Published online Aug 19, 2024. doi: 10.5498/wjp.v14.i8.1190
Revised: July 3, 2024
Accepted: July 12, 2024
Published online: August 19, 2024
Processing time: 82 Days and 19 Hours
The aging of the population has become increasingly obvious in recent years, and the incidence of cerebral infarction has shown an increasing trend annually, with high death and disability rates.
To analyze the effects of infarct location and volume on cognitive dysfunction in elderly patients with acute insular cerebral infarction.
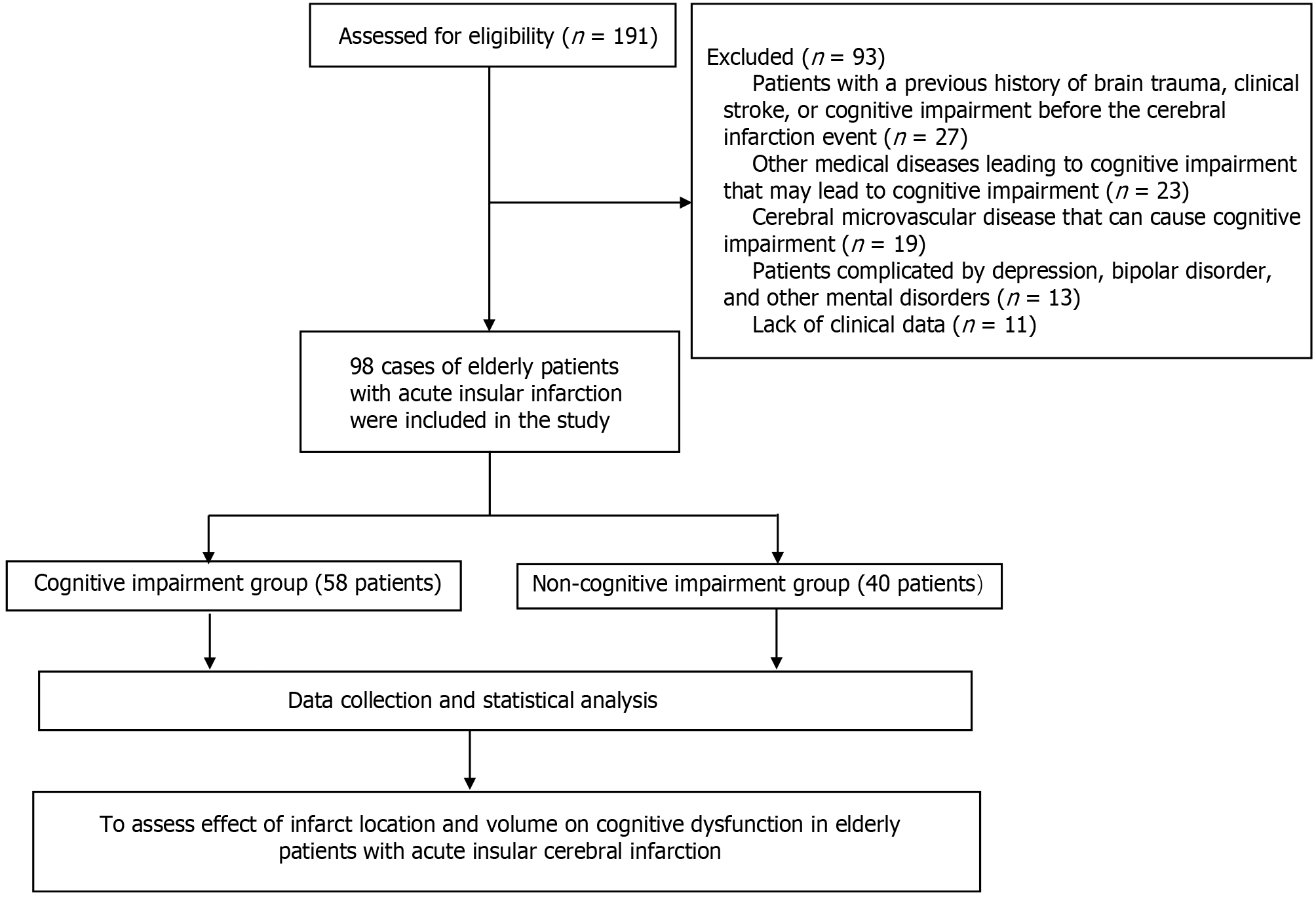
Between January 2020 and December 2023, we treated 98 cases of elderly acute insula, patients with cerebral infarction in the cerebral infarction acute phase (3-4 weeks) and for the course of 6 months in Montreal Cognitive Assessment Scale (MoCA) for screening of cognition. Notably, 58 and 40 patients were placed in the cognitive impairment group and without-cognitive impairment group, respec
The number of patients with cognitive impairment in the basal ganglia and thalamus was significantly higher than that without cognitive impairment (P < 0.05). The total infarct volume in the cognitive impairment group was higher than that in the non-cognitive impairment group, and the difference was statistically significant (P < 0.05). The infarct volumes at different sites in the cognitive impairment group was higher than in the non-cognitive impairment group (P < 0.05). In the cognitive impairment group, the infarct volumes in the basal ganglia, thalamus, and mixed lesions were negatively correlated with the total MoCA score, with correlation coefficients of -0.67, -0.73, and -0.77, respectively.
In elderly patients with acute insular infarction, infarction in the basal ganglia, thalamus, and mixed lesions were more likely to lead to cognitive dysfunction than in other areas, and patients with large infarct volumes were more likely to develop cognitive dysfunction. The infarct volume in the basal ganglia, thalamus, and mixed lesions was significantly negatively correlated with the MoCA score.
Core Tip: With the increasingly obvious aging of the population, the incidence of cerebral infarction is increasing year by year, which seriously leads to patient death. Most survivors suffer from sequelae such as cognitive dysfunction, aphasia and paralysis. This study aims to investigate the different clinical manifestations of patients with acute island infarction from the perspective of infarct location and volume, and mainly explore the influence of infarct location and volume on cognitive dysfunction in elderly patients with acute island infarction, hoping to evaluate cognitive function score, infarct location and volume. This can provide a basis for the clinical diagnosis and treatment of acute island infarction.
- Citation: Liang FF, Liu XX, Liu JH, Gao Y, Dai JG, Sun ZH. Effect of infarct location and volume on cognitive dysfunction in elderly patients with acute insular cerebral infarction. World J Psychiatry 2024; 14(8): 1190-1198
- URL: https://www.wjgnet.com/2220-3206/full/v14/i8/1190.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i8.1190
Cerebral infarction, or ischemic stroke[1], refers to a disturbance of the blood supply to the brain for various reasons such as local ischemia and hypoxia in the brain, resulting in necrosis and softening of the brain tissue, and corresponding clinical symptoms and even death in severe cases[2]. The incidence and mortality of cerebral infarction is relatively high worldwide, accounting for 87.00% of the total incidence of cerebral strokes[3]. In China, the annual incidence of cerebral infarction is 150 per 100000[4]. With the rapid development of China's national economy, the aging of the population has become increasingly obvious in recent years, and the incidence of cerebral infarction has shown an increasing trend annually, with high death and disability rates[5]. Most survivors suffer from sequelae, such as cognitive dysfunction, aphasia, and paralysis, which seriously affect their quality of life.
Cognitive dysfunction after cerebral infarction is a common complication of stroke. It is reported that cerebral infarction will increase the risk of cognitive impairment by at least 26-27 times[6], and the incidence of cognitive impairment after cerebral infarction is 10%-40%, which is higher than that of the general population[7]. At present, the pathogenesis of cognitive dysfunction after cerebral infarction is not fully defined[8]. Studies have shown that patients with mild cognitive impairment or dementia are more prone to cognitive decline after cerebral infarction, and their rehabilitation process is relatively slow[9]. In addition, comorbidity is one of the important factors affecting cognitive function after cerebral infarction. Common comorbidity, such as hypertension, cardiovascular disease and diabetes, may aggravate the degree of nerve injury and ischemia in cerebral infarction and then affect the rehabilitation and recovery of cognitive function[10,11]. Drug therapy may also affect cognitive function after cerebral infarction. Some drugs may affect the cognitive function of patients, especially some drugs that affect neurotransmitters or brain function, such as sedatives and antidepressants. At the same time, the adverse reactions and side effects of drugs may also affect the cognitive function of patients, which needs to be fully considered during treatment[12]. In addition, different infarct locations and volumes of acute cerebral infarction may lead to different clinical manifestations, and there is a certain relationship with cognitive dysfunction[13]. Other studies have confirmed that insular cortex infarction is a special sign of cardiovascular autonomic function in patients with acute cerebral infarction, mostly in the middle cerebral artery blood supply area[14]. The insula is a highly ischemic region valuable for predicting cognitive dysfunction, motor ability, and aphasia in patients with cerebral infarction[15].
Therefore, this study aimed to investigate the different clinical manifestations of patients with acute insular infarction from the perspective of infarct location and volume, and mainly explore the impact of infarct location and volume on cognitive dysfunction in elderly patients with acute insular infarction, in the hope that the cognitive function of patients with acute insular infarction can be predicted clinically by assessing the cognitive function scores, infarct location, and infarct volume. This can provide a basis for the clinical diagnosis and treatment of acute insular infarctions.
Overall, 98 elderly patients with acute insular infarction who were treated at the Department of Neurology of our hospital between January 2020 and December 2023 were selected from the medical records system. The participants were divided into two groups according to the Montreal Cognitive Assessment Scale (MoCA)[16]. Individuals with a MoCA score ≥ 26 were placed into the non-cognitive impairment group (n = 40) and individuals with a MoCA score < 26 group (n = 58) were placed into the cognitive impairment group.
The inclusion criteria[17] were as follows: (1) Age ≥ 60 years; (2) Patients with acute insular infarction diagnosed by craniocerebral magnetic resonance imaging (MRI); (3) Patients with a stable condition and who can cooperate with relevant examinations; and (4) Patients with complete case data and follow-up data.
The exclusion criteria[18] were as follows: (1) Patients with a previous history of brain trauma, clinical stroke, or cognitive impairment before the cerebral infarction event; (2) Other medical diseases leading to cognitive impairment (such as hypothyroidism, vitamin B12 deficiency, and severe infection) or neurological diseases (Parkinson’s disease, Alzheimer’s disease, Lewy body dementia, brain atrophy, and cerebral amyloidosis) that may lead to cognitive impairment; (3) Cerebral microvascular disease that can cause cognitive impairment; (4) Patients complicated by depression, bipolar disorder, and other mental disorders; and (5) Lack of clinical data.
This study was approved by the Research Ethics Committee, and all patients were informed and voluntarily participated in the study.
Location screening of cerebral infarction and calculation of the cerebral infarction volume were conducted based on MRI detection[19]: A head MRI was performed to identify the location of cerebral infarction lesions in the acute stage of cerebral infarction (3-4 weeks post-cerebral infarction) and 6 months later.
Statistics on the volume of cerebral infarction: The volume of cerebral infarction was based on the MRI results[20]. Two attending or deputy chief physicians of radiology independently read the head MRI images that met the image quality standards using a double-blind method for independent analysis. If the opinions were divided, a third deputy chief physician of radiology participated until a consensus was reached. The location of the infarct was recorded in patients with acute cerebral infarction, and the infarct volume was measured using fluid-attenuated inversion recovery images at 6 months of the disease course. Post-processing software was used to outline the infarction contour of each layer of cerebral infarction in the sequence related to the head, and the area could be automatically calculated and accumulated layer-by-layer. Finally, the volume of the cerebral infarction was obtained by multiplying the sum of layer thickness and layer distance. The sites of the two MRI infarcts in the same patient were compared, and patients with new infarcts were excluded. Using the results of the volume measured by MRI at 6 months, the infarct volume of all patients in each group was added according to the infarct site to obtain the total volume value of each infarct volume in the groups with and without cognitive impairment, and a comparison was conducted between the groups.
Cognitive function assessment[21]: The patients’ cognitive function was evaluated using the MoCA score. The MoCA score is composed of 12 items, with one point deducted for 12 years of education or less, and a maximum total score of 30. It mainly covers seven cognitive domains, which are subdivided into visual space, executive function, naming, attention, memory, computation, repetition, verbal fluency, abstraction, delayed recall, and orientation, and takes 10–15 min to complete.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0. The MoCA score results were represented by mean ± SD and t-test. The χ2 test was used to analyze the data. The correlation between the infarct location, infarct volume, and MoCA score was tested using the rank-sum and Spearman’s rank correlation coefficient tests. A P value < 0.05 indicated that the difference was statistically significant. If the correlation coefficient |r| was greater than 0.6, it was considered that a significant correlation was present between them.
A research flowchart is presented in Figure 1. Among the 58 patients with cognitive impairment, the average age was 65.84 ± 8.13 years, including 41 males and 17 females, 23 patients with a history of smoking, 31 patients with a history of drinking, 27 patients with hypertension, and 15 patients with diabetes, and the average length of education were 5.78 ± 4.72 years. There were 52 right-handed and six left-handed cases. Among the 40 patients in the cognitively normal group, the average age was 63.19 ± 7.57 years, with 28 males and 12 females, 16 patients with a history of smoking, 27 patients with a history of drinking, 19 patients with hypertension, and nine patients with diabetes, and the average length of education were 6.12 ± 4.95 years. There were no statistical differences in the above general data between the two groups (P > 0.05), as shown in Table 1.
| Variable | Cognitive impairment group, n = 58 | Non-cognitive impairment group, n = 40 | t/χ2 | P value |
| Sex | 0.345 | 0.557 | ||
| Male | 41 | 28 | ||
| Female | 17 | 12 | ||
| Age in years | 65.84 ± 8.13 | 63.19 ± 7.57 | 1.673 | 0.692 |
| Smoking history | 0.082 | 0.783 | ||
| Yes | 23 | 16 | ||
| No | 35 | 24 | ||
| Drinking history | 0.367 | 0.674 | ||
| Yes | 31 | 27 | ||
| No | 27 | 23 | ||
| Complicated with hypertension | 0.815 | 0.363 | ||
| Yes | 27 | 19 | ||
| No | 31 | 21 | ||
| Complicated with diabetes | 0.459 | 0.582 | ||
| Yes | 15 | 9 | ||
| No | 43 | 31 | ||
| Years of education | 5.78 ± 4.72 | 6.12 ± 4.95 | 3.683 | 0.062 |
| Handedness | 0.175 | 0.684 | ||
| Right-handed | 52 | 36 | ||
| Left-handed | 6 | 4 |
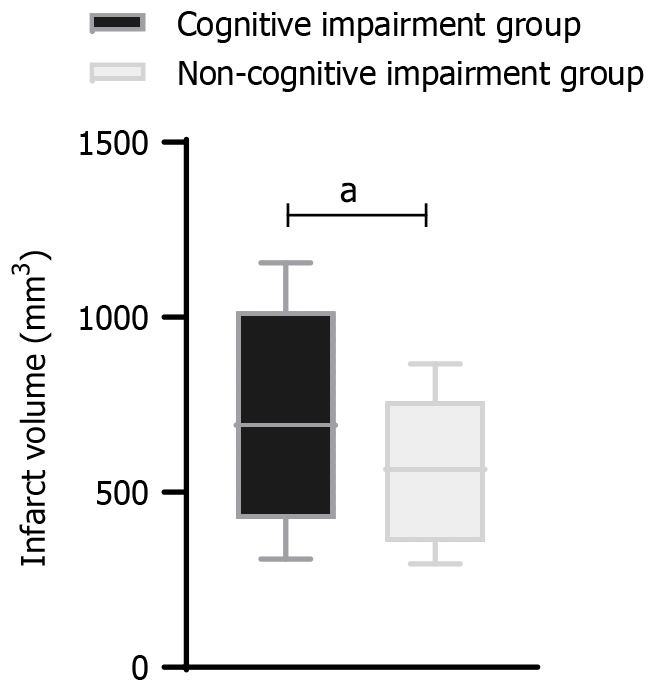
The infarct volume in the cognitive impairment group [711.6 mm3 (309.3, 1156.0)] was significantly larger than that in the non-cognitive impairment group [566.0 mm3 (295.1, 866.9); P < 0.05)], as shown in Figure 2.
The comparison of the number of infarct sites (basal ganglia, cortex, brainstem, cerebellum, thalamus, and mixed lesions) between the two groups showed that the number of infarct sites in the thalamus and basal ganglia in the cognitive impairment group was significantly higher than that in the group without cognitive impairment, and the difference was statistically significant (P < 0.05), as shown in Table 2.
| Variable | Cognitive impairment group, n = 58 | Non-cognitive impairment group, n = 40 | χ2 | P value |
| Basal ganglia | 41 (70.69) | 14 (35.23) | 12.245 | < 0.001 |
| Cortical layer | 5 (9.39) | 5 (11.95) | 0.081 | 0.776 |
| Brain stem | 4 (6.56) | 4 (9.00) | 0.31 | 0.86 |
| Cerebellum | 5 (9.00) | 4 (11.00) | 0.015 | 0.902 |
| Thalamus | 23 (39.66) | 7 (17.13) | 5.471 | 0.019 |
| Mix | 7 (12.83) | 6 (15.69) | 0.177 | 0.674 |
The infarct volumes at different infarct sites in the cognitive impairment group was higher than that in the non-cognitive impairment group, and the differences between the two groups were statistically significant (P < 0.05), as shown in Table 3.
| Variable | Cognitive impairment group, n = 58 | Non-cognitive impairment group, n = 40 | t value | P value |
| Basal ganglia | 48.55 ± 1.32 | 24.76 ± 1.01 | 96.161 | < 0.001 |
| Cortical layer | 194.26 ± 3.75 | 110.02 ± 2.64 | 122.577 | < 0.001 |
| Brain stem | 112.23 ± 3.64 | 72.65 ± 2.03 | 62.346 | < 0.001 |
| Cerebellum | 81.05 ± 2.02 | 31.86 ± 1.04 | 141.47 | < 0.001 |
| Thalamus | 15.87 ± 1.02 | 7.32 ± 0.57 | 48.045 | < 0.001 |
| Mixed lesions | 289.84 ± 8.13 | 163.19 ± 6.53 | 81.932 | < 0.001 |
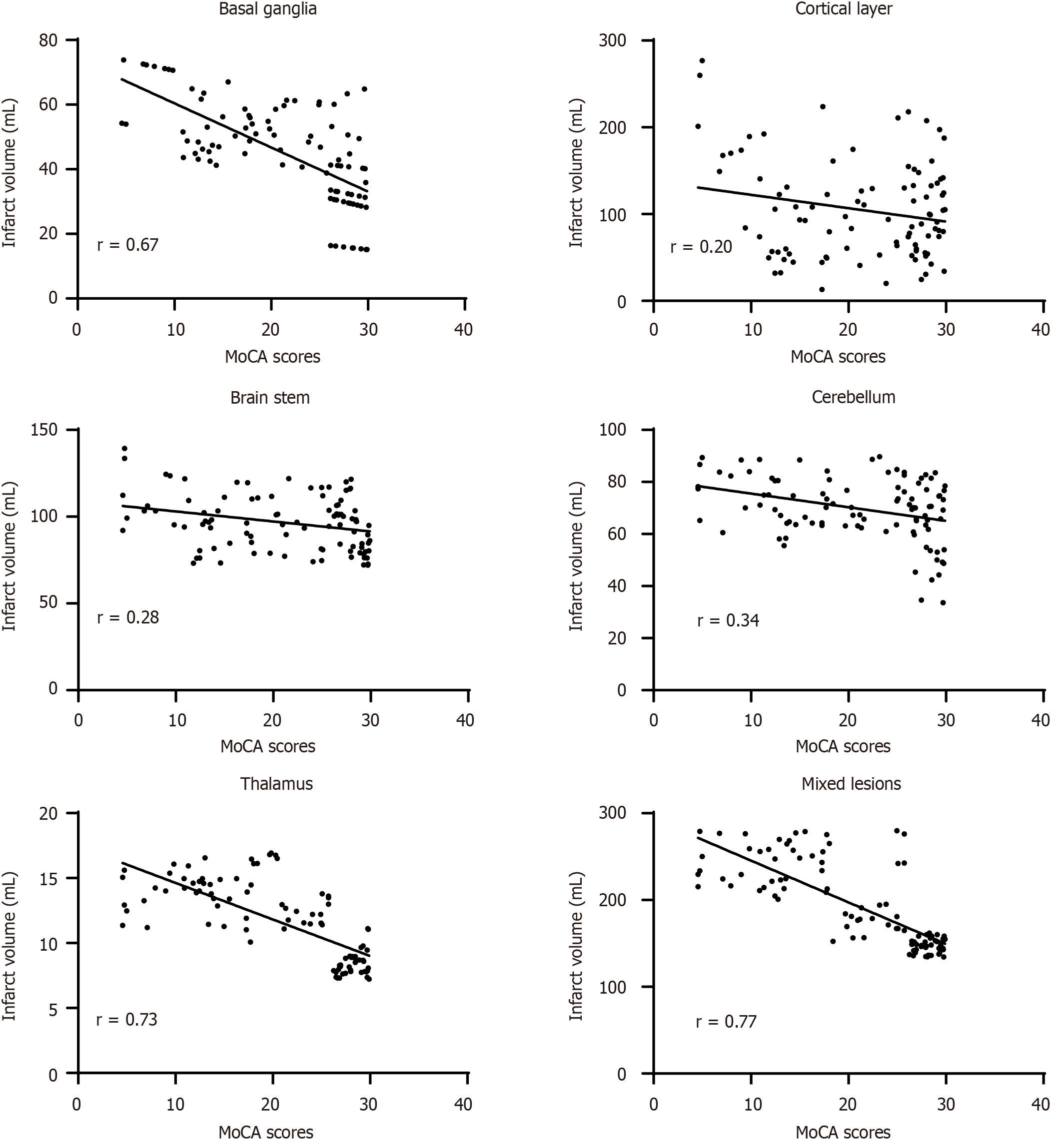
As shown in Figure 3, the MoCA score of elderly patients with acute insular infarction has a strong negative correlation with the infarct volume of the basal ganglia, thalamus, and mixed lesions, and the correlation coefficients r were -0.67,
It is reported that compared with the Mini-Mental State Examination, the MoCA score is recommended to be used for patients in Asia with an education period of < 6 years[22]. The average education period of the cognitive impairment group in this study was 5.78 ± 4.72 years, so this article used the MoCA score.
Few studies have examined the relationship among the infarct location, infarct volume, and cognitive impairment in elderly patients with acute insular infarction[23-25]. The infarction sites of patients with acute insular infarction mainly involved the basal ganglia, cortex, brainstem, cerebellum, thalamus, and mixed lesions among these sites, with the highest incidence in the basal ganglia (41 cases in the cognitive impairment group and 14 cases in the non-cognitive impairment group), followed by the thalamus (23 cases in the cognitive impairment group and seven cases in the non-cognitive impairment group). This is consistent with the results of Jones et al[26], and the difference in the incidence in the basal ganglia and thalamus between the two groups was statistically significant (P < 0.05)[27].
In addition, the results of this study showed that the occurrence of cognitive impairment in patients with cerebral infarction was related to the infarction volume. The infarction volumes in the basal ganglia, cortex, brainstem, cerebellum, thalamus, and mixed lesions in the cognitive impairment group were larger than those in the non-cognitive impairment group, indicating that these areas were related to cognitive impairment. However, Zhou et al[28] believed that the multiple infarction lesions and specific infarction types were important risk factors for cognitive impairment in patients with a cerebral infarction recovery period. The results of the present study confirm this conclusion. This may be because the cerebral cortex is mainly responsible for cognitive and emotional learning and other advanced functions, and patients with cortical infarction are prone to cognitive dysfunction[29].
Further correlation analysis showed that the infarct volumes of basal ganglia, thalamus and mixed lesion in the cognitive impairment group were strongly correlated with MoCA scores (r = -0.67, -0.73, -0.77), while the infarct volumes of cortex, brainstem and cerebellum were weakly correlated with MoCA scores (r = -0.20, -0.28, -0.34). As the conclusion of the current research on the correlation between the infarct location, infarct volume, and cognitive impairment was controversial. Peng et al[17] showed that thalamic infarction is an independent risk factor for cognitive impairment following cerebral infarction. Li et al[30] believed that the location of cerebral infarction was an independent risk factor for cognitive impairment. The results of this study are basically consistent with those of Peng et al[18] and Li et al[30].
This study had a few limitations. First, this was a single-center retrospective study and the number of patients was small, which limited the generalizability and extension of the results. Second, previous studies have shown that male sex, hypertension, diabetes mellitus, and low education are independent risk factors for cognitive impairment in cerebral infarction. However, this study appropriately controlled for these factors to make them comparable, such that it can obtain more accurate results of infarction location and infarction volume on cognitive impairment. Nevertheless, there may also be some potential biases that may affect the conclusions of the study. Third, this study did not conduct a long-term follow-up and lacked data on the long-term prognosis of the patients. Another limitation of this study is that a control group of elderly individuals without cerebral infarction has not been added, and further consideration will be given to adding this control group in the later stage to verify the accuracy of the results. In the future, it will be necessary to conduct a multicenter prospective epidemiological study with a large sample size to fully reflect the impact of infarction location and volume on cognitive dysfunction in elderly patients with acute insular infarction.
In summary, the total infarct volume of patients with acute insular infarction has a significant impact on cognitive impairment. The infarct volume in the basal ganglia, thalamus, and mixed lesions had a significant negative correlation with the MoCA score, whereas the infarct volume in the cortex, brainstem, and cerebellum had no significant correlation with the MoCA score. Therefore, it is necessary to conduct cognitive function assessments for patients with infarction in these key parts of the brain or with a large infarction volume to identify cognitive impairment after cerebral infarction early and to select the appropriate intervention measures.
The authors thank the institution for support and all friends and families.
| 1. | Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, Yuan G, Han H, Chen W, Wei M, Zhang J, Zhou Z, Yao X, Wang G, Song W, Cai X, Nan G, Li D, Wang AY, Ling W, Cai C, Wen C, Wang E, Zhang L, Jiang C, Liu Y, Liao G, Chen X, Li T, Liu S, Li J, Gao F, Ma N, Mo D, Song L, Sun X, Li X, Deng Y, Luo G, Lv M, He H, Liu A, Zhang J, Mu S, Liu L, Jing J, Nie X, Ding Z, Du W, Zhao X, Yang P, Liu L, Wang Y, Liebeskind DS, Pereira VM, Ren Z, Wang Y, Miao Z; ANGEL-ASPECT Investigators. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N Engl J Med. 2023;388:1272-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 458] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 2. | Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, Ren Y, Qian Z, Vaughn MG, McMillin SE, Hay SI, Naghavi M, Cai M, Wang C, Zhang Z, Zhou M, Lin H, Yang Y. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2021;6:e897-e906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 468] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 3. | Thayabaranathan T, Kim J, Cadilhac DA, Thrift AG, Donnan GA, Howard G, Howard VJ, Rothwell PM, Feigin V, Norrving B, Owolabi M, Pandian J, Liu L, Olaiya MT. Global stroke statistics 2022. Int J Stroke. 2022;17:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 4. | Tu WJ, Wang LD; Special Writing Group of China Stroke Surveillance Report. China stroke surveillance report 2021. Mil Med Res. 2023;10:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 5. | Cao S, Teng L, Gao M, Hu S, Xiao S, Chen C, He Y, Cheng S, Xie X. Nonlinear relationship between triglycerides and cognitive function after acute ischemic stroke among older adults. Heliyon. 2024;10:e27943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Lim JS, Lee JJ, Woo CW. Post-Stroke Cognitive Impairment: Pathophysiological Insights into Brain Disconnectome from Advanced Neuroimaging Analysis Techniques. J Stroke. 2021;23:297-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Huang YY, Chen SD, Leng XY, Kuo K, Wang ZT, Cui M, Tan L, Wang K, Dong Q, Yu JT. Post-Stroke Cognitive Impairment: Epidemiology, Risk Factors, and Management. J Alzheimers Dis. 2022;86:983-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 8. | Ryu WS, Schellingerhout D, Hong KS, Jeong SW, Kim BJ, Kim JT, Lee KB, Park TH, Park SS, Park JM, Kang K, Cho YJ, Park HK, Lee BC, Yu KH, Oh MS, Lee SJ, Kim JG, Cha JK, Kim DH, Lee J, Han MK, Park MS, Choi KH, Nahrendorf M, Lee J, Bae HJ, Kim DE. Relation of Pre-Stroke Aspirin Use With Cerebral Infarct Volume and Functional Outcomes. Ann Neurol. 2021;90:763-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Chan ATC, Ip RTF, Tran JYS, Chan JYC, Tsoi KKF. Computerized cognitive training for memory functions in mild cognitive impairment or dementia: a systematic review and meta-analysis. NPJ Digit Med. 2024;7:1. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Ding MY, Xu Y, Wang YZ, Li PX, Mao YT, Yu JT, Cui M, Dong Q. Predictors of Cognitive Impairment After Stroke: A Prospective Stroke Cohort Study. J Alzheimers Dis. 2019;71:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Dhana K, Aggarwal NT, Beck T, Holland TM, Dhana A, Cherian LJ, Desai P, Evans DA, Rajan KB. Lifestyle and Cognitive Decline in Community-Dwelling Stroke Survivors. J Alzheimers Dis. 2022;89:745-754. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Elendu C, Amaechi DC, Elendu TC, Ibhiedu JO, Egbunu EO, Ndam AR, Ogala F, Ologunde T, Peterson JC, Boluwatife AI, Okongko AO, Fatoye JO, Akpovona OL, Onyekweli SO, Temitope AY, Achimugu AO, Temilade AV. Stroke and cognitive impairment: understanding the connection and managing symptoms. Ann Med Surg (Lond). 2023;85:6057-6066. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, Hinman JD, Dichgans M. Post-Stroke Cognitive Impairment and Dementia. Circ Res. 2022;130:1252-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 369] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 14. | Durrani R, Hill MD, Smith EE. Preventing Covert Brain Infarct-Related Cognitive Impairment and Dementia. Can J Neurol Sci. 2020;47:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kitamura J, Ueno H, Nagai M, Hosomi N, Honjo K, Nakamori M, Mukai T, Imamura E, Nezu T, Aoki S, Ohshita T, Nomura E, Wakabayashi S, Maruyama H, Matsumoto M. Blood Pressure Variability in Acute Ischemic Stroke: Influence of Infarct Location in the Insular Cortex. Eur Neurol. 2018;79:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Di Stefano V, De Angelis MV, Montemitro C, Russo M, Carrarini C, di Giannantonio M, Brighina F, Onofrj M, Werring DJ, Simister R. Clinical presentation of strokes confined to the insula: a systematic review of literature. Neurol Sci. 2021;42:1697-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Corral S, Gaspar PA, Castillo-Passi RI, Mayol Troncoso R, Mundt AP, Ignatyev Y, Nieto RR, Figueroa-Muñoz A. Montreal Cognitive Assessment (MoCA) as a screening tool for cognitive impairment in early stages of psychosis. Schizophr Res Cogn. 2024;36:100302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Peng M, Wu B, Wang X, Ding Y, Li Y, Cheng X. Clinical Factors Affecting the Recovery of Sensory Impairment After Cerebral Infarction: A Retrospective Study. Neurologist. 2023;28:117-122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Guglielmi V, Quaranta D, Masone Iacobucci G, Citro S, Scala I, Genovese D, Brunetti V, Marra C, Calabresi P, Della Marca G. Basal ganglia ischaemic infarction after thrombectomy: cognitive impairment at acute stage. Eur J Neurol. 2023;30:3772-3779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Merkler AE, Sigurdsson S, Eiriksdottir G, Safford MM, Phillips CL, Iadecola C, Gudnason V, Weinsaft JW, Kamel H, Arai AE, Launer LJ. Association Between Unrecognized Myocardial Infarction and Cerebral Infarction on Magnetic Resonance Imaging. JAMA Neurol. 2019;76:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Wen H, Lv M. Correlation analysis between serum procalcitonin and infarct volume in young patients with acute cerebral infarction. Neurol Sci. 2021;42:3189-3196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Zhao J, Tang H, Sun J, Wang B, Chen S, Fu Y. Analysis of cognitive dysfunction with silent cerebral infarction: a prospective study in Chinese patients. Metab Brain Dis. 2012;27:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Khaw J, Subramaniam P, Abd Aziz NA, Ali Raymond A, Wan Zaidi WA, Ghazali SE. Current Update on the Clinical Utility of MMSE and MoCA for Stroke Patients in Asia: A Systematic Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Dugger BN, Davis K, Malek-Ahmadi M, Hentz JG, Sandhu S, Beach TG, Adler CH, Caselli RJ, Johnson TA, Serrano GE, Shill HA, Belden C, Driver-Dunckley E, Caviness JN, Sue LI, Jacobson S, Powell J, Sabbagh MN. Neuropathological comparisons of amnestic and nonamnestic mild cognitive impairment. BMC Neurol. 2015;15:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Yeung MK, Chau AK, Chiu JY, Shek JT, Leung JP, Wong TC. Differential and subtype-specific neuroimaging abnormalities in amnestic and nonamnestic mild cognitive impairment: A systematic review and meta-analysis. Ageing Res Rev. 2022;80:101675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Jones RS, Donahue MJ, Davis LT, Pruthi S, Waddle SL, Custer C, Patel NJ, DeBaun MR, Kassim AA, Rodeghier M, Jordan LC. Silent infarction in sickle cell disease is associated with brain volume loss in excess of infarct volume. Front Neurol. 2023;14:1112865. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Lin C, Chatterjee N, Lee J, Harvey R, Prabhakaran S. Predictive value of the combination of lesion location and volume of ischemic infarction with rehabilitation outcomes. Neuroradiology. 2019;61:1131-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Zhou W, Baughman BD, Soman S, Wintermark M, Lazzeroni LC, Hitchner E, Bhat J, Rosen A. Volume of subclinical embolic infarct correlates to long-term cognitive changes after carotid revascularization. J Vasc Surg. 2017;65:686-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Li Y, Geng W, Zhang X, Mi B. Risk factors and characteristics analysis of cognitive impairment in patients with cerebral infarction during recovery period. Int J Neurosci. 2024;1-6. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Li T, Bao X, Li L, Qin R, Li C, Wang X. Heart failure and cognitive impairment: A narrative review of neuroimaging mechanism from the perspective of brain MRI. Front Neurosci. 2023;17:1148400. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |