Published online Dec 28, 2020. doi: 10.5500/wjt.v10.i12.392
Peer-review started: June 22, 2020
First decision: October 6, 2020
Revised: November 23, 2020
Accepted: December 8, 2020
Article in press: December 8, 2020
Published online: December 28, 2020
Processing time: 184 Days and 13 Hours
The aim of this minireview is to compare various pancreas transplantation exocrine drainage techniques i.e., bladder vs enteric. Both techniques have different difficulties and complications. Numerous comparisons have been made in the literature between exocrine drainage techniques throughout the history of pancreas transplantation, detailing complications and their impact on graft and patient survival. Specific emphasis has been made on the early postoperative management of these complications and the related surgical infections and their consequences. In light of the results, a number of bladder-drained pancreas grafts required conversion to enteric drainage. As a result of technical improvements, outcomes of the varied enteric exocrine drainage techniques (duodenojejunostomy, duodenoduodenostomy or gastric drainage) have also been discussed i.e., assessing specific risks vs benefits. Pancreatic exocrine secretions can be drained to the urinary or intestinal tracts. Until the late 1990s the bladder drainage technique was used in the majority of transplant centers due to ease of monitoring urine amylase and lipase levels for evaluation of possible rejection. Moreover, bladder drainage was associated at that time with fewer surgical complications, which in contrast to enteric drainage, could be managed with conservative therapies. Nowadays, the most commonly used technique for proper driving of exocrine pancreatic secretions is enteric drainage due to the high rate of urological and metabolic complications associated with bladder drainage. Of note, 10% to 40% of bladder-drained pancreata eventually required enteric conversion at no detriment to overall graft survival. Various surgical techniques were originally described using the small bowel for enteric anastomosis with Roux-en-Y loop or a direct side-to-side anastomosis. Despite the improvements in surgery, enteric drainage complication rates ranging from 2%-20% have been reported. Treatment depends on the presence of any associated complications and the condition of the patient. Intra-abdominal infection represents a potentially very serious problem. Up to 30% of deep wound infections are associated with an anastomotic leak. They can lead not only to high rates of graft loss, but also to substantial mortality. New modifications of established techniques are being developed, such as gastric or duodenal exocrine drainage. Duodenoduodenostomy is an interesting option, in which the pancreas is placed behind the right colon and is oriented cephalad. The main concern of this technique is the challenge of repairing the native duodenum when allograft pancreatectomy is necessary. Identification and prevention of technical failure remains the main objective for pancreas transplantation surgeons. In conclusion, despite numerous techniques to minimize exocrine pancreatic drainage complications e.g., leakage and infection, no universal technique has been standardized. A prospective study/registry analysis may resolve this.
Core Tip: A review of recent post-transplant complications regarding bladder drainage (urologic complications), enteric drainage (leak), surgical infections and abdominal compartment syndrome. Although safe and effective, bladder drainage brings metabolic and urologic complications; therefore, physiologic enteric drainage is preferred. Nevertheless, intra-abdominal infections and laparotomies arising from complications may result in significant graft loss. New modifications of established techniques are being developed, such as gastric or duodenal exocrine drainage. Donor-related factors, preservation injury, and surgical techniques should be managed to minimize adverse post-transplant events.
- Citation: Ferrer-Fàbrega J, Fernández-Cruz L. Exocrine drainage in pancreas transplantation: Complications and management. World J Transplant 2020; 10(12): 392-403
- URL: https://www.wjgnet.com/2220-3230/full/v10/i12/392.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i12.392
The first successful human pancreas transplant was performed on December 17, 1966 at the University of Minnesota[1]. Since then, a variety of pancreas transplant surgical techniques have evolved, as is evident from the wide ranging contributions provided by transplant groups throughout the world[2,3]. Regarding exocrine pancreas drainage, outcomes have improved notably since the first physiologic enteric procedures designed to divert pancreatic ductal secretions. Previously, drainage of exocrine secretions was via the gut or in the urinary bladder. The bladder drainage (BD) technique was used in most transplant centers until the late 1990s[3]. The duodenocystostomy contributed extraordinarily to the viability of pancreas transplants, as it allowed monitoring of pancreatic allograft function via urinary amylase measurements together with directed biopsy of duodenal mucosa and/or pancreas graft by cystoscopy[4-6] . Moreover, BD was associated at that time with fewer surgical complications when compared with enteric drainage[3]. However, this approach brings with it morbidity since it creates a nonphysiologic condition from which specific metabolic and urologic complications may arise. As a result of these common complications, the majority of centers now utilize enteric drainage, currently considered the optimal method in the management of pancreatic exocrine secretions[7]. Given the success of the enteric drainage, limited progress has thus been made in the field of BD in terms of novel surgical approaches. Notwithstanding, BD is still a considered alternative in selected cases when allograft duodenum viability is doubtful due to reperfusion injury, or an increased likelihood of acute rejection in the case of solitary pancreas transplantation[7].
Taking into account the historic importance of BD in pancreas transplantation, and because it remains a preferred option at specific centers, some immediate post-operative complications have been outlined below.
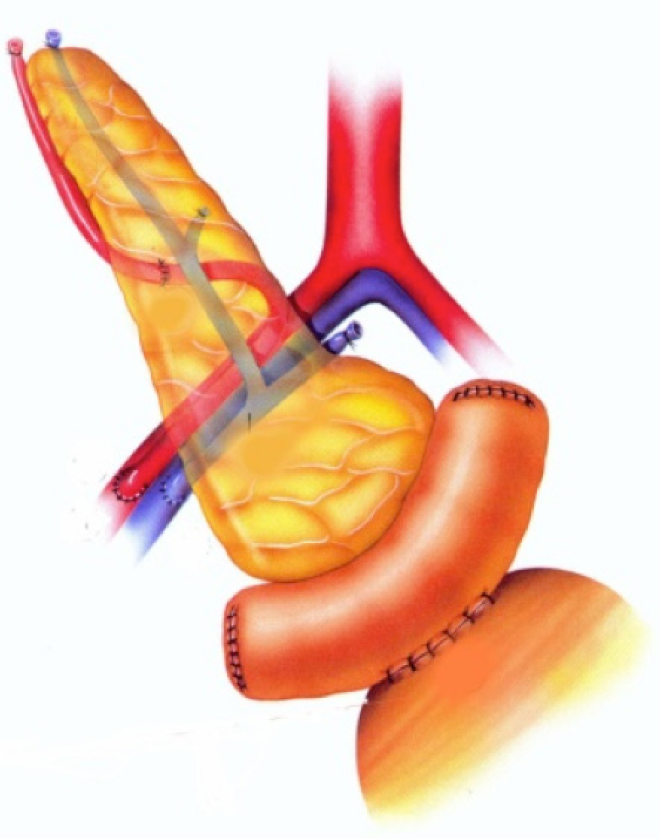
Using this technique, the graft duodenum is, in effect an exocrine conduit and is anastomosed to the bladder by the classic double-layer hand sewn technique (Figure 1) or using a circular stapler[8,9].
Consequently, metabolic issues arise from urinary loss of pancreatic juice, together with its alkaline content. In spite of most patients compensating with increased hydration and bicarbonate supplementation, hyperchloremic metabolic acidosis and dehydration may occur[6]. In an attempt to minimize protein and bicarbonate loss from the allograft duodenal mucosa, the length of donor duodenum transplanted with the pancreas has been progressively shortened over time[7].
In addition, urologic complications have been reported in the literature[6,10-18] including: Hematuria (16%); duodenal segment leaks (14%); reflux pancreatitis (11%); recurrent urinary tract infections (10%); urethritis (3%); and urethral stricture/disruption (3%). Also, abnormal pre-transplant urodynamic tests may increase the incidence of urologic complications[19]. Despite the high incidence of urologic complications, the patient and graft survival rates are not affected[3].
Regarding vesical bleeding, early post-transplant hematuria is frequent and is usually self-limited. This development is due to bladder surgical manipulation in addition to duodenal mucosal bleeding. In some recipients, with severe postoperative hematuria, management includes the institution of a continuous bladder irrigation regimen. If bleeding persists, cystoscopy is needed.
In contrast to enteric drainage, bladder drainage does not interfere with native bowel integrity. Consequently, graft leaks related to the latter generally have a decreased rate of life-threatening infectious complications and a less serious clinical course[3,7]. Nascent leaks (≤ 4 wk post-transplant) typically occur at the duodenocystostomy; while later leaks (> 4 wk post-transplant) usually originate from the donor duodenum, in some cases from the lateral duodenal staple line or when caused by ulcers, from the general area of the duodenal segment[18,20-21]. Initially, postoperative patients may complain of abdominal discomfort, or an asymptomatic rise in pancreatic enzymes may occur. Furthermore, urine production may decrease and serum creatinine levels may increase, although symptoms usually improve after Foley catheter placement. Low-pressure cystography or abdominal computed tomography (CT) with retrograde bladder contrast is used in the diagnosis of bladder-drained graft leaks. Extended bladder decompression is the treatment usually employed. This involves Foley catheterization together with percutaneous drainage of associated intraabdominal fluid collections (nascent leaks). High volume or infected postoperative leakage in bladder-drained recipients exhibiting peritonitis may need relaparotomy and surgical repair. In the case of significant compromise of the duodenal stump, a transplant pancreatectomy should be considered. Late-onset duodenocystostomy/duodenal segment leaks typically call for conversion from bladder to enteric drainage, regardless of etiology[21].
Sollinger et al[18] previously described a condition known as reflux pancreatitis, defined by the following criteria: (1) Rapid onset of lower abdominal pain situated around the pancreatic graft; (2) Increased serum amylase; (3) No leakage; (4) Pancreas edema, without evidence of abscess/fluid collection on computed tomography scan; and (5) 24 h resolution of symptoms following Foley catheter placement. Graft pancreatitis is most likely caused by reflux of urine into the pancreatic duct during the micturition high-pressure phase, when detrusor pressures exceed those existing in the pancreatic duct (10-12 cm water)[13,22]. Stephanian et al[23] speculated that this process may be exacerbated by sphincter of Oddi incompetence or through stagnation of exocrine secretions in a neuropathic bladder. As suggested by Fernandez-Cruz et al[24] the Oddi sphincter may be occluded secondary to rejection, and may result in enzyme release and graft pancreatitis. It has been reported that obstruction arising from calculous formation in the duodenal segment, could result in reflux pancreatitis[25]. Repeated episodes of reflux pancreatitis are possible, but in these cases an intensive search for a small leak has to be initiated. Therapy consisting of Foley drainage for several days is usually successful[18]. Antibiotics are indicated if urinary tract infection is present[26].
In diabetic patients, urinary tract infections are common, although most of these respond to appropriate antibiotic management. Contributing factors include prolonged catheter drainage, alkaline urine secondary to bicarbonate excretion from the exocrine pancreas, mucosal damage, and the presence of a diabetic neuropathic bladder with incomplete emptying[18]. Moreover, it has been previously documented that non-absorbable sutures and staples can be a focus for urinary calculi in bladder-drained pancreas transplantation[27]. Persistent urinary tract infections call for further study, such as cystoscopic examination with the view to excluding underlying lower urinary tract pathologies.
Other specific complications of BD such as urethritis, urethral strictures, and disruptions, are most likely due to activation of pancreatic enzymes in the bladder. It was speculated that a higher content of trypsinogen in the pancreatic secretions of certain recipients is responsible for this complication[18]. Despite urethral complications being largely restricted to male patients, they could also present in females[23]. They are initially manifested by pain and discomfort during urination. Initial conservative treatment consists of placement of a Foley catheter for several weeks. If symptoms recur, urethrogram and/or cystoscopy should be performed. Disruptions of the urethra with massive extravasation will respond with complete resolution after enteric drainage. Due to the above complications, conversion from bladder to enteric drainage may be deemed necessary[28-31]. The reported conversion rate from BD to enteric drainage ranges from 10% to 40%[28-38].
A recent study by Riad et al[39] looked into the link between enteric conversion and the resulting pancreas graft and patient outcomes by analyzing 593 recipients with bladder-drained pancreata, the majority of which received solitary transplants and 70 received simultaneous pancreas-kidney transplants. It was concluded that enteric conversion was associated with increased risk of rejection, but not graft loss or mortality. A longer interval from engraftment to conversion appears favorable. In addition, in a University of Wisconsin study[30], some 162 (41.9%) out of 386 bladder-drained pancreata eventually required enteric conversion, 29 (17.9%) within the first year, and without affecting the overall graft survival. In this series, there were no known exocrine content leaks, and the majority of the post-conversion surgical complications were managed conservatively or via percutaneous measures. Regrettably, in this setting, serious morbidity can occur[39] and consequently management of surgical complications can be challenging. In the case of enterovesical fistula formation described elsewhere[5,40], various options can be considered, including: Constructing a Roux-en-Y duodeno-enteric anastomosis in addition to bladder repair; donor duodenum-bladder reconnection, including resection and reanastomosis of the small bowel segment involved; or to perform a graft pancreatectomy. However, it is important to keep in mind that some factors (patient immunosuppressed status, the increased possibility of local contamination, and the elevated risk of duodenal segment ischemia) increase the surgical management failure rate, resulting in a graft pancreatectomy. All attempts should therefore be made to treat these patients conservatively.
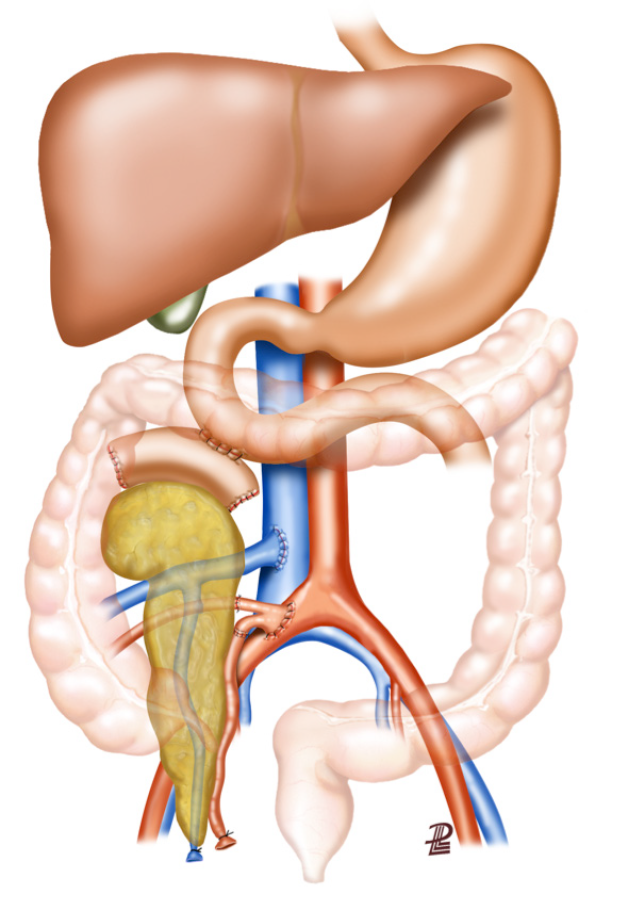
Various technical contributions in the search for the perfect technique for endogenous replacement of beta cell function and proper driving of exocrine secretions have been proposed. Despite leaks from the allograft duodenum having been reported in 5%-20% of bladder-drained and 5%-8% of bowel-drained pancreas transplants[41-43], some studies have shown no consistent differences in outcomes for bladder-drained or enteric-drained pancreas transplants with either portal or systemic venous drainage. In the modern era, pancreas transplantation with primary enteric exocrine drainage and systemic or portal venous anastomosis is performed in most cases[44]. A variety of techniques have been described using different small bowel sites for the enteric anastomosis[7,45]. Some groups prefer to use a Roux-en Y loop, while other surgeons choose direct anastomosis[2] (Figure 2). Most commonly, the duodenum-jejunum anastomosis is performed using a 2-layer, hand-sewn technique to create a hemostatic closure[46]. Although bleeding rates may be higher, techniques using either the circular or linear stapler were also described with the view to simplifying enteric anastomosis[47,48].
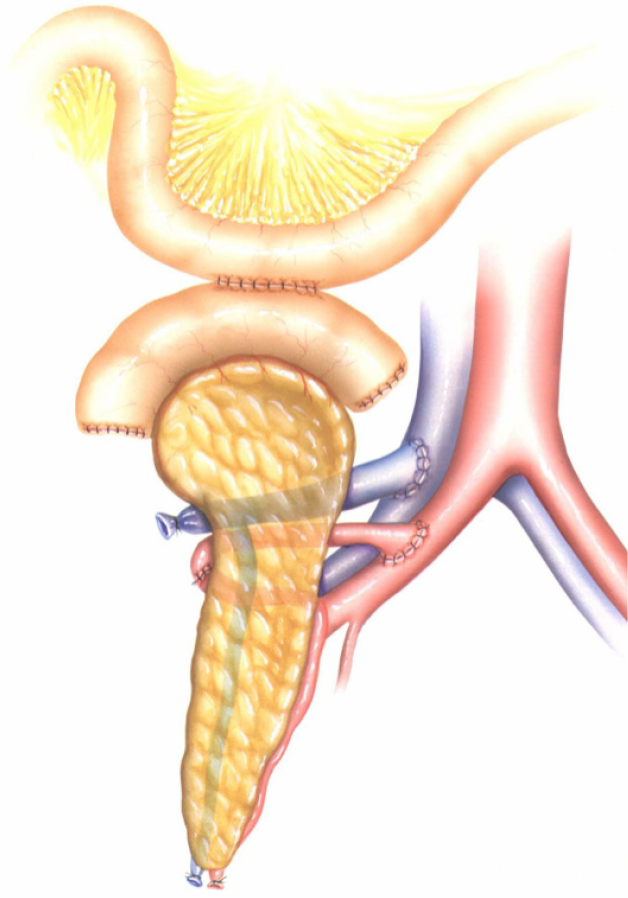
Newer techniques include drainage into native duodenum[49,50] and stomach[51]. Theoretically, the duodenoduodenostomy technique (Figure 3) (side-to-side anastomosis between the duodenal segment and the lower knee of the recipients’ duodenum with a 2.5- to 3.0-cm longitudinal duodenotomy) could facilitate intervention such as stenting of the pancreatic duct in cases of exocrine leakage among other advantages such as facilitating endoscopic access to the site of exocrine drainage for biopsies of the pancreas graft, when indicated[50].
The well tolerated gastric drainage procedure provides excellent patient and graft survival[3] as the third portion of the donor duodenum is anastomosed in two layers to the greater curvature of the stomach. Access to graft duodenum and pancreas via endoscopy is novel and straightforward[51].
A multitude of variations have arisen in the search for the ideal technique to address the problems associated with the management of pancreatic exocrine secretions. With increasing experience, the incidence of postoperative complications[3,52] (2%-10%) has notably reduced in recent years, with less than 1% of grafts lost due to this cause.
However, enteric leakage continues to be a significant source of morbidity[53]. Fistulas arising in the 3 mo following surgery are typically a result of ischemia or technical issues, while later fistulas usually arise from infections or acute rejection. This event represents the second cause of relaparotomy following hemorrhage[21].
Enteric leakage usually shares signs and symptoms characteristic of intestinal perforation, including abdominal pain, fever, nausea and vomiting, tachycardia, leukocytosis, peritonitis, or sepsis[53]. In unclear cases, radiographic imaging often provides confirmatory evidence, with CT imaging with oral contrast being the most useful. Findings may comprise intraperitoneal fluid, extraluminal air, and contrast extravasation[54].
Resulting treatment depends on the type of exocrine secretion derivation and the severity of the leak. In the present setting, early enteric leakage almost always requires relaparotomy with an anastomotic revision, with treatment depending on leakage extent and graft duodenum condition. When anastomosis is performed between the graft duodenum and the recipient jejunum, simple oversewing may be sufficient for small leaks with limited abdominal contamination in a patient hemodynamically stable. In the patient with a leak located adjacent to a staple line, performing a repair by limited duodenal resection with a GIA stapler could be indicated. Should part of the duodenum be compromised, the portion in question may be resected and the remaining duodenum shortened. If the original anastomosis has been performed in a side-to-side fashion, a Roux-en-Y limb may be created with the aim of diverting the intestinal stream away from the graft[53]. A total duodenectomy offers another management approach for duodenal leaks[55-57]. Having said this, these procedures are generally reserved for stable patients with limited abdominal contamination. On rare occasions, pancreatic head resection and subsequent duct-to-intestine anastomosis may be performed[58]. A graft pancreatectomy is the preferred choice in cases of: Significant leakage with sepsis or severe peritonitis; the presence of devitalized tissue; patient instability[59]. In one of the largest single-centre series, Sollinger et al[33], described a leakage rate of 5.7% in 610 enterically drained transplants, of which up to 50% resulted in pancreas graft loss. No consistent reports demonstrated the benefit of using octreotide in drained patients to promote fistula closure[60,61].
Interestingly, in 2005, Boggi et al[62] described a modified technique with portal-enteric drainage performed via a transperitoneal approach, with the pancreas graft placed into a fully retroperitoneal position. A duodenojejunostomy was performed side-by-side together with a Roux-en-Y jejunal limb. An advantage of “retroperitoneal” graft location behind the right colon is that, instead of causing peritonitis, potentially septic complications remain localized as peripancreatic fluid collections. In view of this, other surgeons have adopted this technique, employing a systemic venous drainage in the inferior vena cava[63,64].
Similarly, when duodenoduodenostomy is performed, the pancreas allograft is inserted vertically with the head upright and placed in a retrocolic position, behind the recipient´s bowel. In selected cases, this method can facilitate conservative management of an enteric leak, largely due to a decreased likelihood of intraperitoneal contamination. In allograft pancreatectomies, concerns persist regarding the safety of these maneuvers in the setting of local inflammation/sepsis in spite of recommendations for closure of the opening on the native duodenum[3]. Lindah et al[50], assessed safety profiles with duodenoduodenostomy vs duodenojejunostomy procedures. The percentage of anastomotic leakage was 5% and 4%, respectively. When a pancreatectomy is needed, adequate mobilization of the duodenum is mandatory with the aim of achieving a tension-free closure. To avoid stenosis the hole is closed transversely to the longitudinal duodenotomy using a single full-layer absorbable synthetic monofilament 4-0 suture (e.g., Biosyn, MonoPlus). There has been no need for duodenal exclusion or gastrectomy. In the series published by Walter et al[65], anastomotic insufficiency (1.6% vs 7%) and relaparotomy (41% vs 48%) occurred more frequently in the duodenojejunostomy group, whereas gastrointestinal bleeding (11% vs 3%) occurred more often in the duodenoduodenostomy group. The resulting hole in the recipient duodenum was, in all cases, initially treated using a transverse, two-layer, interrupted polydioxanone (3-0 or 4-0) suture. Following pancreatectomy and duodenum oversewing, three patients developed duodenal suture insufficiencies with consecutive duodenal leak, treated with a Roux-en-Y duodenojejunostomy, forming a side-to-side anastomosis. The third patient was treated conservatively with a longer-term intra-abdominal drain.
Currently, duodenoduodenostomy is a feasible and safe method i.e., equivalent to other classic techniques, extending the viability of anastomotic sites, especially in the case of re-transplanted recipients.
In an attempt to minimize early bowel leaks, special attention should be paid to the detection and control of potential risk factors for both donor and recipient i.e. those arising from donor hemodynamic instability, ischemia-reperfusion and preservation trauma (preservation solution/warm-cold ischemia), back-table preparation of the graft or other technical issues[21].
As demonstrated, leaks continue to be a persistent issue, especially as they represent a significant risk factor for intraabdominal abscesses, a third of which are associated with an anastomotic leak (duodenoenterostomy or duodenocystostomy)[21]. Infections are frequent in this group of transplant recipients, playing a central role in patient and graft survival, with diabetes, surgery, and immunosuppression as predisposing factors. Consistent with other studies reporting a proportion of patients affected by bacterial infections ranging from 51% to 95%[66-68], Bodro et al[69] demonstrated urinary tract infection as the most frequent (61%), followed by abdominal and surgical site infection.
Intra-abdominal infections represent a potentially serious problem, leading to not only high rates of graft loss, but substantial mortality[21,70]. Infections usually occur within 30 days of transplant, with bacterial etiology being more common than fungal abscesses. Intraabdominal infection risk factors comprise: Older donor age; retransplantation; pre-transplant peritoneal dialysis; extended preservation time; graft pancreatitis; and immunosuppression with sirolimus[21,71,72].
As treatment largely depends on the infection type (diffuse peritonitis vs localized abscess) any diagnostic processes must detail both the extent and nature of any infection. Equally, other vascular and intestinal complications must be ruled out. The clinical presentation of intra-abdominal infection is similar (and closely related) to enteric leakage. Abdominal CT with oral and iv contrast (for bladder-drained grafts, and retrograde bladder contrast also used to rule out leakage from the duodenovesical anastomosis) is the preferred choice for diagnosis.
Associated complications and the condition of the patient notably affect treatment decisions. A stable patient with a localized abscess can generally be treated with percutaneous fluid drainage. Relaparotomy is mandatory should conservative treatment fail, patients develop widespread infection, or recipients deteriorate or become clinically unstable[70]. Ideally, cultures are recommended in order to determine appropriate antimicrobial therapy. It should be noted that intra-abdominal infection might predispose to pseudoaneurysm, more so when in close proximity to the vascular anastomosis[53].
Moreover, a cytomegalovirus (CMV) infection risk of approximately 15% exists, mostly as a result of potent antilymphocyte drugs. CMV infection is often associated with increases in mortality, rejection rates, and late duodenal leaks[73]. Prophylaxis schemes (against bacterial, viral, and fungal infections), established from the moment of intervention by the transplant groups, have managed to reduce its incidence in the short term.
Although there is a lack of reported cases in the literature concerning abdominal compartment syndrome (ACS) in pancreas transplantation, aspects of abdominal compartment mechanics have been depicted in some publications regarding liver transplant and hepato-biliary-pancreatic (HBP) surgery[74-76]. Systemic inflammatory response syndrome and the toll of surgical trauma promote ACS, leading to renal and respiratory failure in addition to other risk factors[77]. The World Society of ACS defines intra-abdominal hypertension (IAH) as a sustained or repeated pathological elevation of intra-abdominal pressure (IAP) of 12 mmHg[78]. In these cases, raised IAH levels act to exacerbate decreases in visceral perfusion in hypotensive patients with visceral vasoconstriction[79].
IAH is especially worrying in cases of severe acute pancreatitis and for patients with post-operative abdominal morbidity. IAP monitoring is indicated for gravely ill patients, with management ideally following general principles and standard recommendations of other cases. Factors such as adequate analgesia, neuromuscular blockade and mechanical ventilation may promote abdominal compliance. Gastrointestinal distension can be mitigated through the use of promotility agents and nasogastric and colonic decompression. In refractory cases, drainage of intra-abdominal fluid collections is the initial invasive procedure to consider[80]. Negative pressure wound therapy has also been shown to be a safe treatment and can increase the chance for early fascial closure in septic patients. However, few data are available regarding the specific setting of transplantation[81].
As happens in other surgical scenarios, the implementation of robotic technology shows promise in the sense that it could decrease complications rates, thus improving the postoperative course of pancreas transplantation. Further study in this field is required to determine to what extent robotic pancreas transplantation could be beneficial for diabetic patients requiring beta-cell replacement[82-84].
Nowadays, the most commonly used technique for proper driving of exocrine pancreatic secretions is enteric drainage due to the high rate of urological and metabolic complications associated with bladder drainage. Despite numerous techniques to minimize exocrine pancreatic drainage complications e.g., leakage and infection, no universal technique has been standardized. A prospective study or large registry analysis may resolve this controversy.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ciancio G, Cimen SG, Montemurro N, Stratta RJ S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827-837. [PubMed] |
| 2. | Di Carlo V, Castoldi R, Cristallo M, Ferrari G, Socci C, Baldi A, Molteni B, Secchi A, Pozza G. Techniques of pancreas transplantation through the world: an IPITA Center survey. Transplant Proc. 1998;30:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Laftavi MR, Gruessner A, Gruessner R. Surgery of pancreas transplantation. Curr Opin Organ Transplant. 2017;22:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Sutherland DE, Gruessner RW, Gruessner AC. Pancreas transplantation for treatment of diabetes mellitus. World J Surg. 2001;25:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | West M, Gruessner AC, Metrakos P, Sutherland DE, Gruessner RW. Conversion from bladder to enteric drainage after pancreaticoduodenal transplantations. Surgery. 1998;124:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Sollinger HW, Odorico JS, Knechtle SJ, D'Alessandro AM, Kalayoglu M, Pirsch JD. Experience with 500 simultaneous pancreas-kidney transplants. Ann Surg. 1998;228:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | El-Hennawy H, Stratta RJ, Smith F. Exocrine drainage in vascularized pancreas transplantation in the new millennium. World J Transplant. 2016;6:255-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Pescovitz MD, Dunn DL, Sutherland DE. Use of the circular stapler in construction of the duodenoneocystostomy for drainage into the bladder in transplants involving the whole pancreas. Surg Gynecol Obstet. 1989;169:169-171. [PubMed] |
| 9. | Gruessner RW. Recipient procedures. In: Gruessner RW, Sutherland DE. Transplantation of the pancreas. New York: Springer-Verlag Inc, 2004: 150-178. [DOI] [Full Text] |
| 10. | Torigian DA, Banner MP, Ramchandani P. Imaging urologic complications of pancreas transplantation with bladder drainage. Clin Imaging. 2000;24:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Stratta RJ, Sindhi R, Sudan D, Jerius JT, Radio SJ. Duodenal segment complications in vascularized pancreas transplantation. J Gastrointest Surg. 1997;1:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Henry ML, Elkhammas EA, Bumgardner GL, Pelletier RP, Ferguson RM. Outcome of 300 consecutive pancreas-kidney transplants. Transplant Proc. 1998;30:291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Del Pizzo JJ, Jacobs SC, Bartlett ST, Sklar GN. Urological complications of bladder-drained pancreatic allografts. Br J Urol. 1998;81:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Gettman MT, Levy JB, Engen DE, Nehra A. Urological complications after kidney-pancreas transplantation. J Urol. 1998;159:38-42; discussion 42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Medina Polo J, Morales JM, Blanco M, Aguirre JF, Andrés A, Díaz R, Jiménez C, Leiva O, Meneu JC, Moreno E, Pamplona M, Passas J, Rodríguez A, de la Rosa F. Urological complications after simultaneous pancreas-kidney transplantation. Transplant Proc. 2009;41:2457-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Sudan D, Sudan R, Stratta R. Long-term outcome of simultaneous kidney-pancreas transplantation: analysis of 61 patients with more than 5 years follow-up. Transplantation. 2000;69:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Sollinger HW, Ploeg RJ, Eckhoff DE, Stegall MD, Isaacs R, Pirsch JD, D'Alessandro AM, Knechtle SJ, Kalayoglu M, Belzer FO. Two hundred consecutive simultaneous pancreas-kidney transplants with bladder drainage. Surgery. 1993;114:736-743; discussion 743. [PubMed] |
| 18. | Sollinger HW, Messing EM, Eckhoff DE, Pirsch JD, D'Alessandro AM, Kalayoglu M, Knechtle SJ, Hickey D, Belzer FO. Urological complications in 210 consecutive simultaneous pancreas-kidney transplants with bladder drainage. Ann Surg. 1993;218:561-8; discussion 568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Blanchet P, Droupy S, Eschwege P, Hammoudi Y, Durrbach A, Charpentier B, Benoit G. Urodynamic testing predicts long-term urological complications following simultaneous pancreas-kidney transplantation. Clin Transplant. 2003;17:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Nath DS, Gruessner A, Kandaswamy R, Gruessner RW, Sutherland DE, Humar A. Late anastomotic leaks in pancreas transplant recipients - clinical characteristics and predisposing factors. Clin Transplant. 2005;19:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Troppmann C. Surgical complications. In: Gruessner RWG, Sutherland DER. Pancreas transplantation. New York: Springer, 2004: 206-237. |
| 22. | deGroat WC, Booth AM. Physiology of the urinary bladder and urethra. Ann Intern Med. 1980;92:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Stephanian E, Gruessner RW, Brayman KL, Gores P, Dunn DL, Najarian JS, Sutherland DE. Conversion of exocrine secretions from bladder to enteric drainage in recipients of whole pancreaticoduodenal transplants. Ann Surg. 1992;216:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Fernandez-Cruz L, Sabater L, Gilabert R, Ricart MJ, Saenz A, Astudillo E. Native and graft pancreatitis following combined pancreas-renal transplantation. Br J Surg. 1993;80:1429-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Wengrovitz M, Jamwenko MV, Gifford RRM, Sechtin AG, Mandell MJ, Yang HC. Stone formation as a cause of allograft pancreatitis in the recipient of a combined kidney and pancreas transplant. Clin Transplant. 1990;4:117-119. |
| 26. | Hickey DP, Bakthavatsalam R, Bannon CA, O'Malley K, Corr J, Little DM. Urological complications of pancreatic transplantation. J Urol. 1997;157:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Hakim NS, Gruessner AC, Papalois BE, Troppmann C, Dunn DL, Sutherland DE, Gruessner RW. Duodenal complications in bladder-drained pancreas transplantation. Surgery. 1997;121:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Gruessner RW, Stephanian E, Dunn DL, Gruessner AC, Najarian JS, Sutherland DE. Cystoenteric conversion after whole pancreaticoduodenal transplantation: indications, risk factors, and outcome. Transplant Proc. 1993;25:1179-1181. [PubMed] |
| 29. | Senaratne NV, Norris JM. Bladder vs enteric drainage following pancreatic transplantation: How best to support graft survival? Int J Surg. 2015;22:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Adler JT, Zaborek N, Redfield RR 3rd, Kaufman DB, Odorico JS, Sollinger HW. Enteric conversion after bladder-drained pancreas transplantation is not associated with worse allograft survival. Am J Transplant. 2019;19:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Choi JY, Jung JH, Kwon HW, Shin S, Kim YH, Han DJ. Does Enteric Conversion Affect Graft Survival After Pancreas Transplantation with Bladder Drainage? Ann Transplant. 2018;23:89-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Van der Werf WJ, Odorico JS, D'Alessandro AM, Knechtle SJ, Pirsch JD, Kalayoglu M, Sollinger HW. Enteric conversion of bladder-drained pancreas allografts: experience in 95 patients. Transplant Proc. 1998;30:441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Sollinger HW, Odorico JS, Becker YT, D'Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg. 2009;250:618-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 34. | Sindhi R, Stratta RJ, Lowell JA, Sudan D, Cushing KA, Castaldo P, Jerius JT. Experience with enteric conversion after pancreatic transplantation with bladder drainage. J Am Coll Surg. 1997;184:281-289. [PubMed] |
| 35. | Fernandez-Cruz L, Ricart MJ, Astudillo E, Sabater L, Fondevila C, Prados M. Enteric drainage as primary procedure and after cystoenteric conversion in whole pancreaticoduodenal transplantation. Transplant Proc. 1997;29:643-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | van de Linde P, van der Boog PJ, Baranski AG, de Fijter JW, Ringers J, Schaapherder AF. Pancreas transplantation: advantages of both enteric and bladder drainage combined in a two-step approach. Clin Transplant. 2006;20:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Kaplan AJ, Valente JF, First MR, Demmy AM, Munda R. Early operative intervention for urologic complications of kidney-pancreas transplantation. World J Surg. 1998;22:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Connolly EM, Baktavatsalam R, O'Malley K, Little DM, Hickey DP. Enteric conversion after bladder-drained pancreatic transplantation; a simple and safe salvage procedure. Eur J Surg. 2001;167:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Riad SM, Keys DO, Jackson S, Vakil V, Berglund D, Matas A, Finger EB, Kandaswamy R. Enteric Conversion of Bladder-drained Pancreas as a Predictor of Outcomes in Almost 600 Recipients at a Single Center. Transplant Direct. 2020;6:e550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Akateh C, Rajab A, Henry M, El-Hinnawi A. Enterovesical Fistula After Enteric Conversion of a Bladder-Drained Pancreatic Allograft: A Case Report. Exp Clin Transplant. 2019;17:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP, Gruessner AC, Najarian JS. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 427] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 42. | Sugitani A, Gritsch HA, Shapiro R, Bonham CA, Egidi MF, Corry RJ. Surgical complications in 123 consecutive pancreas transplant recipients: comparison of bladder and enteric drainage. Transplant Proc. 1998;30:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Corry RJ, Chakrabarti P, Shapiro R, Jordan ML, Scantlebury VP, Vivas CA. Comparison of enteric vs bladder drainage in pancreas transplantation. Transplant Proc. 2001;33:1647-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Siskind EJ, Amodu LI, Pinto S, Akerman M, Jonsson J, Molmenti EP, Ortiz J. Bladder Versus Enteric Drainage of Exocrine Secretions in Pancreas Transplantation: A Retrospective Analysis of the United Network for Organ Sharing Database. Pancreas. 2018;47:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Boggi U, Amorese G, Marchetti P. Surgical techniques for pancreas transplantation. Curr Opin Organ Transplant. 2010;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Rogers J, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Stratta RJ. Pancreas transplantation with portal venous drainage with an emphasis on technical aspects. Clin Transplant. 2014;28:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Fridell JA, Milgrom ML, Henson S, Pescovitz MD. Use of the end-to-end anastomotic circular stapler for creation of the duodenoenterostomy for enteric drainage of the pancreas allograft [corrected]. J Am Coll Surg. 2004;198:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Lam VW, Wong K, Hawthorne W, Ryan B, Lau H, Robertson P, Allen RD, Pleass H. The linear cutting stapler for enteric anastomosis: a new technique in pancreas transplantation. Transpl Int. 2006;19:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Ferrer J, Molina V, Rull R, López-Boado MÁ, Sánchez S, García R, Ricart MJ, Ventura-Aguiar P, García-Criado Á, Esmatjes E, Fuster J, Garcia-Valdecasas JC. Pancreas transplantation: Advantages of a retroperitoneal graft position. Cir Esp. 2017;95:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Lindahl JP, Horneland R, Nordheim E, Hartmann A, Aandahl EM, Grzyb K, Haugaa H, Kjøsen G, Åsberg A, Jenssen T. Outcomes in Pancreas Transplantation With Exocrine Drainage Through a Duodenoduodenostomy Versus Duodenojejunostomy. Am J Transplant. 2018;18:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Linhares MM, Beron RI, Gonzalez AM, Tarazona C, Salzedas A, Rangel EB, Sá JR, Melaragno C, Goldman SM, Souza MG, Sato NY, Matos D, Lopes-Filho GJ, Medina JO. Duodenum-stomach anastomosis: a new technique for exocrine drainage in pancreas transplantation. J Gastrointest Surg. 2012;16:1072-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Moya-Herraiz A, Muñoz-Bellvis L, Ferrer-Fábrega J, Manrique Municio A, Pérez-Daga JA, Muñoz-Casares C, Alarcó-Hernández A, Gómez-Gutiérrez M, Casanova-Rituerto D, Sanchez-Bueno F, Jimenez-Romero C, Fernández-Cruz Pérez L. Cooperative Study of the Spanish Pancreas Transplant Group (GETP): Surgical Complications. Cir Esp. 2015;93:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Goodman J, Becker YT. Pancreas surgical complications. Curr Opin Organ Transplant. 2009;14:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Lall CG, Sandrasegaran K, Maglinte DT, Fridell JA. Bowel complications seen on CT after pancreas transplantation with enteric drainage. AJR Am J Roentgenol. 2006;187:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Orsenigo E, Cristallo M, Socci C, Castoldi R, Fiorina P, Invernizzi L, Caldara R, Secchi A, Di Carlo V. Successful surgical salvage of pancreas allograft. Transplantation. 2003;75:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Uva PD, Villavicencio Fornaciari S, Giunippero AE, Cabrera IC, Casadei DH. Case report: pancreas graft with a duodenal complication rescued using total duodenectomy. Transplant Proc. 2014;46:3068-3071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Boggi U, Vistoli F, Del Chiaro M, Moretto C, Croce C, Signori S, D'Imporzano S, Amorese G, Campani D, Calabrese F, Capocasale E, Marchetti P. Total duodenectomy with enteric duct drainage: a rescue operation for duodenal complications occurring after pancreas transplantation. Am J Transplant. 2010;10:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Reddy MS, White SA, Jaques BC, Torpey N, Manas DM. Pancreas graft salvage using pancreatico-duodenectomy with enteric drainage. Am J Transplant. 2007;7:2422-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Al-Adra D, McGilvray I, Goldaracena N, Spetzler V, Laurence J, Norgate A, Marquez M, Greig P, Sapisochin G, Schiff J, Singh S, Selzner M, Cattral M. Preserving the Pancreas Graft: Outcomes of Surgical Repair of Duodenal Leaks in Enterically Drained Pancreas Allografts. Transplant Direct. 2017;3:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Hesse UJ, Meester D, Troisi R, Cathenis K, Lameire N, Hemptinne B. The use of low dose octreotide prophylaxis in pancreatic transplants with enteric drainage. Results of a prospective randomized single center trial. Clin Transplant 2005; 19: 299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Benedetti E, Coady NT, Asolati M, Dunn T, Stormoen BM, Bartholomew AM, Vasquez EM, Pollak R. A prospective randomized clinical trial of perioperative treatment with octreotide in pancreas transplantation. Am J Surg. 1998;175:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Boggi U, Vistoli F, Signori S, Del Chiaro M, Campatelli A, Amorese G, Marciano E, Coppelli A, Tregnaghi C, Rizzo G, Marchetti P, Mosca F. A technique for retroperitoneal pancreas transplantation with portal-enteric drainage. Transplantation. 2005;79:1137-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Kahn J, Iberer F, Kniepeiss D, Duller D, Jakoby E, Tscheliessnigg K. Retroperitoneal pancreas transplantation with systemic-enteric drainage--case report. Clin Transplant. 2008;22:674-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Ono S, Kuroki T, Kitazato A, Adachi T, Chen YY, Chen SC, Eguchi S, Shyr YM, Wang SE. Simultaneous pancreas and kidney composite graft transplantation with retroperitoneal systemic-enteric drainage. Ann Transplant. 2014;19:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Walter M, Jazra M, Kykalos S, Kuehn P, Michalski S, Klein T, Wunsch A, Viebahn R, Schenker P. 125 Cases of duodenoduodenostomy in pancreas transplantation: a single-centre experience of an alternative enteric drainage. Transpl Int. 2014;27:805-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Herrero-Martínez JM, Lumbreras C, Manrique A, San-Juan R, García-Reyne A, López-Medrano F, Lizasoain M, de Dios B, Andrés A, Jiménez C, Gutiérrez E, Moreno E, Aguado JM. Epidemiology, risk factors and impact on long-term pancreatic function of infection following pancreas-kidney transplantation. Clin Microbiol Infect. 2013;19:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Rostambeigi N, Kudva YC, John S, Mailankody S, Pedersen RA, Dean PG, Prieto M, Cosio FG, Kremers WK, Walker RC, Abraham RS, Stegall MD. Epidemiology of infections requiring hospitalization during long-term follow-up of pancreas transplantation. Transplantation. 2010;89:1126-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Michalak G, Kwiatkowski A, Czerwinski J, Chmura A, Wszola M, Nosek R, Ostrowski K, Danielewicz R, Lisik W, Adadynski L, Małkowski P, Fesolowicz S, Bieniasz M, Kasprzyk T, Durlik M, Walaszewski J, Rowinski W. Surgical complications of simultaneous pancreas-kidney transplantation: a 16-year-experience at one center. Transplant Proc. 2005;37:3555-3557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Bodro M, Ferrer J, Ricart MJ, Sanclemente G, Linares L, Cervera C, Cofan F, Ventura-Aguiar P, Lopez-Boado MÁ, Marco F, Fuster J, García-Valdecasas JC, Moreno A. Epidemiology, risk factors, and impact of bacterial infections on outcomes for pancreatic grafts. Clin Transplant. 2018;32:e13333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant. 2010;15:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Troppmann C, Pierce JL, Gandhi MM, Gallay BJ, McVicar JP, Perez RV. Higher surgical wound complication rates with sirolimus immunosuppression after kidney transplantation: a matched-pair pilot study. Transplantation. 2003;76:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Humar A, Kandaswamy R, Drangstveit MB, Parr E, Gruessner AG, Sutherland DE. Prolonged preservation increases surgical complications after pancreas transplants. Surgery. 2000;127:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A; Transplantation Society International CMV Consensus Group. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 567] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 74. | Biancofiore G, Bindi ML, Romanelli AM, Boldrini A, Consani G, Bisà M, Filipponi F, Vagelli A, Mosca F. Intra-abdominal pressure monitoring in liver transplant recipients: a prospective study. Intensive Care Med. 2003;29:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Kathemann S, Dohna-Schwake C, Paul A, Hoyer PF, Gerner P. Intraabdominal Pressure Monitoring in Pediatric Liver Transplant Recipients: A Useful Tool for the Detection of Abdominal Compartment Syndrome. Transplantation. 2012;94:665. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 76. | Bressan AK, Kirkpatrick AW, Ball CG. Abdominal intra-compartment syndrome - a non-hydraulic model of abdominal compartment syndrome due to post-hepatectomy hemorrhage in a man with a localized frozen abdomen due to extensive adhesions: a case report. J Med Case Rep. 2016;10:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Bressan AK, Ball CG. Intra-abdominal hypertension and abdominal compartment syndrome in acute pancreatitis, hepato-pancreato-biliary operations and liver transplantation. Anaesthesiol Intensive Ther. 2017;49:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, Sugrue M, Cheatham M, Ivatury R, Ball CG, Reintam Blaser A, Regli A, Balogh ZJ, D'Amours S, Debergh D, Kaplan M, Kimball E, Olvera C; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 856] [Article Influence: 71.3] [Reference Citation Analysis (2)] |
| 79. | Basu A. Elevated intra-abdominal pressure is a "second-hit". World J Surg. 2010;34:1144; author reply 1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Malbrain ML, Peeters Y, Wise R. The neglected role of abdominal compliance in organ-organ interactions. Crit Care. 2016;20:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Hobeika C, Allard MA, Bucur PO, Naili S, Sa Cunha A, Cherqui D, Castaing D, Adam R, Vibert E. Management of the Open Abdomen after Liver Transplantation. World J Surg. 2017;41:3199-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Boggi U, Signori S, Vistoli F, D'Imporzano S, Amorese G, Consani G, Guarracino F, Marchetti P, Focosi D, Mosca F. Laparoscopic robot-assisted pancreas transplantation: first world experience. Transplantation. 2012;93:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Tzvetanov I, D'Amico G, Bejarano-Pineda L, Benedetti E. Robotic-assisted pancreas transplantation: where are we today? Curr Opin Organ Transplant. 2014;19:80-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Yeh CC, Spaggiari M, Tzvetanov I, Oberholzer J. Robotic pancreas transplantation in a type 1 diabetic patient with morbid obesity: A case report. Medicine (Baltimore). 2017;96:e5847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |