Published online Mar 18, 2021. doi: 10.5500/wjt.v11.i3.54
Peer-review started: September 6, 2020
First decision: December 1, 2020
Revised: December 25, 2020
Accepted: February 19, 2021
Article in press: February 19, 2021
Published online: March 18, 2021
Processing time: 192 Days and 14.4 Hours
Heart transplant recipients are at higher risk of developing skin cancer than the general population due to the long-term immunosuppression treatment. Cancer has been reported as one of the major causes of morbidity and mortality for patients after heart transplantation. Among different types of skin cancers, cutaneous squamous cell carcinoma (cSCC) is the most common one, which requires timely screening and better management.
To identify risk factors and predict the incidence of cSCC for heart transplant recipients.
We retrospectively analyzed adult heart transplant recipients between 2000 and 2015 extracted from the United Network for Organ Sharing registry. The whole dataset was randomly divided into a derivation set (80%) and a validation set (20%). Uni- and multivariate Cox regression were done to identify significant risk factors associated with the development of cSCC. Receiver operating charac-teristics curves were generated and area under the curve (AUC) was calculated to assess the accuracy of the prediction model. Based on the selected risk factors, a risk scoring system was developed to stratify patients into different risk groups. A cumulative cSCC-free survival curve was generated using the Kaplan-Meier method for each group, and the log-rank test was done to compare the inter-group cSCC rates.
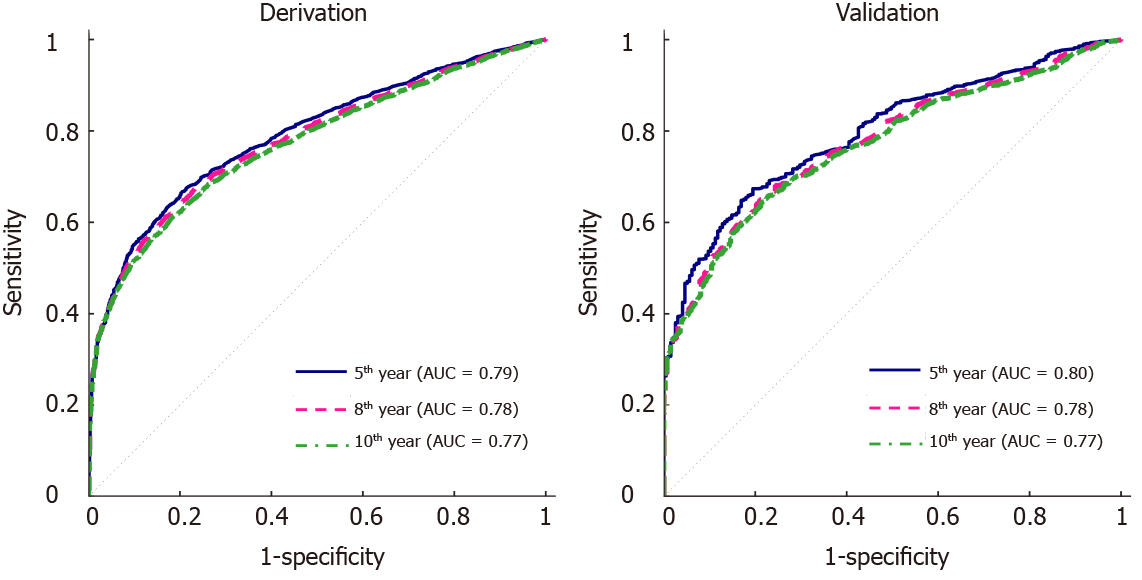
There were 23736 heart-transplant recipients during the study period, and 1827 of them have been reported with cSCC. Significant predictors of post-transplant cSCC were older age, male sex, white race, recipient and donor human leukocyte antigen (HLA) mismatch level, malignancy at listing, diagnosis with restrictive myopathy or hypertrophic myopathy, heart re-transplant, and induction therapy with OKT3 or daclizumab. The multivariate model was used to predict the 5-, 8- and 10-year incidence of cSCC and respectively provided AUC of 0.79, 0.78 and 0.77 in the derivation set and 0.80, 0.78 and 0.77 in the validation set. The risk scoring system assigned each patient with a risk score within the range of 0-11, based on which they were stratified into 4 different risk groups. The predicted and observed 5-year probability of developing cSCC match well among different risk groups. In addition, the log-rank test indicated significantly different cSCC-free survival across different groups.
A risk prediction model for cSCC among heart-transplant recipients has been generated for the first time. It offers a c-statistic of ≥ 0.77 in both derivation and validation sets.
Core Tip: We retrospectively analyzed 23736 heart-transplant recipients between 2010 and 2015. Eight risk factors associated with post-transplant cutaneous squamous cell carcinoma were identified, including older age, male sex, lower human leukocyte antigen mismatch level, white race, malignancy at listing, diagnosis with restrictive myopathy or hypertrophic myopathy, heart re-transplant, induction therapy with OKT3 or daclizumab. A multivariate risk prediction model was developed with c-statistics of ≥ 0.77 in both derivation and validation sets. A risk scoring system was designed to stratify patients into 4 risk groups based on their total risk scores. The predicted and observed 5-year probability of developing cutaneous squamous cell carcinoma match well among different risk groups.
- Citation: Nair N, Hu Z, Du D, Gongora E. Risk prediction model for cutaneous squamous cell carcinoma in adult cardiac allograft recipients. World J Transplant 2021; 11(3): 54-69
- URL: https://www.wjgnet.com/2220-3230/full/v11/i3/54.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i3.54
Skin cancer has been reported as one of the major causes of morbidity and mortality in heart transplantation recipients[1]. The incidence rate of nonmelanoma and melanoma skin cancers, especially cutaneous squamous cell carcinoma (cSCC), is significantly higher in heart transplant recipients than the general population with equivalent age and gender[2].
Multiple studies have been done to investigate the risk of skin cancer in heart transplant recipients[1], and factors including male gender, older age, white race, greater sunlight exposure were commonly identified to be associated with a high risk of post-transplant skin cancer[3-6]. Although risk factors have been characterized, few stratification models have been developed to predict the incidence of skin cancer after transplantation. Accurately stratifying the risk of skin cancer has been a challenge that prevents the development of evidence-based screening recommendations. In addition, most of the existing studies investigated the risk factors of several skin cancers collectively. The risk of cSCC, the most common skin cancer among heart transplant recipients, has not been exclusively assessed for a large patient population.
In this study, we sought to develop a risk prediction model for cSCC after heart transplantation using a national organ transplant database, i.e., the United Network for Organ Sharing (UNOS). The model aims to stratify patients into different risk groups regarding the development of cSCC post-transplantation and provides a useful tool for pre-transplant counseling and post-transplant surveillance and management.
The data consisted of 23736 adults (aged ≥ 18 years) heart transplant recipients between 2000 and 2015 were extracted from the UNOS registry of thoracic organ transplantation database. Patients who were listed for and received multi-organ transplantation were excluded from this study. Information on patient characteristics, cancer history, induction therapy, and other risk predictors were extracted for each transplant event, which includes age, sex, race, primary diagnosis, patient’s malig-nancy status at listing and at transplant, patient’s emergency status at transplant, donor’s cancer history, the recipient and donor human leukocyte antigen (HLA) Mismatch level, recipient’s most recent tests before transplant for panel-reactive antibody (PRA) against Class I and Class II antigens, induction with different types of drugs including thymoglobulin, ATGAM, OKT3, daclizumab, basiliximab, and alemtuzumab. cSCC event was determined by the post-transplant follow-up of malignancy status. Time to cSCC development was calculated as days between transplantation and the first reported incidence of cSCC or the last follow-up.
The data was randomly divided into a derivation set (80%) and a validation set (20%). All variables were compared between the derivation and validation sets as well as between the cancer and non-cancer groups (Table 1). Continuous variables were reported as mean (standard deviation), and categorical variables were summarized as percentages. Categorical variables and continuous variables were compared using χ2test and Wilcoxon rank-sum test, respectively.
| Total (n = 23736) | Derivation group (n = 18989) | Validation group (n = 4747) | P value for derivation vs validation groups | cSCC positive (n = 1827) | cSCC negative (n = 21909) | P value for cSCC positive vs cSCC negative | |
| Age | 52.1 (12.6)1 | 52.1 (12.6)1 | 52.3 (12.6)1 | 0.293 | 59.1 (7.76)1 | 51.6 (12.7)1 | < 0.001 |
| Female | 24.5 | 24.7 | 23.7 | 0.159 | 9.58 | 25.8 | < 0.001 |
| HLA mismatch level | 4.67 (1.02)1 | 4.67 (1.02)1 | 4.68 (1.01)1 | 0.966 | 4.59 (1.05)1 | 4.68 (1.01)1 | < 0.001 |
| PRA against Class I antigens | 5.36 (16.3)1 | 5.41 (16.4)1 | 5.16 (15.6)1 | 0.960 | 3.61 (13.2)1 | 5.51 (16.5)1 | < 0.001 |
| PRA against Class II antigens | 3.95 (14.3)1 | 3.98 (14.4)1 | 3.83 (14.0)1 | 0.404 | 2.85 (12.2)1 | 4.04 (14.5)1 | 0.003 |
| Race | |||||||
| White | 71.4 | 71.4 | 71.6 | 0.716 | 97.0 | 69.3 | < 0.001 |
| Black | 17.6 | 17.6 | 17.5 | 0.906 | 0.712 | 19.0 | < 0.001 |
| Hispanic | 7.26 | 7.26 | 7.27 | 0.989 | 1.81 | 7.72 | < 0.001 |
| Other | 3.70 | 3.74 | 3.54 | 0.514 | 0.493 | 3.97 | < 0.001 |
| Diagnosis | |||||||
| Dilated myopathy | 82.1 | 82.1 | 82.0 | 0.884 | 81.5 | 82.1 | 0.515 |
| Restrictive myopathy | 2.22 | 2.27 | 2.00 | 0.261 | 2.24 | 2.21 | 0.932 |
| Heart re-transplant | 2.63 | 2.58 | 2.84 | 0.301 | 2.68 | 2.62 | 0.883 |
| Coronary artery disease | 4.47 | 4.43 | 4.61 | 0.582 | 6.51 | 4.30 | < 0.001 |
| Hypertrophic myopathy | 1.92 | 1.90 | 2.00 | 0.636 | 1.70 | 1.94 | 0.475 |
| Valvular heart disease | 2.01 | 2.10 | 1.69 | 0.0716 | 2.35 | 1.99 | 0.282 |
| Congenital heart defect | 2.46 | 2.48 | 2.42 | 0.835 | 0.985 | 2.59 | < 0.001 |
| Other | 2.23 | 2.18 | 2.44 | 0.272 | 2.03 | 2.25 | 0.532 |
| Donor cancer history | |||||||
| No | 98.1 | 98.1 | 98.0 | 0.566 | 98 | 98.1 | 0.764 |
| Yes | 1.60 | 1.56 | 1.73 | 0.422 | 1.81 | 1.58 | 0.457 |
| Unknown | 0.282 | 0.29 | 0.253 | 0.669 | 0.164 | 0.292 | 0.322 |
| Malignancy at listing | |||||||
| No | 92.7 | 92.8 | 92.5 | 0.476 | 90.3 | 92.9 | < 0.001 |
| Yes | 5.83 | 5.80 | 5.94 | 0.708 | 7.72 | 5.67 | < 0.001 |
| Unknown | 1.45 | 1.42 | 1.58 | 0.416 | 1.97 | 1.41 | 0.055 |
| Malignancy at transplant | |||||||
| No | 98.1 | 98.1 | 97.8 | 0.15 | 97.4 | 98.1 | 0.039 |
| Yes | 0.421 | 0.416 | 0.442 | 0.802 | 0.712 | 0.397 | 0.046 |
| Unknown | 1.51 | 1.45 | 1.75 | 0.136 | 1.86 | 1.48 | 0.204 |
| Donor skin cancer history | |||||||
| No | 97.4 | 97.4 | 97.2 | 0.571 | 97.6 | 97.3 | 0.518 |
| Yes | 0.139 | 0.147 | 0.105 | 0.486 | 0.164 | 0.137 | 0.764 |
| Unknown | 2.50 | 2.46 | 2.65 | 0.454 | 2.24 | 2.52 | 0.462 |
| Patient status at transplant | |||||||
| Status 1A | 46.4 | 46.6 | 45.5 | 0.213 | 38.2 | 47.0 | < 0.001 |
| Status 1B | 37.6 | 37.4 | 38.3 | 0.268 | 40.6 | 37.3 | 0.006 |
| Status 2 | 16.0 | 16.0 | 16.2 | 0.817 | 21.2 | 15.6 | < 0.001 |
| Induction with thymoglobulin | 14.6 | 14.7 | 14.1 | 0.335 | 14.4 | 14.6 | 0.819 |
| Induction with ATGAM | 5.02 | 5.11 | 4.66 | 0.201 | 5.15 | 5.01 | 0.795 |
| Induction with OKT3 | 2.32 | 2.29 | 2.44 | 0.517 | 5.42 | 2.06 | < 0.001 |
| Induction with daclizumab | 8.30 | 8.43 | 7.77 | 0.142 | 12.2 | 7.98 | < 0.001 |
| Induction with basiliximab | 17.5 | 17.4 | 18.0 | 0.321 | 12.6 | 17.9 | < 0.001 |
| Induction with alemtuzumab | 1.56 | 1.56 | 1.81 | 0.116 | 1.48 | 1.57 | 0.771 |
Uni- and multivariate Cox regression analyses were done to assess the association of different risk factors with post-transplant cSCC, and p-values, hazard ratios and their confidence intervals were reported. Variables with small P values (< 0.1) in the univariate analysis were selected as inputs to the multivariate analysis. Stepwise forward selection was done to select the final multivariate model. The multivariate model was used to predict the probability of developing cSCC in 5, 8, and 10 years after heart transplantation. The model accuracy was assessed using receiver operating characteristics (ROC) curves and area under curves (AUCs). Based on the hazard ratio, a risk score was assigned to each significant variable (P value < 0.05), and the sum of all scores predicted the risk of a recipient developing cSCC after heart transplantation. The risk scoring system was validated by comparing the predicted and observed probability of developing cSCC 5 years after transplantation across different risk groups. The cumulative cSCC-free survival curves of different risk groups were derived using the Kaplan-Meier method, and the log-rank test was done to quantitatively assess the difference of cSCC risk. All the analysis was performed using MATLB software from MathWorks, Inc.
Table 1 provides the summary of all variables between the derivation and validation cohorts as well as between the cancer and non-cancer groups. No significant differences were observed between the derivation and validation groups for all factors. Within the study population, 1827 recipients (7.70%) developed cSCC whereas 21909 recipients (92.30%) were not reported with the event. Patients in the cSCC positive group were older, had a higher percentage of male sex and white race, had a lower level of recipient and donor HLA mismatch level, had a lower level of PRA against Class I and Class II antigens. The cSCC positive group had a higher percentage of patients who had coronary artery disease at listing, and a lower percentage of patients who had congenital heart defect at listing. More patients in the cSCC positive group had malignancy at listing and at transplantation. Patients in the cSCC positive group were less likely to be in status 1A and more likely in status 1B or status 2. In addition, recipients with post-transplant cSCC were more likely to be inducted with OKT3 or daclizumab while less likely to be inducted with basiliximab.
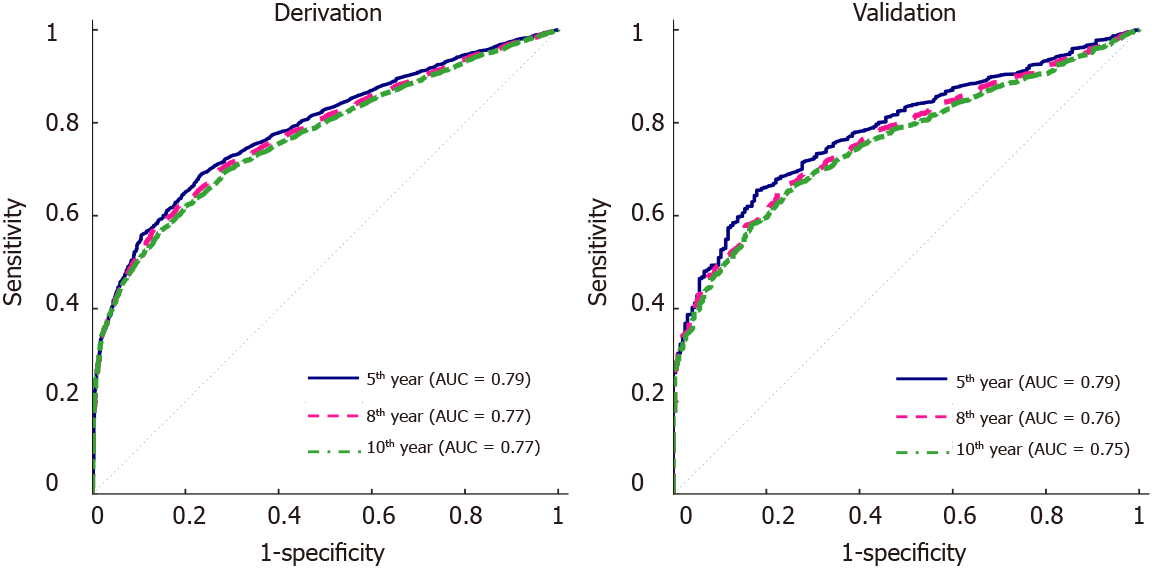
Table 2 gives a summary of the univariate Cox regression analysis, where 10 variables were significant (P < 0.05). These variables include age, sex, race, HLA mismatch level, PRA against Class I antigens, PRA against Class II antigens, diagnosis of coronary artery disease or congenital heart disease, patient’s malignancy status at listing, and at transplant, and OKT3. The final multivariate model had 8 variables (Table 3), including age, sex, HLA mismatch level, race, malignancy at listing, diagnosis at listing, and induction with OKT3 or daclizumab. ROC curves for the 5-year, 8-year and 10-year post-transplant cSCC prediction provided AUCs of 0.79, 0.78, 0.77 respectively in the derivation set and 0.80, 0.78, 0.77 respectively in the validation set (Figure 1).
| Covariates | Hazard ratio (95%CI) | P value |
| Age | 1.08 (1.07-1.09) | < 0.001 |
| Female | 0.310 (0.260-0.370) | < 0.001 |
| HLA mismatch level | 0.914 (0.870-0.960) | < 0.001 |
| PRA against Class I antigens | 0.994 (0.990-0.998) | 0.006 |
| PRA against Class II antigens | 0.994 (0.989-0.999) | 0.012 |
| Race | ||
| White | 1 | - |
| Black | 0.0390 (0.0221-0.068) | <0.001 |
| Hispanic | 0.178 (0.120-0.265) | <0.001 |
| Other | 0.108 (0.0512-0.226) | <0.001 |
| Diagnosis | ||
| Dilated myopathy | 1 | - |
| Restrictive myopathy | 1.38 (0.985-1.93) | 0.061 |
| Heart re-transplant | 1.12 (0.807-1.55) | 0.500 |
| Coronary artery disease | 1.49 (1.22-1.82) | < 0.001 |
| Hypertrophic myopathy | 0.923 (0.630-1.35) | 0.681 |
| Valvular heart disease | 1.16 (0.842-1.59) | 0.368 |
| Congenital heart defect | 0.393 (0.232-0.666) | 0.001 |
| Other | 1.01 (0.695-1.47) | 0.951 |
| Donor cancer history | ||
| No | 1 | - |
| Yes | 1.28 (0.883-1.84) | 0.195 |
| Unknown | 0.997 (0.321-3.10) | 0.997 |
| Malignancy at listing | ||
| No | 1 | - |
| Yes | 1.72 (1.43-2.09) | < 0.001 |
| Unknown | 0.983 (0.667-1.45) | 0.930 |
| Malignancy at transplant | ||
| No | 1 | 0 |
| Yes | 2.55 (1.48-4.41) | 0.001 |
| Unknown | 0.791 (0.528-1.18) | 0.255 |
| Donor skin cancer history | ||
| No | 1 | - |
| Yes | 1.06 (0.265-4.24) | 0.935 |
| Unknown | 0.631 (0.439-0.906) | 0.013 |
| Patient status at transplant | ||
| Status 1A | 1 | - |
| Status 1B | 1.07 (0.950-1.20) | 0.274 |
| Status 2 | 0.983 (0.854-1.13) | 0.805 |
| Induction with thymoglobulin | 1.05 (0.911-1.22) | 0.481 |
| Induction with ATGAM | 0.980 (0.784-1.22) | 0.857 |
| Induction with OKT3 | 1.59 (1.27-2.01) | < 0.001 |
| Induction with daclizumab | 1.16 (0.995-1.36) | 0.057 |
| Induction with basiliximab | 1.08 (0.927-1.26) | 0.322 |
| Induction with alemtuzumab | 1.18 (0.773-1.80) | 0.444 |
| Covariates | Hazard ratio (95%CI) | P value |
| Age | 1.068 (1.062-1.075) | < 0.001 |
| Female | 0.412 (0.344-0.494) | < 0.001 |
| HLA mismatch level | 0.951 (0.905-0.999) | 0.043 |
| Race | ||
| White | 1 | - |
| Black | 0.124 (0.059-0.261) | < 0.001 |
| Hispanic | 0.058 (0.033-0.102) | < 0.001 |
| Other | 0.229 (0.154-0.340) | < 0.001 |
| Diagnosis | ||
| Dilated myopathy | 1 | - |
| Restrictive myopathy | 1.869 (1.333-2.619) | < 0.001 |
| Heart re-transplant | 1.711 (1.231-2.378) | 0.001 |
| Coronary artery disease | 1.144 (0.935-1.400) | 0.192 |
| Hypertrophic myopathy | 1.596 (1.087-2.345) | 0.017 |
| Valvular heart disease | 1.159 (0.842-1.596) | 0.364 |
| Congenital heart defect | 1.106 (0.649-1.886) | 0.710 |
| Other | 1.381 (0.9477-2.012) | 0.093 |
| Malignancy at listing | ||
| No | 1 | - |
| Yes | 1.593 (1.315-1.930) | < 0.001 |
| Unknown | 0.982 (0.666-1.448) | 0.926 |
| Induction with OKT3 | 1.380 (1.095-1.739) | 0.006 |
| Induction with daclizumab | 1.371 (1.173-1.603) | < 0.001 |
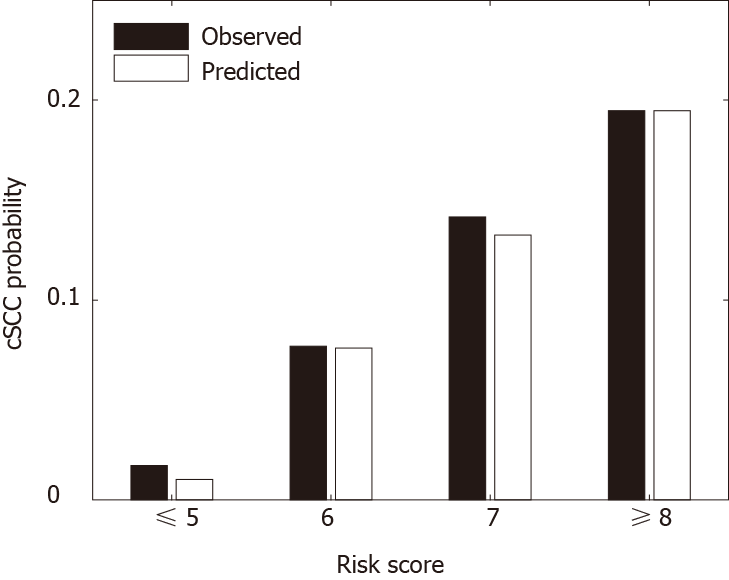
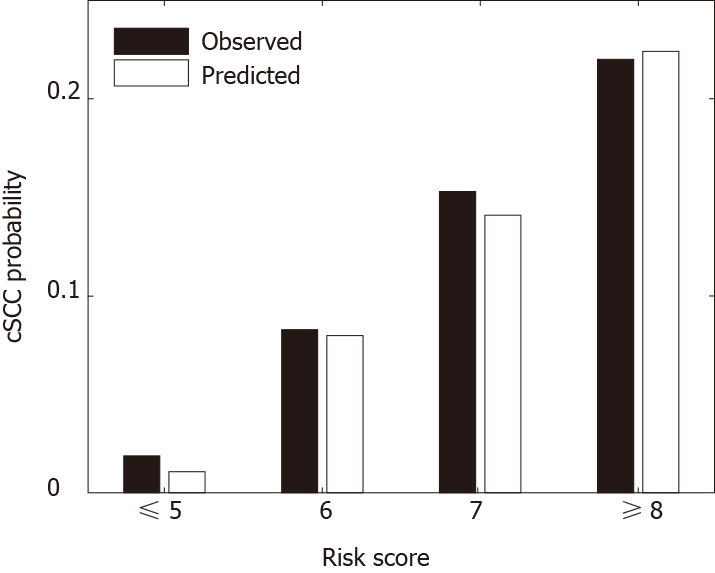
Table 4 provides the risk scores derived based on the multivariate model to predict the risk of developing cSCC 5 years after heart transplantation. The scoring system can classify patients into 4 risk groups: very low-risk group (score ≤ 5, n = 12383), low-risk group (score = 6, n = 6162), medium-risk group (score = 7, n = 4371), high-risk group (score ≥ 8, n = 820). Figure 2 shows the predicted and observed probabilities of developing cSCC 5 years after heart transplantation, which match well across different riskgroups. Patients in the high-risk group (score ≥ 8) had a higher probability (11-fold higher) of developing cSCC after transplant than patients in the very low-risk group (score ≤ 5).
| Covariates | Category | Score |
| Age | 18-40 | 0 |
| 40-60 | 1 | |
| > 60 | 2 | |
| Sex | Female | 0 |
| Male | 2 | |
| HLA mismatch level | > 5 | 0 |
| ≤ 5 | 1 | |
| Race | White | 2 |
| Other | 0 | |
| Diagnosis | Restrictive myopathy | 1 |
| Heart re-transplant | 1 | |
| Hypertrophic myopathy | 1 | |
| Other | 0 | |
| Malignancy at listing | No | 0 |
| Yes | 1 | |
| Unknown | 0 | |
| Induction with OKT3 | No | 0 |
| Yes | 1 | |
| Induction with daclizumab | No | 0 |
| Yes | 1 |
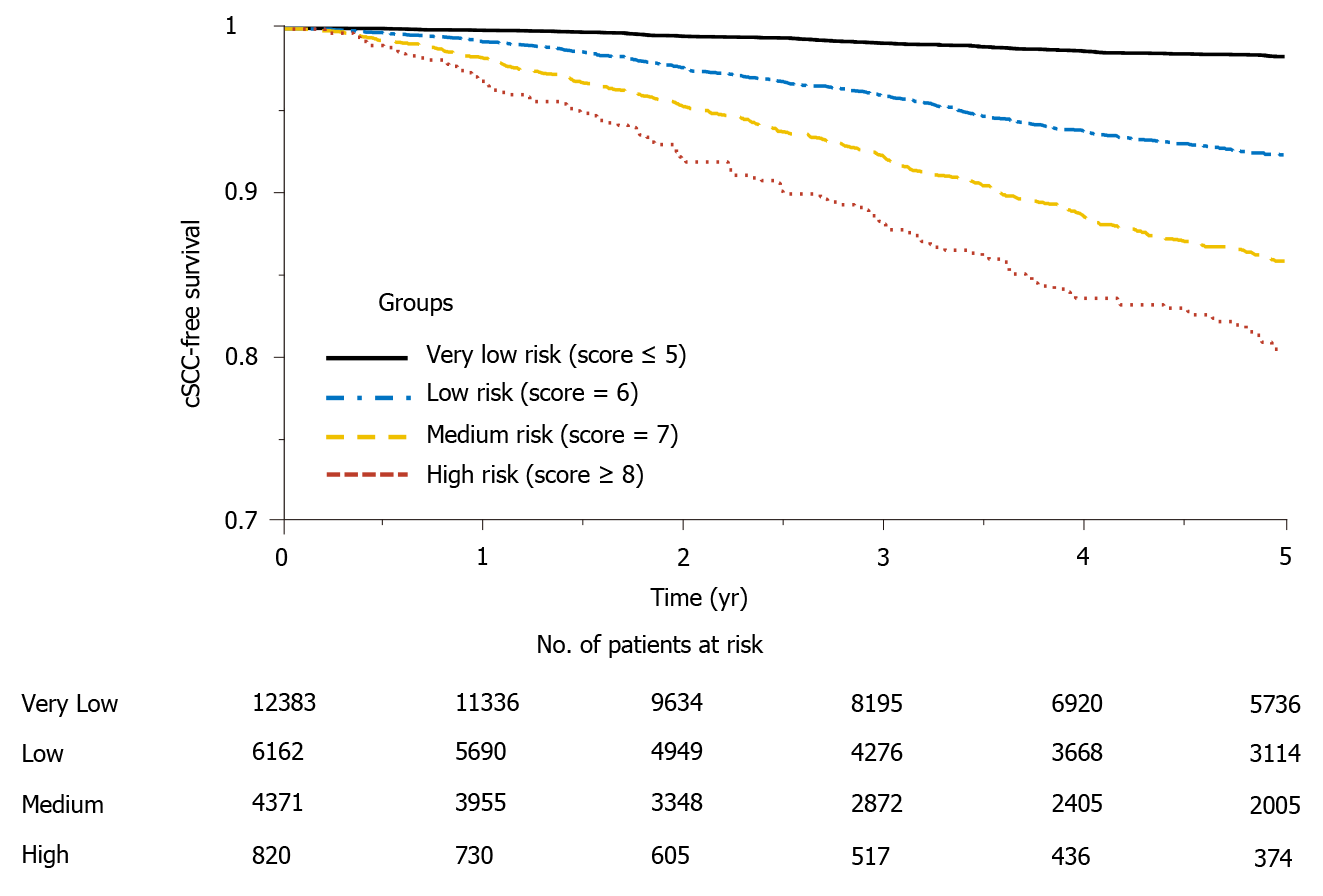
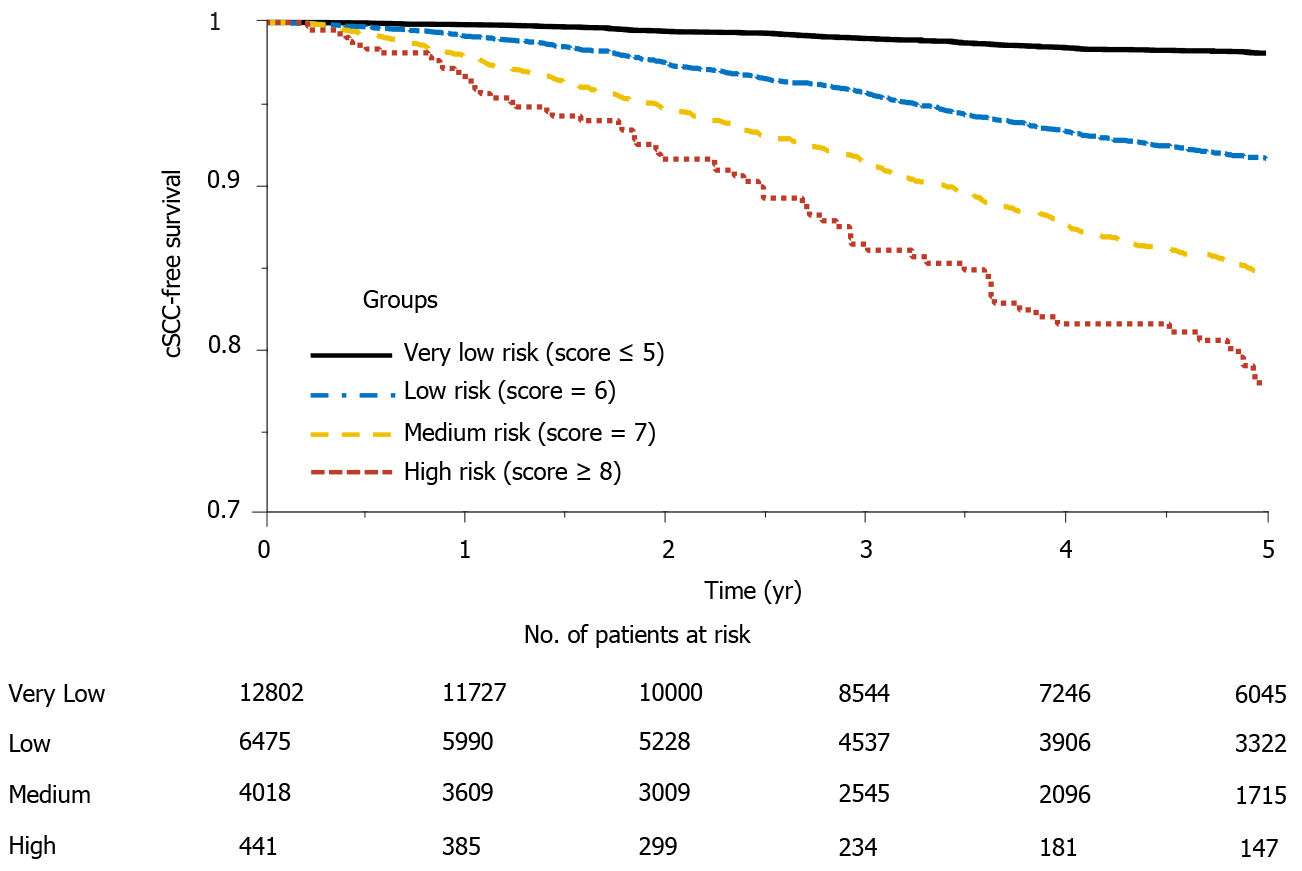
Figure 3 shows the Kaplan Meier estimator of the cSCC-free survival curve and risk table for each risk group. It shows that the probability of developing cSCC in the very low-risk group is significantly lower than that of the high-risk group, and about 20% of the subjects in the high-risk group developed cSCC 5 years after transplantation. In addition, log-rank test was performed to test the null hypothesis that there was no difference regarding the occurrence probability of cSCC among the four groups. The results in Table 5 show that the risk of developing cSCC in high-risk group is greater than that in the low and medium-risk groups. Significant differences (P value < 0.001) were observed between every two groups. The cSCC risk in the high-risk group is respectively 9.16-fold, 2.18-fold, and 1.28-fold higher than that of the very low-risk, low-risk, and medium-risk group; the risk of the medium-risk group is respectively 7.12-fold and 1.69-fold higher than that of the very low-risk and low-risk group, and the risk of the low-risk group is 4.19-fold higher than that of the very low-risk group.
| Group | P value | Hazard ratio (95%CI) |
| Low vs very low | < 0.001 | 4.19 (3.66-4.78) |
| Medium vs very low | < 0.001 | 7.12 (6.18-8.21) |
| Medium vs low | < 0.001 | 1.69 (1.52-1.88) |
| High vs very low | < 0.001 | 9.16 (6.23-13.5) |
| High vs low | < 0.001 | 2.18 (1.74-2.72) |
| High vs medium | 0.004 | 1.28 (1.07-1.54) |
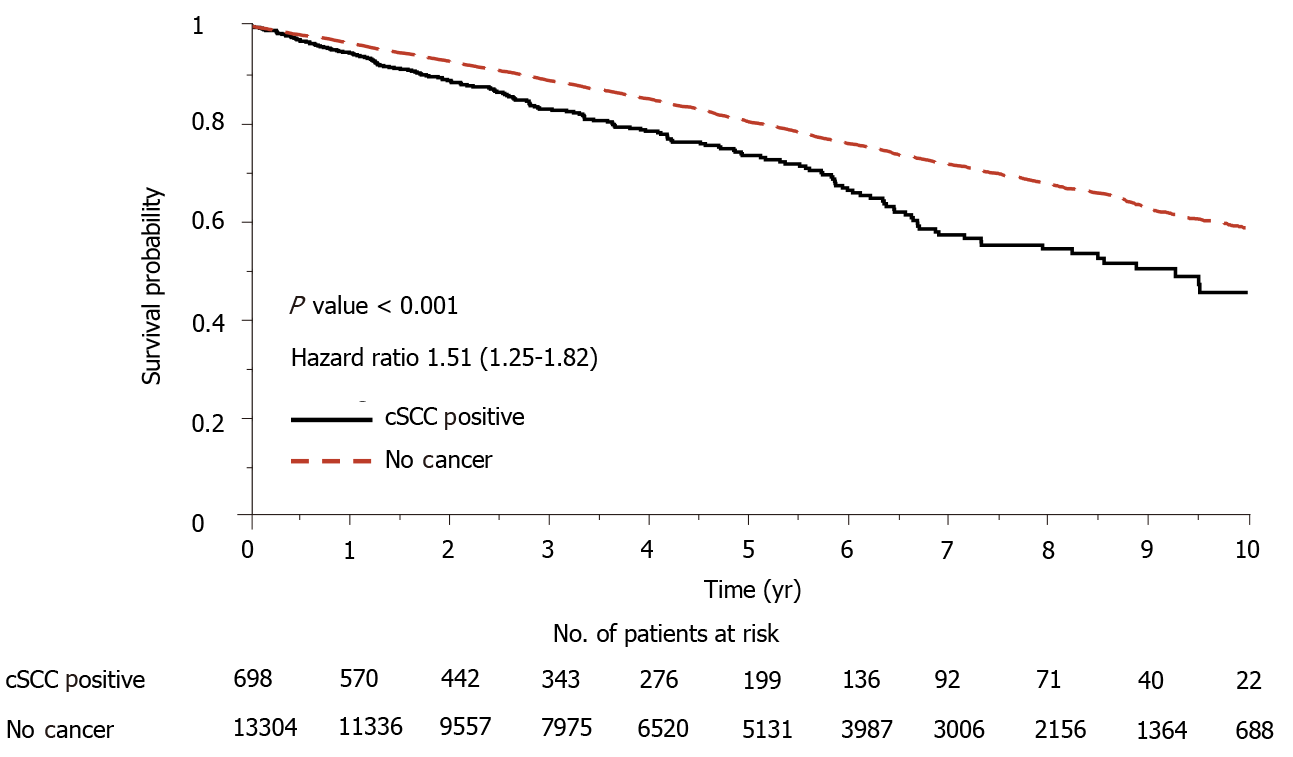
Most of the registry data including UNOS database showed that heart-transplant recipients with skin cancer revealed significantly lower overall survival than the recipients with no skin cancer. To demonstrate the consistency of our dataset, the survival experience of these two groups of patients were compared using landmark analysis[7]. Median time from the date of transplantation to cSCC was taken as the landmark time point. Kaplan Meier survival curves of the two groups were displayed in Figure 4. The log-rank test demonstrates a significant difference between the two groups and the mortality risk of the group with skin cancer is 1.51-fold greater than its counterpart.
Since induction drugs of OKT3 and daclizumab are not used currently, additional analysis without these two drugs was conducted. The analysis followed the same procedure as described in the Statistical Analysis section. The multivariate model excluding OKT3 and daclizumab was given in Table 6, which had six variables, including age, sex, HLA mismatch level, race, diagnosis at listing, and malignancy at listing. None of the rest of the induction drugs were significant and selected in the multivariate model. The AUCs for 5-year, 8-year, and 10-year post-transplant cSCC prediction were 0.79, 0.77, 0.77 respectively in the derivation set and 0.79, 0.76, 0.75 respectively in the validation set (Figure 5). Eliminating OKT3 and daclizumab slightly affected the AUCs (decreased by 0.01-0.02) in the validation set compared to the model with OKT3 and daclizumab. In addition, a new risk stratification model without OKT3 and daclizumab was developed, and the risk scores were given in Table 7. The scoring system without OKT3 and daclizumab divided patients into 4 risk groups: very low-risk group (score ≤ 5), low-risk group (score = 6), medium-risk group (score = 7), high-risk group (score ≥ 8). The predicted and observed probabilities of developing cSCC 5 years after transplant in different risk groups were shown in Figure 6, and the Kaplan Meier estimator of the cSCC-free survival curve was given in Figure 7. Further, log-rank test was done to compare the risk between different groups where patients were divided using the new scoring system, and significant differences were observed between every two groups (Table 8). The new stratification model without induction drugs provided comparable results to the model with OKT3 and daclizumab.
| Covariates | Hazard ratio (95%CI) | P value |
| Age | 1.068 (1.062-1.075) | < 0.001 |
| Female | 0.412 (0.344-0.494) | < 0.001 |
| HLA mismatch level | 0.948 (0.903-0.996) | 0.034 |
| Race | ||
| White | 1 | - |
| Black | 0.126 (0.060-0.265) | < 0.001 |
| Hispanic | 0.058 (0.033-0.102) | < 0.001 |
| Other | 0.228 (0.154-0.339) | < 0.001 |
| Diagnosis | ||
| Dilated myopathy | 1 | - |
| Restrictive myopathy | 1.897 (1.354-2.658) | < 0.001 |
| Heart re-transplant | 1.703 (1.226-2.366) | 0.002 |
| Coronary artery disease | 1.135 (0.927-1.389) | 0.219 |
| Hypertrophic myopathy | 1.589 (1.082-2.334) | 0.018 |
| Valvular heart disease | 1.156 (0.840-1.592) | 0.373 |
| Congenital heart defect | 1.098 (0.645-1.872) | 0.730 |
| Other | 1.329 (0.913-1.935) | 0.138 |
| Malignancy at listing | ||
| No | 1 | - |
| Yes | 1.589 (1.312-1.925) | < 0.001 |
| Unknown | 0.983 (0.666-1.449) | 0.930 |
| Covariates | Category | Score |
| Age | 18-40 | 0 |
| 40-60 | 1 | |
| > 60 | 2 | |
| Sex | Female | 0 |
| Male | 2 | |
| HLA mismatch level | > 5 | 0 |
| ≤ 5 | 1 | |
| Race | White | 2 |
| Other | 0 | |
| Diagnosis | Restrictive myopathy | 1 |
| Heart re-transplant | 1 | |
| Hypertrophic myopathy | 1 | |
| Other | 0 | |
| Malignancy at listing | No | 0 |
| Yes | 1 | |
| Unknown | 0 |
| Group | P value | Hazard ratio (95%CI) |
| Low vs very low | < 0.001 | 3.97 (3.51-4.50) |
| Medium vs very low | < 0.001 | 6.80 (5.86-7.90) |
| Medium vs low | < 0.001 | 1.70 (1.52-1.90) |
| High vs very low | < 0.001 | 10.1 (5.41-18.8) |
| High vs low | < 0.001 | 2.48 (1.78-3.47) |
| High vs medium | 0.003 | 1.41 (1.09-1.83) |
cSCC is a predominant skin malignancy among heart transplant recipients. Studies have been done to investigate the risk factors of post-transplant cSCC, but risk stratification and prediction have not been examined in the literature. This study conducted a retrospective study of the post-transplant event of cSCC for a large cohort of heart transplant patients in the UNOS registry and developed a risk score model to stratify patients into different risk groups.
In the univariate analysis, PRA against Class I and Class II antigens were identified as significant factors, but they were not significant in the multivariable analysis. Coronary artery disease was a risk factor in univariate analysis but was not selected in the multivariate model. The univariate analysis also identified congenital heart defect as a protective factor, but the observation did not hold up in multivariate analysis. The possible reason is that these two diseases are strongly correlated with patient age, thus the inclusion of age in the multivariate model eliminated the influence of these two diseases.
Eight predictors, including age, gender, HLA mismatch level, race, patient’s malignancy at listing, patient’s diagnosis at listing, induction therapy with OKT3 or daclizumab were selected in the final multivariate model. Among these predictors, older age, male sex, and white race have been previously reported as significant risk factors in many studies[3,8,9]. In addition, the multivariate model included the HLA mismatch level as a protective factor for cSCC, which is consistent with the observation in a recent study on the relationship between the HLA antigen mismatch level and the skin cancer incidence after heart and lung transplantation[10]. Heart re-transplant was identified as a significant risk factor as compared to dilated myopathy, which matches with a previous report that suggested re-transplant was a risk factor vs cardiomyopathy[11]. The multivariate model also showed that patients diagnosed with restrictive myopathy or hypertrophic myopathy before transplant had a higher risk of developing cSCC than patients who had other types of conditions. Recipients’ malignancy status is an indication of patients’ cancer history, which has been reported as a risk factor for skin cancer development in various studies[12,13], and was also identified as a risk factor for heart-transplant recipients in this study. In addition, the multivariate analysis revealed that induction therapy with OKT3 resulted in an increased incidence of cSCC, which is consistent with the observation reported in a previous study on a small cohort of heart transplant patients[3]. Our analysis also found that induction with daclizumab significantly (P value < 0.001) increased the risk of post-transplant cSCC.
The risk score separated patients into four risk groups (Figure 2), and the observed and predicted probabilities of developing cSCC 5 years after transplantation in very low-risk, low-risk, medium-risk, and high-risk groups were 0.017 vs 0.010, 0.077 vs 0.076, 0.142 vs 0.133 and 0.195 vs 0.195, respectively. The cumulative incidence probability of post-transplant cSCC was compared between different risk groups (Figure 3). For the high-risk group, the cumulative incidence rate increased significantly with respect to time. The one-, three-, and five-year incidence probabilities in the high-risk group were 0.03, 0.12, and 0.19, respectively. The significant differences in the cumulative incidence rates among different risk groups show the effectiveness of the proposed risk stratification model. Furthermore, cSCC greatly increased the mortality after heart transplantation with a hazard ratio of 1.51 (P value < 0.001) (Figure 4), which shows the importance of early screening and identification of cSCC among heart-transplant recipients.
The study has limitations which are discussed here. Firstly, this is a retrospective study using a single data source for the derivation and the validation cohorts. Missing data and poor data quality are generally recognized as drawbacks of retrospective studies. Thus, the results will need to be replicated in a separate patient population and ideally prospectively. Secondly, sunshine exposure has been identified as a risk factor for skin cancer but was not included in the current study. Ultraviolet exposure information such as latitude, average daily total global solar radiation, or patients' reports of previous sun exposure was used in many studies to assess the risk of ultraviolet exposure on skin cancer. However, it was previously reported that such information was not reliable biomarkers of ultraviolet radiation[9], and these data were not reported in the UNOS database.
In addition, the UNOS database contains missing and inaccurate reporting. Some posttransplant malignancy forms submitted to the Organ Procurement Transplant Network registry have been reported to be incomplete[9,14]. To minimize the possible bias due to incomplete reports, our analysis only used patient records with a clear indication of post-transplant malignancy status. That is, the records with unknown post-transplant malignancy status were excluded for the analysis.
In conclusion, this study developed a risk prediction model for post-transplant cSCC using a group of basic demographic and clinical parameters that can be estimated in every local center. The model provides a simple tool to aid clinical judgment for pre-transplant counseling and post-transplant health management. Identification of high-risk patients can facilitate the diagnosis of skin cancer in an early stage and potentially reduce morbidity and mortality after heart transplantation.
Heart transplant recipients are at higher risk of developing skin cancer than the general population due to the long-term immunosuppression treatment. Cancer has been reported as one of the major causes of morbidity and mortality for patients after heart transplantation.
Cutaneous squamous cell carcinoma (cSCC) is reported as the most common skin cancer in adult heart transplant recipients. This study was initiated to develop a risk stratification model using the United Network for Organ Sharing database in order to identify important risk factors and predict post-transplant incidence of cSCC. Among the different types of skin cancers, cSCC is the most common type of cancer. Timely screening and better management would help in prevention of long-term complications.
To identify risk factors and predict the incidence of cSCC for heart transplant recipients. Develop a risk prediction model for cSCC.
The whole dataset was randomly divided into a derivation set (80%) and a validation set (20%). Uni- and multivariate Cox regression were done to identify significant risk factors associated with the development of cSCC. Receiver operating characteristics curves were generated and area under the curve (AUC) was calculated to assess the accuracy of the prediction model.
Of the 23736 heart-transplant recipients in the database during the study period, 1827 were reported to have cSCC. Significant predictors of post-transplant cSCC were older age, male sex, white race, recipient and donor human leukocyte antigen mismatch level, malignancy at listing, a diagnosis of restrictive myopathy or hypertrophic myopathy, re-transplantation of the heart, and induction therapy with OKT3 or daclizumab. The multivariate model was used to predict the 5-, 8- and 10-year incidence of cSCC and respectively provided AUC of 0.79, 0.78, and 0.77 in the derivation set and 0.80, 0.78, and 0.77 in the validation set. The risk scoring system assigned each patient with a risk score within the range of 0-11. Based on the scores they were stratified into 4 different risk groups. The predicted and observed 5-year probability of developing cSCC match well among different risk groups. In addition, the log-rank test indicated significantly different cSCC-free survival across different groups.
A risk prediction model for cSCC among heart-transplant recipients has been generated for the first time. It offers a c-statistic of ≥ 0.77 in both derivation and validation sets.
Using a risk prediction score for screening of adult cardiac allograft recipients for early detection of cSCC can become a reality. The risk prediction score can be further validated in independent data sets in the future. Identification of risk factors is an important step towards the prevention of cSCC in this population.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: International Society for Heart and Lung Transplantation, No. 30681; and American College of Cardiology, No. 817696.
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carbone J, Greenway SC S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Jäämaa-Holmberg S, Salmela B, Lemström K, Pukkala E, Lommi J. Cancer incidence and mortality after heart transplantation - A population-based national cohort study. Acta Oncol. 2019;58:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Alam M, Brown RN, Silber DH, Mullen GM, Feldman DS, Oren RM, Yancy CW; Cardiac Transplant Research Database Group. Increased incidence and mortality associated with skin cancers after cardiac transplant. Am J Transplant. 2011;11:1488-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Molina BD, Leiro MG, Pulpón LA, Mirabet S, Yañez JF, Bonet LA, Vilchez FG, Delgado JF, Manito N, Rábago G, Arizón JM, Romero N, Roig E, Blasco T, Pascual D, de la Fuente L, Muñiz J. Incidence and risk factors for nonmelanoma skin cancer after heart transplantation. Transplant Proc. 2010;42:3001-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Brewer JD, Colegio OR, Phillips PK, Roenigk RK, Jacobs MA, Van de Beek D, Dierkhising RA, Kremers WK, McGregor CG, Otley CC. Incidence of and risk factors for skin cancer after heart transplant. Arch Dermatol. 2009;145:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Caforio AL, Fortina AB, Piaserico S, Alaibac M, Tona F, Feltrin G, Pompei E, Testolin L, Gambino A, Volta SD, Thiene G, Casarotto D, Peserico A. Skin cancer in heart transplant recipients: risk factor analysis and relevance of immunosuppressive therapy. Circulation. 2000;102:III222-III227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | España A, Redondo P, Fernández AL, Zabala M, Herreros J, Llorens R, Quintanilla E. Skin cancer in heart transplant recipients. J Am Acad Dermatol. 1995;32:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 940] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 8. | Alam M, Silber D, Mullen M, Brown R, Yancy C, Feldman D, Oren R, Kirklin J. Cutaneous malignancies after cardiac transplantation-risk factors and effect on late survival. J Heart Lung Transplant. 2005;24:S46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, Bibee K, Breithaupt A, Cannon J, Chen A, Cheng JY, Chiesa-Fuxench Z, Colegio OR, Curiel-Lewandrowski C, Del Guzzo CA, Disse M, Dowd M, Eilers R Jr, Ortiz AE, Morris C, Golden SK, Graves MS, Griffin JR, Hopkins RS, Huang CC, Bae GH, Jambusaria A, Jennings TA, Jiang SI, Karia PS, Khetarpal S, Kim C, Klintmalm G, Konicke K, Koyfman SA, Lam C, Lee P, Leitenberger JJ, Loh T, Lowenstein S, Madankumar R, Moreau JF, Nijhawan RI, Ochoa S, Olasz EB, Otchere E, Otley C, Oulton J, Patel PH, Patel VA, Prabhu AV, Pugliano-Mauro M, Schmults CD, Schram S, Shih AF, Shin T, Soon S, Soriano T, Srivastava D, Stein JA, Sternhell-Blackwell K, Taylor S, Vidimos A, Wu P, Zajdel N, Zelac D, Arron ST. Incidence of and Risk Factors for Skin Cancer in Organ Transplant Recipients in the United States. JAMA Dermatol. 2017;153:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 10. | Gao Y, Twigg AR, Hirose R, Roll GR, Nowacki AS, Maytin EV, Vidimos AT, Rajalingam R, Arron ST. Association of HLA Antigen Mismatch With Risk of Developing Skin Cancer After Solid-Organ Transplant. JAMA Dermatol. 2019;155:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Youn JC, Stehlik J, Wilk AR, Cherikh W, Kim IC, Park GH, Lund LH, Eisen HJ, Kim DY, Lee SK, Choi SW, Han S, Ryu KH, Kang SM, Kobashigawa JA. Temporal Trends of De Novo Malignancy Development After Heart Transplantation. J Am Coll Cardiol. 2018;71:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Kransdorf E, Patel J, Kittleson M, Czer L, Chang D, Dimbil S, Levine R, Hsu A, Davis T, Norland K, Trento A, Kobashigawa JA. Does a History of Malignancy Prior to Heart-transplant Increase Post-transplant Risk? J Heart Lung Transplant. 2018;37:S421. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Jambusaria-Pahlajani A, Crow LD, Lowenstein S, Garrett GL, Melcher ML, Chan AW, Boscardin J, Arron ST. Predicting skin cancer in organ transplant recipients: development of the SUNTRAC screening tool using data from a multicenter cohort study. Transpl Int. 2019;32:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Garrett GL, Yuan JT, Shin TM, Arron ST; Transplant Skin Cancer Network (TSCN). Validity of skin cancer malignancy reporting to the Organ Procurement Transplant Network: A cohort study. J Am Acad Dermatol. 2018;78:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |