Copyright
©The Author(s) 2020.
World J Transplant. Nov 28, 2020; 10(11): 330-344
Published online Nov 28, 2020. doi: 10.5500/wjt.v10.i11.330
Published online Nov 28, 2020. doi: 10.5500/wjt.v10.i11.330
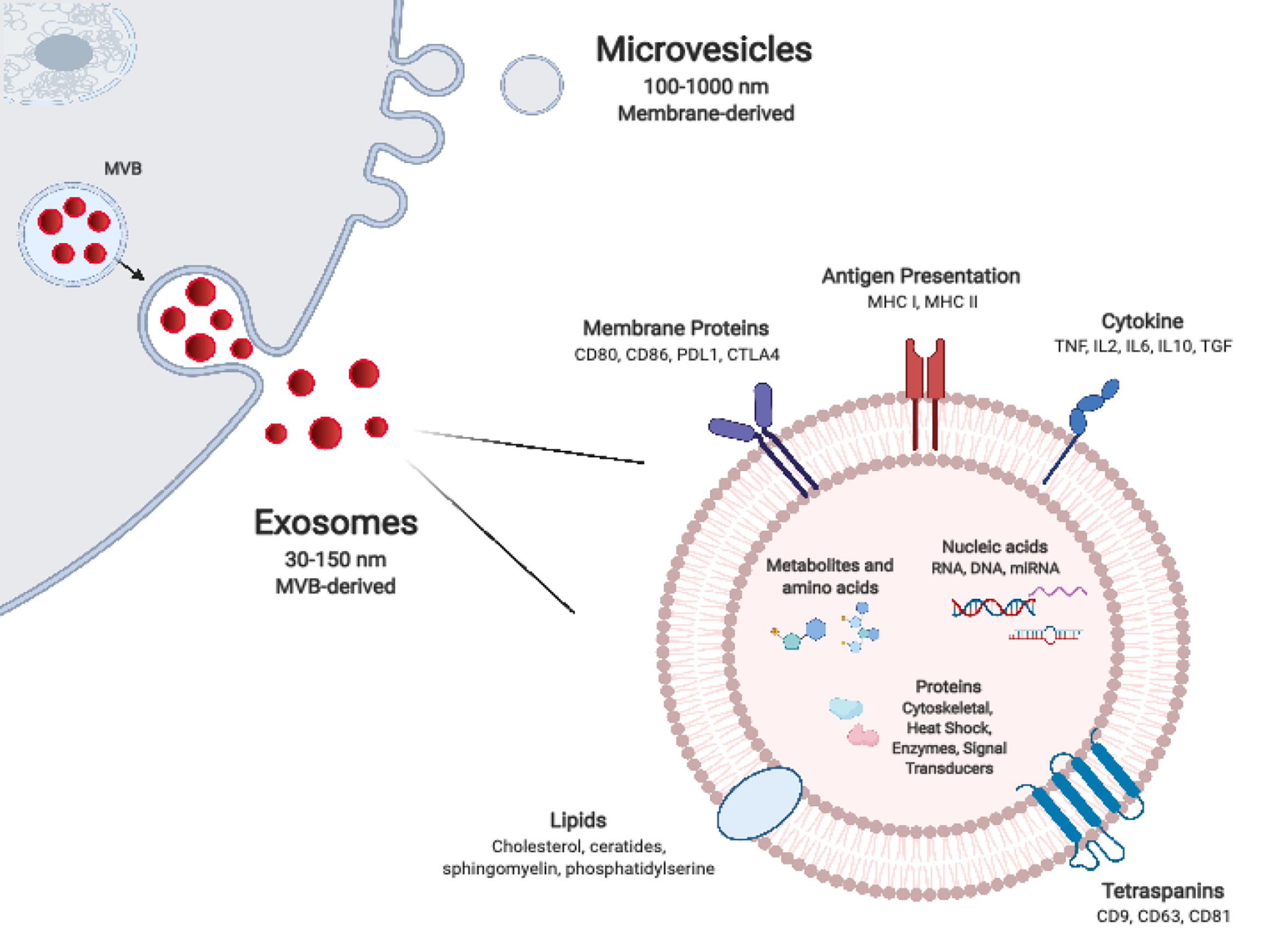
Figure 1 Extracellular vesicle biogenesis and composition.
Exosomes are generated by inward budding of endosomal membrane which result in the formation of intra-luminal vesicles (ILVs) within multivesicular bodies (MVB). ILVs are released from MVBs as exosomes upon MVB fusion with the plasma membrane. Exosomes are smaller and more uniform in size in comparison to microvesicles, which form by directly pinching-off from the plasma membrane. The molecular composition of extracellular vesicles, which includes nucleic acids, proteins, and lipids, is dependent on their particular mode of biogenesis in addition to their parental cell of origin and its activation state. MHC: Major histocompatibility; PDL1: Programmed Death-Ligand 1; TGF: Transforming growth factor; CTLA4: Cytotoxic lymphocyte antigen 4; MVB: Multivesicular bodies; IL: Interleukin.
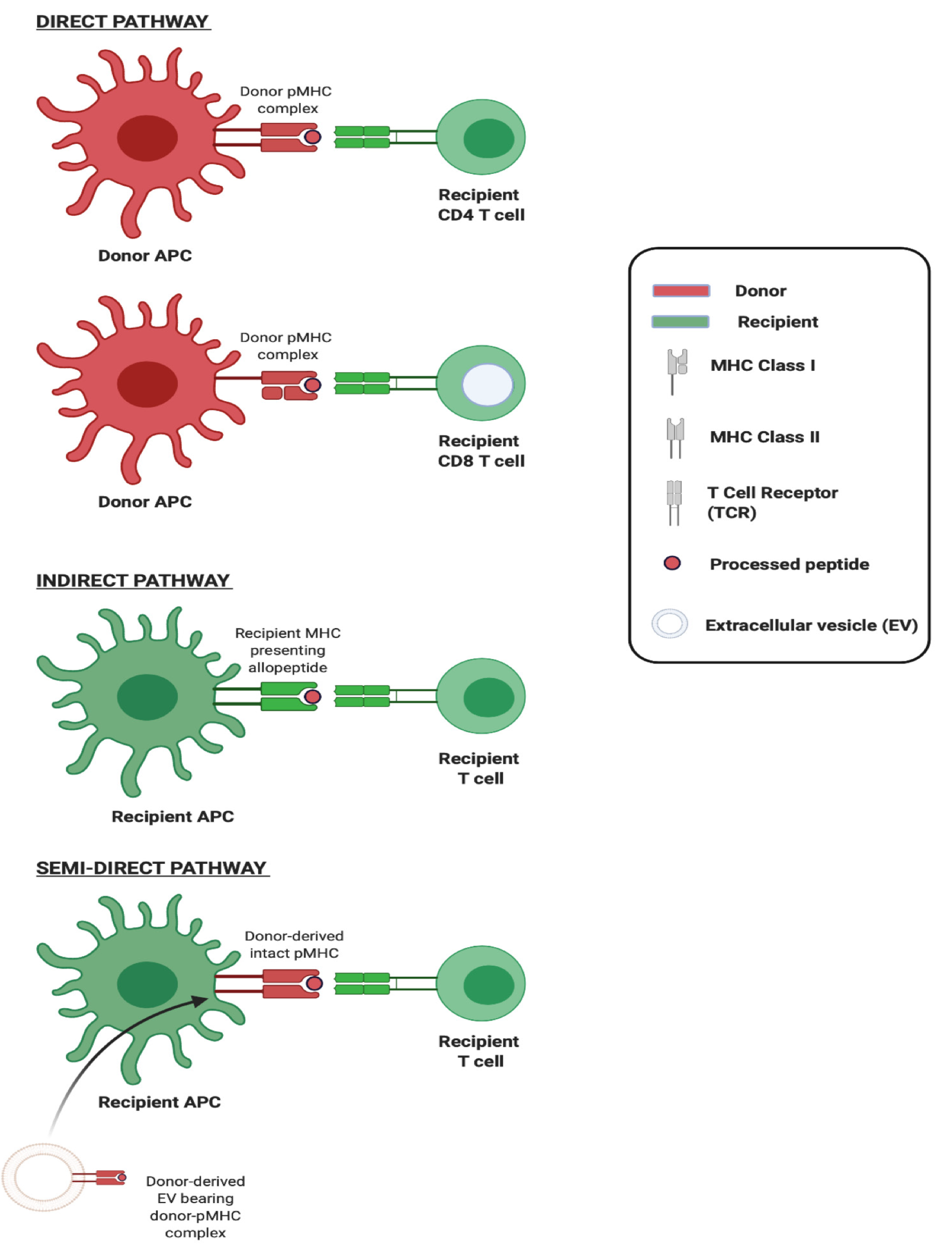
Figure 2 Three pathways of allorecognition.
Schematic illustration of the three major pathways of allorecognition: Direct, indirect, and semidirect. In the direct pathway, intact non-self major histocompatibility (MHC) Class I and Class II on donor antigen-presenting cells (APCs) activates CD8 and CD4 T cells respectively. In indirect recognition, recipient APCs present processed donor allogeneic peptides in the context of self-MHC to recipient T cells. In the semidirect pathway, recipient APCs are cross-dressed with donor MHC, acquired from donor-origin extracellular vesicles for instance, which upon encounter activates recipient T cells. Created with BioRender.com. APC: Antigen-presenting cell; MHC: Major histocompatibility; EV: Extracellular vesicle.
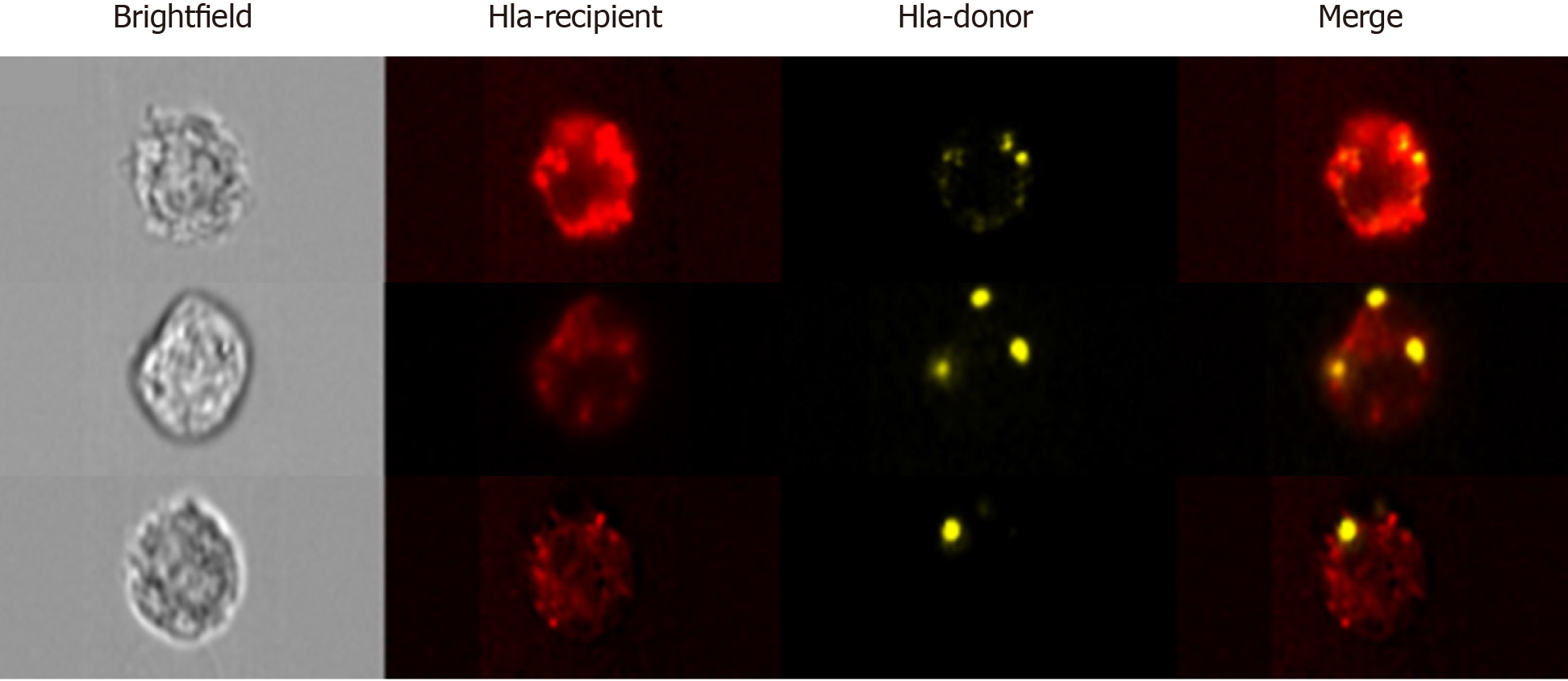
Figure 3 Advanced imaging flow cytometry by ImageStreamx.
Analysis by ImageStreamx (ISx) enables the accurate detection of particles of diameter as low as 20 nm, including small extracellular vesicles. Furthermore, the combination of microscopic imaging with fluorescence detection enables the morphometric and photometric assessment of whole cells. This is of particular utility in assessing major histocompatibility cross-dressing. Representative images acquired by ISx of three recipient cells [bearing recipient human lymphocyte antigen (HLA), red] cross-dressed with donor-HLA (yellow) following liver transplantation. The discrete foci of donor alloantigen point to the vesicular nature of transfer.
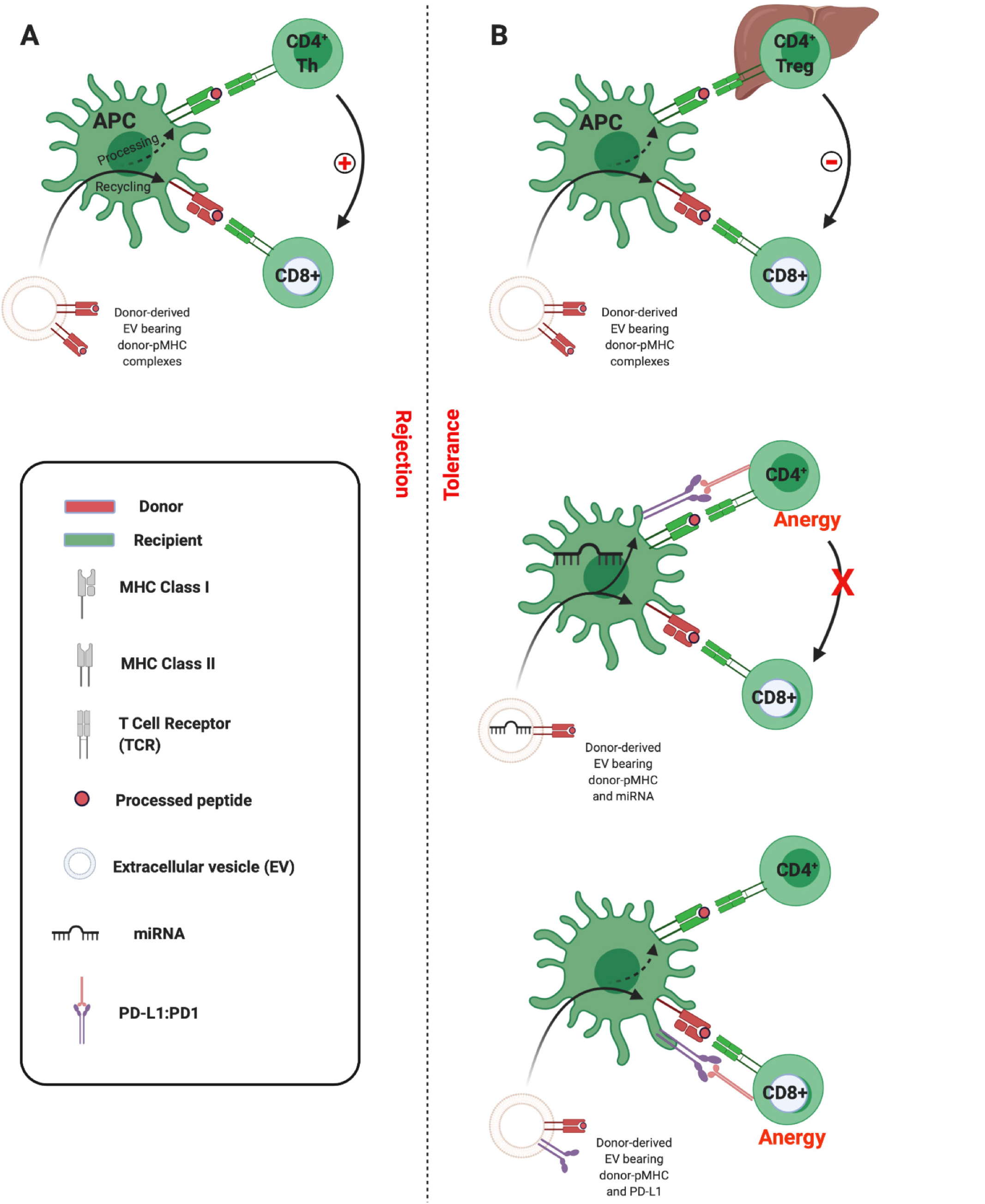
Figure 4 Three-cell model of semi-direct allorecognition.
A: Adaptive CD8 T cell immunity is the principle arm of the cellular alloimmune response, but its development requires help. This can be provided by CD4 T cells that recognise alloantigen indirectly. Extracellular vesicle (EV) cross-dressing of recipient antigen-presenting cells (APCs) can precipitate the simultaneous presentation of intact donor peptide-major histocompatibility complex (pMHC) and of processed alloantigen on self-MHC. The resultant cooperation that can occur between CD4 T cells and CD8 effector cells enables delivery of the essential help for generating the cytotoxic alloresponses forming the basis for allograft rejection; B: Under certain conditions, within the hepatic microenvironment for instance, it is possible that similar co-presentation of EV-derived alloantigen can promote CD4 regulatory T cell (Treg) suppression of effector T cells and promotion of tolerance (upper panel). Tolerance to alloantigen may also occur as a consequence of EV co-transport of nucleic acids triggering recipient APCs to upregulate immunoinhibitory molecules such as Programmed Death-Ligand 1 (middle panel), or indeed due to the tandem transfer of such intact immunoinhibitory molecules which then colocalise at the immunological synapse (lower panel). Created with BioRender.com. PD-L1: Programmed Death-Ligand 1; EV: Extracellular vesicle; APC: Antigen presenting cell; pMHC: Peptide-major histocompatibility complex.
- Citation: Mastoridis S, Martinez-Llordella M, Sanchez-Fueyo A. Extracellular vesicles as mediators of alloimmunity and their therapeutic potential in liver transplantation. World J Transplant 2020; 10(11): 330-344
- URL: https://www.wjgnet.com/2220-3230/full/v10/i11/330.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i11.330