Published online Mar 19, 2025. doi: 10.5498/wjp.v15.i3.100103
Revised: December 21, 2024
Accepted: January 22, 2025
Published online: March 19, 2025
Processing time: 104 Days and 20.9 Hours
Traumatic brain injury (TBI) poses a considerable risk to human health. After TBI, individuals are susceptible to a range of psychiatric disorders, with depression being a primary complication. Selective serotonin reuptake inhibitors (SSRIs) are frequently used in the treatment of depression; however, their efficacy in addres
To investigate the efficacy of SSRIs in the treatment of MDD after TBI.
A comprehensive search across multiple databases was conducted following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, encompassing studies published until May 2024. This review focused on studies that examined the efficacy of SSRIs in the treatment of MDD following TBI. Studies were assessed based sample size, treatment duration, treatment methodologies, severity of brain injury, assessment techniques, and drug response. A random-effects model was used to derive the summary effect size.
Eight studies compared the reduction in depression scores in patients with MDD after TBI and SSRI treatment. The eight studies did not exhibit heterogeneity (I2 = 38%). The depression score for MDD after TBI in the SSRI group decreased more than that in the control group [odds ratio (OR) 1.68, 95%CI: 1.09-2.58, P = 0.02]. The adverse reactions after treatment included diarrhea, dizziness, dry mouth, nausea, or vomiting. There was no difference in the incidence of adverse reactions after treatment between the two groups (OR 1.16, 95%CI: 0.78-1.73, P = 0.46]. These studies did not show significant heterogeneity (I2 = 44%).
SSRIs may be effective in treating patients with MDD after TBI. Adequately powered, randomized, controlled trials are required to confirm these findings.
Core Tip: Results of this meta-analysis indicate that selective serotonin reuptake inhibitors have advantages over placebos for the treatment of major depressive disorder after Traumatic brain injury. The incidence of adverse reactions after treatment did not differ substantially between the two groups. However, these studies did not exhibit heterogeneity, although the sample sizes of all studies included in this meta-analysis were relatively small (n < 100). This may have resulted in an overestimation of the treatment effects in small trials. Future prospective studies with larger sample sizes are needed to verify the results of the present study.
- Citation: Gao RX, Zhang XN, Zhu P. Selective serotonin reuptake inhibitors in the treatment of major depressive disorder after brain trauma: Systematic review and meta-analysis. World J Psychiatry 2025; 15(3): 100103
- URL: https://www.wjgnet.com/2220-3206/full/v15/i3/100103.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i3.100103
Traumatic brain injury (TBI) is a condition that threatens human health. More than 50 million individuals are affected by TBI annually and approximately half of the world's population may experience TBI at least once during their lifetime[1]. The main type of damage caused by TBI is the destruction of the brain tissue structure that occurs immediately after injury; however, persistent pathological changes are more serious after injury. TBI can cause neurological dysfunction and death of nerve and glial cells, leading to serious consequences or even death. The age range of patients exhibits a bimodal distribution, with a higher probability of occurrence between 14 and 25 years of age and after 70 years[2]. There are several causes of TBI; the primary being falls, which are common among the elderly and children, followed by blunt trauma, and motor vehicle accidents. Patients with TBI who experience falls and motor vehicle accidents account for > 50% of all cases[3]. TBI not only threatens human life and health but also imposes an economic burden on society, re
Patients are prone to various mental illnesses after TBI, depression being one of the main complications[4]. Depression is characterized by low mood, decreased willpower, and lack of pleasure. Other symptoms include changes in body weight and appetite, insomnia, drowsiness, restlessness, fatigue, and suicidal tendencies[5]. In recent years, with the accelerated pace of life and increased social pressure, the annual incidence of depression has increased. According to the World Health Organization, depression is expected to become the second most harmful disease after heart disease by 2020. Major depressive disorder (MDD) is a common form of depression that affects millions of individuals globally[6].
A study evaluated and followed up patients 2 weeks after TBI and 1, 6, and 12 months later; patients were found to be more likely to experience depression after TBI[7]. Many clinical studies have reported that depression is associated with TBI; however, the actual incidence of depression after TBI reported in different studies remains quite different. For example, the prevalence of depression 1 year after TBI is 15.3%-18.0%, the incidence of depression 1 year after TBI is 53.1%, and as high as 61.0% 8 years after TBI. The probability of a high incidence of depression in patients with TBI varies from 15%-77%[8].
Sertraline, citalopram, and other selective serotonin reuptake inhibitors (SSRIs) have the potential to improve mood and cognition[9-11]. In one study, 16 patients with post-TBI depression were treated with sertraline for 8 weeks, and all subjects exhibited substantial improvements in sleep, mood, social function, and work ability, demonstrating that the drug was effective in treating depression in patients with TBI. Compared to non-TBI depression, tricyclic antidepressants are less effective in relieving post-TBI depressive symptoms and cause more adverse reactions. The effectiveness of norepinephrine-dopamine reuptake inhibitors remains controversial because these drugs can lower the seizure threshold. SSRIs are first-line drugs for treating major depressive symptoms[12]; however, their effectiveness in treating major depression after TBI remains unclear.
To clarify the clinical effects of SSRIs on MDD after TBI, we conducted a systematic review and meta-analysis of studies investigating SSRIs for the treatment of MDD after TBI.
This meta-analysis evaluated all studies investigating SSRIs in patients with MDD after TBI according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Two authors independently reviewed all studies. Published and unpublished studies were considered. A systematic electronic literature search of the PubMed, Embase, Web of Science, EBSCO, and the Cochrane Library databases, from inception up to May 2024, was performed using the following combined MeSH heading and keyword search terms: (traumatic brain injury OR concussion OR closed head injury OR TBI) AND (depression OR depressive disorder). In the case of MeSH headings, the search included the heading term (e.g., “concussion”) and was mapped to include any terms that appeared below it in the corresponding MeSH table (e.g., “post-concussive syndrome”, “intracranial hemorrhage”, “traumatic intracerebral hemorrhage”). The search was limited to clinical trials involving adult participants published in English. The reference list of each full text used for the review, as well as any existing reviews identified, were manually searched to identify additional potentially eligible citations not found in the electronic database search.
The following inclusion criteria were applied to ensure that relevant studies were selected. We included studies that measured depressive symptoms as a secondary outcome to include more studies in an area of research that has been exhausted. Unpublished and ongoing studies were not retrieved and were therefore excluded. These studies were not restricted to specific settings such as secondary care. The inclusion criteria for the current meta-analysis were: Age ≥ 18 years; clinical trials measuring the effects of SSRI treatments on depression after TBI; diagnosis of depression before the start of the study; and the sample included participants with TBI of any severity (mild, moderate, or severe).
Using EndnoteX9 (Clarivate, London, United Kingdom) for literature management, irrelevant, duplicate, and nonexperimental (particularly animal-based) studies were excluded. Subsequently, the titles and abstracts were thoroughly reviewed to exclude studies that failed to fulfill the inclusion criteria. The refined literature pool was scrutinized in detail to ensure a comprehensive methodological approach. The full texts of the retrieved studies were read for secondary screening and a third researcher resolved any disagreements through consensus discussion. Two researchers indepen
Review Manager version 5.4 (The Cochrane Collaboration) was used for the meta-analysis. Measurement data were transformed with mean difference into effect size, and count data were transformed with odds ratios (OR). Cochran’s Q statistic was used to test for heterogeneity among the included studies, and the Higgins I2 test was used to evaluate the differences among effect sizes. When the I2 value exceeded 50%, heterogeneity among studies was considered statistically significant. Parallel subgroup and sensitivity analyses excluded obvious heterogeneity from the meta-analysis. A fixed-effects model was used for meta-analyses when heterogeneity among the studies did not reach statistical significance. Differences with P < 0.05 were considered to be statistically significant.
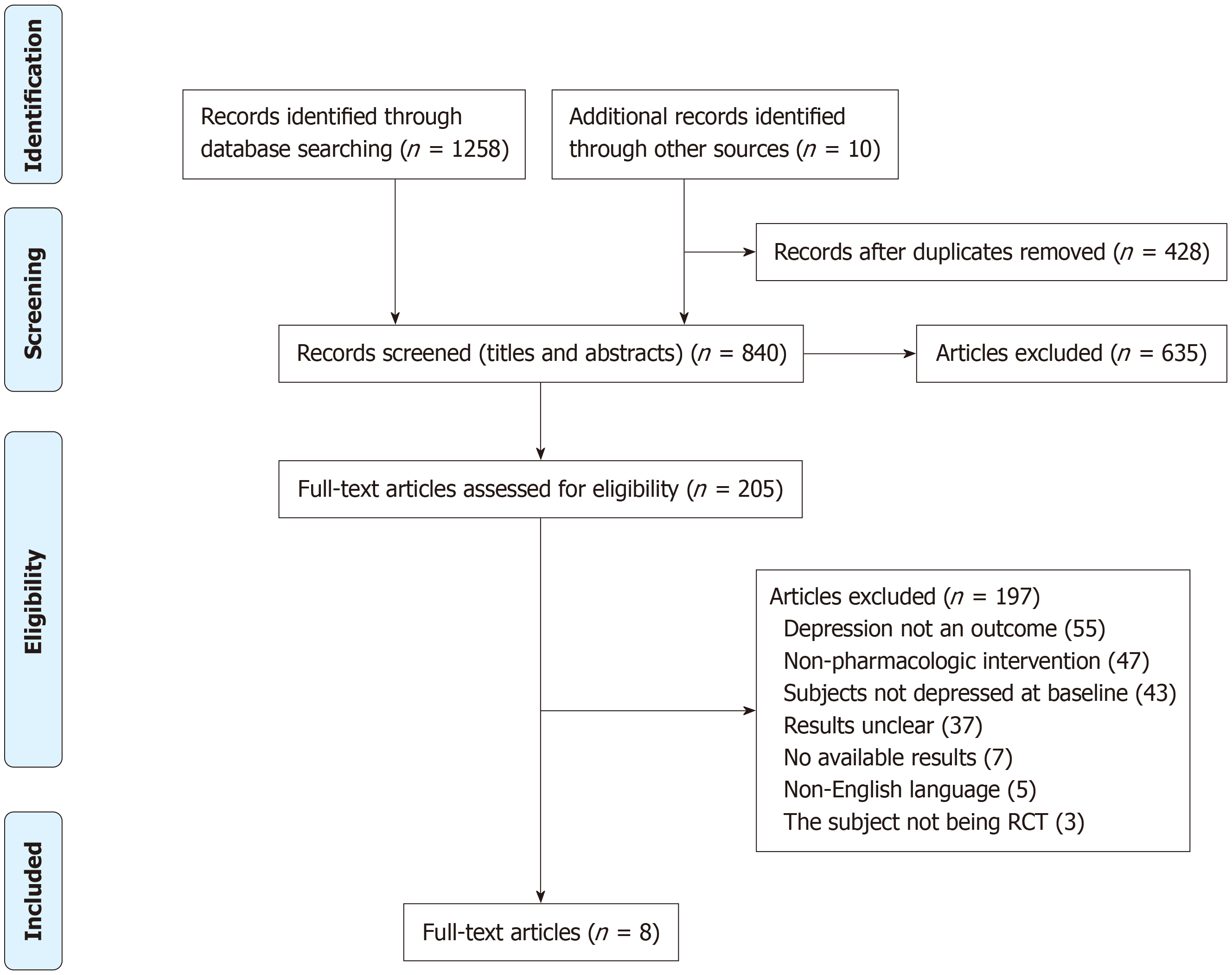
A flow diagram illustrating the study selection process is shown in Figure 1. After the initial title/abstract inspection, 428 duplicates and 635 studies were excluded. After further reading of the full texts, 197 studies that did not meet the screening criteria were excluded; these included 55 studies without a depression outcome, 47 that were not pharmacological interventions, 43 where participants did not have depressive symptoms at baseline, seven with no outcome, five that were not in English, and three that were not randomized, controlled trials. Eight studies were ultimately included in the analysis.
The characteristics of the studies included in this review are summarized in Table 1[8,11,13-18]. The primary antidepre
| Ref. | Country | Antidepressant(s) | Weeks treated | n | SSRI (n) | Control (n) | Time since injury | Severity of injury | Outcome measure |
| Ansari et al[8], 2014 | India | Sertraline | 24 | 80 | 40 | 40 | > 2 weeks | Mild and moderate | PHD-9 |
| Ashman et al[13], 2009 | United States | Sertraline | 10 | 41 | 22 | 19 | 17 years | Mild to severe | HAM-D |
| Fann et al[14], 2000 | United States | Sertraline | 8 | 30 | 15 | 15 | 10.6 months | Mild | HAM-D |
| Fann et al[15], 2017 | United States | Sertraline | 12 | 53 | 24 | 29 | 4.6 months | Mild to severe | HAM-D |
| Jorge et al[11], 2016 | Argentina | Sertraline | 24 | 94 | 48 | 46 | - | Severe | GCS |
| Lee et al[16], 2005 | Korea | Methylphenidate and sertraline | 4 | 30 | 20 | 10 | 32.2 days | Mild to moderate | HAM-D |
| Rao et al[17], 2016 | United States | Escitalopram | 12 | 14 | 8 | 6 | Not reported | Not reported | MADRS |
| Rapoport et al[18], 2008 | Canada | Citalopram | 10 | 54 | 54 | Self-control | < 1 year | Mild to severe | HAM-D |
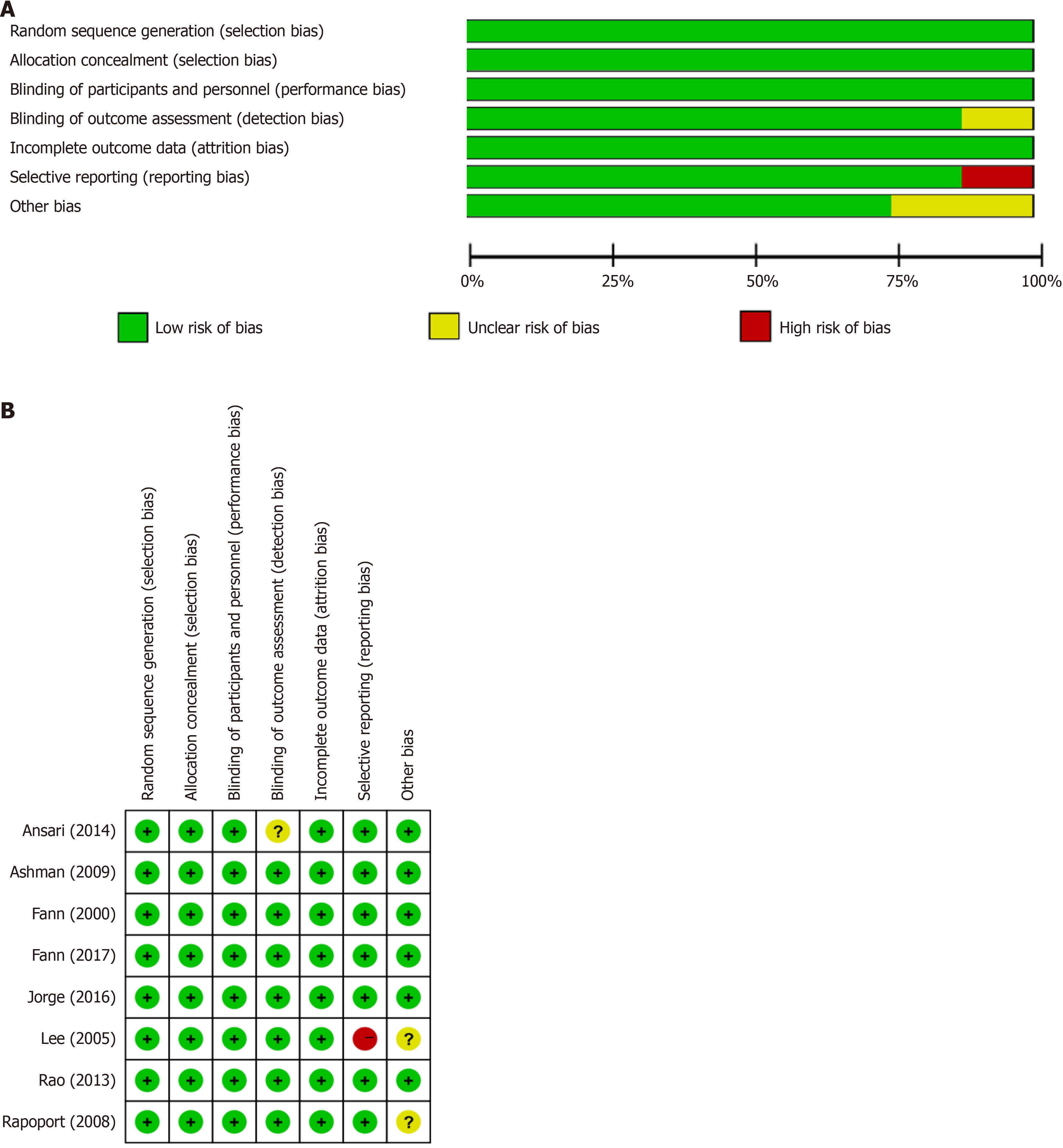
This analysis compared the baseline conditions of patients in eight studies. Figure 2 present tables outlining the "Risk of Bias in Included Studies”. Detailed descriptions of the study characteristics can be found in the section titled "Characteristics of Included Studies”. Several trials have shown a high risk of bias, primarily due to performance bias, allocation concealment, self-reported outcomes, random sequence generation, and inadequate protection against other biases. The challenges posed by unsegregated participants, age, and multiple outcome measures increased the risk of bias in most of the included randomized clinical trials.
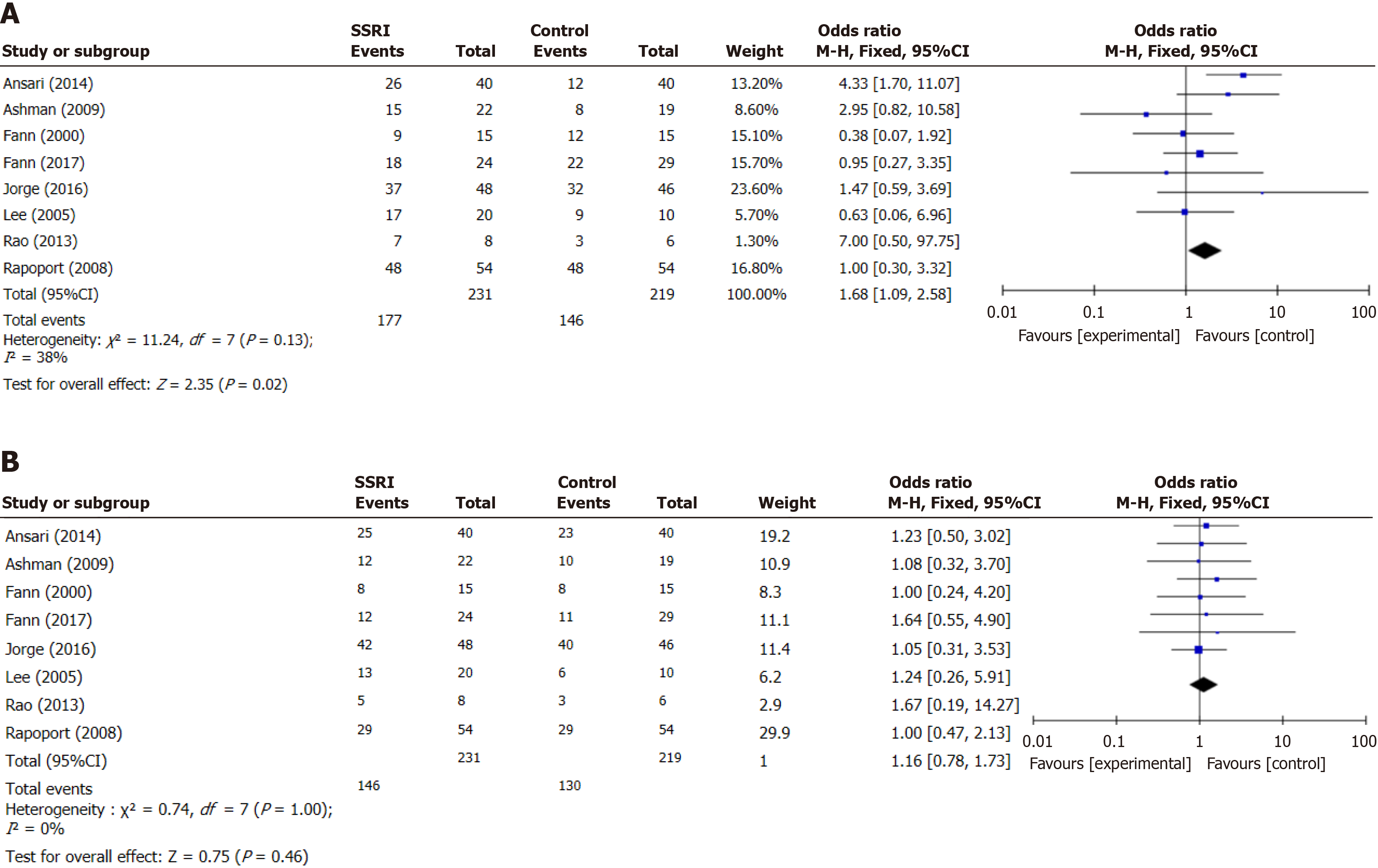
Eight studies compared the reduction in depression scores in patients with MDD after SSRI treatment following TBI. The included studies did not exhibit heterogeneity (I2 = 38%). The depression score of MDD after TBI in the SSRI group decreased more than that in the control group (OR 1.68, 95%CI: 91.09-2.58, P = 0.02; Figure 3A).
The adverse reactions after treatment included diarrhea, dizziness, dry mouth, nausea, or vomiting. There was no statistical difference between the two groups in the incidence of adverse reactions after treatment (OR 1.16, 95%CI: 0.78-1.73, P = 0.46; Figure 3B). Analysis revealed no significant heterogeneity (I2 = 0%).
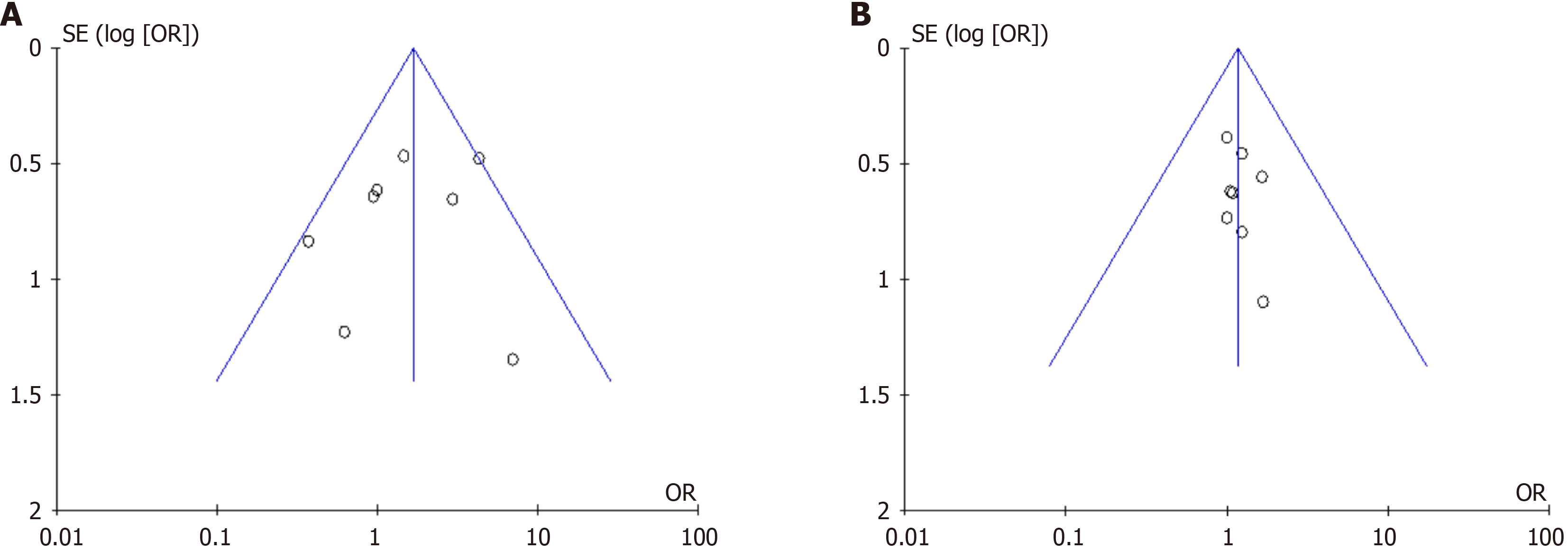
The publication bias of each result was assessed using funnel plots, which indicated good symmetry and scatter points evenly distributed in the funnel, indicating that publication bias did not significantly affect the results of the meta-analysis (Figure 4). The I2 value in each figure was less than 50%, indicating that there was no heterogeneity among the studies.
MDD is a common complication after TBI. Although the specific causes of depression after TBI are not fully understood, studies have shown that multiple brain regions, pathways, and neurotransmitters are inextricably linked[19]. The current diagnostic criteria for severe depression after TBI need to be improved, and the treatment methods for severe depression after TBI remain in their infancy. Currently, the treatment of severe depression after TBI is very difficult because of its complex mechanism(s); therefore, it is necessary to identify key targets and therapeutic drugs for severe depression after TBI from multiple sources[20]. SSRIs are the first-line treatment in clinical practice. The therapeutic effects of SSRIs in patients with MDD after TBI has been investigated[21]. The present investigation systematically reviewed and analyzed these findings.
Antidepressants may be associated with reduced depressive symptoms after TBI[15,22]. Our meta-analysis indicated that SSRI treatment for TBI reduced depression scores post-MDD (OR 1.63, 95%CI: 1.12-2.38, P = 0.01), indicating that SSRIs were more effective than the control in treating post-TBI MDD. This finding is consistent with that of previous studies. This was further supported by a randomized, controlled trial in which both the placebo and experimental groups exhibited substantial reductions in depressive symptoms over the course of the study[15]. Further research is needed to determine the SSRIs that yield better results in patients with post-TBI MDD.
Our meta-analysis consistently demonstrated no statistically significant differences in the incidence of diarrhea, dizziness, dry mouth, nausea, or vomiting between the SSRI and control groups. The incidence of adverse reactions after treatment was similar between the two groups (OR 1.16, 95%CI: 0.78-1.73, P = 0.46). Analysis indicated no heterogeneity
The present meta-analysis has some limitations. First, only eight studies were included and the sample sizes were relatively small (n < 100). This may have resulted in an overestimation of treatment effects. Second, although the heterogeneity among the included studies was low, different doses and drug treatments may have affected the summary results, and further subdivision of the treatment effects of different SSRIs should be considered in the future. Finally, early use of SSRIs may have a better effect on TBI, and future studies should explore this possibility.
The meta-analysis indicates that SSRIs were slightly superior to the placebo in the treatment of post-TBI MDD. Larger, prospective studies in well-defined populations of patients with TBI are warranted to elucidate treatment effects and the relationship between post-TBI MDD and individual SSRIs.
| 1. | Egodage T, Patel PP. Updates in traumatic brain injury management: brain oxygenation, middle meningeal artery embolization and new protocols. Trauma Surg Acute Care Open. 2024;9:e001382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Marrone F, Zavatto L, Allevi M, Di Vitantonio H, Millimaggi DF, Dehcordi SR, Ricci A, Taddei G. Management of Mild Brain Trauma in the Elderly: Literature Review. Asian J Neurosurg. 2020;15:809-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Magyar-Sumegi ZD, Stankovics L, Lendvai-Emmert D, Czigler A, Hegedus E, Csendes M, Toth L, Ungvari Z, Buki A, Toth P. Acute neuroendocrine changes after traumatic brain injury. Brain Spine. 2024;4:102830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Oft HC, Simon DW, Sun D. New insights into metabolism dysregulation after TBI. J Neuroinflammation. 2024;21:184. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Monroe SM, Harkness KL. Major Depression and Its Recurrences: Life Course Matters. Annu Rev Clin Psychol. 2022;18:329-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 260] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 6. | Liu Y, Jing Y, Gao Y, Li M, Qin W, Xie Y, Zhang B, Li J. Exploring the correlation between childhood trauma experiences, inflammation, and brain activity in first-episode, drug-naive major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Barker-Collo S, Jones A, Jones K, Theadom A, Dowell A, Starkey N, Feigin VL. Prevalence, natural course and predictors of depression 1 year following traumatic brain injury from a population-based study in New Zealand. Brain Inj. 2015;29:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ansari A, Jain A, Sharma A, Mittal RS, Gupta ID. Role of sertraline in posttraumatic brain injury depression and quality-of-life in TBI. Asian J Neurosurg. 2014;9:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Conroy SK, Brownlowe KB, McAllister TW. Depression Comorbid With Stroke, Traumatic Brain Injury, Parkinson's Disease, and Multiple Sclerosis: Diagnosis and Treatment. Focus (Am Psychiatr Publ). 2020;18:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Agarwal CD, Palka JM, Gajewski AJ, Khan DA, Brown ES. The efficacy of citalopram or escitalopram in patients with asthma and major depressive disorder. Ann Allergy Asthma Immunol. 2024;132:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Jorge RE, Acion L, Burin DI, Robinson RG. Sertraline for Preventing Mood Disorders Following Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Psychiatry. 2016;73:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Carotenuto A, Fasanaro AM, Manzo V, Amenta F, Traini E. Association Between the Cholinesterase Inhibitor Donepezil and the Cholinergic Precursor Choline Alphoscerate in the Treatment of Depression in Patients with Alzheimer's Disease. J Alzheimers Dis Rep. 2022;6:235-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Ashman TA, Cantor JB, Gordon WA, Spielman L, Flanagan S, Ginsberg A, Engmann C, Egan M, Ambrose F, Greenwald B. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil. 2009;90:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Fann JR, Bombardier CH, Temkin N, Esselman P, Warms C, Barber J, Dikmen S. Sertraline for Major Depression During the Year Following Traumatic Brain Injury: A Randomized Controlled Trial. J Head Trauma Rehabil. 2017;32:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Lee H, Kim SW, Kim JM, Shin IS, Yang SJ, Yoon JS. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol. 2005;20:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Rao V. Lexapro for the treatment of traumatic brain injury (TBI) depression & other psychiatric conditions. [accessed 2025 Jan 21]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT01368432 ClinicalTrials.gov Identifier: NCT01368432. |
| 18. | Rapoport MJ, Chan F, Lanctot K, Herrmann N, McCullagh S, Feinstein A. An open-label study of citalopram for major depression following traumatic brain injury. J Psychopharmacol. 2008;22:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Das M, Mayilsamy K, Tang X, Han JY, Foran E, Willing AE, Mohapatra SS, Mohapatra S. Pioglitazone treatment prior to transplantation improves the efficacy of human mesenchymal stem cells after traumatic brain injury in rats. Sci Rep. 2019;9:13646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Yang XJ, Zhao BC, Li J, Shi C, Song YQ, Gao XZ, Jiang HL, Yu QY, Liang XC, Feng SX, Li X, Sun Y, Li YH, Wang YP, Bao T, Zhang ZJ. Serum NLRP3 Inflammasome and BDNF: Potential Biomarkers Differentiating Reactive and Endogenous Depression. Front Psychiatry. 2022;13:814828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Janssen-Aguilar R, Rojas P, Ruiz-Sánchez E, Rodriguez-Violante M, Alcántara-Flores YM, Crail-Meléndez D, Cervantes-Arriaga A, Sánchez-Escandón Ó, Ruiz-Chow ÁA. Naturalistic Study of Depression Associated with Parkinson's Disease in a National Public Neurological Referral Center in Mexico. Brain Sci. 2022;12:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Salter KL, McClure JA, Foley NC, Sequeira K, Teasell RW. Pharmacotherapy for Depression Posttraumatic Brain Injury: A Meta-analysis. J Head Trauma Rehabil. 2016;31:E21-E32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |