Published online Aug 19, 2024. doi: 10.5498/wjp.v14.i8.1216
Revised: July 15, 2024
Accepted: July 17, 2024
Published online: August 19, 2024
Processing time: 55 Days and 20.6 Hours
Alzheimer's disease (AD), characterized by the ongoing deterioration of neural function, often presents alongside depressive features and greatly affects the quality of life of individuals living with the condition. Although several treatment methods exist, their efficacy is limited. In recent years, repetitive transcranial magnetic stimulation (rTMS) utilizing the theta burst stimulation (TBS) mode, specifically the intermittent TBS (iTBS), has demonstrated promising therapeutic potential in the management of neuropsychiatric disorders.
To examine the therapeutic efficacy of iTBS mode of rTMS for treating depressive symptoms in patients with AD.
This retrospective study enrolled 105 individuals diagnosed with AD with depressive symptoms at Huzhou Third Municipal Hospital, affiliated with Huzhou University, between January 2020 and December 2023. Participants received standard pharmacological interventions and were categorized into control (n = 53) and observation (n = 52) groups based on treatment protocols. The observation group received iTBS mode of rTMS, while the control group received pseudo-stimulation. A comparative analysis evaluated psychological well-being, adverse events, and therapeutic at initiation of hospitalization (T0) and 15 days post-treatment (T1).
At T1, both groups exhibited a marked reduction in self-rating depression scale and Hamilton depression scale scores compared to T0. Furthermore, the observa
The iTBS model of rTMS effectively treated AD with depression, improving depressive symptoms and cognitive function in patients without serious adverse reactions, warranting clinical consideration.
Core Tip: Alzheimer's disease (AD) predominantly impacts brain neurons, being a chronic neurodegenerative condition. With disease progression, many patients with AD experience depressive symptoms, significantly impeding their lives while imposing a substantial strain on families and society. Therefore, this study investigated the clinical effects of repetitive transcranial magnetic stimulation-intermittent theta burst stimulation (iTBS-rTMS) on patients with AD experiencing depressive symptoms. Our findings suggest iTBS-rTMS significantly improves depressive symptoms and shows promise in enhancing cognitive function, offering a novel approach to managing comorbid depression in patients with AD and promoting the development of the field of neuroscience.
- Citation: Jin X, Xu CY, Fei JF, Fang Y, Sun CH. Alzheimer's disease with depressive symptoms: Clinical effect of intermittent theta burst stimulation repetitive transcranial magnetic stimulation. World J Psychiatry 2024; 14(8): 1216-1223
- URL: https://www.wjgnet.com/2220-3206/full/v14/i8/1216.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i8.1216
Alzheimer's disease (AD) is a prevalent form of dementia that manifests as gradual deterioration of cognitive and behavioral skills due to degenerative lesions in the central nervous system. The core clinical symptoms include a decline in memory, comprehension, computation, orientation, self-care ability, and other aspects that are often accompanied by a decline in both cognitive and physical functions. It is characterized by insidious onset and progressive exacerbation[1,2]. With the progression of global population aging, there is an increasing concern about AD, which accounts for 60% to 80% of all dementia types[3]. Studies have shown that AD is associated with depression during disease progression[4]. Depressive symptoms accelerate the decline of cognitive function and self-care ability in patients with AD, profoundly impacting their overall quality of life and leading to heightened mortality and suicide rates[5]. Consequently, treating and managing depressive symptoms among patients with AD is crucial and should not be ignored. In the past, patients with AD and mood disorders were mainly treated with antidepressant medications, which could improve their mood, but were prone to side effects, leading to poor adherence to treatment[6,7]. Today, the effective treatment of AD is still a major problem plaguing scholars at home and abroad; therefore, actively seeking safe and effective alternative or adjunctive therapeutic measures for patients with AD is of great practical significance.
Repetitive transcranial magnetic stimulation (rTMS) is an electrophysiological therapy known for its precise positioning, minimal invasiveness, and painlessness, thereby effectively alleviating clinical symptoms in patients with depression[8]. However, this treatment also has shortcomings, such as a long stimulation time and treatment course, resulting in relatively low efficiency and low compliance. Theta burst stimulation (TBS) represents an innovative type of rTMS. Its advantage is that it can excite the cerebral cortex for a longer time under smaller stimulation intensities and times. Compared with conventional rTMS stimulation, this is more in line with the neuron discharge mode[9]. At the same time, according to the different stimulation and intermittent times, as well as the distinct impacts on cortical excitability, TBS can be categorized into intermittent TBS (iTBS), which facilitates excitatory outcomes, and continuous TBS, which results in inhibitory effects. It has been found that rTMS in the TBS mode is effective and safe for the treatment of anxiety, depression, and hallucinations[10]. However, there are no reports on the use of non-continuous TBS with rTMS for the treatment of patients with AD experiencing depressive symptoms. Based on this, the present study selected 105 patients with AD and depressive symptoms as research participants, aiming to investigate the application effect of the non-continuous theta short-burst rapid pulse mode of rTMS, with a view of providing a reference for the popularization of the application of the TBS mode.
The clinical data of 105 patients with AD accompanied by depressive symptoms admitted to the Huzhou Third Municipal Hospital, The Affiliated Hospital of Huzhou University, between January 2020 and December 2023, were selected and divided into control group (n = 53) and observation group (n = 52) according to the different treatment modalities.
Inclusion criteria: (1) Participants must meet the clinical diagnostic criteria of AD[11] and the diagnostic criteria of depression, specifically AD combined with depressive symptoms; (2) Cranial magnetic resonance imaging or computed tomography suggesting diffuse cerebral cortical atrophy; (3) Mini-mental state examination (MMSE) scores for illiterate people ≤ 17 points, primary school group ≤ 20 points, secondary school and above group ≤ 24 points; (4) Cornell scale for depression in dementia score ≥ 8 points; (5) No history of epilepsy or other psychiatric disorders; (6) Aged 55-85 years old; (7) Absence of other systemic and organ pathologies; (8) Normal literacy; and (9) Stable vital signs.
Exclusion criteria: (1) Those who were receiving treatment related to this disease; (2) Those who were depressed due to other diseases or emotional factors; (3) Those who had other combinations that may cause cognitive dysfunction; (4) Those who had severe cardiovascular and cerebrovascular diseases or hepatic and renal insufficiency; (5) Those who had severe dementia; (6) Those who had unauthorized use of other treatment protocols related to this disease during the course of treatment; and (7) Those who actively withdrew from treatment.
Both groups of patients were treated with conventional medication, and the observation group received treatment with the non-sustained theta short-burst rapid pulse mode of rTMS. A rTMS therapeutic instrument was used, with the frequency set at 1 Hz and the stimulation intensity at 80% of the motor threshold (MT). The main stimulation site was the dorsolateral prefrontal cortex (DLPFC) of the bilateral frontal lobes. A quiet treatment place was chosen, maintaining the room temperature at 16-23 °C. Patients assumed a flat or lateral position, with the treatment coil centered in the temporal cortex. It was adjusted to the non-sustained TBS mode for treatment, with the stimulation site and volume adjusted according to the amplitude of the waveform of the action evoked potentials during treatment. The first-day parameter was 80% MT, and on the next day, it was 100% MT. The stimulation site was the left DLPFC. The inter-cluster frequency was 5 Hz, the series stimulation time was 2 s, the series interval time was 8 s, the number of pulses in the cluster was 3, and the intra-cluster frequency was 50 Hz. The total number of pulses administered was 600, totaling 3 min and 12 s per session, conducted twice a day. During treatment, both the patients and operators wore earplugs. Each course of treatment lasted for 5 days, with a 1-day interval between each course, totaling two courses of treatment.
The control group received pseudostimulation treatment, and the implementation of the treatment protocol was consistent with that of the observation group. During the treatment, the stimulation magnetic head was turned 180°.
The Observation indicators are as follows: (1) The self-rating depression scale (SDS) was used to assess the depressed mood of both groups of patients on the day of admission (T0) and 15 days after treatment (T1). A total of 20 items were investigated, and each item was scored on a 4-level scale. A score of ≥ 53 was established as the cut-off value, with higher scores indicating more pronounced symptoms in the patient; (2) The Hamilton depression scale (HAMD) was used to assess the patients’ depressive mood at T0 and T1. The scale consists of 17 items, 10 items scored 0-4 points, 7 items scored 0-2 points, and the total score was 0-54, with < 7 points classified as no symptoms of depression, > 17 points classified as moderate symptoms of depression, and > 24 points classified as severe symptoms of depression[12]; (3) The cognitive functioning of patients at T0 and T1 was assessed using the MMSE, which comprises 7 dimensions covering 30 items, with a score range of 0-30. A score of ≥ 27 suggests normal cognitive functioning, with higher scores indicating better cognitive performance[13]; (4) Clinical efficacy was determined by evaluating the change in HAMD-14 score, the HAMD scale score reduction rate was calculated as [(T0 score - T1 score)/T0 score] × 100% - cured: Score reduction rate > 75%; Apparent effect: 50% ≤ score reduction rate ≤ 75%; Effective: 25% ≤ score reduction rate < 50%; and Ineffective: Score reduction rate < 25%; and (5) Adverse reactions during treatment, including tingling sensation, panic, and headache, were recorded in both groups.
Statistical analysis was performed using Statistical Product and Service Solutions version 20.0. Continuous data were represented as mean ± SD with a t-test; discrete data were presented as frequencies and percentages [n (%)] and compared using the χ2 test. Statistical significance was set at P < 0.05.
There were no differences in sex, age, disease duration, or education level between the two groups of patients included in the statistical analysis (P = 0.496; P = 0.210; P = 0.576; and P = 0.868, respectively), as shown in Table 1.
| Groups | Sex | Age (mean ± SD, years) | Course (mean ± SD, year) | Standard of culture | |||
| Males | Females | Primary and below | Middle school | College and above | |||
| Control group (n = 53) | 29 (54.72) | 24 (45.28) | 72.26 ± 6.17 | 5.26 ± 1.02 | 12 (22.64) | 18 (33.96) | 23 (43.40) |
| Observation group (n = 52) | 25 (48.08) | 27 (51.92) | 73.85 ± 6.52 | 5.15 ± 1.10 | 10 (19.23) | 17 (32.69) | 25 (48.08) |
| χ2/t | 0.463 | -1.261 | 0.561 | 0.284 | |||
| P value | 0.496 | 0.210 | 0.576 | 0.868 | |||
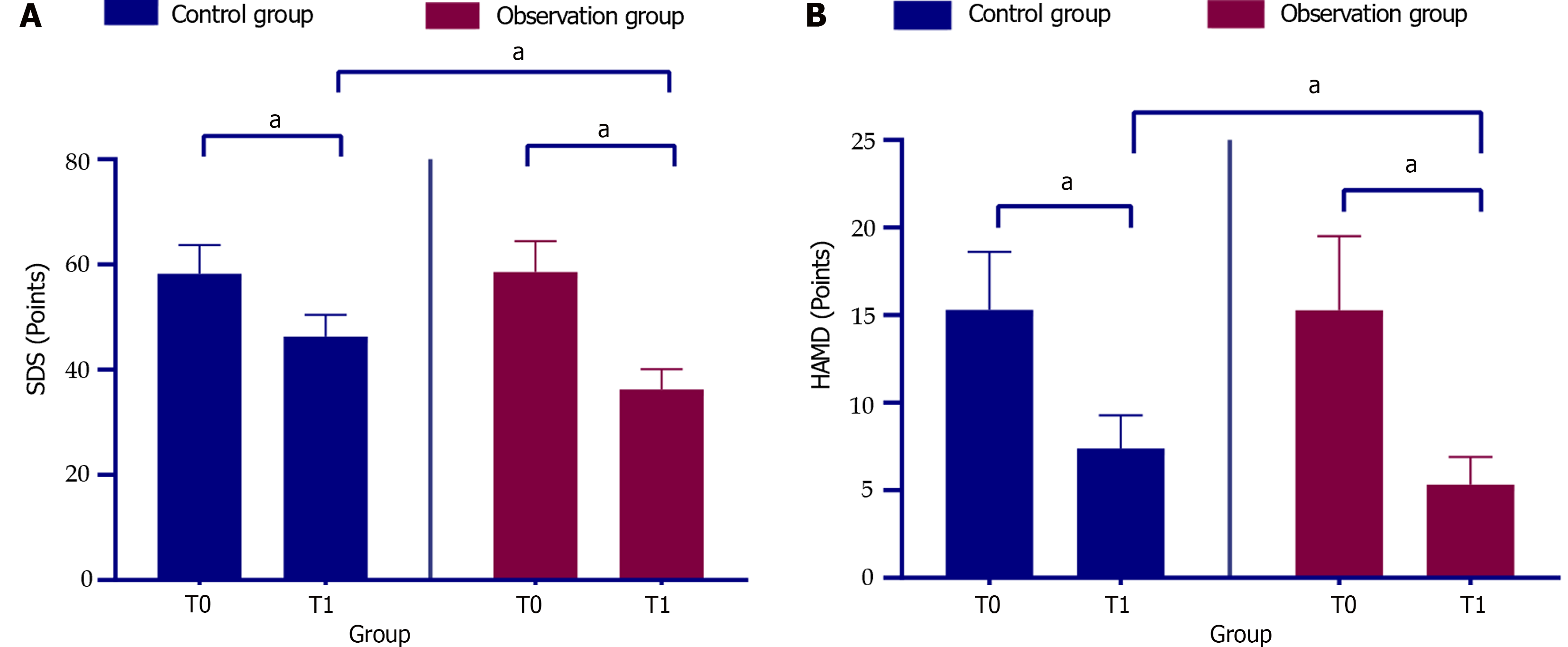
At T1, both groups exhibited a marked reduction in SDS and HAMD scores compared to at T0 (P < 0.05), with the observation group showing a more pronounced decrease than the control group (P < 0.05, Figure 1).
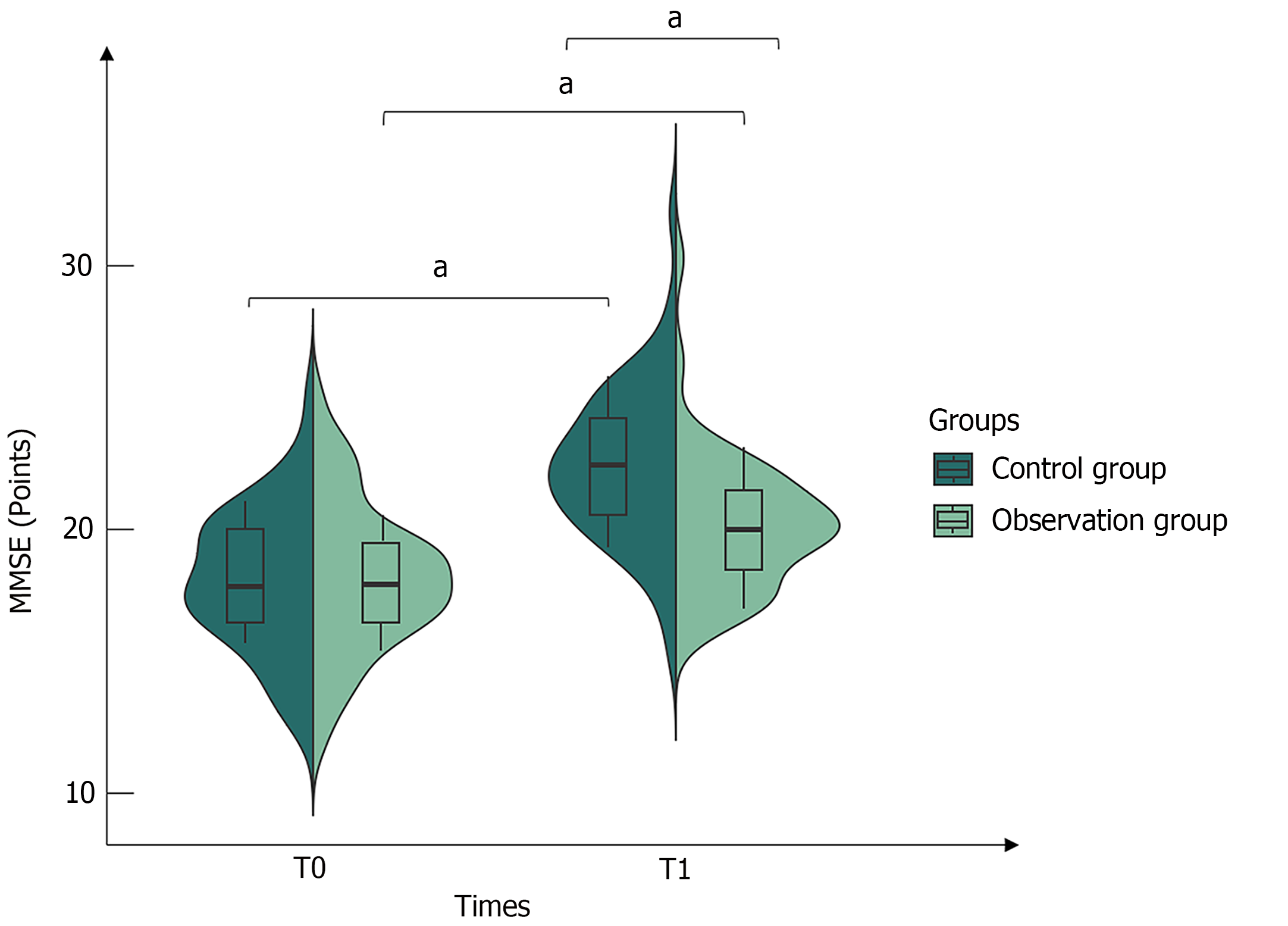
After receiving different therapeutic measures, by the time of the T1, the MMSE scores for both groups had shown a pivotal increase from T0 (P < 0.05). Furthermore, the observation group had a greater degree of improvement than the control group (P < 0.05, Figure 2).
In the control group, 14 patients had ineffective treatment effects, whereas in the observation group, the number was reduced to 5. The number of cases displaying ineffective treatment outcomes in the observation group was notably fewer compared to the control group (P < 0.05), as shown in Table 2.
| Groups | Recovery | Effectual | In force | Null and void | Overall effectiveness |
| Control group (n = 53) | 7 (13.21) | 13 (24.52) | 19 (35.85) | 14 (26.42) | 39 (73.58) |
| Observation group (n = 52) | 10 (19.23) | 17 (32.69) | 20 (38.46) | 5 (9.62) | 47 (90.38) |
| χ2 | 4.998 | ||||
| P value | 0.025 |
The variation in the occurrence of adverse reactions was comparably minimal across both groups under investigation (χ2 = 0.485, P = 0.486), as shown in Table 3.
| Groups | Tingling | Burning pain | Palpitation | Headache | Footing |
| Control group (n = 53) | 1 (1.89) | 2 (3.77) | 1 (1.89) | 0 (0.00) | 4 (7.55) |
| Observation group (n = 52) | 2 (3.85) | 1 (1.92) | 1 (1.92) | 2 (3.85) | 6 (11.54) |
AD is one of the most common diseases leading to the loss of daily living ability in the elderly. It is characterized by full-blown dementia, manifesting as personality and behavioral changes and a persistent decline in intellectual cognition. Currently, it is clinically incurable[14,15]. According to a survey, the prevalence of AD in China is approximately 3.94%, with the number of patients reaching 9.83 million. AD imposes a serious disease burden, bringing heavy medical, care, and economic burdens to families and society[16]. AD and depression share some common pathological characteristics[17], prompting researchers to focus on their co-occurrence and the development of depressive symptoms as the main risk factors[18]. Surveys have shown that approximately 50% of patients with AD have anxiety and depression with cognitive and behavioral changes, which can exacerbate the AD condition and affect the treatment of AD to a certain extent[19]. The high morbidity and disability rates of AD have made it a major public health problem and a growing social and family burden and medical problem worldwide, and the prevention and treatment of AD have become a major issue in modern medicine.
Previous studies have found that rTMS induces brain functional remodeling through magnetic field-induced currents, improves brain metabolism and synaptic plasticity, regulates interconnections between neurons, and effectively alters cerebral cortical excitability. These changes improve cognitive function and psycho-behavioral symptoms in patients with AD, thus providing new hope for the rehabilitation treatment of AD[20,21]; however, the specific mechanism of its action is not yet completely clear and lacks profound theoretical support. Non-sustained theta short burst rapid pulse mode, also known as intermittent theta rhythm stimulation, is a newly developed high-frequency treatment mode in recent years. It is a clumped stimulation modality simulating the physiological action potential burst discharge in the human hippocampus, and it has good effects on depressive symptoms. Additionally, it features low treatment time and high efficiency[22]. In this study, we showed that, at T1, the HAMD and SDS scores of the observation group were signifi
The mechanisms by which depression affects cognitive function in patients with AD are not clearly established and still need to be further explored. Chen et al[23] conducted a meta-analysis that found depression to be associated with frontal-limbic circuits, with the prefrontal cortex identified as the command-and-control center of the brain, reflecting higher executive functions, cognitive-functional behaviors, and attention. Therefore, the effect of depressive symptoms on cognitive function in patients with AD may be related to the prefrontal cortex. In this study, iTBS with rTMS for patients with AD with comorbid depression showed that the improvement in MMSE scores in the observation group was better than those in the control group, suggesting improved cognitive function among patient pairs, similar to the study by Wu et al[24]. As the pathogenesis of AD is more complex and closely related to the damage to the patient’s cerebral cortex or hippocampal functional areas, brain tissue metabolic abnormalities, and neurotransmitter disorders, the reduction of acetylcholinergic neurons and the decrease in transmitter levels can lead to impaired cognitive function[25]. We speculate that the TBS mode of rTMS may produce excitatory euthanasia in the prefrontal cortex, accelerating cerebral metabolic activity, promoting interaction between the cerebral cortex and subnuclei, and improving the function and structure of neural cells. These effects may improve the cognitive function of the patients and alleviate the symptoms of AD. Moreover, rTMS can directly stimulate the cerebral cortex, hippocampus, and striatum, which can effectively regulate cerebral microcellularity and improve metabolism. Furthermore, it promotes the release of neurotransmitters such as acetylcholine to a certain extent; this can significantly increase the content of acetylcholine in the brain tissue of patients, thereby enhancing the function of cholinergic neurons and improving the symptoms of cognitive impairment.
In addition, Lowe et al[26] compared the effects of continuous theta rhythm stimulation of rTMS with those of standard rTMS and found that the pulses delivered through the scalp interacted with neural firing. They concluded that iTBS was more in line with the rhythmic firing pattern of the brain itself. Some studies have pointed out that rTMS has better applications in the treatment of AD, depression, and stroke and is considered satisfactory for addressing various neurological disorders[27]. In this study, the observation group demonstrated a more effective treatment success rate than the control group, with no serious adverse events recorded in either group, indicating that the iTBS mode of rTMS is effective, safe, and reliable in the treatment of AD with depression. However, this study is a single-center study, and the sample size is small; hence, the results of the study have certain limitations. Therefore, it is necessary to carry out large-sample, multi-center, long-term follow-up clinical trials in the future to further explore the mechanism of iTBS mode of rTMS in the treatment of AD with depressive symptoms so as to provide stronger support for clinical practice and patient treatment.
The treatment of AD with depressive symptoms using the iTBS mode of rTMS has obvious advantages in improving depressive symptoms, mental status, and quality of life, thus enhancing the overall effectiveness of rTMS treatment, this is worthy of promotion and application.
| 1. | Hazar N, Seddigh L, Rampisheh Z, Nojomi M. Population attributable fraction of modifiable risk factors for Alzheimer disease: A systematic review of systematic reviews. Iran J Neurol. 2016;15:164-172. [PubMed] |
| 2. | Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, Nixon RA, Jones DT. Alzheimer disease. Nat Rev Dis Primers. 2021;7:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 1269] [Article Influence: 317.3] [Reference Citation Analysis (1)] |
| 3. | Xing Y, Li P, Jia Y, Zhang K, Liu M, Jiang J. Association of inflammatory bowel disease and related medication exposure with risk of Alzheimer's disease: An updated meta-analysis. Front Aging Neurosci. 2022;14:1082575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 5. | Yang H, Hong W, Chen L, Tao Y, Peng Z, Zhou H. Analysis of risk factors for depression in Alzheimer's disease patients. Int J Neurosci. 2020;130:1136-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Sharma K. Cholinesterase inhibitors as Alzheimer's therapeutics (Review). Mol Med Rep. 2019;20:1479-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 7. | Esumi S, Ushio S, Zamami Y. Polypharmacy in Older Adults with Alzheimer's Disease. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Brandt SJ, Oral HY, Arellano-Bravo C, Plawecki MH, Hummer TA, Francis MM. Repetitive Transcranial Magnetic Stimulation as a Therapeutic and Probe in Schizophrenia: Examining the Role of Neuroimaging and Future Directions. Neurotherapeutics. 2021;18:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Marron EM, Viejo-Sobera R, Quintana M, Redolar-Ripoll D, Rodríguez D, Garolera M. Transcranial magnetic stimulation intervention in Alzheimer's disease: a research proposal for a randomized controlled trial. BMC Res Notes. 2018;11:648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Bation R, Magnin C, Poulet E, Mondino M, Brunelin J. Intermittent theta burst stimulation for negative symptoms of schizophrenia-A double-blind, sham-controlled pilot study. NPJ Schizophr. 2021;7:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12014] [Cited by in RCA: 11042] [Article Influence: 788.7] [Reference Citation Analysis (0)] |
| 12. | Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 801] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 13. | Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56757] [Cited by in RCA: 60701] [Article Influence: 1214.0] [Reference Citation Analysis (0)] |
| 14. | Roquet A, Michel BF, Lemaire P. Alzheimer's disease disrupts domain-specific and domain-general processes in numerosity estimation. J Clin Exp Neuropsychol. 2020;42:690-709. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Badji A, de la Colina AN, Boshkovski T, Sabra D, Karakuzu A, Robitaille-Grou MC, Gros C, Joubert S, Bherer L, Lamarre-Cliche M, Stikov N, Gauthier CJ, Cohen-Adad J, Girouard H. A Cross-Sectional Study on the Impact of Arterial Stiffness on the Corpus Callosum, a Key White Matter Tract Implicated in Alzheimer's Disease. J Alzheimers Dis. 2020;77:591-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, Tang Y, Qin Q, Wang F, Qiao Y, Shi S, Wang YJ, Du Y, Zhang J, Zhang J, Luo B, Qu Q, Zhou C, Gauthier S, Jia J; Group for the Project of Dementia Situation in China. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 503] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 17. | Botto R, Callai N, Cermelli A, Causarano L, Rainero I. Anxiety and depression in Alzheimer's disease: a systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol Sci. 2022;43:4107-4124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 18. | Ai PH, Chen S, Liu XD, Zhu XN, Pan YB, Feng DF, Chen S, Xu NJ, Sun S. Paroxetine ameliorates prodromal emotional dysfunction and late-onset memory deficit in Alzheimer's disease mice. Transl Neurodegener. 2020;9:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Orgeta V, Tabet N, Nilforooshan R, Howard R. Efficacy of Antidepressants for Depression in Alzheimer's Disease: Systematic Review and Meta-Analysis. J Alzheimers Dis. 2017;58:725-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 20. | Chou YH, Ton That V, Sundman M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2020;86:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 21. | Antczak J, Kowalska K, Klimkowicz-Mrowiec A, Wach B, Kasprzyk K, Banach M, Rzeźnicka-Brzegowy K, Kubica J, Słowik A. Repetitive transcranial magnetic stimulation for the treatment of cognitive impairment in frontotemporal dementia: an open-label pilot study. Neuropsychiatr Dis Treat. 2018;14:749-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Bulteau S, Laurin A, Pere M, Fayet G, Thomas-Ollivier V, Deschamps T, Auffray-Calvier E, Bukowski N, Vanelle JM, Sébille V, Sauvaget A. Intermittent theta burst stimulation (iTBS) versus 10 Hz high-frequency repetitive transcranial magnetic stimulation (rTMS) to alleviate treatment-resistant unipolar depression: A randomized controlled trial (THETA-DEP). Brain Stimul. 2022;15:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 23. | Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer's disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener. 2021;16:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 24. | Wu X, Ji GJ, Geng Z, Zhou S, Yan Y, Wei L, Qiu B, Tian Y, Wang K. Strengthened theta-burst transcranial magnetic stimulation as an adjunctive treatment for Alzheimer's disease: An open-label pilot study. Brain Stimul. 2020;13:484-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Li Q, Wu X, Xu L, Chen K, Yao L; Alzheimer's Disease Neuroimaging Initiative. Classification of Alzheimer's Disease, Mild Cognitive Impairment, and Cognitively Unimpaired Individuals Using Multi-feature Kernel Discriminant Dictionary Learning. Front Comput Neurosci. 2017;11:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Lowe CJ, Manocchio F, Safati AB, Hall PA. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: A systematic review and meta-analysis. Neuropsychologia. 2018;111:344-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Bailey NW, Hoy KE, Rogasch NC, Thomson RH, McQueen S, Elliot D, Sullivan CM, Fulcher BD, Daskalakis ZJ, Fitzgerald PB. Differentiating responders and non-responders to rTMS treatment for depression after one week using resting EEG connectivity measures. J Affect Disord. 2019;242:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |