Published online Feb 19, 2025. doi: 10.5498/wjp.v15.i2.100214
Revised: November 26, 2024
Accepted: December 17, 2024
Published online: February 19, 2025
Processing time: 157 Days and 7 Hours
Schizophrenia (SZ), a chronic and widespread brain disorder, presents with complex etiology and pathogenesis that remain inadequately understood. Despite the absence of a universally recognized endophenotype, peripheral blood mono
To preliminarily investigate potential pathogenic mechanisms and identify novel biomarkers for SZ.
PBMCs from SZ patients were subjected to integrative transcriptomic and proteomic analyses to uncover differentially expressed genes (DEGs) and differentially expressed proteins while mapping putative disease-associated signaling pathways. Key findings were validated using western blot (WB) and real-time fluorescence quantitative PCR (RT-qPCR). RNAi-lentivirus was employed to transfect rat hippocampal CA1 neurons in vitro, with subsequent verification of target gene expression via RT-qPCR. The levels of neuronal conduction proteins, including calmodulin-dependent protein kinase II (caMKII), CREB, and BDNF, were assessed through WB. Apoptosis was quantified by flow cytometry, while cell proliferation and viability were evaluated using the Cell Counting Kit-8 assay.
The integration of transcriptomic and proteomic analyses identified 6079 co-expressed genes, among which 25 DEGs were significantly altered between the SZ group and healthy controls. Notably, haptoglobin (HP), lactotransferrin (LTF), and SERPING1 exhibited marked upregulation. KEGG pathway enrichment analysis implicated neuroactive ligand-receptor interaction pathways in disease pathogenesis. Clinical sample validation demonstrated elevated protein and mRNA levels of HP, LTF, and SERPING1 in the SZ group compared to controls. WB analysis of all clinical samples further corroborated the significant upregulation of SERPING1. In hippocampal CA1 neurons transfected with lentivirus, reduced SERPING1 expression was accompanied by increased levels of CaMKII, CREB, and BDNF, enhanced cell viability, and reduced apoptosis.
SERPING1 may suppress neural cell proliferation in SZ patients via modulation of the CaMKII-CREB-BDNF signaling pathway.
Core Tip: The strength of this study lies in the integration of transcriptomics and proteomics data from the same sample source (peripheral blood mononuclear cells) to better observe mRNA-protein inconsistencies and mRNA-protein correlations, thus explaining the biology of schizophrenia in a holistic manner. Proteomics and transcriptomics correlation analyses provide a panoramic view of the expression profile of schizophrenia, enabling complementarity and integration at the transcriptional and protein levels. Again, this study is one of the few to investigate the role of the SERPING1 gene in neural cell proliferation and apoptosis.
- Citation: Li F, Ren X, Liu JX, Wang TD, Wang B, Wei XB. Integrative transcriptomic and proteomic analysis reveals that SERPING1 inhibits neuronal proliferation via the CaMKII-CREB-BDNF pathway in schizophrenia. World J Psychiatry 2025; 15(2): 100214
- URL: https://www.wjgnet.com/2220-3206/full/v15/i2/100214.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i2.100214
Schizophrenia (SZ) is a complex mental illness characterized by positive symptoms such as hallucinations, delusions, and speech disturbances, alongside negative symptoms including hypobulia, anhedonia, and social withdrawal, as well as cognitive deficits. Its progression typically spans three stages: prodromal (pre-psychotic), psychotic, and chronic phases[1,2]. Diagnostic approaches rely exclusively on clinical manifestations, as no auxiliary tests or reliable biomarkers are available[3], creating significant challenges in early detection, disease stratification, therapy selection, and prognosis. This diagnostic limitation often leads to misdiagnosis, delayed diagnosis, ineffective treatment, or relapse. The poorly understood pathogenesis of SZ is linked to neurodevelopmental abnormalities, genetic predispositions, and the cumu
Genome-wide association studies have identified over 100 SZ-associated risk genes[11], encompassing key functional categories including neuronal, immune, and histone-related pathways[12]. However, the mechanisms through which these genes contribute to SZ pathogenesis remain poorly understood. Current SZ research primarily relies on post-mortem tissues, blood samples, and patient-derived induced pluripotent stem cells. While post-mortem samples provide valuable insights, their acquisition and preservation present significant logistical hurdles compared to the relative ease of obtaining blood samples. iPSC models are instrumental for investigating fundamental neural cell processes but are less effective for case-control studies due to statistical constraints and challenges in discerning individual-level cellular phenotypes[13]. Blood samples, by contrast, reflect immune system dynamics and provide indirect evidence linking central nervous system activity in SZ to alterations in peripheral blood gene expression[14]. Among blood-derived components, peripheral blood mononuclear cells (PBMCs) are widely utilized in SZ research due to their proven sensitivity and specificity as biomarkers across a range of diseases[15,16]. This study integrated transcriptomics and proteomics analyses of PBMCs from SZ patients with in vitro experiments to offer preliminary insights into SZ pathogenesis.
SZ group: This study involved 150 SZ inpatients, comprising 6 participants for omics analysis and 150 for western blot (WB) detection, recruited from Hainan Anning Hospital between March and July 2022. Data collected included demographic and clinical parameters such as age, age of onset, gender, SZ subtype, family history, and head imaging results, with detailed clinical profiles of those included in the omics analysis provided in Table 1. Eligibility was determined by an SZ diagnosis based on International Classification of Diseases-10 criteria, independently confirmed by two trained specialists. Exclusion criteria encompassed intellectual disabilities, other psychiatric disorders, neurological conditions, significant systemic illnesses (e.g., cancer, leukemia), severe infections (e.g., acquired immune deficiency syndrome), and pregnancy or lactation.
| SZ | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
| Gender | Male | Male | Male | Female | Female | Female |
| Age | 36 | 25 | 19 | 16 | 29 | 36 |
| Age of onset | 24 | 19 | 17 | 15 | 20 | 28 |
| Disease classification | Undifferentiated type | Undifferentiated type | Paranoid type | Undifferentiated type | Paranoid type | Undifferentiated type |
| Family history | No | No | No | No | No | No |
| Imaging examination | Abnormal | Abnormal | Normal | Abnormal | Normal | Normal |
Healthy control group: A cohort of 150 healthy controls (HC) from Haikou People's Hospital, matched by age and gender to the SZ group, was selected during the same timeframe. Participants were required to meet the following inclusion criteria: absence of personal or familial history of mental disorders, no intellectual disability, no prior or current alcohol, drug, or substance abuse, no chronic illnesses, no history of head trauma or neurological surgeries, and not being pregnant or lactating.
Neuronal cells in the CA1 region of the hippocampus of male healthy SD rats: The study was reviewed and approved by the Biomedical Ethics Committee of Haikou Hospital affiliated with Xiangya School of Medicine, Central South University (Haikou People's Hospital), under ethics approval number 2021- (Ethics Review)-263 (Ethical Statement 1). Additional approval was granted by the Biomedical Ethics Committee of Hainan Anning Hospital, referenced as approval number 2022- (Ethics Review)-2 (Ethical Statement 2). Written informed consent was obtained from participants or their guardians, with documentation validated by the respective Medical Ethics Committees before the commencement of the study. The experiment does not involve animal experiments and is based on rat neuronal cells sourced from the Animal Experiment Center of the School of Pharmacy, Hainan University, China.
Collection and PBMC extraction of clinical samples: Fresh fasting venous whole blood was collected from participants using 9 mL sodium citrate anticoagulant vacuum tubes (sodium citrate: blood ratio = 1:9). PBMCs were isolated via lymphocyte separation solution, followed by washing with PBS. After discarding the supernatant, the PBMCs were harvested and preserved at -80 °C.
RNA extraction and sequencing: PBMCs were ground in liquid nitrogen, transferred to 1.5 mL centrifuge tubes, and processed for RNA extraction using the Trizol method. RNA concentration and purity were assessed with an enzyme-labeled instrument, and high-quality RNA was preserved at -80 °C. mRNA was enriched via magnetic beads conjugated with Oligo (dT) targeting the poly-A structure at the 3' end, followed by reverse transcription into cDNA for library construction. Fragment size (25-1000 bp) and concentration (0.1-50 ng/µL) were verified using the DNA 1000 assay kit (Agilent Technology, China). Sequencing of qualified libraries was conducted on the Illumina HiSeq Xten platform. Raw data underwent quality control and alignment with the reference genome, with unqualified sequences filtered to obtain high-quality reads. HISAT2 (https://daehwankimlab.github.io/hisat2/) was utilized for genome alignment. Gene expression levels were quantified using featureCounts (http://bioinf.wehi.edu.au/featureCounts/) to compute FPKM values, enabling comparative analysis of read counts between samples. Differential expression analysis was performed using DESeq2 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html) and edgeR (https://bioconductor.org/packages/release/bioc/html/edgeR.html). Hypothesis testing results were adjusted for multiple comparisons to yield P-adj values, with DEGs defined by P-adj < 0.05 and |log2foldchange| > 1.
Quantitative analysis of data-independent acquisition proteomics: PBMCs were lysed in SDT buffer, subjected to ultrasonication, and heated at 100 °C for 15 minutes. After centrifugation at 14000 g for 40 minutes, protein concentration in the supernatant was determined using a BCA protein assay kit (Bio-Rad). Equivalent aliquots from each sample were combined to form a pooled sample, which was fractionated by HPRP and analyzed via LC-MS/MS in QE-HFX_DDA mode. The DDA library database was generated using Spectronaut Pulsar X software (https://biognosys.com/resources/spectronaut-manual/). Individual samples were then processed in LC-MS/MS data-independent acquisition (DIA) mode, with the DDA database employed for qualitative and quantitative assessments.
Integrative transcriptomics and proteomics analysis: The integrative analysis was performed using R (https://www.r-project.org/) and associated tools, including Blast2 (https://www.blast2go.com/), to derive insights from trans
Validation of protein expression by WB in clinical samples: Total protein extracted from PBMCs was resolved by SDS-PAGE and transferred onto PVDF membranes (Merck Millipore, Bedford, MA, United States). Membranes were blocked with 5% non-fat milk in PBST and incubated overnight at 4 °C with primary antibodies (anti-HP, anti-LTF, and anti-SERPING1, Abcam, United Kingdom). Secondary antibody incubation at room temperature for 40 minutes preceded visualization using an ECL kit (Beyotime, Shanghai, China). GAPDH was utilized as the internal control, and ImageJ software was used for grayscale analysis to quantify relative protein expression levels.
Real-time fluorescence quantitative PCR detection of expression in clinical samples: Total RNA extracted from PBMCs served as the template for cDNA synthesis following the Thermo real-time fluorescence quantitative PCR (RT-qPCR) Kit protocol. Quantification of mRNA expression was conducted via real-time fluorescence qPCR using TransGen Biotech® SybrGreen qPCR SuperMix. Primer sequences specific to the target genes were detailed in Table 2. Relative mRNA expression levels were calculated using the 2-ΔΔCT method (Livak method).
| ID | Forward primer (5'- > 3') | Reverse primer (5'- > 3') |
| GAPDH | TGTTCGTCATGGGTGTGAAC | ATGGCATGGACTGTGGTCAT |
| HP | AATGTGAAGCAGATGACGGC | GATCCCAGTCGCATACCAGG |
| LTF | TTGTCTTCCTCGTCCTGCTG | GGGAGCCAAAGAGCTGGAAT |
| SEEPING1 | CTCCTACCCAGCCCACTACT | CCAAGTTGGCGTCACTGTTG |
Rat hippocampal tissue isolation and cell culture: Healthy male SD rats (250 ± 20 g) were housed in a controlled environment (21 ± 2 °C, 40%-70% humidity) with regular rodent chow and clean bedding. Anesthesia was administered using 0.3% sodium pentobarbital (1 mL/100 g), followed by perfusion and fixation with physiological saline and 4% paraformaldehyde. After dissection, the hippocampus was isolated and stored at -80 °C. CA1 region tissues were washed in D-Hanks solution, minced, and digested with 0.25% trypsin at 37 °C to obtain cell clusters or single cells. Following centrifugation at 500 rpm for 5 minutes, the supernatant was removed, and the cells were resuspended in complete neuron culture medium. The cell density, adjusted to 2 × 106 cells/mL, was determined using a hemocytometer. Cells were seeded into culture flasks and maintained at 37 °C in a 5% CO2 incubator.
Construction of RNAi-silencing lentivirus: The SERPING1 gene sequence (GENE ID: 2182) was retrieved from NCBI, and interference targets were designed as outlined in Table 3. The Plko.1-EGFP-PURO vector served as the cloning backbone, utilizing Age I (ACCGGT) and EcoR I (GAATTC) as restriction sites. Recombinant plasmids were amplified and purified using the OMEGA Endotoxin-Free Plasmid Mini Kit. The A260/280 ratio and plasmid concentration were quantified, and samples were stored at -20 °C. Serum-free, antibiotic-free DMEM (1 mL) was prepared for transfection, into which lentiviral packaging and expression plasmids were combined with the transfection reagent. The solution was vortexed and incubated at room temperature for 30 minutes to facilitate polymer formation. The polymer mixture was then gradually added to 293T cells cultured in fresh medium, followed by incubation. Viral supernatants were subsequently harvested, centrifuged, filtered, concentrated, purified, aliquoted, and stored at -80 °C. Lentivirus-infected 293T cells underwent flow cytometry and fluorescence microscopy analysis to assess lentiviral packaging titers. The corresponding results are presented in Supplementary material.
| RNAi target | Target sequence |
| SEEPING1-sh1 | ACACTGTTTTGCAGCTTTTAT |
| SEEPING1-sh2 | AACCAGTGGATCCACCAAAAT |
| SEEPING1-sh3 | AGTCTTCATGGGCCGTGTATA |
Lentiviral infection of target cells (neuronal cells in the CA1 region of the rat hippocampus): Lentiviral transfection in target cells (neuronal cells within the CA1 region of the rat hippocampus) was initiated by diluting 50 μL of si-SERPING1 Lentivirus in Opti-Medium and mixing gently via pipetting. Separately, 1.2 μL of Lipofectamine 2000 was diluted in 50 μL of Opti-Medium, mixed gently by pipetting, and allowed to stand at room temperature for 5 minutes. The Lentivirus solution and diluted transfection reagent were combined, mixed gently, and left to stand at room temperature for 20 minutes to form transfection complexes. A volume of 100 μL of these complexes was added to each well of a 24-well cell culture plate, followed by gentle rocking to ensure even distribution. The plate was incubated at 37 °C in a 5% CO2 atmosphere for 24-48 hours, with the active transfection phase lasting 4-6 hours.
RT-qPCR detection of SEEPING1 expression in neuronal cells in the CA1 region of rat hippocampus: Neuronal cells were transfected with Lentivirus in four groups: negative control (NC), si-SERPING1#1, si-SERPING1#2, and si-SERPING1#3, corresponding to three distinct silencing targets. After 4-6 hours of transfection, RNA extraction was performed, followed by cDNA synthesis using the Thermo qPCR RT Kit. Real-time fluorescence-based quantitative PCR was carried out using TransGen Biotech® SybrGreen qPCR SuperMix, with the following primer sequences designed for the rat SERPING1 gene:
Forward primer (5'- > 3'): CGCCTCTCTGAGCCTGTATG
Reverse primer (5'- > 3'): TCAGTTCCAACACCGTCTCG
The relative expression of the target gene mRNA was calculated using the 2-ΔΔCT method (Livak method).
WB detection of neuronal expression of neural-related conductance proteins calcium/calmodulin-dependent protein kinase II, cAMP response element-binding protein, brain-derived neurotrophic factor in neuronal cells of CA1 region of rat hippocampus: Lentivirus transfection was conducted across three groups: Control, NC, and si-SERPING1#1, which demonstrated the highest silencing efficiency among the tested candidates. WB detection was performed 4-6 hours post-transfection, following the procedural details specified in the previous WB detection.
Apoptosis of target cells detected by flow cytometry: Rat hippocampal neurons in the logarithmic growth phase were seeded into 6-well culture plates at a density of 7 × 105 cells per well, with 2 mL of medium per well. Lentivirus transfection was conducted across the Control, NC, and si-SERPING1-1 groups. Following 4-6 hours of transfection, cells were harvested by centrifugation, rinsed twice with pre-chilled PBS (4 °C), and resuspended in 500 μL of binding buffer to achieve a final concentration of 106 cells/mL. Subsequently, 100 μL of the suspension was transferred to a 5 mL flow cytometry tube, supplemented with 5 μL Annexin V-APC and mixed thoroughly, followed by 5 μL Propidium Iodide. The mixture was incubated at room temperature in the dark for 15 minutes. Apoptotic analysis was performed using a flow cytometer as per the Biolegend Annexin V-FITC/PI double-staining apoptosis detection kit protocol.
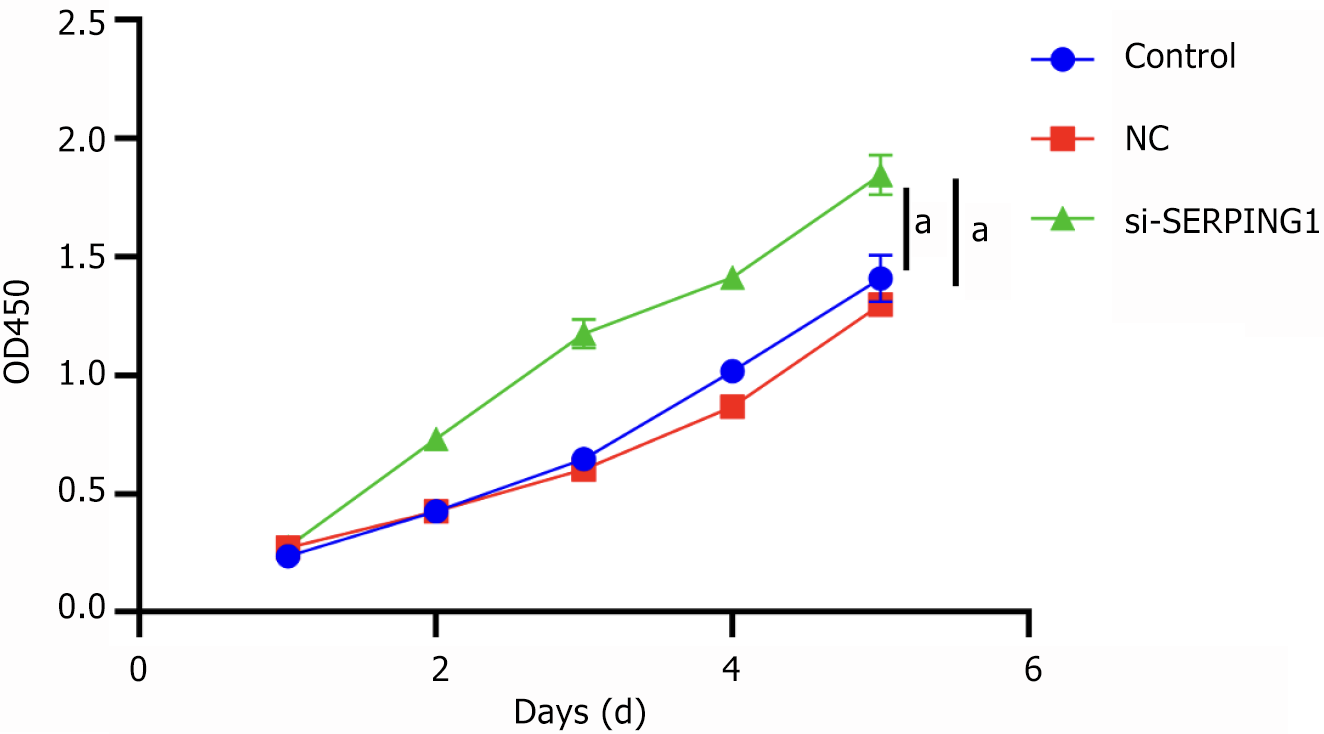
Proliferation of target cells detected by Cell Counting Kit-8: The viability of rat hippocampal neurons was evaluated using the Cell Counting Kit-8 (CCK-8, Solarbio, Beijing, China). Lentivirus transfection was applied to three groups: Control, NC, and si-SERPING1-1. Following transfection, cells from each group were trypsinized and seeded into 96-well plates at a density of 8000 cells per well. After stabilization, CCK-8 assays were performed on days 1, 2, 3, 4, and 5. At each time point, 10 μL of CCK-8 solution was added per well, and cells were incubated at 37 °C in a 5% CO2 atmosphere for 1-4 hours. The optical density (OD) at 450 nm was then determined using a microplate reader (ELx800, BioTech, VT, United States). OD measurements were recorded, and a growth curve was constructed with time as the x-axis and OD values as the y-axis.
Data analysis and visualization were conducted using GraphPad Prism 9 software. Results were presented as mean ± SD. Two-group comparisons were performed using multiple t-tests, while one-way analysis of variance (ANOVA) was used for comparisons among multiple groups. Statistical evaluations included χ² tests where applicable. A P value < 0.05 was considered indicative of statistical significance.
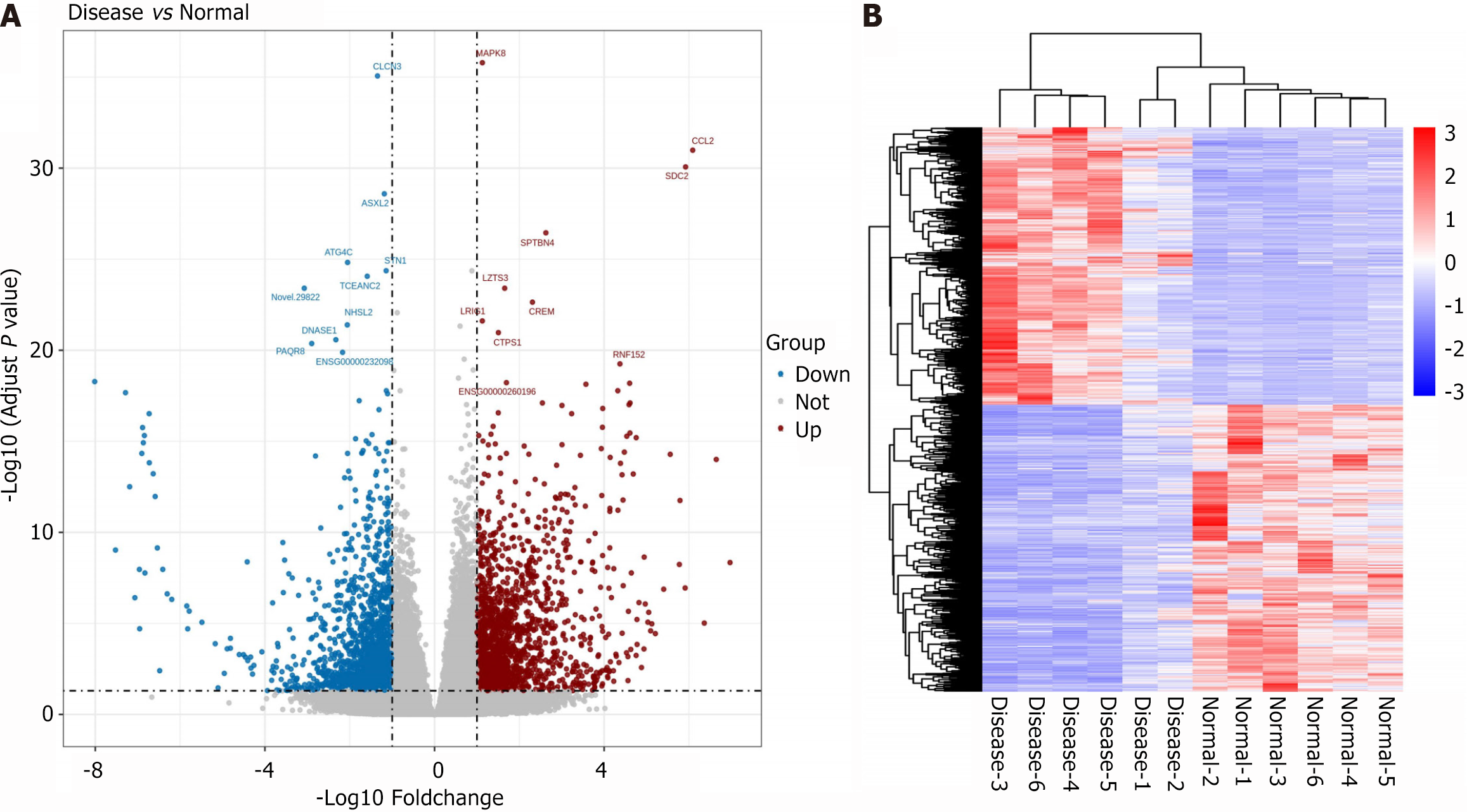
A total of 3317 differentially expressed genes (DEGs) were identified, including 1635 upregulated and 1682 downregulated genes. The 10 most statistically significant DEGs are illustrated in Figure 1A, with detailed information provided in Table 4. Clustering analysis revealed that the DEGs distinctly separated SZ patients from HCs, indicating substantial changes in PBMC gene expression among SZ patients (Figure 1B).
| Gene_id | Symbol | Disease_read count | Normal_read count | Log2FC | P-adj |
| ENSG00000107643 | MAPK8 | 2141.92 | 980.48 | 1.1278 | 1.63E-36 |
| ENSG00000108691 | CCL2 | 965.58 | 14.1 | 6.0888 | 1.04E-31 |
| ENSG00000169439 | SDC2 | 726.54 | 12.05 | 5.9209 | 8.61E-31 |
| ENSG00000160460 | SPTBN4 | 100.36 | 16.26 | 2.626 | 3.57E-27 |
| ENSG00000088899 | LZTS3 | 436.07 | 138.93 | 1.6541 | 3.95E-24 |
| ENSG00000095794 | CREM | 1760.64 | 355.27 | 2.3089 | 2.31E-23 |
| ENSG00000144749 | LRIG1 | 1648.48 | 755.18 | 1.127 | 2.46E-22 |
| ENSG00000171793 | CTPS1 | 1093.29 | 384.58 | 1.5064 | 1.08E-21 |
| ENSG00000176641 | RNF152 | 182.81 | 8.89 | 4.3723 | 5.64E-20 |
| ENSG00000260196 | - | 163.81 | 50.63 | 1.6952 | 6.05E-19 |
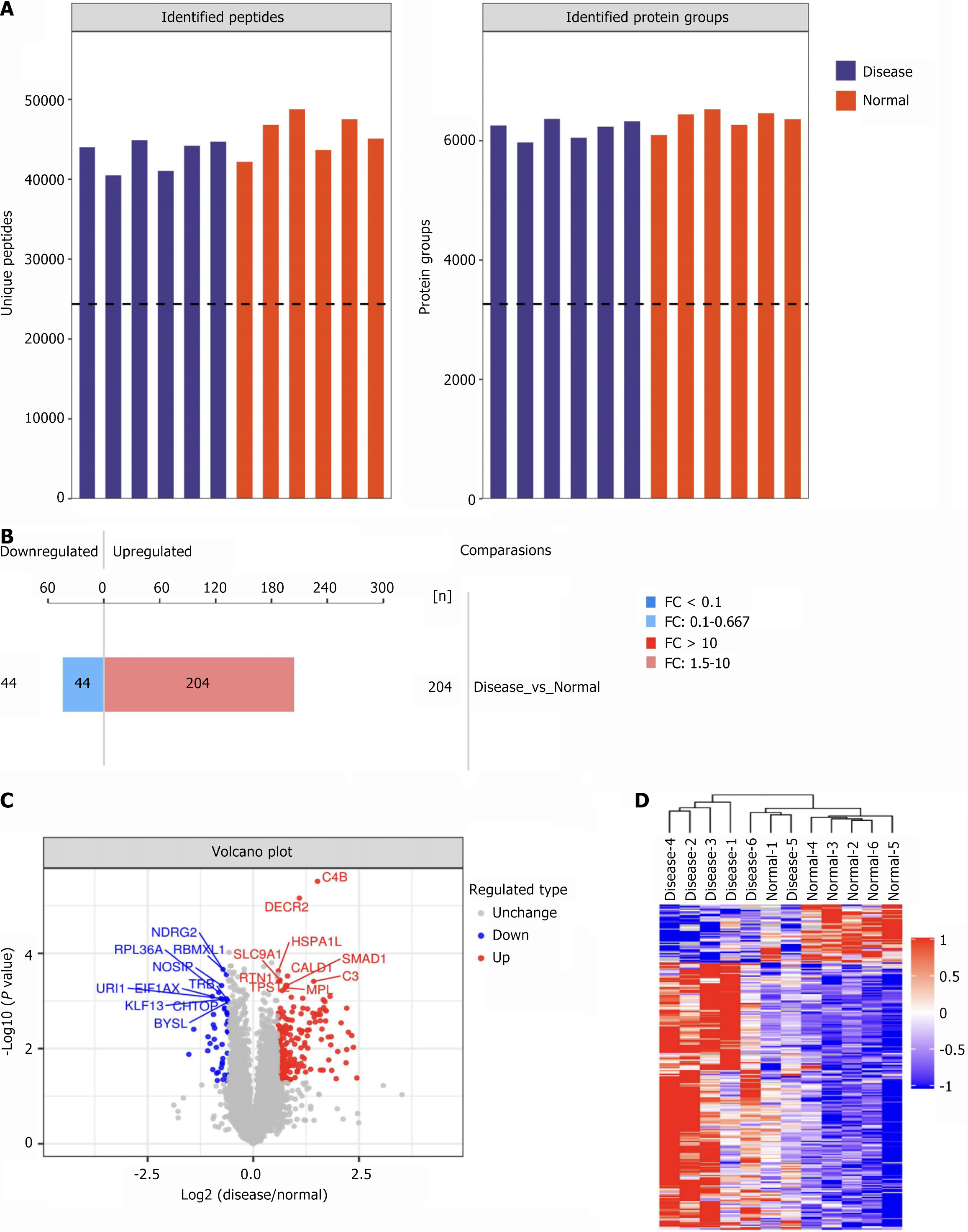
DIA-based quantitative proteomics was conducted for the SZ and HC groups. The total proteins and peptides identified in each sample are represented in a bar chart (Figure 2A). Differential analysis was performed to identify differentially expressed proteins (DEPs) between the groups, applying thresholds of fold change > 1.5 (upregulated) or < 0.67 (downregulated) with a P value < 0.05. This analysis identified 204 upregulated and 44 downregulated proteins (Figure 2B). The top 10 most significantly upregulated and downregulated DEPs are annotated in Figure 2C. Clustering analysis of DEP expression patterns effectively discriminated between SZ and HC groups, reflecting marked alterations in PBMC protein expression profiles in SZ patients (Figure 2D).
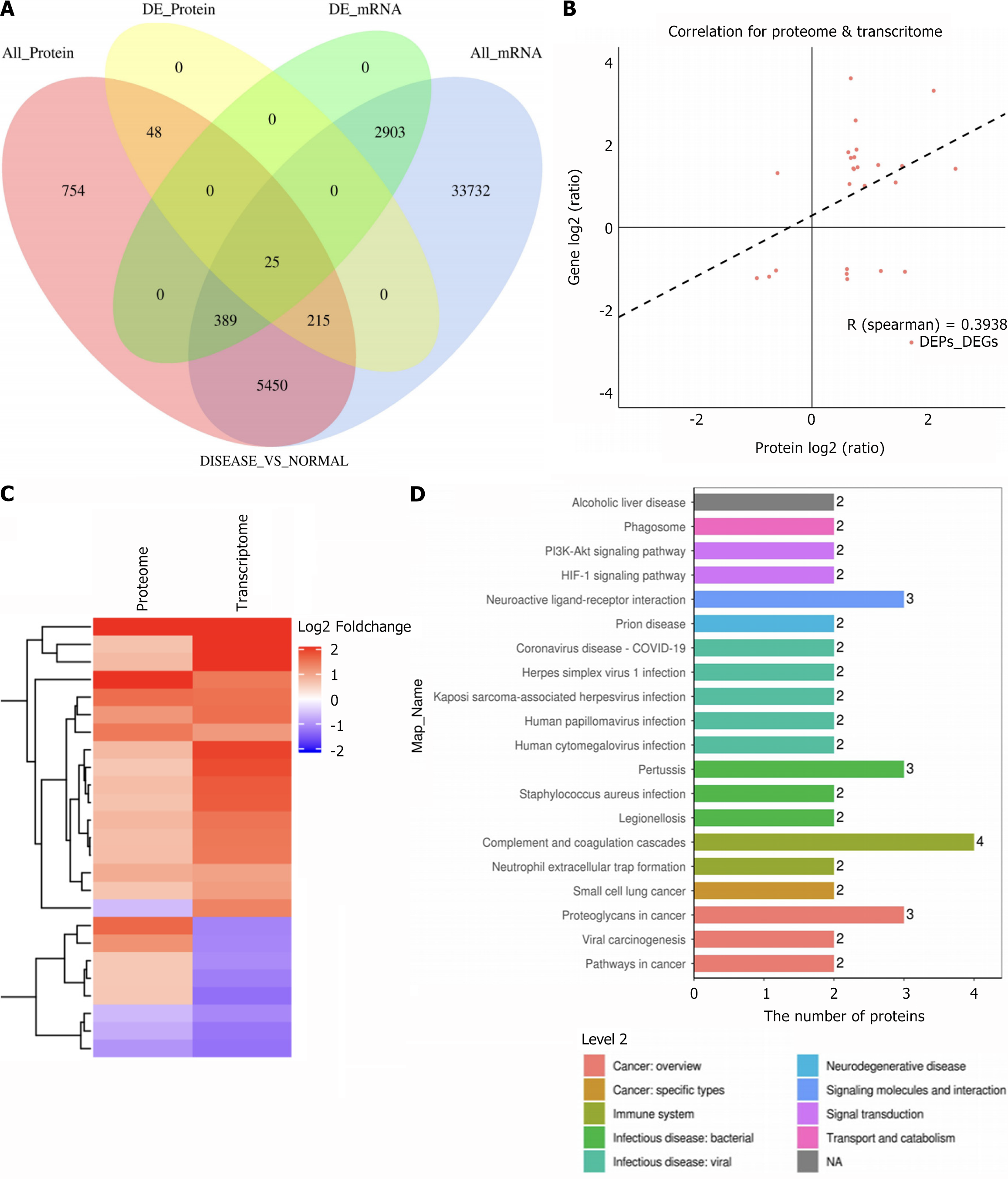
Integration of transcriptomic and proteomic data revealed 6079 commonly expressed genes, including 25 DEGs differentiating SZ from HC groups (Figure 3A). Among these, haptoglobin (HP), lactotransferrin (LTF), and SERPING1 showed significant upregulation (Table 5). A Spearman correlation coefficient of 0.3928 was observed between significant DEGs and DEPs, indicating high data reliability (Figure 3B). Clustering analysis provided a visual representation of differential expression patterns for mRNAs and DEPs (Figure 3C). KEGG enrichment analysis highlighted the neuroactive ligand-receptor interaction pathway as potentially influential in SZ progression (Figure 3D). Based on these insights, subsequent studies prioritized the neuro-associated conduction protein pathway for further mechanistic exploration.
| Gene_id | DEGs | Protein_id | Protein_log2FC | Gene_log2FC |
| ENSG00000257017 | HP | P00738 | 2.449962325 | 1.4294 |
| ENSG00000012223 | LTF | P02788 | 2.077758566 | 3.3348 |
| ENSG00000091513 | TF | P02787 | 1.587407166 | -1.0837 |
| ENSG00000149131 | SERPING1 | P05155 | 1.538266732 | 1.4989 |
| ENSG00000125730 | C3 | P01024 | 1.428640888 | 1.0951 |
| ENSG00000106804 | C5 | P01031 | 1.176272232 | -1.0651 |
| ENSG00000197249 | SERPINA1 | P01009 | 1.13095551 | 1.5216 |
| ENSG00000177697 | CD151 | P48509 | 0.89951464 | 1.0137 |
| ENSG00000122862 | SRGN | P10124 | 0.77902409 | 1.4672 |
| ENSG00000124762 | CDKN1A | P38936 | 0.760160059 | 1.8984 |
| ENSG00000079308 | TNS1 | Q9HBL0 | 0.745846947 | 2.6092 |
| ENSG00000137507 | LRRC32 | Q14392 | 0.722864535 | 1.7123 |
| ENSG00000064651 | SLC12A2 | P55011 | 0.716578449 | 1.4223 |
| ENSG00000138448 | ITGAV | P06756 | 0.706188096 | 1.4381 |
| ENSG00000108405 | P2RX1 | P51575 | 0.662687029 | 1.6951 |
| ENSG00000145685 | LHFPL2 | Q6ZUX7 | 0.662220906 | 3.638 |
| ENSG00000151327 | FAM177A1 | Q8N128 | 0.640431398 | 1.0575 |
| ENSG00000173083 | HPSE | Q9Y251 | 0.620238989 | 1.8333 |
| ENSG00000139970 | RTN1 | Q16799 | 0.601847201 | -1.2624 |
| ENSG00000235194 | PPP1R3E | Q9H7J1 | 0.597450245 | -1.0182 |
| ENSG00000204390 | HSPA1 L | P34931 | 0.593410635 | -1.1346 |
| ENSG00000108175 | ZMIZ1 | Q9ULJ6 | -0.587965548 | 1.3241 |
| ENSG00000166965 | RCCD1 | A6NED2 | -0.613166445 | -1.0529 |
| ENSG00000139193 | CD27 | P26842 | -0.730964915 | -1.2033 |
| ENSG00000168310 | IRF2 | P14316 | -0.940903659 | -1.239 |
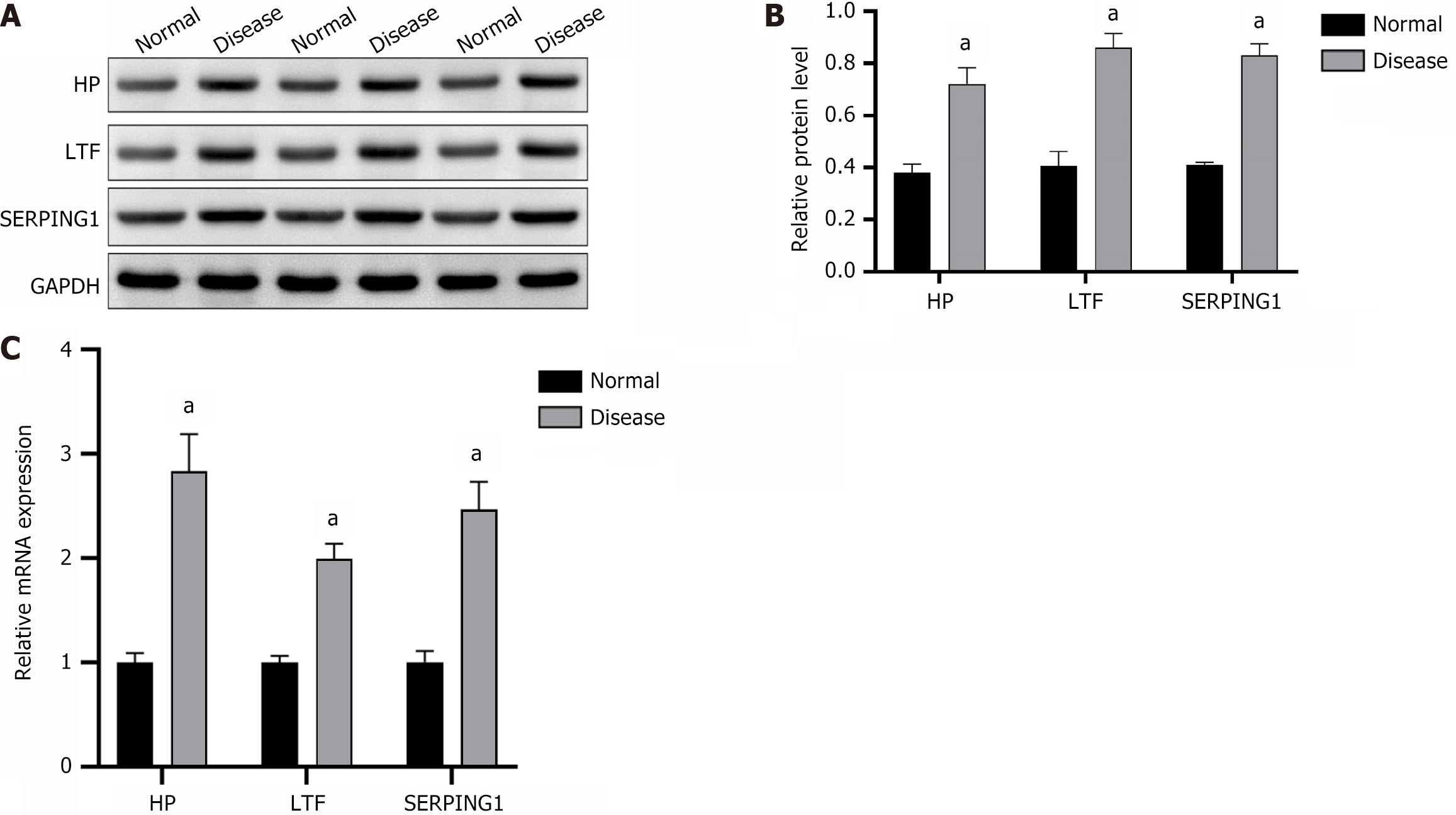
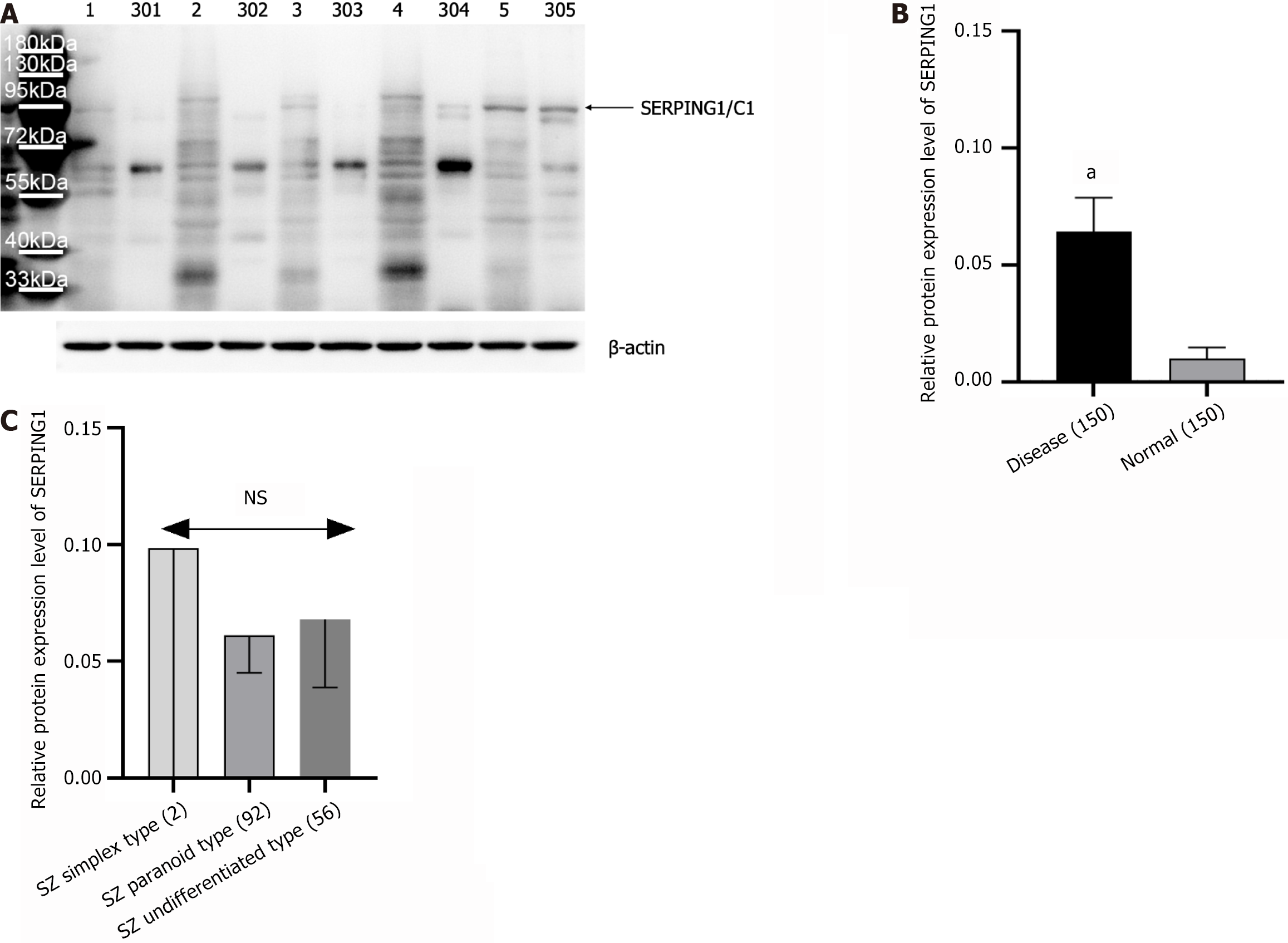
Protein expression levels of HP, LTF, and SERPING1 in PBMCs from clinical samples were analyzed via WB, revealing significant upregulation, consistent with findings from the integrated transcriptomics and proteomics analysis. Comparative analysis showed markedly higher expression of HP, LTF, and SERPING1 proteins in the SZ group relative to the HC group (Figure 4A and B). Similarly, RT-qPCR results confirmed a significant increase in mRNA expression of HP, LTF, and SERPING1 in the SZ group compared to the HC group (Figure 4C).
The study analyzed SERPING1 protein expression in 150 age- and gender-matched SZ patients and 150 HCs using WB, with representative results displayed in Figure 4A. Compared to the HC group, SERPING1 protein levels were markedly elevated in the SZ cohort (Figure 5A and B). Among the 150 SZ patients, the sample included 2 cases of the simple type, 56 of the undifferentiated type, and 92 of the paranoid type. A one-way ANOVA examining SERPING1 protein expression across these SZ subtypes showed no statistically significant differences (Figure 5C).
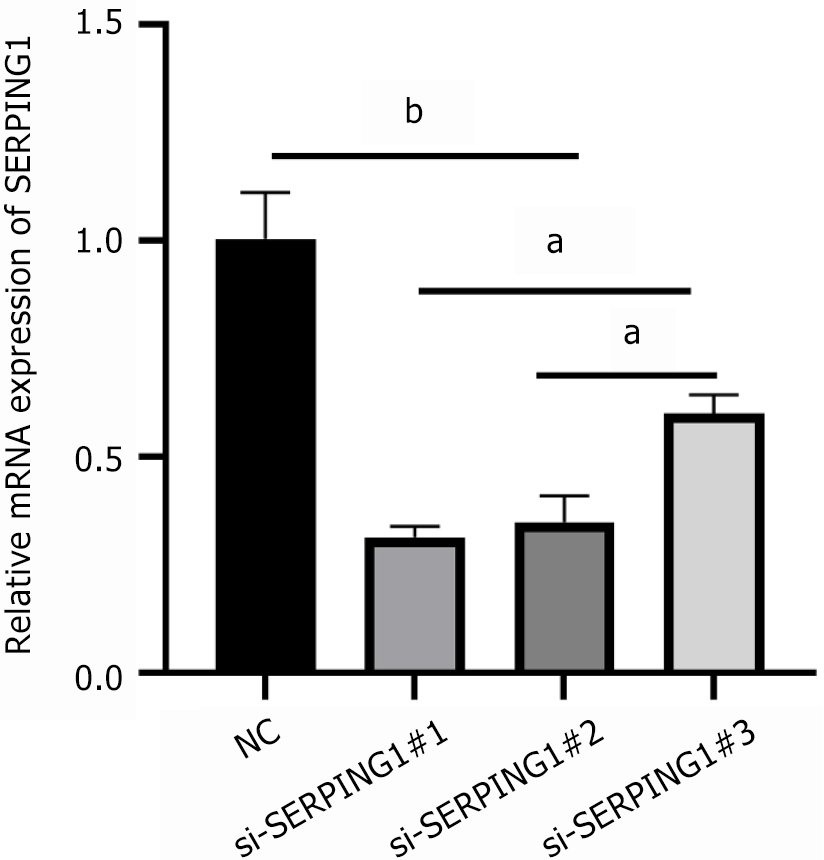
Lentiviral transfection with pre-constructed RNAi constructs effectively suppressed SERPING1 mRNA expression across all three silencing targets (si-SERPING1#1, si-SERPING1#2, and si-SERPING1#3) compared to the NC group (si-NC). Among the targets, si-SERPING1#1 demonstrated the greatest silencing efficiency (Figure 6) and was consequently chosen for further experimentation.
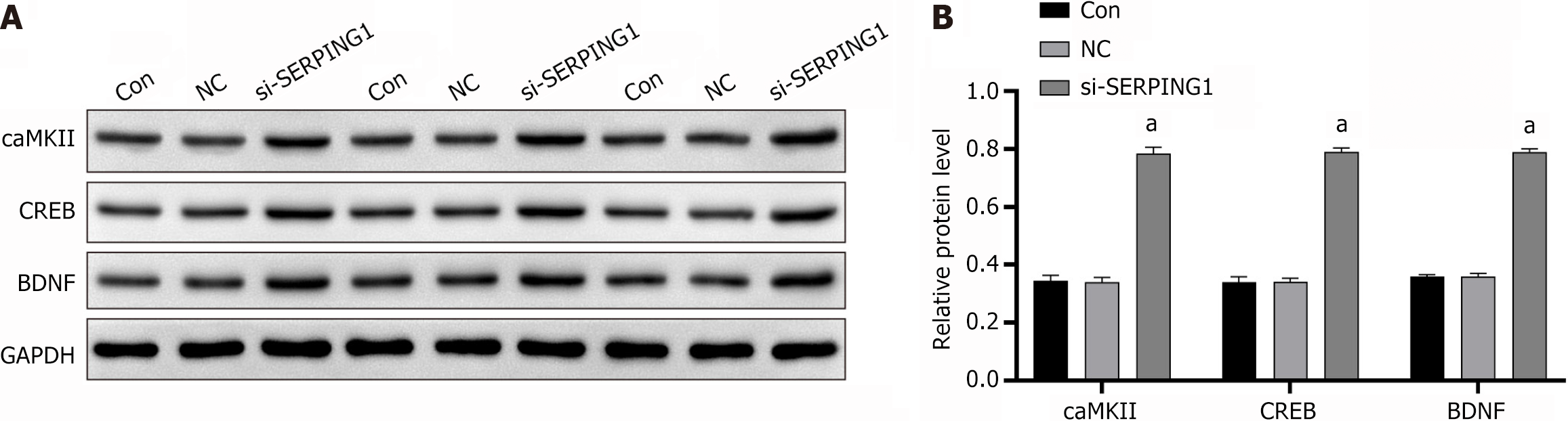
Transfection of target cells with the si-SERPING1#1 silencing lentivirus resulted in a marked upregulation of neuro-associated signaling proteins, including calcium/calmodulin-dependent protein kinase II (caMKII), cAMP response element-binding protein (CREB), and brain-derived neurotrophic factor (BDNF), when compared to the si-NC group (Figure 7).
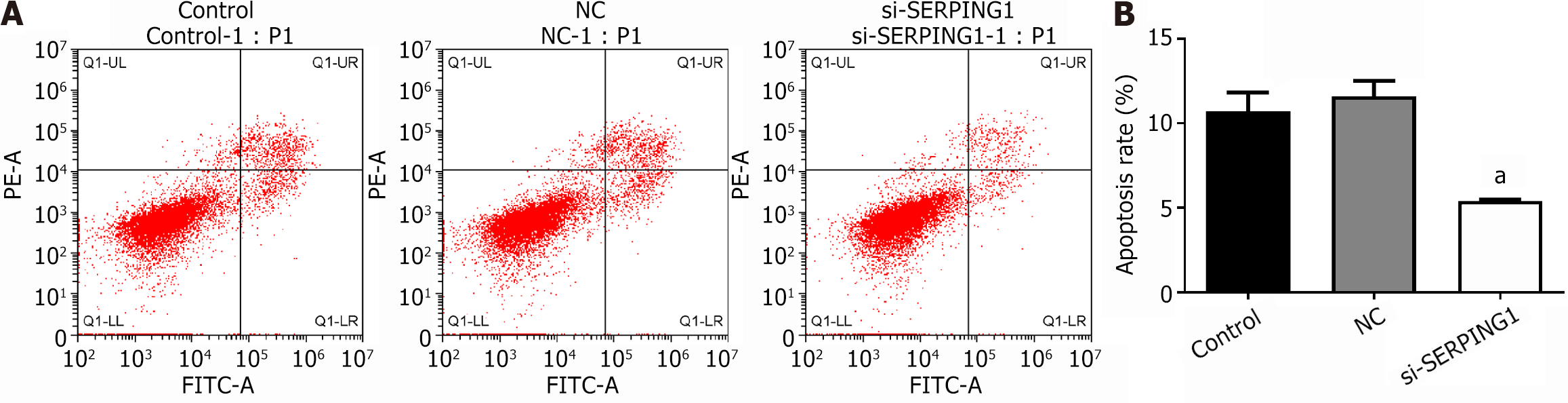
Transfection of target cells with the si-SERPING1#1 silencing lentivirus resulted in a marked reduction in apoptosis rates and an enhancement in cell viability relative to the NC group (Figures 8 and 9). The data suggest that SERPING1 silencing enhances the proliferation of rat hippocampal neurons while suppressing apoptosis.
The pursuit of reliable biomarkers for early SZ diagnosis remains a significant challenge. To date, the identification of blood-based biomarkers for major psychiatric disorders has been limited and insufficient for clinical application[17]. Research has demonstrated that PBMCs and brain tissues exhibit similar expression profiles in certain signaling and metabolic pathways[18]. Furthermore, patients experiencing first-episode SZ have shown abnormal expression of synaptic genes, which are closely linked to the disorder, in PBMCs[19]. A study by Kozłowska et al[20] on a SZ case-control cohort revealed that PBMCs were actively involved in cytokine production when stimulated by both spontaneous mechanisms and phytohemagglutinin (PHA), suggesting that immune system dysfunction may underlie the patho
The SERPING1 gene, located at 11q12-q13.1 on chromosome 11, encodes the plasma protease C1 inhibitor (C1-Inh), a key regulator of the classical complement and coagulation pathways. By targeting these systems, C1-Inh mitigates inflammatory and coagulation responses[22]. Research on SERPING1 has primarily centered on hereditary angioedema, where C1-Inh deficiency underpins the pathogenesis of types I and II hereditary angioedema[23]. Multiple variants of SERPING1 have been identified, including single nucleotide polymorphisms, small insertions or deletions, and larger structural changes such as insertions or duplications[24]. This study observed elevated SERPING1 expression in SZ but did not explore its structural variations, leaving the potential presence of pathogenic variants in SZ for future investigation. Cooper et al[25] performed a quantitative blood protein analysis in first-episode SZ patients, reporting significant upregulation of hemoglobin and C1-Inh, consistent with the proteomics findings presented here. Transcriptomic studies on post-mortem brain tissues from SZ patients revealed increased expression of immune-related and inflammatory genes, including SERPING1, implicating enhanced complement cascade activity in SZ pathophysiology[26]. Additionally, Allswede et al[27] analyzed PBMC samples from 129 adult SZ twins in Sweden, demonstrating that peripheral mRNA expression of complement genes, including SERPING1 and C5, uniquely correlated with variations in frontal cortical thickness.
The HP gene, located on chromosome 16 (16q22), is part of a multigene family comprising two alleles, HP1 and HP2, which encode the HP1 and HP2 proteins, respectively[28]. Research by Wan et al[29] identified altered HP protein expression and distinct genotype distributions in SZ patients within the Chinese Han population, suggesting a potential link between HP and SZ. Lee et al[30] reported increased HP gene expression in individuals with first-episode psychiatric disorders. The LTF gene, located on chromosome 3 (3p21) and also referred to as lactoferrin, belongs to the transferrin family. LTF encodes a multifunctional protein involved in antibacterial, antiviral, and anti-inflammatory processes, regulation of lipid metabolism, oxidative stress prevention, immune modulation, cell growth, and various enzymatic reactions[31]. Hällgren et al[32] observed significantly elevated serum LTF levels in SZ patients, independent of antipsychotic treatment. In this study, LTF exhibited substantial upregulation in both transcriptomic and proteomic analyses, highlighting its potential significance in SZ. However, the limited research on LTF in SZ emphasizes the need for further investigation, which may provide valuable insights into its role in SZ pathophysiology and guide future research directions.
The BDNF gene, located on chromosome 11p13 and spanning 70 kb with 11 exons[33], exhibits reduced expression in both first-episode and chronic SZ patients, suggesting its potential as a biomarker for cognitive impairment in SZ[34]. Polymorphisms in the BDNF gene have been linked to negative symptoms of SZ in studies conducted on the Han Chinese population[35]. Neurotrophic factors, including BDNF, are integral to neurodevelopment and synaptic plasticity[36,37]. The cAMP response CREB, a key regulator of neurotrophic responses, binds to specific sequences in the BDNF promoter in its phosphorylated form, thereby modulating transcription[38]. Guo et al[39] reported a correlation between increased BDNF mRNA expression and elevated phosphorylated CREB levels. In the hippocampus, calcium/CaMKII is critical for learning and memory consolidation. Evidence suggests that diminished CaMKII activity underlies widespread brain dysfunction, contributing to neuropsychiatric conditions such as addiction, SZ, depression, epilepsy, and other neurodevelopmental disorders, likely due to disrupted glutamate signaling and impaired neural plasticity[40]. Research on CaMKIIα heterozygous knockout mice has identified EEG and behavioral changes characteristic of SZ subtypes and intellectual disability[41]. Rodent studies have highlighted the therapeutic potential of enhancing CaMKII activity to ameliorate cognitive deficits associated with SZ[42]. Silencing SERPING1 significantly elevated the expression of neuronal pathway proteins, including CaMKII, CREB, and BDNF, suggesting its involvement in SZ through the CaMKII-CREB-BDNF signaling cascade and its potential as a novel target for SZ diagnosis and treatment. Supporting this mechanism, Lee et al[43] demonstrated that the atypical antipsychotic olanzapine promoted BDNF gene transcription by activating CREB via the PKA, PI3K, PKC, and CaMKII pathways, conferring neuroprotective effects. Cognitive impairments in SZ patients are closely associated with synaptic dysfunction. A study simulating synaptic damage in rats linked neuronal injury to the CaM/CaMKII/CREB/BDNF pathway, implicating postsynaptic ion channels and synaptic plasticity-related proteins[44]. Furthermore, theobromine treatment improved working memory in rats by upregulating the CaMKII/CREB/BDNF pathway in the medial prefrontal cortex[45]. While most research on this signaling cascade centers on depression, studies specifically addressing its role in SZ remain limited.
WB was employed to analyze SERPING1 protein expression in PBMCs from 150 SZ patients and 150 HCs, with comparisons across distinct disease subtypes. The analysis revealed significantly elevated SERPING1 protein levels in SZ patients relative to HCs. However, no notable differences were observed among the subtypes, possibly due to the limited sample size. SZ, a heterogeneous disorder influenced by polygenic and multifactorial contributions, does not adhere to Mendelian inheritance patterns. The observed disconnect between genotype and phenotype may account for the absence of a significant association between SERPING1 protein levels and clinical subtypes. An RNAi-silenced lentivirus targeting the SERPING1 gene was constructed and transfected into rat neuronal cells, resulting in markedly enhanced neuronal cell proliferation and reduced apoptosis. This intervention also increased the expression of channel proteins CaMKII, CREB, and BDNF, highlighting the neuroprotective role of the CaMKII-CREB-BDNF signaling pathway. Few studies have explored the influence of the SERPING1 gene on neuronal proliferation and apoptosis, placing this research within a nascent area of investigation. Study limitations include the exclusive enrollment of recurrent SZ patients undergoing long-term antipsychotic therapy, which may have introduced confounding variables into the peripheral blood omics analysis. Additionally, the relatively small cohort size may have led to selection bias, restricting the broader applicability of the findings to the general SZ population.
This study employed integrated transcriptomic and proteomic analyses to reveal significant upregulation of HP, LTF, and SERPING1 in PBMCs from SZ patients. Clinical sample validation further confirmed elevated SERPING1 expression levels. Silencing SERPING1 led to a pronounced increase in neural pathway-associated channel proteins, including CaMKII, CREB, and BDNF. Functional assays indicated that SERPING1 exerted anti-proliferative and pro-apoptotic effects on neuronal cells. These results suggest that SERPING1 may regulate neuronal cell proliferation via the CaMKII-CREB-BDNF pathway, potentially contributing to the development and progression of SZ. This discovery offers a foundation for further research and highlights potential therapeutic targets for the disease.
Acknowledgment is extended to the Hainan Province Clinical Medical Center for its support and to the reviewers for their valuable insights.
| 1. | Insel TR. Rethinking schizophrenia. Nature. 2010;468:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1255] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 2. | Zhao C, Habtewold TD, Naderi E, Liemburg EJ; GROUP Investigators, Bruggeman R, Alizadeh BZ. Association of clinical symptoms and cardiometabolic dysregulations in patients with schizophrenia spectrum disorders. Eur Psychiatry. 2023;67:e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Momtazmanesh S, Zare-Shahabadi A, Rezaei N. Cytokine Alterations in Schizophrenia: An Updated Review. Front Psychiatry. 2019;10:892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 4. | Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 798] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 5. | Yue W, Yang Y, Zhang Y, Lu T, Hu X, Wang L, Ruan Y, Lv L, Zhang D. A case-control association study of NRXN1 polymorphisms with schizophrenia in Chinese Han population. Behav Brain Funct. 2011;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Altamura AC, Pozzoli S, Fiorentini A, Dell'osso B. Neurodevelopment and inflammatory patterns in schizophrenia in relation to pathophysiology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, Palmer E, Karwath A, Barsky A, Gkoutos GV, Wood S, Barnes NM, David AS, Donohoe G, Neill JC, Deakin B, Khandaker GM, Upthegrove R; PIMS Collaboration. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry. 2022;79:498-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 168] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 8. | Sullivan CR, O'Donovan SM, McCullumsmith RE, Ramsey A. Defects in Bioenergetic Coupling in Schizophrenia. Biol Psychiatry. 2018;83:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Hoirisch-Clapauch S, Amaral OB, Mezzasalma MA, Panizzutti R, Nardi AE. Dysfunction in the coagulation system and schizophrenia. Transl Psychiatry. 2016;6:e704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6882] [Cited by in RCA: 5702] [Article Influence: 518.4] [Reference Citation Analysis (0)] |
| 12. | Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 587] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 13. | De Los Angeles A, Fernando MB, Hall NAL, Brennand KJ, Harrison PJ, Maher BJ, Weinberger DR, Tunbridge EM. Induced Pluripotent Stem Cells in Psychiatry: An Overview and Critical Perspective. Biol Psychiatry. 2021;90:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Comes AL, Papiol S, Mueller T, Geyer PE, Mann M, Schulze TG. Proteomics for blood biomarker exploration of severe mental illness: pitfalls of the past and potential for the future. Transl Psychiatry. 2018;8:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Mosallaei M, Ehtesham N, Rahimirad S, Saghi M, Vatandoost N, Khosravi S. PBMCs: a new source of diagnostic and prognostic biomarkers. Arch Physiol Biochem. 2022;128:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Ni P, Zhou C, Liang S, Jiang Y, Liu D, Shao Z, Noh H, Zhao L, Tian Y, Zhang C, Wei J, Li X, Yu H, Ni R, Yu X, Qi X, Zhang Y, Ma X, Deng W, Guo W, Wang Q, Sham PC, Chung S, Li T. YBX1-Mediated DNA Methylation-Dependent SHANK3 Expression in PBMCs and Developing Cortical Interneurons in Schizophrenia. Adv Sci (Weinh). 2023;10:e2300455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Mohammadi A, Rashidi E, Amooeian VG. Brain, blood, cerebrospinal fluid, and serum biomarkers in schizophrenia. Psychiatry Res. 2018;265:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Song X, Liu Y, Pu J, Gui S, Zhong X, Chen X, Chen W, Chen X, Chen Y, Wang H, Cheng K, Zhao L, Xie P. Transcriptomics Analysis Reveals Shared Pathways in Peripheral Blood Mononuclear Cells and Brain Tissues of Patients With Schizophrenia. Front Psychiatry. 2021;12:716722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Zhang T, Tang Y, Yang X, Wang X, Ding S, Huang K, Liu Y, Lang B. Expression of GSK3β, PICK1, NEFL, C4, NKCC1 and Synaptophysin in peripheral blood mononuclear cells of the first-episode schizophrenia patients. Asian J Psychiatr. 2021;55:102520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Kozłowska E, Żelechowska P, Wysokiński A, Rasmus P, Łucka A, Brzezińska-Błaszczyk E. In vitro cytokine synthesis in unstimulated and mitogen-stimulated peripheral blood mononuclear cells from individuals with schizophrenia. J Investig Med. 2019;67:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Petrikis P, Polyzou A, Premeti K, Roumelioti A, Karampas A, Georgiou G, Grigoriadis D, Leondaritis G. GSK3β and mTORC1 Represent 2 Distinct Signaling Markers in Peripheral Blood Mononuclear Cells of Drug-Naive, First Episode of Psychosis Patients. Schizophr Bull. 2022;48:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Karnaukhova E. C1-Inhibitor: Structure, Functional Diversity and Therapeutic Development. Curr Med Chem. 2022;29:467-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Ren Z, Zhao S, Li T, Wedner HJ, Atkinson JP. Insights into the pathogenesis of hereditary angioedema using genetic sequencing and recombinant protein expression analyses. J Allergy Clin Immunol. 2023;151:1040-1049.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Steiner UC, Keller M, Schmid P, Cichon S, Wuillemin WA. Mutational spectrum of the SERPING1 gene in Swiss patients with hereditary angioedema. Clin Exp Immunol. 2017;188:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Cooper JD, Ozcan S, Gardner RM, Rustogi N, Wicks S, van Rees GF, Leweke FM, Dalman C, Karlsson H, Bahn S. Schizophrenia-risk and urban birth are associated with proteomic changes in neonatal dried blood spots. Transl Psychiatry. 2017;7:1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Lindholm Carlström E, Niazi A, Etemadikhah M, Halvardson J, Enroth S, Stockmeier CA, Rajkowska G, Nilsson B, Feuk L. Transcriptome Analysis of Post-Mortem Brain Tissue Reveals Up-Regulation of the Complement Cascade in a Subgroup of Schizophrenia Patients. Genes (Basel). 2021;12:1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Allswede DM, Zheutlin AB, Chung Y, Anderson K, Hultman CM, Ingvar M, Cannon TD. Complement Gene Expression Correlates with Superior Frontal Cortical Thickness in Humans. Neuropsychopharmacology. 2018;43:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | di Masi A, De Simone G, Ciaccio C, D'Orso S, Coletta M, Ascenzi P. Haptoglobin: From hemoglobin scavenging to human health. Mol Aspects Med. 2020;73:100851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 29. | Wan C, La Y, Zhu H, Yang Y, Jiang L, Chen Y, Feng G, Li H, Sang H, Hao X, Zhang G, He L. Abnormal changes of plasma acute phase proteins in schizophrenia and the relation between schizophrenia and haptoglobin (Hp) gene. Amino Acids. 2007;32:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Lee J, Goh LK, Chen G, Verma S, Tan CH, Lee TS. Analysis of blood-based gene expression signature in first-episode psychosis. Psychiatry Res. 2012;200:52-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Kowalczyk P, Kaczyńska K, Kleczkowska P, Bukowska-Ośko I, Kramkowski K, Sulejczak D. The Lactoferrin Phenomenon-A Miracle Molecule. Molecules. 2022;27:2941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 32. | Hällgren R, Venge P, Wistedt B. Elevated serum levels of lactoferrin and eosinophil cationic protein in schizophrenic patients. Br J Psychiatry. 1982;140:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Maisonpierre PC, Le Beau MM, Espinosa R 3rd, Ip NY, Belluscio L, de la Monte SM, Squinto S, Furth ME, Yancopoulos GD. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics. 1991;10:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 396] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Yang Y, Liu Y, Wang G, Hei G, Wang X, Li R, Li L, Wu R, Zhao J. Brain-derived neurotrophic factor is associated with cognitive impairments in first-episode and chronic schizophrenia. Psychiatry Res. 2019;273:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Ping J, Zhang J, Wan J, Huang C, Luo J, Du B, Jiang T. A Polymorphism in the BDNF Gene (rs11030101) is Associated With Negative Symptoms in Chinese Han Patients With Schizophrenia. Front Genet. 2022;13:849227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 36. | Landeira BS, Santana TTDS, Araújo JAM, Tabet EI, Tannous BA, Schroeder T, Costa MR. Activity-Independent Effects of CREB on Neuronal Survival and Differentiation during Mouse Cerebral Cortex Development. Cereb Cortex. 2018;28:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Kitagawa H, Sugo N, Morimatsu M, Arai Y, Yanagida T, Yamamoto N. Activity-Dependent Dynamics of the Transcription Factor of cAMP-Response Element Binding Protein in Cortical Neurons Revealed by Single-Molecule Imaging. J Neurosci. 2017;37:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Wang H, Xu J, Lazarovici P, Quirion R, Zheng W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front Mol Neurosci. 2018;11:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 39. | Guo H, Cheng Y, Wang C, Wu J, Zou Z, Niu B, Yu H, Wang H, Xu J. FFPM, a PDE4 inhibitor, reverses learning and memory deficits in APP/PS1 transgenic mice via cAMP/PKA/CREB signaling and anti-inflammatory effects. Neuropharmacology. 2017;116:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 40. | Robison AJ. Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci. 2014;37:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Featherstone RE, Shimada T, Crown LM, Melnychenko O, Yi J, Matsumoto M, Tajinda K, Mihara T, Adachi M, Siegel SJ. Calcium/calmodulin-dependent protein kinase IIα heterozygous knockout mice show electroencephalogram and behavioral changes characteristic of a subpopulation of schizophrenia and intellectual impairment. Neuroscience. 2022;499:104-117. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Yabuki Y, Wu L, Fukunaga K. Cognitive enhancer ST101 improves schizophrenia-like behaviors in neonatal ventral hippocampus-lesioned rats in association with improved CaMKII/PKC pathway. J Pharmacol Sci. 2019;140:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Lee JG, Cho HY, Park SW, Seo MK, Kim YH. Effects of olanzapine on brain-derived neurotrophic factor gene promoter activity in SH-SY5Y neuroblastoma cells. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Xi YD, Ding J, Han J, Zhang DD, Liu JM, Feng LL, Xiao R. The effect of soybean isoflavone on the dysregulation of NMDA receptor signaling pathway induced by β-amyloid peptides 1-42 in rats. Cell Mol Neurobiol. 2015;35:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Islam R, Matsuzaki K, Sumiyoshi E, Hossain ME, Hashimoto M, Katakura M, Sugimoto N, Shido O. Theobromine Improves Working Memory by Activating the CaMKII/CREB/BDNF Pathway in Rats. Nutrients. 2019;11:888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |