Published online Nov 28, 2020. doi: 10.5500/wjt.v10.i11.330
Peer-review started: August 31, 2020
First decision: October 23, 2020
Revised: November 6, 2020
Accepted: November 17, 2020
Article in press: November 17, 2020
Published online: November 28, 2020
Processing time: 82 Days and 2.8 Hours
Extracellular vesicles (EVs) are a heterogenous group of nanosized, membrane-bound particles which are released by most cell types. They are known to play an essential role in cellular communication by way of their varied cargo which includes selectively enriched proteins, lipids, and nucleic acids. In the last two decades, wide-ranging evidence has established the involvement of EVs in the regulation of immunity, with EVs released by immune and non-immune cells shown to be capable of mediating immune stimulation or suppression and to drive inflammatory, autoimmune, and infectious disease pathology. More recently, studies have demonstrated the involvement of allograft-derived EVs in alloimmune responses following transplantation, with EVs shown to be capable of eliciting allograft rejection as well as promoting tolerance. These insights are necessitating the reassessment of standard paradigms of T cell alloimmunity. In this article, we explore the latest understanding of the impact of EVs on alloresponses following transplantation and we highlight the recent technological advances which have enabled the study of EVs in clinical transplantation. Furthermore, we discuss the rapid progress afoot in the development of EVs as novel therapeutic vehicles in clinical transplantation with particular focus on liver transplantation.
Core Tip: Extracellular vesicles (EVs) are key contributors to T cell alloimmunity through the transfer of major histocompatibility alloantigens to host antigen presenting cells (APCs) thereby initiating alloresponses and acute rejection. Strong circumstantial evidence suggests that under certain conditions EV-mediated cross-dressing of recipient APCs can also tolerance responses and allay allograft rejection–for instance in the context of liver transplantation. We anticipate improved mechanistic understanding of these processes will facilitate design of novel EV therapies in transplantation. A number of clinical trials assessing the safety and efficacy of EVs are underway. The substantial developments in engineered Good Manufacture Practices-grade EVs hold promise for novel EV-therapeutics in transplantation and beyond.
- Citation: Mastoridis S, Martinez-Llordella M, Sanchez-Fueyo A. Extracellular vesicles as mediators of alloimmunity and their therapeutic potential in liver transplantation. World J Transplant 2020; 10(11): 330-344
- URL: https://www.wjgnet.com/2220-3230/full/v10/i11/330.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i11.330
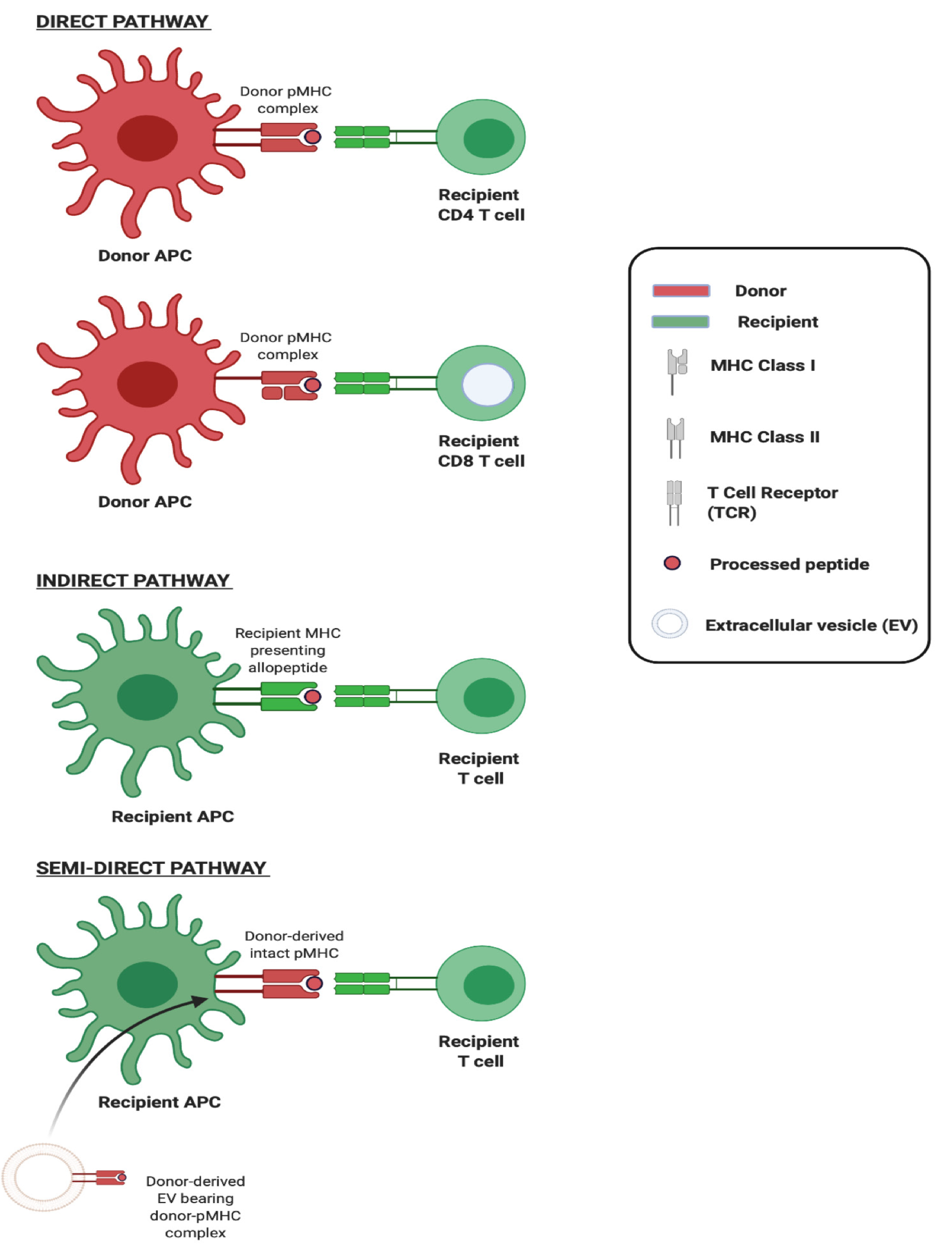
The adaptive immune response to an allograft is initiated upon activation of T lymphocytes recognising donor major histocompatibility (MHC) antigens principally via two distinct mechanisms which can occur concurrently but differ in the origin of antigen presenting cell (APC) and in their contribution to the alloresponse over time (Figure 1). The first of these, direct allorecognition, occurs without the need for antigen processing by APCs, and involves the interaction of recipient T cells with intact allogeneic MHC-peptide complexes (pMHC) displayed on the surface of transplanted cells. It has been widely accepted, until recently, that ‘passenger leukocytes’, dendritic cells (DCs) in particular, transported within transplanted tissues and trafficking to recipient secondary lymphoid organs (SLOs) are primarily responsible for triggering the recipient immune response via the direct pathway[1]. The second, indirect allorecognition, occurs upon recipient T cell recognition of processed donor peptides presented by recipient antigen presenting cells. Given that thymic selection of T cells is not directed either in favour or against any given non-self MHC, the frequency of T cells recognising intact allogeneic MHC can be as high as 10% of the total population and so the direct pathway is considered the driving force behind acute allograft rejection[2,3]. In contrast, the frequency of T cells exhibiting alloreactivity to any given allopeptide which is processed and subsequently presented by APCs is low (< 1/100000) and so, though this indirect pathway is less likely to be pivotal in acute rejection, there is circumstantial evidence of its role in governing alloantibody production and chronic rejection[4].
Recent studies have called into question the centrality of passenger leukocytes in the generation of the direct alloresponse following transplantation. Mounting data from both vascularised and non-vascularised animal models demonstrate that in the early post-transplant period few if any such cells are found in SLOs[5,6]. Rather, within hours of transplantation, a far greater number of recipient APCs carry intact allogeneic MHC on their surface capable of being presented directly, without further antigen processing, to cognate T cells. As we will show, recent work demonstrates that the presence of donor MHC on host-APCs is in large part attributable to extracellular vesicles (EVs) released by the allograft. Here, we review current understanding of the role of EVs in the transfer of donor MHC following transplantation, and we assess the impact on graft rejection and tolerance. Drawing on this, we go on to consider the potential of EVs as therapeutic vehicles in transplantation with reference to the significant progress afoot in this area of novel biotherapeutics.
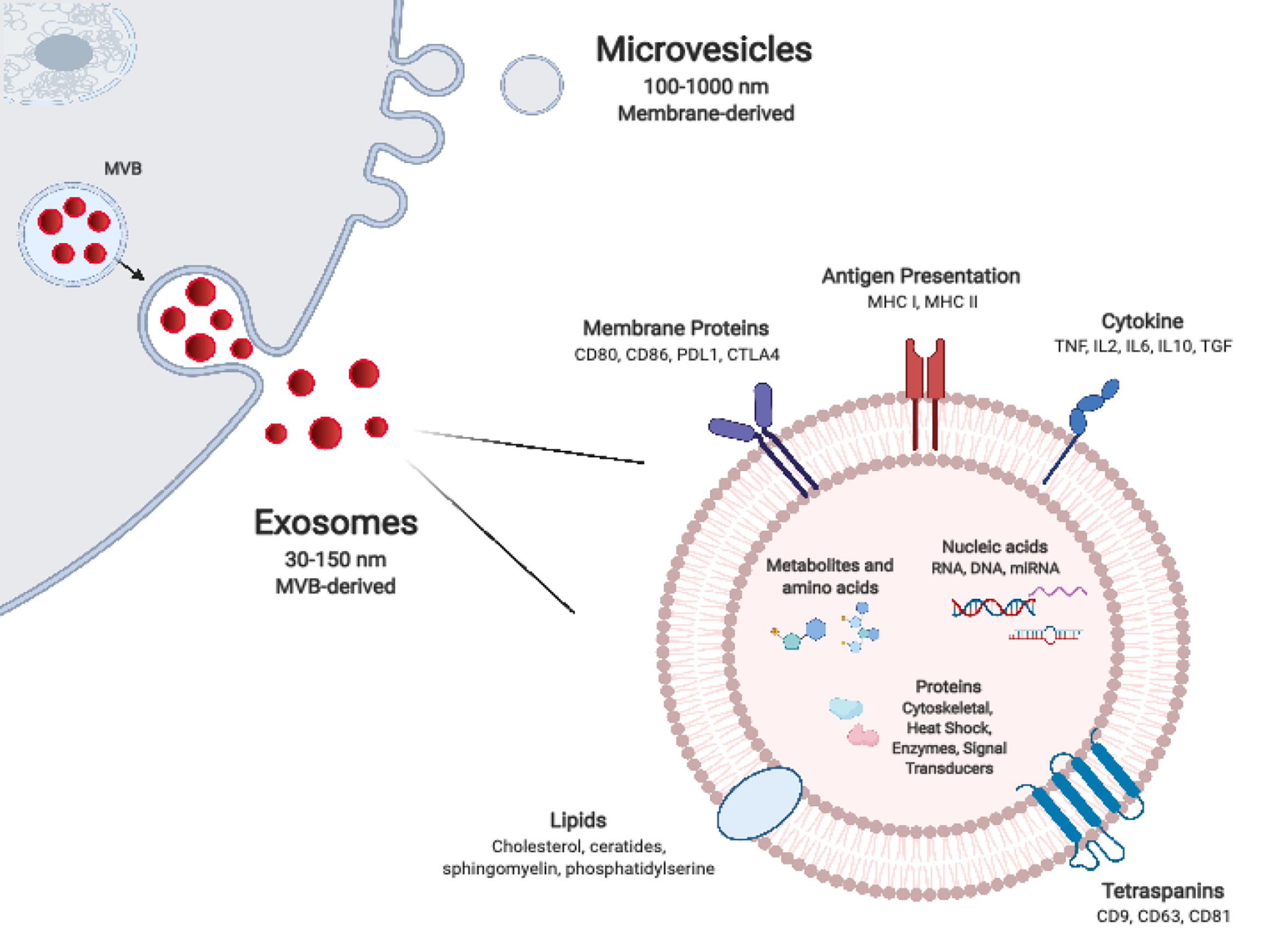
Most cells, including graft parenchymal, endothelial, and immune cells, release nanosized particles delimited by a lipid bilayer membrane which have come to be known collectively as EVs. Owing to their small size, durability, and capacity to transport a variety of biomolecules, EVs function as important mediators of intercellular communication, across a spectrum of tissues and biofluids. EV subtypes, have been categorised variably according to their particular mode of biogenesis, size, morphological characteristics, and/or cell of origin. With the expansion of tools and assays for their isolation, characterisation, and functional assessment, their classification and nomenclature continues to evolve[7-9]. Exosomes are the smallest of described EV subtype, with a diameter of 30-150 nm, and are formed within the lumens of multivesicular bodies (MVBs). The mechanisms responsible for their formation are now well understood and involve the Endosomal Sorting Complex Required for Transport (ESCRT), as well as ESCRT-independent mechanisms such as the tetraspanin family of proteins. The precise complement of these and other proteins likely affects the final composition of released exosomes (Figure 1). Microvesicles are larger, between 100-1000 nm in diameter, and form by pinching off directly from the plasma membrane. This outward budding is heavily dependent on the molecular composition of the plasma membrane. Apoptotic bodies, which tend to be larger still (up to 2000 nm in diameter), are also formed directly from the plasma membrane, however this occurs specifically at the time of apoptosis of the parental cell. Differences in their mode of biogenesis govern to a certain extent the size, cargo repertoire, and morphological features of EV subtypes. The repertoire of cargo of microvesicles is thought to reflect the parental cell of origin more closely than exosomes which undergo more selective enrichment. Though exosome and microvesicle biogenesis occurs at distinct sites within the cell and by different modes, in broad terms there is substantial overlap in the sorting machineries involved as well as in basic morphologic features such as their size and buoyant density. This can make isolation and distinction between them technically challenging[10-13]. In recent years, ‘omics’ analyses have revealed the diversity of the molecular composition of different EV subsets, of EVs released by different cells, and indeed of EVs release by single cells exposed to different environmental stimuli. Thus, the extensive repertoire of EV proteins, nucleic acids, and lipids is as much a reflection of the parental cell and its particular activation state as it is of the particular mode of EV biogenesis[14].
The exchange of molecules such as antigens and surface immunoglobulins between immune cells was first observed over four decades ago and, following this, the transfer of MHC complexes between leukocytes was described in 1974[15]. In the early 2000s, the acquisition of intact donor-derived allogeneic MHC by recipient APCs, DCs in particular, was described in the context of transplantation[16,17]. These ‘cross-dressed’ APCs, i.e. those host APCs noted to have acquired allogeneic MHC, were demonstrated to have the capacity to activate alloreactive T cells in vitro as well as in vivo, in what represented a novel, third pathway for alloantigen presentation which came to be known as the semi-direct pathway (Figure 2). Cross-dressing was at first understood to be dependent on cell-cell contact, occurring by a process of cell nibbling or trogocytosis. In pivotal work from groups including that of Raposo, it was however noted that among their surface protein cargo, EVs also carry intact MHC class I and class II as well as pMHC[18]. Though it was later established that this conferred to EVs the capacity to activate T cells directly, two seminal studies from 2016 also demonstrated EVs to be responsible for the transfer of intact allogeneic pMHC from the allograft to recipient APCs, and laid bare the biological relevance of this mode of cross-dressing in the generation of alloresponses[5,6].
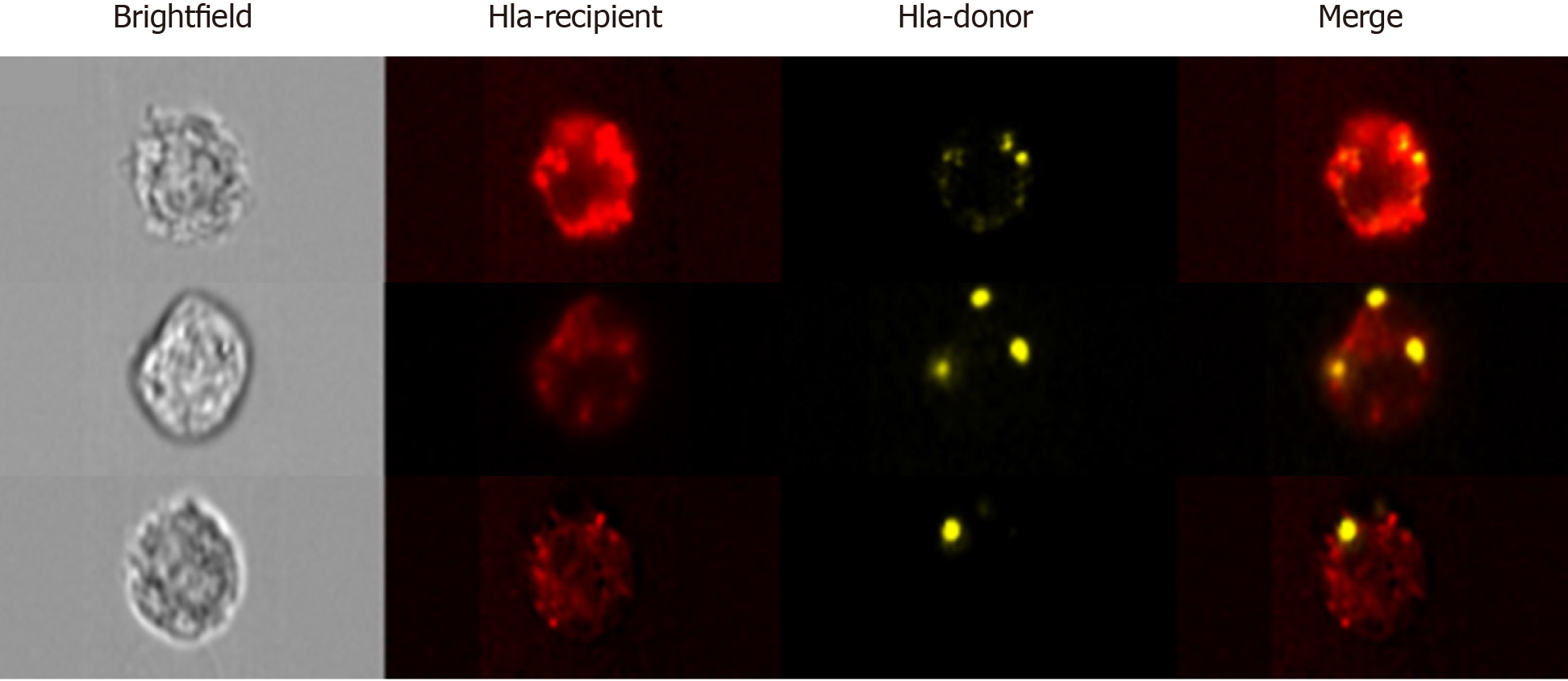
In first of these studies, Benichou and colleagues revisited the passenger leukocyte hypothesis in skin-grafted mice. Using highly sensitive cytometric, microscopic, and genotypic approaches, they confirmed the absence of donor leukocytes in recipient SLOs[6]. Considering that it typically takes 5 d or more for the neolymphangiogenesis required for passenger leukocyte trafficking to occur, the authors argue that it would be counterintuitive to expect this to be the mechanism responsible for the triggering of T cell alloresponses–often detectable within 48 h of transplantation. Rather than finding donor MHC present on passenger leukocytes, what the group observed upon examining recipient SLOs were large numbers of host APCs cross-dressed with donor MHC molecules. Using advanced imaging flow cytometry, a technique which permits the microscopic visualisation of fluorescently labelled flow-sorted single cells (Figure 3), the group were also able to determine that trafficking EVs were the likely source of graft-derived donor MHC. In the second of these reports from the same year, using a murine model of cardiac transplantation, Morelli and colleagues corroborated the paucity of passenger leukocytes in the period after transplantation, but also went a step further in affirming the ultra-structural mechanism of MHC transfer through their use of immuno-electron microscopy. This clearly demonstrated the way in which recipient APCs acquire donor MHC by capturing clusters of EVs bearing the characteristic marker CD63[5].
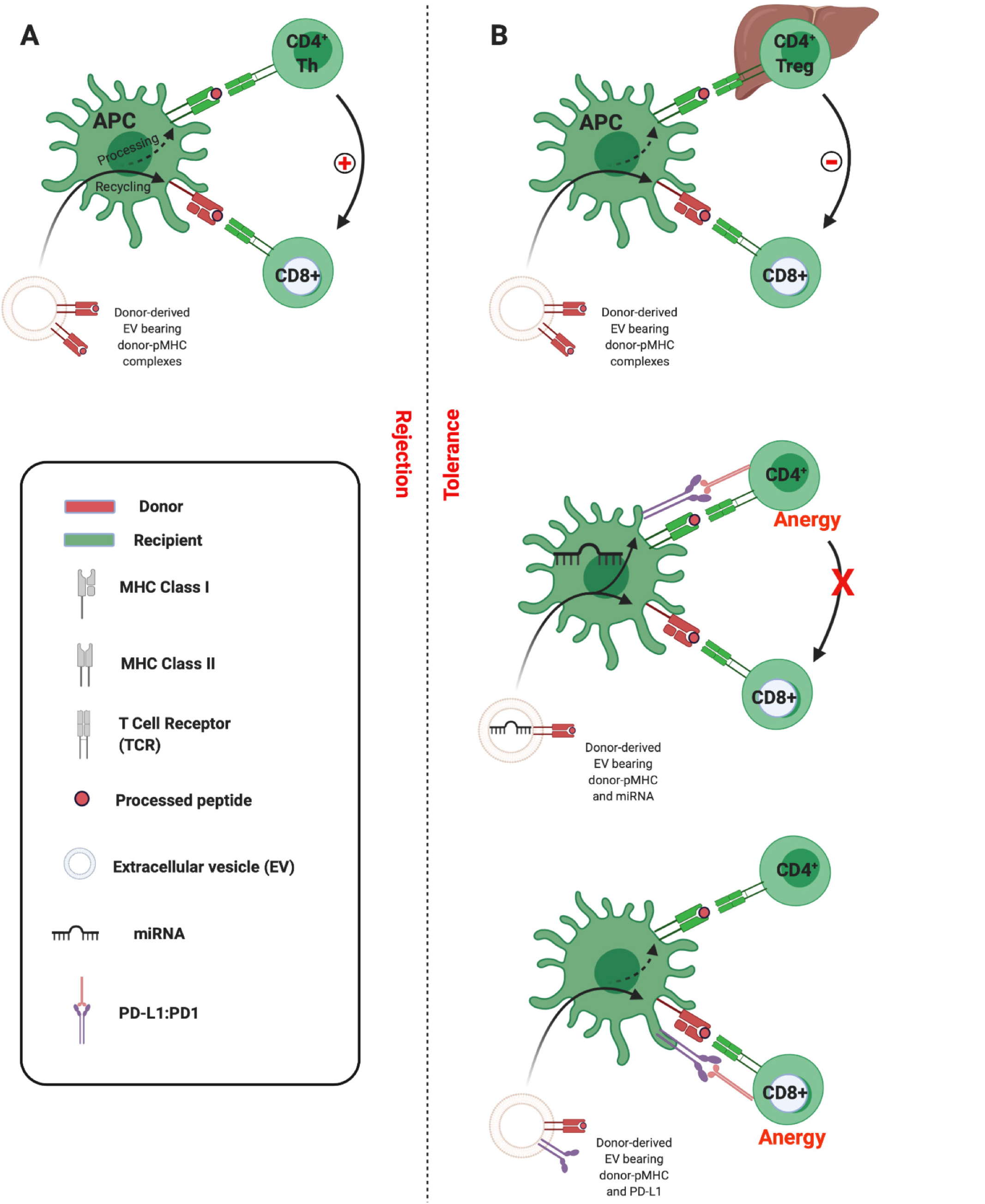
Having confirmed the route of allo-pMHC transfer to recipient SLOs, the researchers went on to demonstrate the centrality of cross-dressed APCs in initiating the alloresponses leading to acute allograft rejection. Flow-sorted conventional DCs cross-dressed by donor EVs were isolated and shown to be capable of the semi-direct priming of alloreactive CD8 T cells, as well as the indirect activation of naïve CD4 T cells in vitro (mixed lymphocyte reactions) and in vivo in mice[5]. These observations are in keeping with the ‘three-cell’ model proposed by Lechler and colleagues in 2004[16]. Adaptive CD8 T cell immunity is the principle arm of the cellular alloimmune response, but its development requires help. This can be provided by CD4 T cells that recognise alloantigen indirectly. According to the three-cell model, cross-dressed APC can indirectly prime an allospecific CD4 T cell which in turn can provide help for the semi-direct activation of CD8 T cells by the same APC (Figure 4A)[1,16]. Corroboration of the salience of crossed-dressed APCs as the main initiators of direct T cell allorecognition was provided when in vivo depletion of recipient DCs was shown to dramatically reduce alloreactive T cell priming and to delay acute rejection in murine heart transplantation[5,19]. Similarly, in skin-grafted mice, Smyth and colleagues show the acquisition of MHC by DCs to be the main source of alloantigen driving cytotoxic responses and alloimmunity[20].
Taken together, these studies in experimental animal models of vascularised and non-vascularised solid organ transplantation support the view that the release of EVs bearing donor MHC and its subsequent presentation by cross-dressed APCs triggers the T-cell alloresponses involved in acute rejection.
The pursuit of non-invasive biomarkers of allograft rejection led to the investigation of EVs from a range of biofluids, employing bulk analyses of their varied cargo, and yielding markers of varying specificity, sensitivity, and utility[21-23]. More recently, in order to achieve allograft-specificity, a number of researchers have turned to investigate EVs bearing donor-human lymphocyte antigen (HLA) in particular as biomarkers of allograft function. In 2016, Gunasekaran and colleagues demonstrated the presence of donor-derived EVs bearing donor HLA in the serum of two transplant recipients undergoing bronchiolitis obliterans syndrome; however, their presence was neither reported nor discussed among the control or acute rejection cohorts studied[24]. The following year, Kim et al[25] investigated the presence of donor-specific EVs bearing donor HLA in a single patient having undergone hand-transplantation[25]. Their data suggested that donor-EVs increased in the serum with worsening clinical rejection. However, this study was significantly limited in its small sample size, the lack of a control group, and its reliance on conventional flow cytometry–a method known to be incapable of detecting EVs less than 200 nm in size, which make up the bulk of EVs. In the same year, Vallabhajosyula and colleagues provided the first comprehensive demonstration of circulating EVs bearing donor HLA in patients having undergone islet transplantation[26]. Allograft-specific EVs bearing donor HLA class I were noted among all of the 5 study participants analysed at a single post-operative time-point. Though the impact of rejection on donor-derived EVs was demonstrated by the group in a murine model of islet transplantation, such analyses were not undertaken in their clinical cohort. EV characterisation was performed using nanoparticle tracking analysis (NTA) by NanoSight which, whilst enabling small EV detection well below the limits of cFCM, achieves only semi-quantitative enumeration of donor-HLA EVs.
These studies, which are among the first attempts to characterise circulating donor-specific EVs, demonstrate the major challenge in the field to find sensitive and robust technological platforms by which to study EVs on a vesicle-by-vesicle basis. This is particularly true for small EVs (sEVs) including exosomes and smaller microvesicles which are less than 200 nm in diameter. Techniques which permit sEV visualization, such as electron microscopy or atomic force microscopy, preclude the analysis of sEVs in large numbers and, in so doing, limit robust statistical assessments. Western blotting, lipidomics, proteomics, and flow cytometry of bead-captured vesicles are useful methods in the analysis of bulk isolates but are unable to distinguish variations in the number of vesicles from changes in molecular composition, and are incapable of multiparametric analysis of single sEVs[27]. Pioneering work, in particular by groups such as that of Lannigan and Erdbrügger, established the potential of imaging flow cytometry (iFCM) using ImageStreamx (ISx) (EMD Millipore) in the characterisation of sEVs. ISx has all the advantages of traditional flow cytometry, including high-throughput and multiparametric analysis, with the added value of providing a microscopic image of individual cells/particles upon which fluorescence can be overlayed (Figure 3)[28-31]. This is achieved using spatially registered charged camera coupled (CCD) which, unlike photomultiplier tubes found on cFCMs, exhibit the larger dynamic range and lower ‘noise’ required for accurate detection of small EVs. Furthermore, the advanced ISx fluidics enable the slower flow rates required for the avoidance of coincident detection of multiple sEVs.
In 2018, our group demonstrated the use of ISx in the multiparametric analysis of circulating small EV subtypes, including exosomes[27]. Furthermore, we set out to explore the utility of the approach in the detection and characterisation of circulating tissue/organ-specific sEVs. The EVs of 3 Liver allograft recipients’ circulating EVs were labelled with a pan-EV marker, a bona fide marker of exosomes (CD63), and probes for donor and recipient HLA. Donor-specific allograft-derived sEVs were confirmed to be detectable in circulation after liver transplantation. Further multiparametric analyses were employed to interrogate gated donor-sEVs for co-stimulatory/inhibitory molecules, thereby providing additional support for the application’s potential for characterisation and functional insights. In a study from 2020, we applied this approach to the detection of allograft-derived EVs in a larger cohort of liver or kidney transplant recipients[32]. Analyses of circulating cross-dressed cells and passenger leukocytes were also performed. We showed, for the first time, that cross-dressed recipient leukocytes can be found in the circulation following liver transplantation and that their numbers far exceed those of passenger leukocytes in keeping with the experimental animal models. The presence of circulating cross-dressed cells coincided with a rise in circulating allograft-derived sEVs in the early post-transplant period. This was a transient phenomenon, with numbers of both circulating donor-sEVs and cross-dressed cells rapidly waning and becoming undetectable by day 30 post-transplant. We speculate that, as shown in murine models, following clinical organ transplantation recipient APC cross-dressing continues to occur in the allograft and/or secondary lymphoid tissues for prolonged periods of time, and detection in circulation wanes[5,6,20,26,33]. For obvious reasons, corroboration of this in clinical contexts presents a challenge given limited availability of such tissues to perform detailed cross-dressing analyses upon. Employing in vitro functional analyses using human cells, we determined that DCs which had undergone EV-mediate MHC cross-dressing acquired the capacity to elicit the proliferation of syngeneic CD8 T cells.
In summary, developments in EV analytic approaches have, in recent years, enabled the description of the kinetics of donor-specific allograft-derived EV release following clinical transplantation, and evidenced the capacity for these to cross-dress recipient APCs through the transfer of donor MHC. Given the pre-eminence of cross-dressed cells in experimental and clinical transplantation and bearing in mind the recognised impact of these on alloresponse generation, it is likely important these pathways be considered when designing tolerance-promoting protocols.
In models of transplantation cross-dressing of APCs with allo-MHC is a highly immunogenic phenomenon. Several factors can govern the nature and magnitude of the immune response induced by any given antigen. The dose, the proximity of other signals, and the state of the presenting cell are among just a few factors which might influence whether the response is directed towards immunity or tolerance. The same might be expected of a given alloantigen transported upon EVs. Whether the alloresponse is directed towards rejection or tolerance might therefore depend on the quantity of EVs released from a given organ, cell of origin, vesicle subtype, other co-transported EV cargo, the state of the APC which acquires it, and the wider context within which the APC presents the antigen. One related consideration is the site at which cross-dressing occurs. While cross-dressed APCs have principally been observed within SLOs, cross-dressing has also been described within allografts themselves. Thus, in rodent models of islet and kidney transplantation, engagement of effector T cells with cross-dressed graft-infiltrating recipient DCs preceded rejection[34]. However, in a mouse model of spontaneous tolerance following MHC-mismatched liver transplantation, recipient DCs cross-dressed with donor EVs markedly suppressed host alloreactive responses[33]. In this model, crossed-dressed DCs constituted approximately 60% of the intrahepatic DC population, expressed high levels of Programmed Death-Ligand 1 (PD-L1), and induced an exhausted phenotype among donor-reactive CD8 T cells.
These studies also highlight the potential for different organs to produce qualitatively different EVs. The PD-1: PD-L1 axis has emerged as a critical inhibitory signalling pathway involved in the regulation of T cell responses and in the maintenance of peripheral tolerance[35]. PD-L1 is particularly highly expressed among liver parenchymal and non-parenchymal cells. It contributes to local protolerogenic pathways essential to the liver-which is seated at the crossroads between the portal venous system and the systemic circulation-to prevent the induction of immunity against innocuous antigens such as intestinal bacterial degradation products and neoantigens arising from metabolic processing[36]. Intrahepatic PD-L1 expression is upregulated following liver transplantation in both mice and humans and has been implicated in the establishment of liver allograft tolerance via inhibition of alloreactive T cell activation and induction of regulatory cell subtypes[33,37,38]. In our analysis of circulating sEVs following clinical liver transplantation, but not kidney transplantation, we observed that donor-derived sEVs carried significantly more PD-L1 than did sEVs of recipient origin. Furthermore, recipient cells which became cross-dressed also exhibited higher levels of PD-L1 than did recipient cells which had not been cross-dressed. PD-L1 was noted to co-localise on the APC surface with donor-HLA, which would be in support of their tandem transport on EVs though other groups have reported global upregulation of PD-L1 (potentially due to EV-miRNA transfer) following cross-dressing[39].
Work from the Burlingham laboratory expands further on the tolerogenic potential of EVs via the upregulation of PD-L1 on DCs. Their work focuses primarily on maternal microchimerism, whereby a tiny population of immune cells are transferred from mother to offspring during pregnancy and breastfeeding and result in the persistent detection of maternal cells throughout adult life[40]. These maternal cells contribute to the induction and maintenance of tolerance against non-inherited maternal antigens (NIMAs) which they bear, including MHC. For example, kidney grafts expressing NIMA-MHC will exhibit longer survival than grafts expressing unrelated MHC. The group demonstrate that the effects of such a small population of maternal cells are mediated and amplified by their avid production of EVs bearing NIMAs which subsequently are taken up by host DCs. The resultant cross-dressed DCs are noted to globally upregulate PD-L1, which the researchers suggest is due to co-transported EV-miRNA, and in doing so inducing NIMA-specific T cell anergy[39,40]. This is of added relevance to our discussion since the establishment of donor chimerism following liver transplantation in particular has long been recognised. Though its beneficial effects on outcome are widely acknowledged, the mechanisms underlying the pro-tolerogenic effect have remained uncertain[41,42].
It would appear then, that under certain circumstances allo-EVs promote tolerance while in others they drive rejection. The three-cell model described above offers a mechanistic framework by which to understand this apparent dichotomy. While allo-MHC transferred intact to an APC will activate CD8 effector T cells via the semi-direct pathway, the fate of processed peptides presented indirectly by the same APCs can result in the recruitment either of CD4 cells which will assist in the activation of the effector cell and drive rejection (Figure 4A), or of CD4 regulatory T cells (Tregs) which will inhibit effector cell activation and so promote tolerance (Figure 4B, upper panel)[43]. Proponents of this model would hold that the propensity towards Treg associations is determined by, for instance, the wider setting in which APC cross-dressing has occurred. In the liver, where there is high expression of molecules such as PD-L1 and anti-inflammatory cytokines such as interleukin (IL)-10, one might expect Treg recruitment to be more likely.
An alternative is that particular EVs are enriched in cargo capable, once transported to APCs, of contributing to the inhibition of T cells. As discussed, this could take the form of intact molecules transported in tandem or of nucleic acids which induce expression of regulatory molecules in recipient cells. Thus, Burlingham et. al. outline a scenario in which certain EVs (they suggest of maternal cell or of liver allograft origin) induce global PD-L1 expression in APCs via the co-transfer of miRNAs. This PD-L1 induces anergy of indirect pathway CD4 T cells, which then fail to help direct pathway CD8 T cells (Figure 4B, middle panel)[39]. In our analyses, we demonstrated that EVs derived from liver transplant recipients were able to transiently inhibit CD8 effector responses following uptake by DCs. Given that we observed allograft-derived EVs to be particularly enriched in PD-L1, and PD-L1 to colocalise with allo-MHC on the cross-dressed APC, it could be the case that effector cell inhibition was due to the proximity of intact, co-transported inhibitory signalling (Figure 4B, lower panel)[32]. These are not, it must be emphasized, mutually exclusive scenarios, and future work should delineate the contribution of both. An understanding of the factors that can tip the balance toward tolerance will likely be critical in the advancement of EV-based immunotherapeutics.
By virtue of their varied bioactive cargo, stability, capacity for tissue-specific targeting, ability to cross biological barriers, and safety profile, EVs have been identified as having significant therapeutic potential. There are currently over ten clinical trials in progress assessing the efficacy and safety of EV therapies[44]. Therapeutic EVs can broadly be subdivided into those derived from unmodified cellular subsets, and those which have been bioengineered.
EV-based therapeutics have, for the most part, turned to the utilisation of EVs derived from stem cell and regulatory cell subsets. Mesenchymal stem cells (MSCs) are among the earliest and most widely employed examples. MSCs were at first believed to mediate protective properties via their capacity to differentiate into and to replace injured tissue. For instance, following cardiac injury, delivered MSCs were understood to ameliorate damage by differentiate into healthy myocardium. However, it has recently been noted that the effects of MSCs are in large part due to their paracrine effects on surrounding tissues which, in part, are mediated by secreted EVs[45-48]. Since this discovery, the capacity for MSC-EVs to attenuate inflammation and to promote tissue regeneration has been demonstrated in pre-clinical models of respiratory, pancreatic, renal, musculoskeletal, neurological, and of liver diseases (reviewed elsewhere[49,50]). The use of MSC-EVs as an alternative to MSCs confers a number of potential advantages including the ability to cross biological barriers, target-specificity, avoidance of entrapment in microvascular beds, stability in storage, reduced potential for phenotypic alteration upon delivery, relatively lower immunogenicity and tumorigenicity, and improved safety profiles on repeated dosing.
Several experimental studies have demonstrated MSC-EVs to play a therapeutic role in liver ischaemia-reperfusion injury (IRI) through regenerative, autophagic, and immunomodulatory processes[51-54]. These rodent models employ variations of in vivo, in situ, vascular occlusion to replicate IRI. It remains to be seen what the impact of such therapies would be on the prolongation of allograft survival in models of liver transplantation. In the clinical context, ex-vivo machine perfusion of organs prior to transplantation under either normothermic (NMP) or hypothermic (HMP) conditions has improved assessment of organ viability, enabled the reconditioning of organs which might otherwise have been discarded, but also provided a platform upon which novel therapeutics can be developed and trialled. Very few studies have investigated the application of EVs in this context; though interest is growing rapidly. While studies have demonstrated beneficial effects of MSC-EVs in rodent models of lung and kidney perfusion, the first such demonstration in liver was by Rigo and colleagues in 2018[55-57]. Using a murine model of ex-vivo NMP, the group demonstrated the favourable outcomes in organs treated with human liver stem cell-derived EVs (HLSC-EVs), in terms of a reduction in histological damage and of enzyme markers of cytolysis. Several limitations are inherent in these studies including not performing onward transplantation to determine the effects on allograft outcomes, providing little mechanistic evidence of the mode by which EVs exert their effect or whether EVs of alternative origin would differ, and the lack of comprehensive uptake and dose-response analyses. Further investigation is warranted in experimental animal models, but it is also anticipated that trials will arise in perfused human organs with onward progression into phase I/II studies[58].
In addition to stem cell derived EVs, it is important to also mention Treg-derived EVs. Progress has been made in the implementation of adoptive Treg cell therapy in a number of scenarios which include type 1 diabetes, rheumatoid arthritis, inflammatory bowel disease, graft-versus-host disease (GvHD) following bone marrow transplantation (BMT), and organ transplant rejection[59,60]. Similar to MSCs, considerable barriers have been faced in the ex-vivo expansion of Treg, in maintaining their phenotypic characteristics once delivered, in delivering sufficient numbers particularly in the context of concomitant immunosuppressive therapies, in their oncogenic potential, and in their immunogenicity[61]. In their seminal paper, Okoye and colleagues showed Tregs to release large quantities of EVs carrying a distinct cargo of miRNA, and went on to demonstrate that blocking the release of these EVs abrogated the Tregs’ ability to suppress Th1 cell proliferation and thereby their immunoregulatory capacity[62]. These findings were independently reasserted by Aiello and colleagues, who also went on to demonstrate the capacity of Treg-EVs to prolong kidney allograft survival in vivo[63]. In recent months, Smyth and colleagues have shown the capacity for Treg-EVs to inhibit T effector cell responses, to affect changes in effector cell cytokine production via cargo miRNAs, and to protect against rejection in a humanised mouse skin transplant model[64].
Studies are lacking which aim specifically to investigate the tolerogenic potential in transplantation of therapeutically delivered EVs which serve to mediate APC cross-dressing. The recent work of Patel et al[65]. serves to demonstrate the potential of such an approach. Donor bone marrow derived EVs bearing allo-MHC were delivered in a non-human primate model of heart and kidney co-transplantation with prior conditioning by thymic irradiation, antithymocyte globulin, and immunosuppression. While design and sample size limit interpretations of functional outcomes, their data shows that delivered EVs are capable of generating stable cross-dressing. They suggest that such EVs might be used in place of whole bone marrow as a tolerance induction strategy and perhaps reduce the need for recipient conditioning[65]. We anticipate that similar approaches might prove more practicable through the development of engineered EVs enriched in specific desired molecules and alloantigens.
Broadly, there are two distinct approaches to selective EV cargo loading: (1) Exogenous, after EV isolation from the parent cell; and (2) Endogenous, during EV biogenesis[66]. Methods to achieve the former include techniques such as electroporation and sonication. Methods towards the latter involve exploiting the parent cell’s EV sorting machinery. Desired cargo can be directly transfected into the parent cell or can be engineered to be stably expressed. Fusion of the therapeutic of interest with molecules enriched in EVs will optimise its loading onto them. While examples of engineering approaches to endogenous EV loading and optimisation of delivery have been comprehensively outlined elsewhere[44], one particularly elegant example is that from Sutaria and colleagues who achieved the 65-fold increase of miRNA-199a-3p by associating its production to Lamp2a within the membrane of EVs produced by a HEK293T cell line[67]. Though no applications of engineered EVs have been reported in the literature with regards to liver IRI or tolerance induction, their recent implementation in diverse inflammatory, autoimmune, and oncological conditions, both in experimental models and in limited clinical trials (Table 1), demonstrate their potential.
| Treatment target | Trial phase | Source of EVs | EV manipulation | Results |
| Pancreatic cancer (NCT03608631) | Phase I | MSC, allogeneic | siRNA direct loading | Not yet recruiting |
| Colon cancer[72] | Phase I | Plant origin | Curcumin direct loading | Active |
| Melanoma[73] | Phase I | Immature DCs, autologous | Tumor antigen (peptide) direct loading | Safe, well tolerated, mixed responses. |
| Non-small cell lung cancer (NCT01159288) | Phase II | Mature DCs, autologous | Tumor antigen (peptide) direct loading | Safe, well tolerated, mixed responses. |
| Non-small cell lung cancer[74] | Phase I | Immature DCs, autologous | Tumor antigen (peptide) direct loading | Safe, well tolerated, mixed responses. |
| Malignant ascites (NCT01854866) | Phase II | Tumor derived | Chemotherapeutic agent loading | Unknown |
| Acute ischaemic stroke (NCT03384433) | Phase I/II | MSCs, allogeneic | miRNA loading | Completed |
Engineered EVs offer significant advantages over alternative synthetic drug delivery systems such as liposomes, nanocapsules, and micelles, which have often proven inefficient, poorly targeted, cytotoxic, and/or immunogenic. Nevertheless, widespread clinical utilisation of engineered EVs also faces a number of obstacles. Among these are: (1) The need for GMP-compliant up-scaling of production and isolation processes; (2) The better understanding of uptake kinetics, targeting, bioavailability, and dosing; and (3) The selection ofappropriate assays and biomarkers for the purpose of monitoring function. The significant progress underway in each of these areas has been reviewed elsewhere[44,68-71].
EVs have emerged as key contributors to T cell alloimmunity. Progress in the accurate identification and analysis of these nano-sized vesicles has confirmed their capacity to transport graft-derived alloantigen to recipient APCs in both experimental models of transplantation and in the clinical setting. While the consequence can be the initiation of strong inflammatory responses leading to acute graft rejection, it is possible in certain settings that tolerogenic responses are mediated and allograft injury allayed. EVs are emerging as potent therapeutic entities with innate potential for use as vehicles for the targeted delivery of small-molecule drugs, nucleic acid species, and therapeutic proteins including alloantigen. Improved understanding of their role in immune homeostasis, tolerance, and rejection, and optimised methods of production make it likely that EVs will serve diverse roles a future platform for biopharmaceuticals in transplantation and beyond.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dazzi F S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant. 2008;13:438-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 413] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352-358. [PubMed] |
| 4. | Zeng F, Morelli AE. Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin Immunopathol. 2018;40:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, Morelli AE. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126:2805-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 6. | Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, Abdi R, Uehara M, Kim JI, Markmann JF, Tocco G, Benichou G. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol. 2016;1:aaf8759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Witwer KW, Théry C. Extracellular vesicles or exosomes? J Extracell Vesicles. 2019;8:1648167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 390] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 8. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach J-M, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Zavec AB, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang Y-T, Chaudhuri AD, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, de Candia P, De Santana EF, De Wever O, del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Rubio APD, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, Andaloussi SE, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG-E, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano S-I, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers E-M, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee M-S, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim S-K, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Meehan KL, Mertens I, Minciacchi VR, Möller A, Jørgensen MM, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Hoen ENN-’, Hooten NN, O'Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostegaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Strandmann von EP, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PRM, Silva AM, Skowronek A, Snyder OL, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, Van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, Van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock ÅM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang J-Y, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7: :1–47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7692] [Article Influence: 1098.9] [Reference Citation Analysis (1)] |
| 9. | Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1880] [Cited by in RCA: 2049] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 10. | Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 11. | Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM, Nawaz M, Akbar N, Couch Y, Makin L, Cooke F, Vettore AL, Batista PX, Freezor R, Pezuk JA, Rosa-Fernandes L, Carreira ACO, Devitt A, Jacobs L, Silva IT, Coakley G, Nunes DN, Carter D, Palmisano G, Dias-Neto E. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 380] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 12. | Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 13. | Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113:E968-E977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2571] [Article Influence: 285.7] [Reference Citation Analysis (0)] |
| 14. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3959] [Cited by in RCA: 4127] [Article Influence: 412.7] [Reference Citation Analysis (0)] |
| 15. | Frelinger JA, Neiderhuber JE, David CS, Shreffler DC. Evidence for the expression of Ia (H-2-associated) antigens on thymus-derived lymphocytes. J Exp Med. 1974;140:1273-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 198] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828-4837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Smyth LA, Herrera OB, Golshayan D, Lombardi G, Lechler RI. A novel pathway of antigen presentation by dendritic and endothelial cells: Implications for allorecognition and infectious diseases. Transplantation. 2006;82:S15-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2293] [Cited by in RCA: 2691] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 19. | Harper SJ, Ali JM, Wlodek E, Negus MC, Harper IG, Chhabra M, Qureshi MS, Mallik M, Bolton E, Bradley JA, Pettigrew GJ. CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc Natl Acad Sci USA. 2015;112:12788-12793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Smyth LA, Lechler RI, Lombardi G. Continuous Acquisition of MHC:Peptide Complexes by Recipient Cells Contributes to the Generation of Anti-Graft CD8+ T Cell Immunity. Am J Transplant. 2017;17:60-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Gonzalez-Nolasco B, Wang M, Prunevieille A, Benichou G. Emerging role of exosomes in allorecognition and allograft rejection. Curr Opin Organ Transplant. 2018;23:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y, Shao Y, Zheng S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 449] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 23. | Morelli AE. Exosomes: From Cell Debris to Potential Biomarkers in Transplantation. Transplantation. 2017;101:2275-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Gunasekaran M, Xu Z, Nayak DK, Sharma M, Hachem R, Walia R, Bremner RM, Smith MA, Mohanakumar T. Donor-Derived Exosomes With Lung Self-Antigens in Human Lung Allograft Rejection. Am J Transplant. 2017;17:474-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Kim JY, Kelesidis T, Yang OO. Detection of Donor-Derived Microparticles in the Peripheral Blood of a Hand Transplant Recipient During Rejection. Transplant Direct. 2017;3:e131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Vallabhajosyula P, Korutla L, Habertheuer A, Yu M, Rostami S, Yuan CX, Reddy S, Liu C, Korutla V, Koeberlein B, Trofe-Clark J, Rickels MR, Naji A. Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue. J Clin Invest. 2017;127:1375-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 27. | Mastoridis S, Bertolino GM, Whitehouse G, Dazzi F, Sanchez-Fueyo A, Martinez-Llordella M. Multiparametric Analysis of Circulating Exosomes and Other Small Extracellular Vesicles by Advanced Imaging Flow Cytometry. Front Immunol. 2018;9:1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Erdbrügger U, Lannigan J. Analytical challenges of extracellular vesicle detection: A comparison of different techniques. Cytometry A. 2016;89:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 29. | Lannigan J, Erdbruegger U. Imaging flow cytometry for the characterization of extracellular vesicles. Methods. 2017;112:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Görgens A, Bremer M, Ferrer-Tur R, Murke F, Tertel T, Horn PA, Thalmann S, Welsh JA, Probst C, Guerin C, Boulanger CM, Jones JC, Hanenberg H, Erdbrügger U, Lannigan J, Ricklefs FL, El-Andaloussi S, Giebel B. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J Extracell Vesicles. 2019;8:1587567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 31. | Erdbrügger U, Rudy CK, Etter ME, Dryden KA, Yeager M, Klibanov AL, Lannigan J. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry A. 2014;85:756-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Mastoridis S, Londoño MC, Kurt A, Kodela E, Crespo E, Mason J, Bestard O, Martínez-Llordella M, Sánchez-Fueyo A. Impact of donor extracellular vesicle release on recipient cell "cross-dressing" following clinical liver and kidney transplantation. Am J Transplant. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, Yokota S, Stolz DB, Ross MA, Morelli AE, Geller DA, Thomson AW. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology. 2018;67:1499-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Hughes AD, Zhao D, Dai H, Abou-Daya KI, Tieu R, Rammal R, Williams AL, Landsittel DP, Shlomchik WD, Morelli AE, Oberbarnscheidt MH, Lakkis FG. Cross-dressed dendritic cells sustain effector T cell responses in islet and kidney allografts. J Clin Invest. 2020;130:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 36. | Mastoridis S, Martinez-Llordella M, Sanchez-Fueyo A. Immunotolerance in Liver Transplantation. Semin Liver Dis. 2017;37:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Shi XL, Mancham S, Hansen BE, de Knegt RJ, de Jonge J, van der Laan LJ, Rivadeneira F, Metselaar HJ, Kwekkeboom J. Counter-regulation of rejection activity against human liver grafts by donor PD-L1 and recipient PD-1 interaction. J Hepatol. 2016;64:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Morita M, Joyce D, Miller C, Fung JJ, Lu L, Qian S. Rejection triggers liver transplant tolerance: Involvement of mesenchyme-mediated immune control mechanisms in mice. Hepatology. 2015;62:915-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Lema DA, Burlingham WJ. Role of exosomes in tumour and transplant immune regulation. Scand J Immunol. 2019;90:e12807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Bracamonte-Baran W, Florentin J, Zhou Y, Jankowska-Gan E, Haynes WJ, Zhong W, Brennan TV, Dutta P, Claas FH, van Rood JJ, Burlingham WJ. Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance. Proc Natl Acad Sci USA. 2017;114:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS, Fung JJ. Chimerism after organ transplantation. Curr Opin Nephrol Hypertens. 1997;6:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Mahr B, Granofszky N, Muckenhuber M, Wekerle T. Transplantation Tolerance through Hematopoietic Chimerism: Progress and Challenges for Clinical Translation. Front Immunol. 2017;8:1762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Benichou G, Wang M, Ahrens K, Madsen JC. Extracellular vesicles in allograft rejection and tolerance. Cell Immunol. 2020;349:104063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 44. | Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev. 2020;S0169-409X(20)30024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 756] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 45. | Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1312] [Cited by in RCA: 1247] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 46. | Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 635] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 47. | Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 491] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 48. | Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 424] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 49. | Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front Cell Dev Biol. 2020;8:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 50. | Willis GR, Kourembanas S, Mitsialis SA. Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency. Front Cardiovasc Med. 2017;4:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 51. | Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y, Wu B, Wang Y, Ai K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 52. | Haga H, Yan IK, Borrelli DA, Matsuda A, Parasramka M, Shukla N, Lee DD, Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl. 2017;23:791-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 53. | Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, Li S, Li H, Chen L, He L, Chen H, Fu H, Zhang Q, Chen G, Yang Y, Zhang Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019;33:1695-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 54. | Yang B, Duan W, Wei L, Zhao Y, Han Z, Wang J, Wang M, Dai C, Zhang B, Chen D, Chen Z. Bone Marrow Mesenchymal Stem Cell-Derived Hepatocyte-Like Cell Exosomes Reduce Hepatic Ischemia/Reperfusion Injury by Enhancing Autophagy. Stem Cells Dev. 2020;29:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 55. | Gregorini M, Corradetti V, Pattonieri EF, Rocca C, Milanesi S, Peloso A, Canevari S, De Cecco L, Dugo M, Avanzini MA, Mantelli M, Maestri M, Esposito P, Bruno S, Libetta C, Dal Canton A, Rampino T. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J Cell Mol Med. 2017;21:3381-3393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Stone ML, Zhao Y, Robert Smith J, Weiss ML, Kron IL, Laubach VE, Sharma AK. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 57. | Rigo F, De Stefano N, Navarro-Tableros V, David E, Rizza G, Catalano G, Gilbo N, Maione F, Gonella F, Roggio D, Martini S, Patrono D, Salizzoni M, Camussi G, Romagnoli R. Extracellular Vesicles from Human Liver Stem Cells Reduce Injury in an Ex Vivo Normothermic Hypoxic Rat Liver Perfusion Model. Transplantation. 2018;102:e205-e210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Dengu F, Abbas SH, Ebeling G, Nasralla D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 59. | Bluestone JA, Tang Q. Treg cells-the next frontier of cell therapy. Science. 2018;362:154-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 60. | Romano M, Tung SL, Smyth LA, Lombardi G. Treg therapy in transplantation: a general overview. Transpl Int. 2017;30:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 61. | Mastoridis S, Issa F, Wood KJ. Novel biomarkers and functional assays to monitor cell-therapy-induced tolerance in organ transplantation. Curr Opin Organ Transplant. 2015;20:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 432] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 63. | Aiello S, Rocchetta F, Longaretti L, Faravelli S, Todeschini M, Cassis L, Pezzuto F, Tomasoni S, Azzollini N, Mister M, Mele C, Conti S, Breno M, Remuzzi G, Noris M, Benigni A. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci Rep. 2017;7:11518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 64. | Tung SL, Fanelli G, Matthews RI, Bazoer J, Letizia M, Vizcay-Barrena G, Faruqu FN, Philippeos C, Hannen R, Al-Jamal KT, Lombardi G, Smyth LA. Regulatory T Cell Extracellular Vesicles Modify T-Effector Cell Cytokine Production and Protect Against Human Skin Allograft Damage. Front Cell Dev Biol. 2020;8:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 65. | Patel PM, Gonzalez-Nolasco BA, Morrissette JA, Prunevieille A, Kaitlan AJ, O JM, Becerra DC, Costa T, Benichou G, Madsen JC. Inducing Donor MHC Chimerism with Bone Marrow Derived Exosomes in Non-Human Primates. J Heart Lung Transpl. 2020;39: :S88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 892] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 67. | Sutaria DS, Jiang J, Elgamal OA, Pomeroy SM, Badawi M, Zhu X, Pavlovicz R, Azevedo-Pouly ACP, Chalmers J, Li C, Phelps MA, Schmittgen TD. Low active loading of cargo into engineered extracellular vesicles results in inefficient miRNA mimic delivery. J Extracell Vesicles. 2017;6:1333882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. 2020;17:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 69. | de Jong OG, Kooijmans SAA, Murphy DE, Jiang L, Evers MJW, Sluijter JPG, Vader P, Schiffelers RM. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc Chem Res 2019; 52: 1761-1770. . [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 70. | Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11:eaav8521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 712] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 71. | Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 488] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 72. | Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 73. | Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 1006] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 74. | Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 678] [Cited by in RCA: 882] [Article Influence: 44.1] [Reference Citation Analysis (0)] |