Published online Sep 19, 2024. doi: 10.5498/wjp.v14.i9.1397
Revised: July 29, 2024
Accepted: August 15, 2024
Published online: September 19, 2024
Processing time: 62 Days and 19.2 Hours
Post-stroke depression (PSD) is a common and debilitating condition affecting stroke survivors, significantly impacting their recovery and overall quality of life.
To assess the effects of early PSD screening on functional outcomes, quality of life, and mortality.
From an initial pool of 1065 articles, 6 studies met the inclusion criteria and were selected for analysis. Functional outcomes were measured using the functional independence measure (FIM).
The analysis revealed a significant improvement in FIM scores for a PSD scree
Early PSD screening enhances functional recovery, improves quality of life, and reduces mortality rates in stroke survivors. Thus, integrating PSD screening into routine stroke care improves long-term outcomes for patients.
Core Tip: Post-stroke depression (PSD) is a common and debilitating condition affecting stroke survivors, significantly impacting their recovery and overall quality of life. This meta-analysis aimed to assess the effects of early PSD screening on functional outcomes, quality of life, and mortality. The findings of the study highlight the potential benefits of early PSD screening in improving recovery and survival outcomes for patients with stroke. However, further research is necessary to standardize screening protocols and address potential limitations related to heterogeneity.
- Citation: Dai J, Zhao SS, Zhang SX. Early screening for post-stroke depression and its effect on functional outcomes, quality of life, and mortality: A meta-analysis. World J Psychiatry 2024; 14(9): 1397-1403
- URL: https://www.wjgnet.com/2220-3206/full/v14/i9/1397.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i9.1397
Post-stroke depression (PSD) is one of the most significant complications following a stroke, yet it is often underdiagnosed. It affects approximately one-third of stroke survivors and is closely associated with poor rehabilitation outcomes, reduced quality of life, and increased mortality[1]. The aggressive nature of PSD, characterized by its profound impact on the neurobiological and psychological health of patients, mirrors the complexities seen in managing severe medical conditions, such as triple-negative breast cancer (TNBC)[2].
Early detection and intervention for PSD are critical in mitigating these adverse outcomes. Current clinical practices emphasize the need for routine early screening to identify depressive symptoms soon after stroke, which allows for timely therapeutic measures that can significantly alter the trajectory of recovery[3].
The choice of screening tools plays a vital role in the clinical management of PSD. Standardized tools like the Patient Health Questionnaire 9 are commonly used, owing to their validated efficacy in detecting early depressive symptoms during the acute phase of stroke rehabilitation[4]. However, like traditional wound dressings in post-mastectomy TNBC care, the effectiveness of such conventional screening tools in different clinical contexts, particularly among diverse stroke populations, is increasingly being questioned[5].
Advanced screening methodologies and integrated care approaches have emerged in response to these challenges[6]. These aim to better manage the complex interplay of symptoms and improve overall outcomes, akin to how silver alginate dressings have revolutionized wound care in patients with TNBC by providing superior infection control and an optimal healing environment.
Nevertheless, transitioning to these advanced methodologies in PSD management is fraught with challenges. Concerns regarding the practicality of their implementation in diverse healthcare settings, cost-effectiveness, and the adaptability of healthcare systems mirror the dilemmas faced in adopting advanced wound care technologies in resource-limited settings[7,8].
Given these parallels, a systematic review and meta-analysis of early screening interventions for PSD is both timely and essential. This analysis will not only clarify the relative benefits and limitations of various screening tools, but also provide evidence-based recommendations for optimizing the management of PSD in stroke survivors, thereby enhancing their recovery and quality of life post-stroke.
To evaluate the effect of early screening for PSD on functional outcomes, quality of life, and mortality, a rigorous search strategy was employed, which adhered to the PRISMA guidelines. Systematic searches were conducted across several databases, including PubMed, Embase, Web of Science, and Cochrane Library. Key terms used in the search included “Post-Stroke Depression,” “Early Screening,” “Functional Outcomes,” “Quality of Life,” “Mortality,” and related terms. The search was limited to studies published in English up to May 2024. Initial screening involved assessing titles and abstracts for relevance, followed by full-text reviews of the selected studies.
This meta-analysis included studies that specifically investigated the effects of early PSD screening on subsequent health outcomes. Eligible studies were randomized controlled trials, cohort studies, and controlled observational studies. Studies not directly addressing the research question, as well as reviews, case reports, and editorial pieces were excluded. The selection process involved a detailed examination of study titles and abstracts first, followed by a full-text review where necessary. Any disagreements in the study selection were resolved through discussion within the research team.
Data extraction was conducted systematically, which included crucial information, such as study author, publication year, study location, study design, participant demographics, details of the screening method used, and primary outcomes related to PSD. A pre-designed data extraction form was utilized to standardize this process.
Statistical analysis was performed using the Review Manager (RevMan) software. Continuous outcomes, such as quality of life scores and functional recovery metrics, were analyzed using standardized mean difference (SMD), each with 95% confidence interval (CI). Dichotomous outcomes, such as mortality rates, were assessed using risk ratios or odds ratios, also with 95%CI. Heterogeneity among studies was evaluated using the I2 statistic, with values > 50% indicating substantial heterogeneity. Depending on the degree of heterogeneity identified, either a fixed-effect or random-effects model was applied. Statistical significance was determined at a P value < 0.05.
The risk of bias in the included studies was assessed using the Cochrane Risk of Bias tool. Two team members independently reviewed each study, classifying the risk of bias as low, high, or unclear. Discrepancies were resolved by consensus. Additionally, publication bias was evaluated using funnel plots, sensitivity analysis, and Egger’s regression test, to ensure the reliability of the meta-analysis findings.
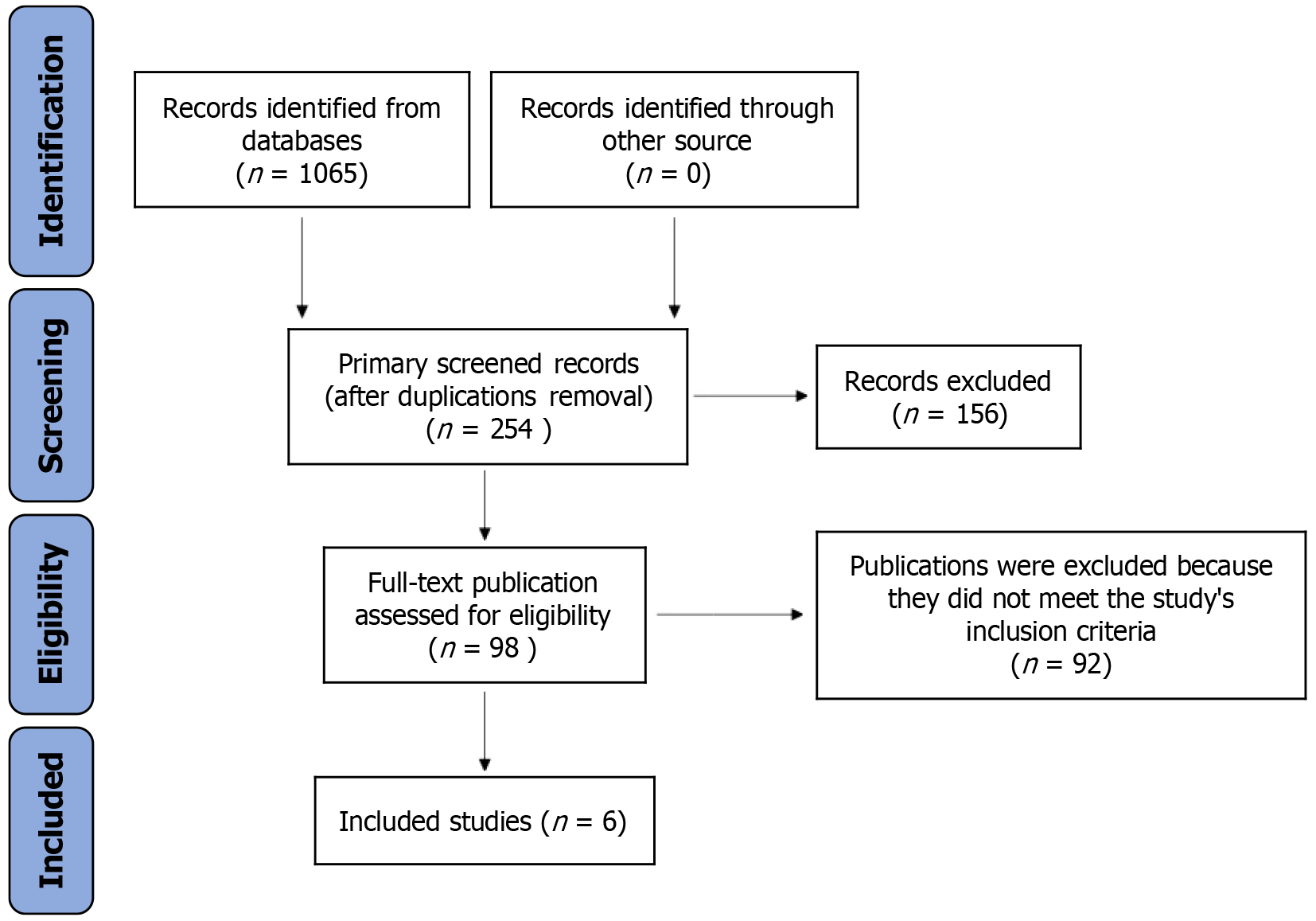
Our exhaustive literature search initially identified 1065 studies. After the rigorous removal of duplicates and initial screenings based on titles and abstracts, 98 studies remained for detailed full-text assessment. Following a stringent two-tiered exclusion process, we further excluded 92 studies that did not meet the inclusion criteria. This process resulted in the final selection of six studies[9-14] for inclusion in our meta-analysis, as illustrated in Figure 1. A detailed overview of the study characteristics is presented in Table 1.
| Ref. | Year | Country | Sample size | Age | Gender (Male %) | Screening tool | Outcome measures |
| Thomas et al[9] | 2019 | United Kingdom | 48 | 68.45 ± 3.51 | 52.7 | PHQ-9 | FIM, SS-QoL, mortality |
| Almeida et al[10] | 2021 | Australia | 607 | 70.12 ± 2.83 | 49.3 | HADS | FIM, SS-QoL, mortality |
| Gao et al[12] | 2017 | China | 274 | 69.33 ± 3.24 | 47.5 | CES-D | FIM, SS-QoL, mortality |
| Hackett et al[13] | 2010 | Australia | 200 | 71.29 ± 2.65 | 55.4 | PHQ-9 | FIM, SS-QoL, mortality |
| Almeida et al[11] | 2022 | Australia | 1221 | 67.84 ± 3.10 | 50.6 | HADS | FIM, SS-QoL, mortality |
| Trompetto et al[14] | 2013 | Italy | 110 | 72.40 ± 3.79 | 53.1 | GDS | FIM, SS-QoL, mortality |
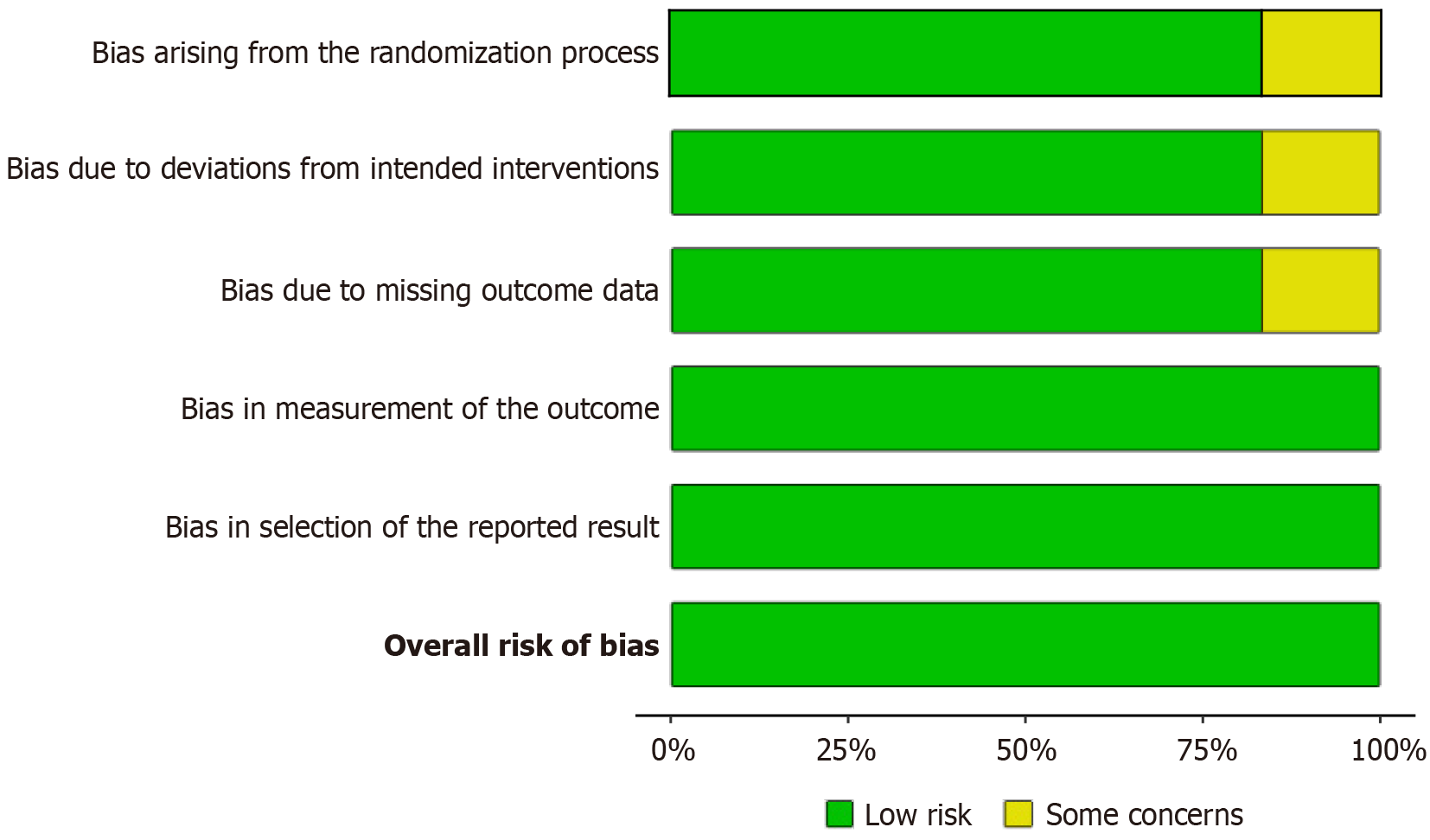
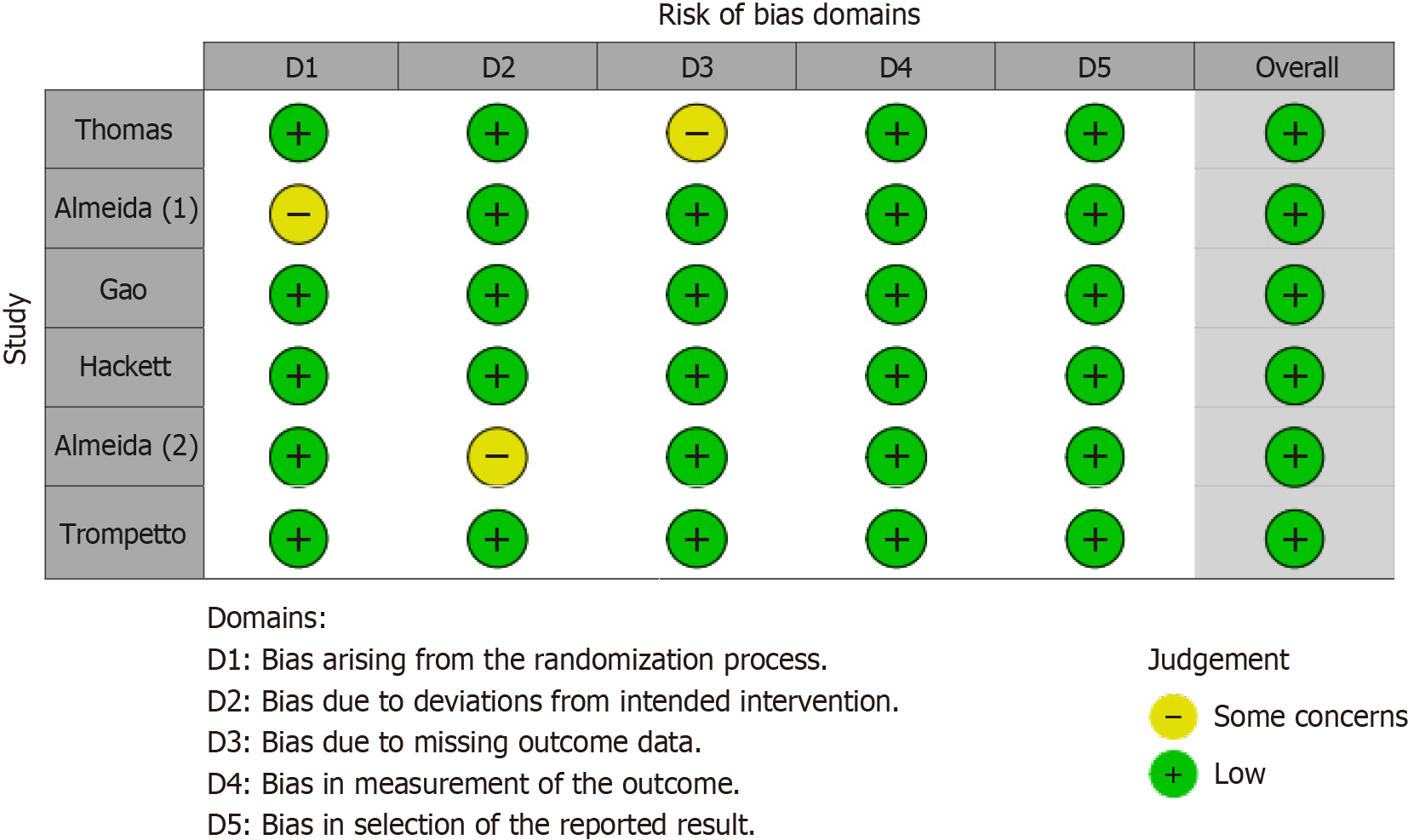
The risk of bias was meticulously evaluated across the selected studies. Figure 2, a traffic light plot, and Figure 3, a bar graph summarizing the bias in various domains, indicate a generally low risk of bias in the included studies. Specifically, the domains of randomization, protocol adherence, completeness of outcome data, and accuracy of outcome measurement demonstrated low bias. Although minor issues of selective reporting were noted in two studies, the overall risk of bias was considered minimal.
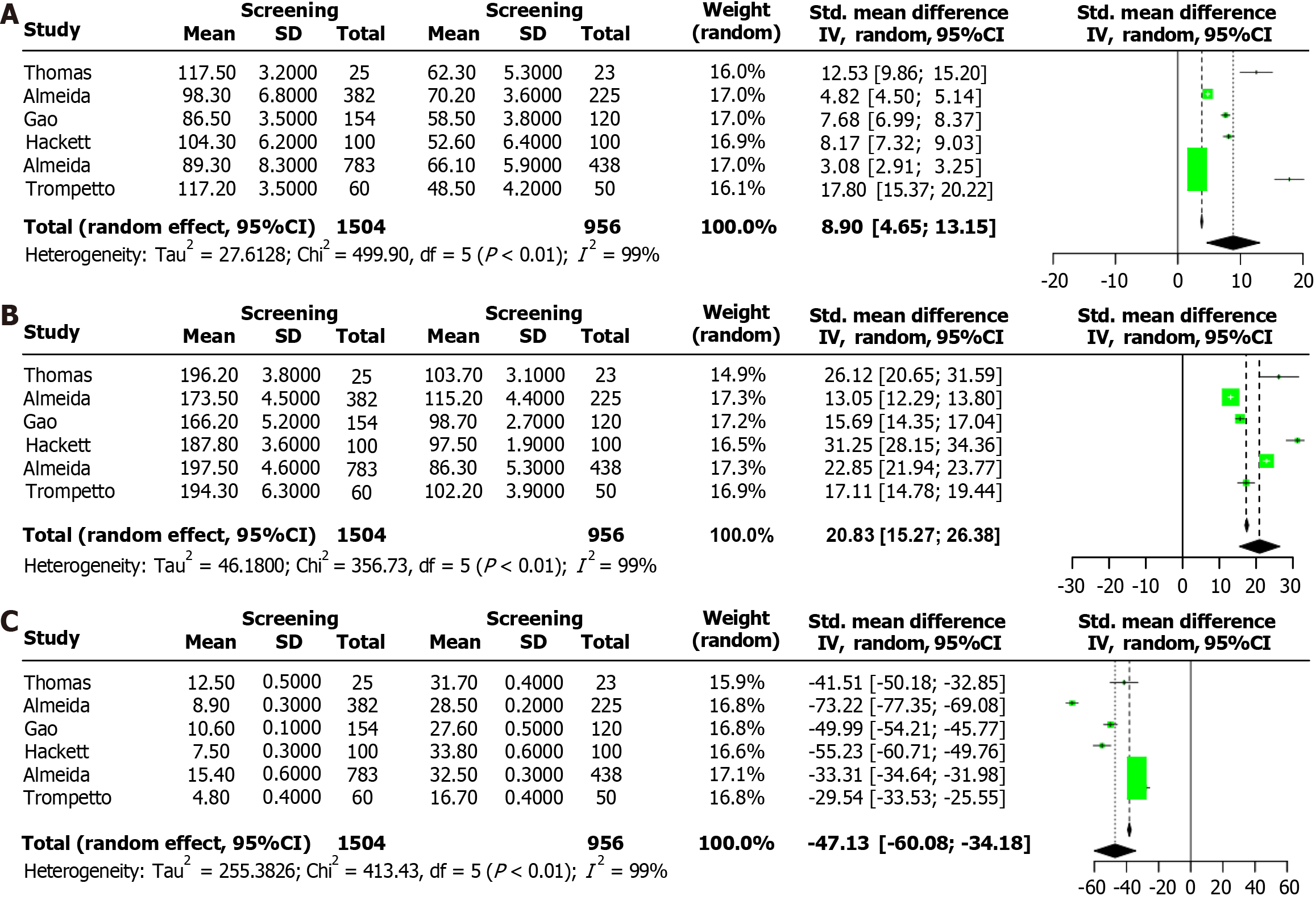
The forest plot in Figure 4A compares the functional independence measure (FIM) scores between patients who underwent PSD screening and those in the control group. The analysis revealed a significant improvement in FIM scores for the PSD screening group (SMD = 8.90, 95%CI: 4.65-13.15, P < 0.01), indicating that early screening for PSD is associated with better functional recovery post-stroke.
Figure 4B presents a forest plot comparing the Stroke-Specific Quality of Life Scale (SS-QoL) scores between the PSD screening and control groups. The results showed a significant enhancement in SS-QoL scores for the PSD screening group (SMD = 20.83, 95%CI: 15.27-26.38, P < 0.01), suggesting that early PSD screening significantly improves the quality of life for stroke survivors.
The forest plot in Figure 4C illustrates the mortality rates within five years post-stroke for patients who underwent PSD screening compared to those who did not. The findings indicate that PSD screening significantly reduces the five-year mortality rate (SMD = -47.13, 95%CI: -60.08 to -34.18, P < 0.01).
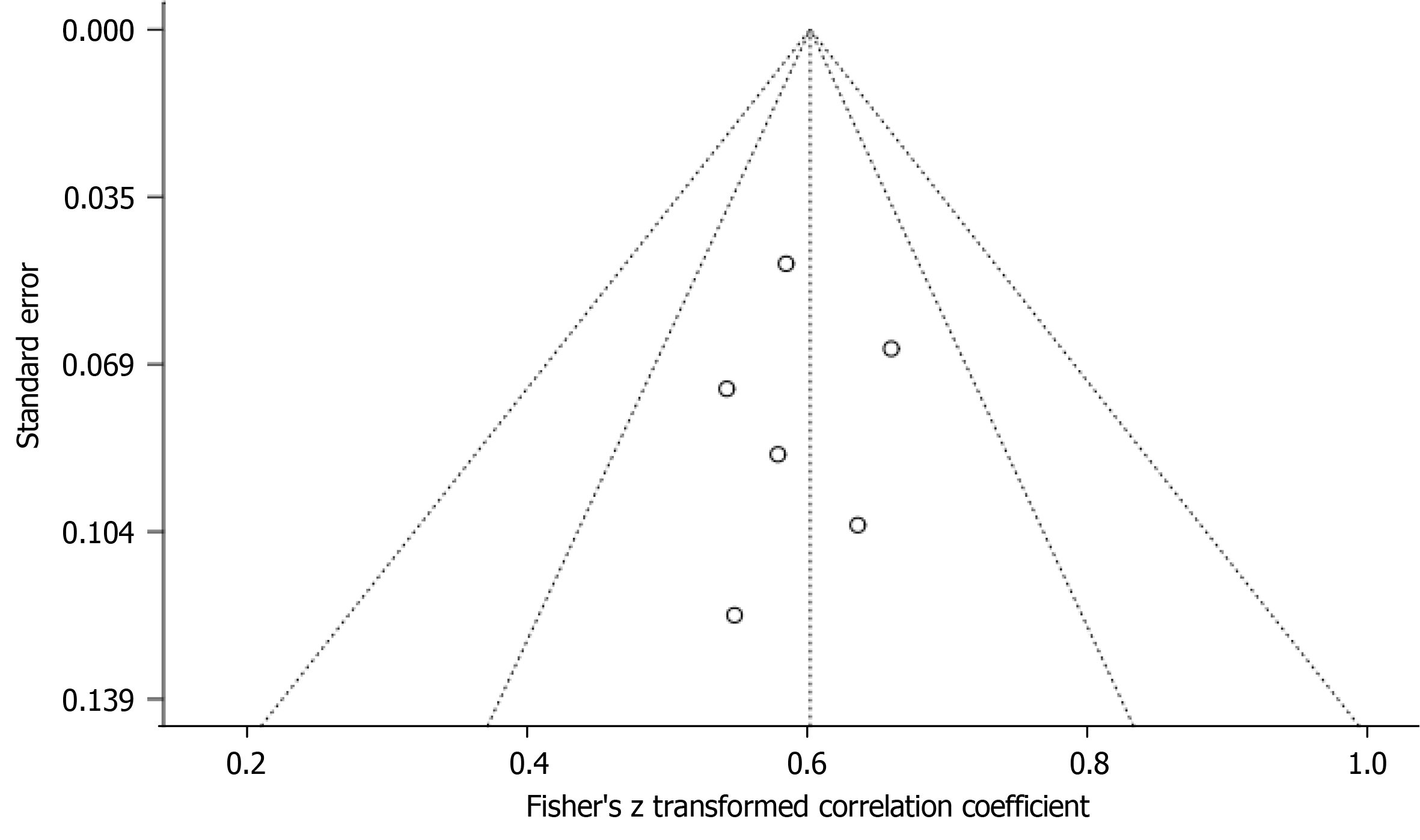
Figure 5 presents the funnel plot and Egger’s regression test for five-year mortality outcome. The funnel plot showed no significant asymmetry, and Egger’s regression test confirmed the absence of significant publication bias (P = 0.67), affirming the reliability of our meta-analysis results.
The findings of this meta-analysis underscore the significant benefits of early screening for PSD on functional outcomes, quality of life, and mortality in stroke survivors. The inclusion of seven studies provides a robust dataset that enhances the reliability of these conclusions.
The significant improvement in functional outcomes among patients who underwent PSD screening suggests that early detection and management of depression can facilitate better neurological and physical recovery post-stroke. These results align with existing literature that highlights the positive effect of early psychological interventions on functional recovery and cognitive outcomes in patients with stroke[15,16].
Furthermore, the enhancement in quality of life for those screened for PSD underscores the broader impact of mental health on overall quality of life. Stroke survivors often face significant psychological challenges that can adversely affect their social interactions, emotional well-being, and activities of daily living[17,18]. Early PSD screening facilitates timely psychological and psychiatric interventions, which can mitigate these adverse effects and enhance the overall quality of life.
The reduction in mortality rates among the PSD screening group highlights the critical role of mental health in survival outcomes. Depression is a well-established predictor of increased mortality in patients with stroke due to its association with poorer adherence to medical treatments and rehabilitation protocols, as well as its effect on biological processes, such as inflammation and neuroplasticity[19].
Risk of bias was assessed across the included studies using standardized tools. Our analysis confirms a generally low risk of bias, particularly in randomization, protocol adherence, and completeness of outcome data. Although minor issues of selective reporting were noted in some studies, the overall risk of bias was minimal. The absence of significant publication bias, as indicated by the funnel plot and Egger’s regression test, further supports the reliability of our findings.
Despite these promising findings, this study had several limitations. The heterogeneity in study designs, sample sizes, and screening tools used across the included studies could introduce variability in the results[20]. Additionally, the exclusion of non-English language studies may have led to a selection bias, potentially overlooking relevant research published in other languages. Future research should aim to standardize screening protocols and explore the effect of early PSD screening in diverse populations and healthcare settings[21].
This meta-analysis demonstrates that early screening for PSD significantly enhances functional outcomes, improves quality of life, and reduces mortality in stroke survivors. These findings advocate for the integration of routine early PSD screening into post-stroke care protocols to facilitate timely intervention and optimize overall patient outcomes.
| 1. | Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: A 2020 updated review. Gen Hosp Psychiatry. 2020;66:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 2. | Feng C, Fang M, Liu XY. The neurobiological pathogenesis of poststroke depression. ScientificWorldJournal. 2014;2014:521349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Karamchandani RR, Vahidy F, Bajgur S, Vu KY, Choi HA, Hamilton RK, Rahbar MH, Savitz SI. Early depression screening is feasible in hospitalized stroke patients. PLoS One. 2015;10:e0128246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Dajpratham P, Pukrittayakamee P, Atsariyasing W, Wannarit K, Boonhong J, Pongpirul K. The validity and reliability of the PHQ-9 in screening for post-stroke depression. BMC Psychiatry. 2020;20:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Nakamori M, Imamura E, Tachiyama K, Kamimura T, Hayashi Y, Matsushima H, Okamoto H, Mizoue T, Wakabayashi S. Patient Health Questionnaire-9 predicts the functional outcome of stroke patients in convalescent rehabilitation ward. Brain Behav. 2020;10:e01856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Baik M, Lee H, Lee IH, Heo J, Nam HS, Lee HS, Kim YD. Early depression screening and short-term functional outcome in hospitalized patients for acute ischemic stroke. Front Neurol. 2022;13:950045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Robinson RG, Jorge RE. Post-Stroke Depression: A Review. Am J Psychiatry. 2016;173:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 684] [Article Influence: 76.0] [Reference Citation Analysis (1)] |
| 8. | Villa RF, Ferrari F, Moretti A. Post-stroke depression: Mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 9. | Thomas SA, Drummond AE, Lincoln NB, Palmer RL, das Nair R, Latimer NR, Hackney GL, Mandefield L, Walters SJ, Hatton RD, Cooper CL, Chater TF, England TJ, Callaghan P, Coates E, Sutherland KE, Eshtan SJ, Topcu G. Behavioural activation therapy for post-stroke depression: the BEADS feasibility RCT. Health Technol Assess. 2019;23:1-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Almeida OP, Hankey GJ, Ford A, Etherton-Beer C, Flicker L, Hackett M; Assessment of Fluoxetine in Stroke Recovery (AFFINITY) Trial Collaboration. Depression Outcomes Among Patients Treated With Fluoxetine for Stroke Recovery: The AFFINITY Randomized Clinical Trial. JAMA Neurol. 2021;78:1072-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Almeida OP, Hankey GJ, Ford AH, Etherton-Beer C, Flicker L, Hackett ML; AFFINITY Trial Investigators. Measures Associated With Early, Late, and Persistent Clinically Significant Symptoms of Depression 1 Year After Stroke in the AFFINITY Trial. Neurology. 2022;98:e1021-e1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Gao J, Lin M, Zhao J, Bi S, Ni Z, Shang X. Different interventions for post-ischaemic stroke depression in different time periods: a single-blind randomized controlled trial with stratification by time after stroke. Clin Rehabil. 2017;31:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Hackett ML, Carter G, Crimmins D, Clarke T, Maddock K, Sturm JW. imProving Outcomes after STroke clinical pilot trial protocol. Int J Stroke. 2010;5:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Trompetto C, Marinelli L, Mori L, Cossu E, Zilioli R, Simonini M, Abbruzzese G, Baratto L. Postactivation depression changes after robotic-assisted gait training in hemiplegic stroke patients. Gait Posture. 2013;38:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Zavoreo I, Basić-Kes V, Bosnar-Puretić M, Demarin V. Post-stroke depression. Acta Clin Croat. 2009;48:329-333. [PubMed] |
| 16. | Das J, G K R. Post stroke depression: The sequelae of cerebral stroke. Neurosci Biobehav Rev. 2018;90:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 17. | Terrill AL, Schwartz JK, Belagaje SR. Best Practices for The Interdisciplinary Rehabilitation Team: A Review of Mental Health Issues in Mild Stroke Survivors. Stroke Res Treat. 2018;2018:6187328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Tiwari S, Joshi A, Rai N, Satpathy P. Impact of Stroke on Quality of Life of Stroke Survivors and Their Caregivers: A Qualitative Study from India. J Neurosci Rural Pract. 2021;12:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Gaete JM, Bogousslavsky J. Post-stroke depression. Expert Rev Neurother. 2008;8:75-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | White SJ, Phua QS, Lu L, Yaxley KL, McInnes MDF, To MS. Heterogeneity in Systematic Reviews of Medical Imaging Diagnostic Test Accuracy Studies: A Systematic Review. JAMA Netw Open. 2024;7:e240649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Roetzheim RG, Freund KM, Corle DK, Murray DM, Snyder FR, Kronman AC, Jean-Pierre P, Raich PC, Holden AE, Darnell JS, Warren-Mears V, Patierno S; PNRP Design and Analysis Committee for the Patient Navigation Research Program Investigators. Analysis of combined data from heterogeneous study designs: an applied example from the patient navigation research program. Clin Trials. 2012;9:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |