.711.9) compared to the D-R- transplants. The aRD between D-R- and D+R+ were 5.3% (95%CI: 4.3%, 6.4%) for PS and 7.1% (95%CI: 5.7%, 8.5%) for DCGS at 3 years (Figure 4A, Table 4).
In the DAA era, 803 pairs of D-R- and D+R+ transplants were generated after matching. HCV+ in donor and recipient marginally significantly increased the mortality (P = 0.049 for log-rank test). The cox proportional hazard model showed a mortality increase by 1.43-fold (HR 1.01.432.04) as compared to the D-R- transplants, with an aRD of 3.3% (95%CI: 0, 6.7%) at 3 years. The 3-year PS were 91.8% and 88.4% for D-R- and D+R+ recipients, respectively. HCV+ in donor and recipient did not statistically significantly affect DCGS (Figure 4B, Table 4).
In the pre-DAA era, HCV+ in the donor had more impact on patient survival than did infection in the recipient (D+R- vs D-R+, adjusted P < 0.001 for log-Rank test, HR1.491.691.83) (Figure 3A and B). After matching, there were 444 pairs of D+R- and D-R+ transplants. Donor HCV+ was associated with 1.36-fold higher mortality (HR 1.161.361.61) and 1.34-fold higher DCGF (HR 1.081.341.67) than recipient HCV+. The aRD between D+R- and D-R+ were 5.4% (95%CI: 2.6%, 8.6%) for PS and 4.8% (95%CI: 1.4%, 8.2%) for DCGS at 3 years (Figure 4A, Table 4).
In the DAA era, 253 pairs of D-R+ and D+R- transplants were identified after matching, with both PS (HR0.521.282.87) and DCGS (HR0.581.734.91) in the matched cohorts being comparable (Figure 4B, Table 4).
In our national study of 177937 DD KT across 25 years, we found a marked increase in HCV+ kidney utilization after DAA availability, initially with KTs of HCV+ kidneys to HCV+ recipients in 2014, followed by a dramatic shift towards transplants into HCV- recipients. This shift in 2016 likely reflects knowledge around the safety of HCV transplants with concurrent use of DAA. Pre-DAA D+R- recipients, despite generally being older, with less dialysis time and higher malignancy prevalence, received younger donors’ kidneys. Interestingly, in the DAA era, recipients’ education level was highest in the D+R- cohort, suggesting superior health literacy potentially facilitating informed consent and appreciation of DAA effects in decreasing HCV+ kidney risks[12]. DGF was reduced in D+R- transplants, despite the longest CIT and higher HLA mismatch compared with other cohorts in both the pre- and post-DAA eras, which could be the result of lower DCD rates and other unmeasured donor factors. In the pre DAA-era, HCV+ in either the donor or recipient of HCV- kidneys was associated with poorer PS and DCGS, but donor HCV+ status impacted PS and DCGS moreso than did recipient HCV+ status. Additionally, donor plus recipient HCV+ (D+R+ vs D-R-) and donor infection in HCV- recipients (D+R- vs D-R-) displayed the largest absolute increase in mortality and DCGF. Importantly, the risks on PS and DCGS associated with HCV+ in donors and/or recipients were no longer statistically significant after the widespread adoption of DAA in 2015, except for a marginally significantly impaired PS in D+R+ vs D-R-, which possessed the largest risk difference in the pre-DAA era.
The presumed risks of viral transmission in HCV+ transplants made these transplants scarce in the pre-DAA era (< 50 annually D+R-)[13-15]. DAAs encouraged broader acceptance of HCV+ candidates and more aggressive utilization of HCV+ kidneys. Two pilot trials in 2017 and 2018 of HCV+ kidney transplants into HCV- recipients found that, despite inevitable HCV transmission, subsequent DAA therapy provided HCV cure in a cost-effective approach that also resulted in well-functioning allografts[16,17]. Similarly, our observational study shows equivalent outcomes between D+R- and D-R- cohorts in the DAA era.
Many studies evaluated the effect of donor HCV+ on KT outcomes prior to the introduction of DAA[13-15], with HCV+ KT improving survival among all patients when compared to staying waitlisted and not receiving a kidney[2]. A single-center analysis summarizing 1990-2007 data compared long-term D+R+ outcomes to D-R+, showing that HCV+ donor status in HCV+ recipients did not significantly influence mortality, graft failure, or liver disease[14]. However, 1995-2008 national registry data showed D+R+ patients had a 2.6-fold higher hazard of joining the liver wait-list (P < 0.001). Nonetheless, the absolute risk difference in subsequently listing for liver transplant was < 2% between recipients of HCV+ and HCV- kidneys[15]. A recent study using 2005-2017 data reported that among HCV+ recipients, receiving an HCV+ kidney was associated with 19% higher mortality (aHR, 1.071.191.32), an effect that disappeared in the DAA era[5]. Our study evaluated the HCV effect of donor separately in HCV+ recipients and HCV- recipients and found similar trends of donor HCV associated PS and DCGS impairment in both recipients groups. with both mortality and DCGF absolute risk differences being larger in HCV- than HCV+ recipients (Table 4).
Two meta-analyses have evaluated the effect of recipient HCV+ status on KT outcomes[18,19], finding HCV+ correlated with increased mortality (aHR: 1.491.852.31, 1.331.691.97) and graft failure (aHR: 1.461.762.11, 1.221.562.00). However, neither distinguished donor HCV status. Our study found that the effect of recipients’ HCV+ status was dramatically modified by the donor’s HCV status—recipient’s HCV+ only impaired transplant outcomes when receiving an HCV-, but not HCV+, kidney. This finding parallels our previous study analyzing outcomes of transplanting the same donor’s pair of kidneys to one HCV+ and to one HCV- recipient[20].
There are several limitations to our study. First, most D+R- patients received transplants in the DAA era with relatively short follow up. Dividing the dataset into pre- and DAA eras resulted in smaller sample sizes. Second, PSM use to eliminate confounders between comparator groups could be biased by unmeasured potential confounders, including HCV genotype, viral load, infection duration and severity, graft rejection, and immunosuppression intensity, none of which is found in the used registry data. Third, we lack viremia data—while most viremic patients are antibody positive, a small portion of antibody positive patients are aviremic. We defined HCV+ by antibody status prior to 2015, and by antibody and NAT results since 2015. Antibody positive aviremic donors or recipients were included as HCV+ in both eras’ analyses, and a miniscule fraction of viremic patients who are antibody negative would have been included in the HCV- cohort in the pre-DAA analysis. Including these patients would yield worse outcomes in the uninfected population, underestimating the difference observed between infected and uninfected groups. Fourth, the registry data does not verify DAA treatment. Fifth, we used a pair matching method to estimate the “average treatment effect in the treated.” Some exposed subjects were excluded from the matched sample because of no available unexposed subjects within the specified caliper distance of the exposed subjects. There might be potential bias generated when unmatched exposed subjects differ systematically from the matched exposed subjects[21]. Other statistical methods, including full matching or inverse probability weighting, with the aim to include all the samples in both groups in comparison could also result in biased estimation due to increased heterogenicity within each group. Lastly, we used single imputation for variables with missingness over 1 percent. Limited impact was found on the magnitude of the hazard ratio or the significance of the findings of DCGS and patient survival, with multiple imputation method.
In conclusion, although HCV+ in either KT donors or recipients negatively impacted PS and DCGS pre-DAA, neither donor nor recipient HCV+ appears to portend worse outcomes in the DAA era, supporting increased utilization of HCV+ kidneys as the standard of care. Given comparable outcomes across all four patient cohorts in the DAA era, a new allocation algorithm, eliminating HCV+ kidneys’ negative influence on the KDRI, is urgently needed to improve utilization and allocation of this under-utilized resource.
While Hepatitis C virus infection (HCV+) kidneys have traditionally been discarded rather than transplanted into HCV- recipients, the introduction of direct-acting antivirals (DAAs) in 2013 revolutionized HCV treatment by consistently achieving sustained virologic responses, opening the door for transplantation of HCV+ organs.
As HCV+ rates in kidney donors and transplant recipients rise, the introduction of DAA may effect transplant outcomes.
To analyze the effects of HCV+ in donors, recipients, or both, on deceased-donor (DD) kidney transplantation (KT) outcomes, and the impact of DAAs on those effects.
The Organ Procurement and Transplantation Network data of adult first solitary DD-KT recipients 1994-2019 were allocated into four groups by donor and recipient HCV+ status. We performed patient survival (PS) and death-censored graft survival (DCGS) pairwise comparisons after propensity score matching to assess the effects of HCV+ in donors and/or recipients, stratifying our study by DAA era to evaluate potential effect modification.
Pre-DAA, for HCV+ recipients, receiving an HCV+ kidney was associated with 1.28-fold higher mortality (HR 1.151.281.42) and 1.22-fold higher death-censored graft failure (HR 1.081.221.39) compared to receiving an HCV- kidney and the absolute risk difference was 3.3% (95%CI: 1.8%-4.7%) for PS and 3.1% (95%CI: 1.2%-5%) for DCGS at 3 years. The HCV dual-infection (donor plus recipient) group had worse PS (0.56-fold) and DCGS (0.71-fold) than the dual-uninfected. Donor HCV+ derived worse post-transplant outcomes than recipient HCV+ (PS 0.36-fold, DCGS 0.34-fold). In the DAA era, the risk associated with HCV+ in donors and/or recipients was no longer statistically significant, except for impaired PS in the dual-infected vs dual-uninfected (0.43-fold).
Prior to DAA introduction, donor HCV+ negatively influenced kidney transplant outcomes in all recipients, while recipient infection only relatively impaired outcomes for uninfected donors. These adverse effects disappeared with the introduction of DAA.
Given comparable outcomes across all four patient cohorts in the DAA era, a new allocation algorithm, eliminating HCV+ kidneys’ negative influence on the KDRI, is urgently needed to improve utilization and allocation of this under-utilized resource.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Balaban HY, Turkey; Wishahi M, Egypt S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Peters TG, Schnitzler MA. The impact of transplantation with deceased donor hepatitis c-positive kidneys on survival in wait-listed long-term dialysis patients. Am J Transplant. 2004;4:2032-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Durand CM, Bowring MG, Thomas AG, Kucirka LM, Massie AB, Cameron A, Desai NM, Sulkowski M, Segev DL. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med. 2018;168:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | Mandal AK, Kraus ES, Samaniego M, Rai R, Humphreys SL, Ratner LE, Maley WR, Burdick JF. Shorter waiting times for hepatitis C virus seropositive recipients of cadaveric renal allografts from hepatitis C virus seropositive donors. Clin Transplant. 2000;14:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Bowring MG, Kucirka LM, Massie AB, Ishaque T, Bae S, Shaffer AA, Garonzik Wang J, Sulkowski M, Desai N, Segev DL, Durand CM. Changes in Utilization and Discard of HCV Antibody-Positive Deceased Donor Kidneys in the Era of Direct-Acting Antiviral Therapy. Transplantation. 2018;102:2088-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct Acting Anti-hepatitis C Virus Drugs: Clinical Pharmacology and Future Direction. J Transl Int Med. 2017;5:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | AASLD/IDSA HCV Guidance. Recommendations for Testing, Managing, and Treating Hepatitis C. Clinical Liver Disease. 2018;12:117-117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Welzel TM, Zeuzem S. Mixing and matching drugs: what makes sense? Clin Liver Dis. 2011;15:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 775] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 10. | Snyder JJ, Salkowski N, Kim SJ, Zaun D, Xiong H, Israni AK, Kasiske BL. Developing Statistical Models to Assess Transplant Outcomes Using National Registries: The Process in the United States. Transplantation. 2016;100:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Sise ME, Wojciechowski D, Chute DF, Gustafson J, Chung RT, Williams WW, Elias N. Process of selecting and educating HCV-uninfected kidney waiting-list candidates for HCV-infected kidney transplantation. Artif Organs. 2019;43:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Bucci JR, Matsumoto CS, Swanson SJ, Agodoa LY, Holtzmuller KC, Peters TG, Abbott KC. Donor hepatitis C seropositivity: clinical correlates and effect on early graft and patient survival in adult cadaveric kidney transplantation. J Am Soc Nephrol. 2002;13:2974-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Morales JM, Campistol JM, Domínguez-Gil B, Andrés A, Esforzado N, Oppenheimer F, Castellano G, Fuertes A, Bruguera M, Praga M. Long-term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipients. Am J Transplant. 2010;10:2453-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Kucirka LM, Peters TG, Segev DL. Impact of donor hepatitis C virus infection status on death and need for liver transplant in hepatitis C virus-positive kidney transplant recipients. Am J Kidney Dis. 2012;60:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N, Wesson R, Reyad A, Naqvi FF, Ostrander D, Sugarman J, Segev DL, Sulkowski M, Desai NM. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med. 2018;168:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 17. | Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, Bloom RD, Nazarian SM, Sawinski D, Porrett P, Naji A, Hasz R, Suplee L, Trofe-Clark J, Sicilia A, McCauley M, Farooqi M, Gentile C, Smith J, Reese PP. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;376:2394-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 18. | Fabrizi F, Martin P, Dixit V, Messa P. Meta-analysis of observational studies: hepatitis C and survival after renal transplant. J Viral Hepat. 2014;21:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Rostami Z, Nourbala MH, Alavian SM, Bieraghdar F, Jahani Y, Einollahi B. The impact of Hepatitis C virus infection on kidney transplantation outcomes: A systematic review of 18 observational studies: The impact of HCV on renal transplantation. Hepat Mon. 2011;11:247-254. [PubMed] |
| 20. | Yuan Q, Hong S, Perez-Ortiz A, Roth E, Chang DC, Madsen JC, Elias N. Effect of Recipient Hepatitis C Status on Outcomes of Deceased Donor Kidney Transplantation. J Am Coll Surg. 2020;230:853-861.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 1112] [Article Influence: 92.7] [Reference Citation Analysis (0)] |