Published online Oct 19, 2024. doi: 10.5498/wjp.v14.i10.1583
Revised: September 3, 2024
Accepted: September 20, 2024
Published online: October 19, 2024
Processing time: 72 Days and 0.2 Hours
Imipenem is a highly effective carbapenem antibiotic, which is widely used in the treatment of many serious bacterial infections. At the same time, it can also cause some adverse reactions, mental abnormalities are the most concerned central nervous system adverse reactions. Different patients respond differently to imipenem, and the effect of imipenem on psychiatric disorders is unclear. Therefore, meta-analysis summarizing the results of multiple previous studies can provide stronger evidence support for clinical guidelines to guide clinical rational use of imipenem to minimize risks.
To systematically review the effects of imipenem on mental-health disorders.
Method in Cochrane Library, Web of science, PubMed, ProQuest, and China’s biomedical literature databases Wanfang, Weipu and China HowNet, the related literatures about the controlled trials of imipenem-induced mental disorders from the establishment of the database to May 2024 were searched; the included literatures were analyzed using RevMan 5.4 software; and the heterogeneity among the studies was also discussed.
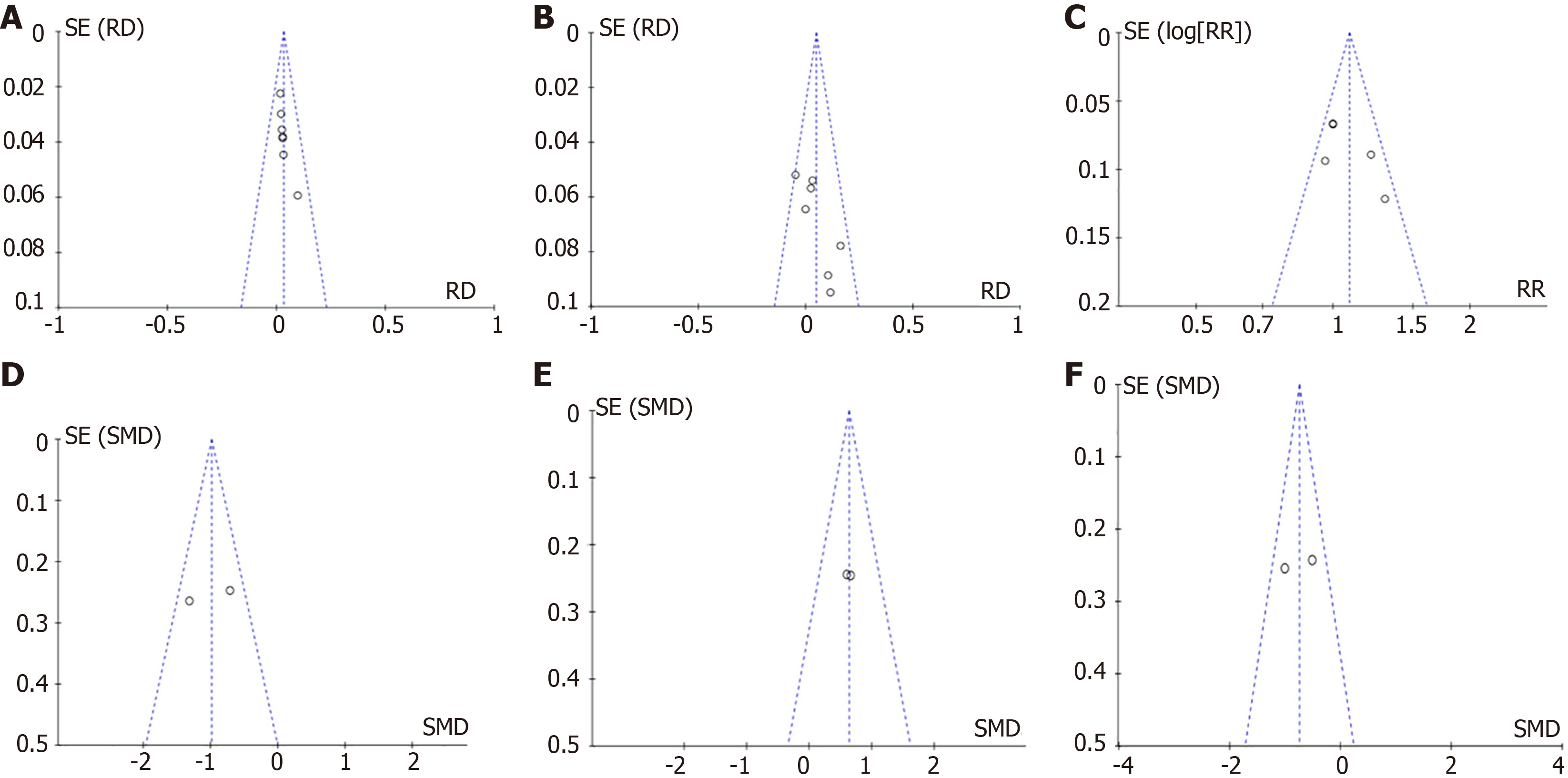
After reviewing the literature published between 2003 and 2017, seven controlled trials with a total of 550 patients were included, with 273 and 277 patients in the control and experimental groups, respectively. The sample size of the study ranged from a minimum of 30 cases to a maximum of 61 cases. Patients in the experimental group were treated with imipenem while the control group was treated with conventional drugs. Meta-analysis showed that the incidence of mental disorders in the experimental group was higher than that in the control group (odds ratio = 3.66, 95% confidence interval: 1.11-12.11, P = 0.030); however, there was no significant difference in the incidence of adverse reactions between the two groups (odds ratio = 0.05, 95% confidence interval: 0.00 to 0.10, P = 0.060). Funnel diagrams showed that the scattered points of each study were symmetrical and distributed in an inverted funnel shape; therefore, there was no publication bias.
Imipenem can cause mental disorders in patients. However, the low quality of the included literature may have affected the final results. Therefore, it is necessary to conduct a high-quality randomized controlled study with multiple samples to further confirm the mechanism of imipenem-induced mental disorders and provide effective guidance for clinical treatment.
Core Tip: Imipenem is widely used in clinical treatments because of its high efficiency, enzyme resistance, and broad-spectrum antibacterial properties. However, reports of its adverse reactions are increasing daily, among which nervous system adverse reactions are the most common, usually showing symptoms similar to those of nervous system diseases, mental disorders, and aggravating the original nervous system diseases. It has been reported that imipenem can cause insomnia, intermittent delirium and unconsciousness, and its nervous system adverse reactions are related to age, renal insufficiency, hypoxemia, and dosage. Although the adverse effects of imipenem on the nervous system have been recognized in reports both at home and abroad, there is still a lack of in-depth and systematic research on its specific mechanism of causing mental disorders. Owing to the differences in the intervention measures and evaluation methods of different studies, the effectiveness and comprehensiveness of existing research results are limited to some extent. Therefore, this study collected and analyzed relevant literature at home and abroad on imipenem-induced mental disorders and deeply explored its potential mechanism, providing a scientific and comprehensive reference for patients to use drugs safely.
- Citation: Zhan ZH, Wang JL, Wang LH, Shen NN, Liu XW, Yu YN, Liu FR. Mechanism of imipenem-induced mental disorder: A meta-analysis. World J Psychiatry 2024; 14(10): 1583-1591
- URL: https://www.wjgnet.com/2220-3206/full/v14/i10/1583.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i10.1583
Imipenem is widely used in clinical treatments because of its high efficiency[1], enzyme resistance, and broad-spectrum antibacterial properties. However, reports of its adverse reactions are increasing daily[2], among which nervous system adverse reactions are the most common, usually showing symptoms similar to those of nervous system diseases, mental disorders, and aggravating the original nervous system diseases[3]. It has been reported that imipenem can cause insomnia, intermittent delirium and unconsciousness, and its nervous system adverse reactions are related to age, renal insufficiency, hypoxemia, and dosage[4]. Although the adverse effects of imipenem on the nervous system have been recognized in reports both at home and abroad, there is still a lack of in-depth and systematic research on its specific mechanism of causing mental disorders. Owing to the differences in the intervention measures and evaluation methods of different studies, the effectiveness and comprehensiveness of existing research results are limited to some extent. Therefore, this study collected and analyzed relevant literature at home and abroad on imipenem-induced mental disorders and deeply explored its potential mechanism, providing a scientific and comprehensive reference for patients to use drugs safely.
The relevant literatures were searched through the network resources of the library and understanding research progress at home and abroad. Database sources: Cochrane Library, Web of Science, PubMed, ProQuest, China Biomedical Literature Database, Wanfang, Weipu, and China HowNet. The search was carried out by combining subject words with free words, and the search was carried out by combining subject words with keywords. The English search words were “Psychosis”, “Imipenem”, “Mechanisms”, and “Pharmaceutic”. Chinese keywords included imipenem, tylenol, mental symptoms, and drug mechanism. If necessary, relevant references were manually searched and tracked. Randomized controlled trials on mental disorders caused by imipenem were collected, and the retrieval time was from the esta
The inclusion criteria were as follows: (1) Randomized controlled trials published at home and abroad on mental disorders caused by imipenem; (2) Imipenem-induced nervous system damage, such as convulsions, epilepsy, and muscle spasm, and the age, course of disease, and education level of the two groups were consistent; (3) Intervention measures: The control group used conventional drugs, while the experimental group added imipenem based on the control group; and (4) The patient suffered from nervous system damage after treatment with imipenem. Exclusion criteria: (1) The content of the article was incomplete; (2) There were duplicate documents, or the documents were summaries, meeting minutes, etc.; (3) The research design lacked rigor; (4) Only one article was published repeatedly; (5) Non-Chinese and English literature; and (6) The sample data were incomplete.
According to the Jadad quality scoring method, the methodological quality of each included article was evaluated individually, including the approval of the ethics committee of each research center, signing of the informed consent form of the participants, scheme design, level of blindness, randomization method, proportion of lost visits, and consistency of comparison baseline. Documents that met the research purpose and admission criteria were screened. Documents inconsistent with this analysis were removed by reading their titles and abstracts. Microsoft Excel was used to extract the literature data, first author, publication date, sample size, intervention methods, and outcome indicators for each experiment. Two researchers who had received complete systematic evaluation training independently screened the literature; differences were resolved through consultation on the basis of screening, and if necessary, a third-party expert with professional knowledge in this field was consulted.
Two researchers independently evaluated the included studies using the Cochrane Bias Risk Assessment Tool Version 5.4[5,6]. The risk of bias was assessed in the included literature. The items were divided into three categories: “high”, “low”, and “unclear”, which mainly included the following seven items: (1) Random allocation method; (2) The allocation scheme was hidden; (3) Adopting blind method to the research object and the implementer of the treatment plan; (4) Adopting blind method to measure the research results; (5) Integrity of the result data; (6) Reporting the research results selectively; and (7) Other sources of bias. If the low risk is fully met, it will be “A”, if it is partially met, it will be “B”, if it is not met, it will be “C”, and all items will be excluded as “high risk bias”.
A meta-analysis was performed using RevMan 5.4, and I2 was used to evaluate the heterogeneity. When I2 < 50% and P > 0.1, heterogeneity was low or nonexistent. The meta-analysis adopted a fixed-effect model. When I2 ≥ 50% and P ≤ 0.1, the heterogeneity was high. The meta-analysis adopted a random effects model and used a funnel diagram to evaluate the bias.
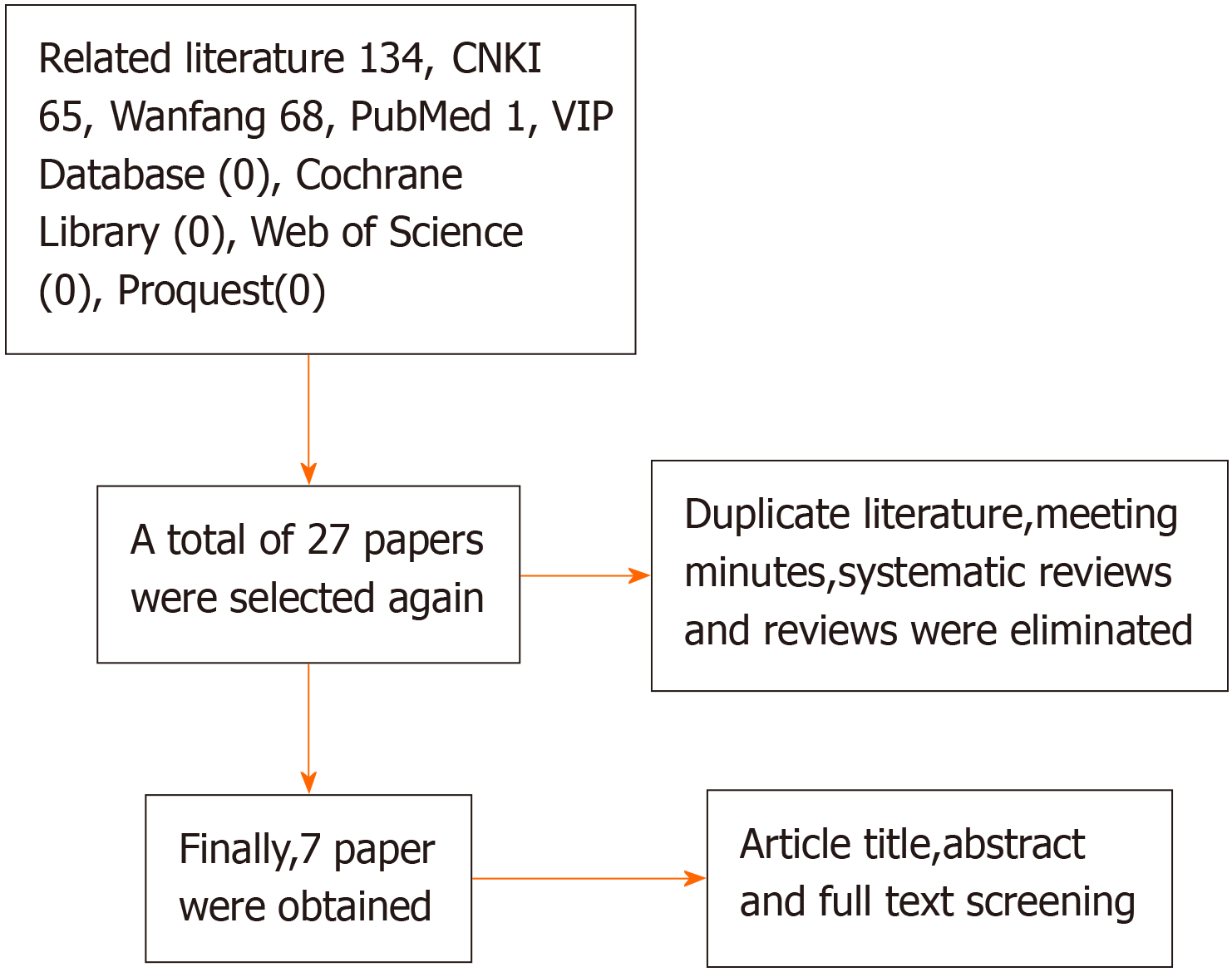
A literature search was conducted using keywords, and 134 related studies were screened. After screening, duplicate studies, meeting records, and systematic reviews were eliminated, and 27 articles were finally obtained. Subsequently, the title, abstract, and full-text content of each document were screened and seven articles were identified. The search process is illustrated in Figure 1.
After reviewing the literature published from 2003 to 2017, seven studies were included, including 550 patients, with 273 and 277 cases in the control and experimental groups, respectively. The sample size ranged from 30 to 61. All patients in the experimental group were treated with imipenem, whereas the control group was treated with conventional drugs. Further details are listed in Table 1[7-13].
| Ref. | Sample size (control group/experimental group) | Grouping mode | Intervention method | Outcome index | |
| Control group | Experimental group | ||||
| Yang[7], 2017 | 35/35 | Randomized controlled trial | Conventional drug therapy | Conventional drugs + imipenem | 1,2,3,4,5,6 |
| Shan et al[8], 2015 | 34/36 | Randomized controlled trial | Conventional drug therapy | Conventional drugs + imipenem | 1,2,3,4,5,6 |
| Chen[9], 2010 | 45/46 | Randomized controlled trial | Conventional drug therapy | Imipenem | 1,2 |
| Xu et al[10], 2005 | 30/30 | Randomized controlled trial | Conventional drug therapy | Imipenem | 1,2,3 |
| Liu and Liu[11], 2004 | 31/31 | Randomized controlled trial | Conventional drug therapy | Imipenem | 1,2,3 |
| Yu et al[12], 2003 | 38/38 | Randomized controlled trial | Conventional drug therapy | Imipenem | 1,2,3 |
| Chen et al[13], 2005 | 61/61 | Randomized controlled trial | Conventional drug therapy | Imipenem | 1,2 |
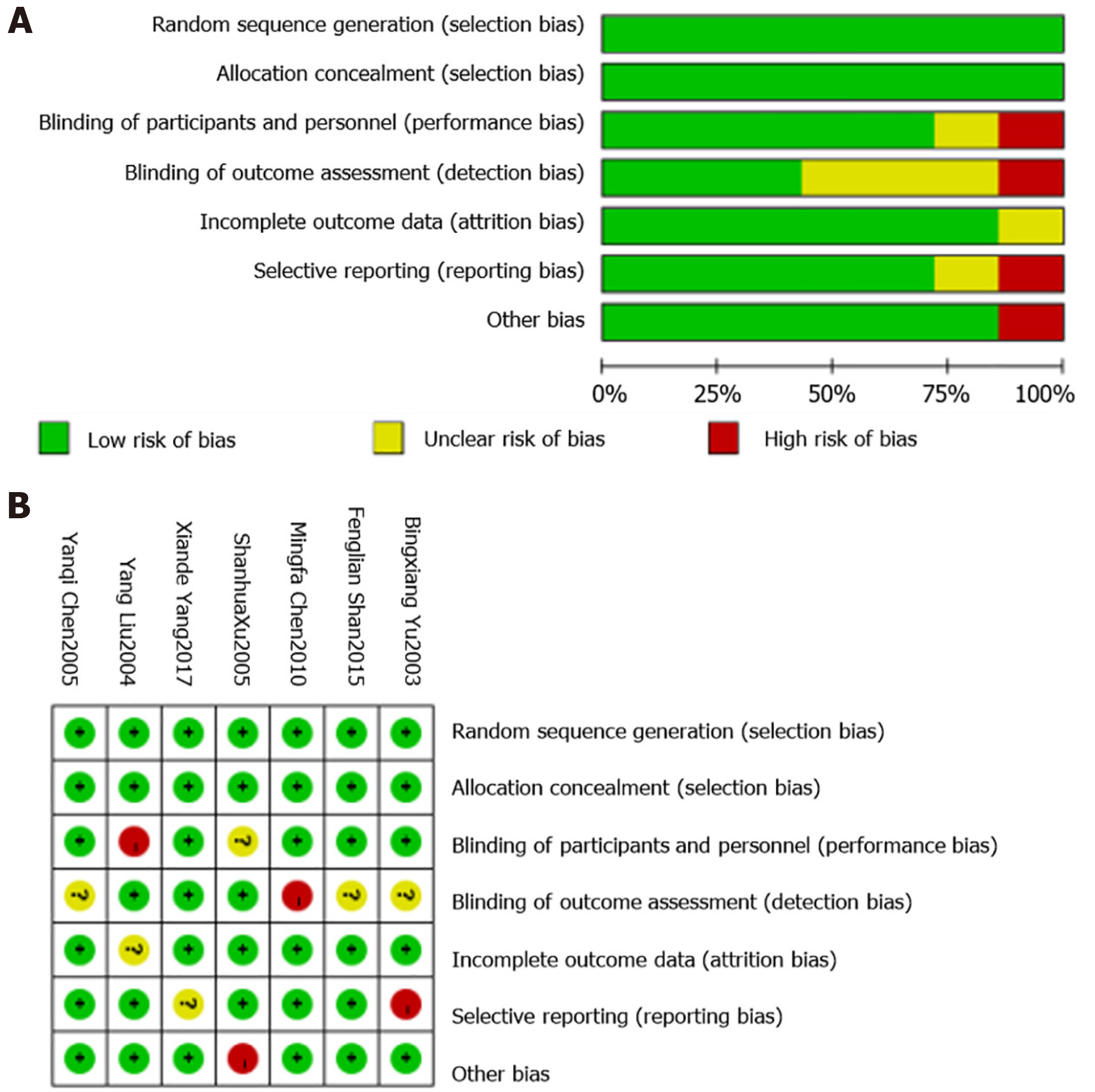
All seven included studies were randomly assigned and classified as randomized controlled experiments. Moreover, none of the studies described the distribution concealment, blind selective reporting, or other potential sources of bias in detail. However, they all mentioned mental disorders and their related adverse reactions. In terms of the therapeutic effect, five articles clearly reported the effective rate of treatment, while the other two articles mentioned the cure time, hospitalization expenses, and remission time. The Cochrane bias risk assessment tool was used to evaluate the quality, and the results are shown in Figure 2.
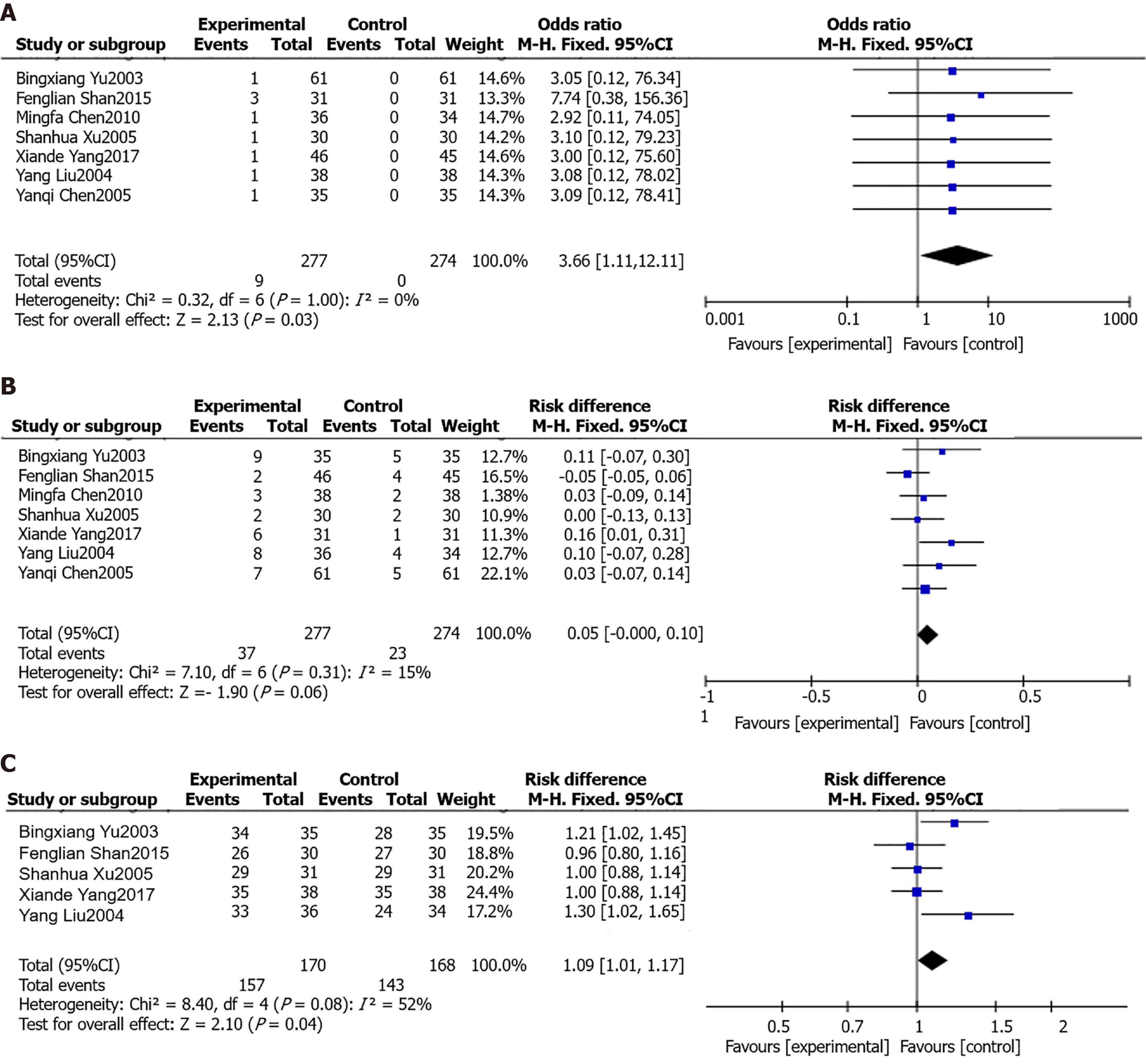
Mental disorders after treatment: Through a comprehensive analysis of seven studies, it was found that patients experienced mental abnormalities after treatment. When comparing the data of the two groups, the results showed that P was 1.00 and I2 was 0%, indicating that there was no obvious heterogeneity. Therefore, the results of the meta-analysis with the fixed-effects model showed that the meta-disorders in the experimental group were significantly higher than those in the control group after treatment [odds ratio (OR) = 3.66,95% confidence interval (CI): 1.11-12.11, P = 0.030], as shown in Figure 3A.
Adverse reaction after treatment: Seven studies analyzed adverse reactions in patients after treatment. When comparing the data of the two groups, it was found that the P value was 0.31 and I2 was 15%, which indicated that the heterogeneity was low. Therefore, the fixed effects model was used for the meta-analysis, and the results showed no significant difference in the incidence of adverse reactions between the two groups after treatment (OR = 0.05, 95%CI: 0.00-0.10, P = 0.060), as shown in Figure 3B.
Effective rate after treatment: Five studies analyzed the total effective rate of treatment, which was calculated as (number of cured cases + number of cases with significant effect + number of effective cases)/total number of cases × 100%. The test results of heterogeneity between the two groups were P = 0.08, I2 = 52%, indicating heterogeneity. A meta-analysis was conducted using the random effects model, and the results showed that after treatment, the efficacy rate of patients in the experimental group was significantly higher than that in the control group (OR = 1.09, 95%CI: 1.01-1.17, P= 0.040), as shown in Figure 3C.
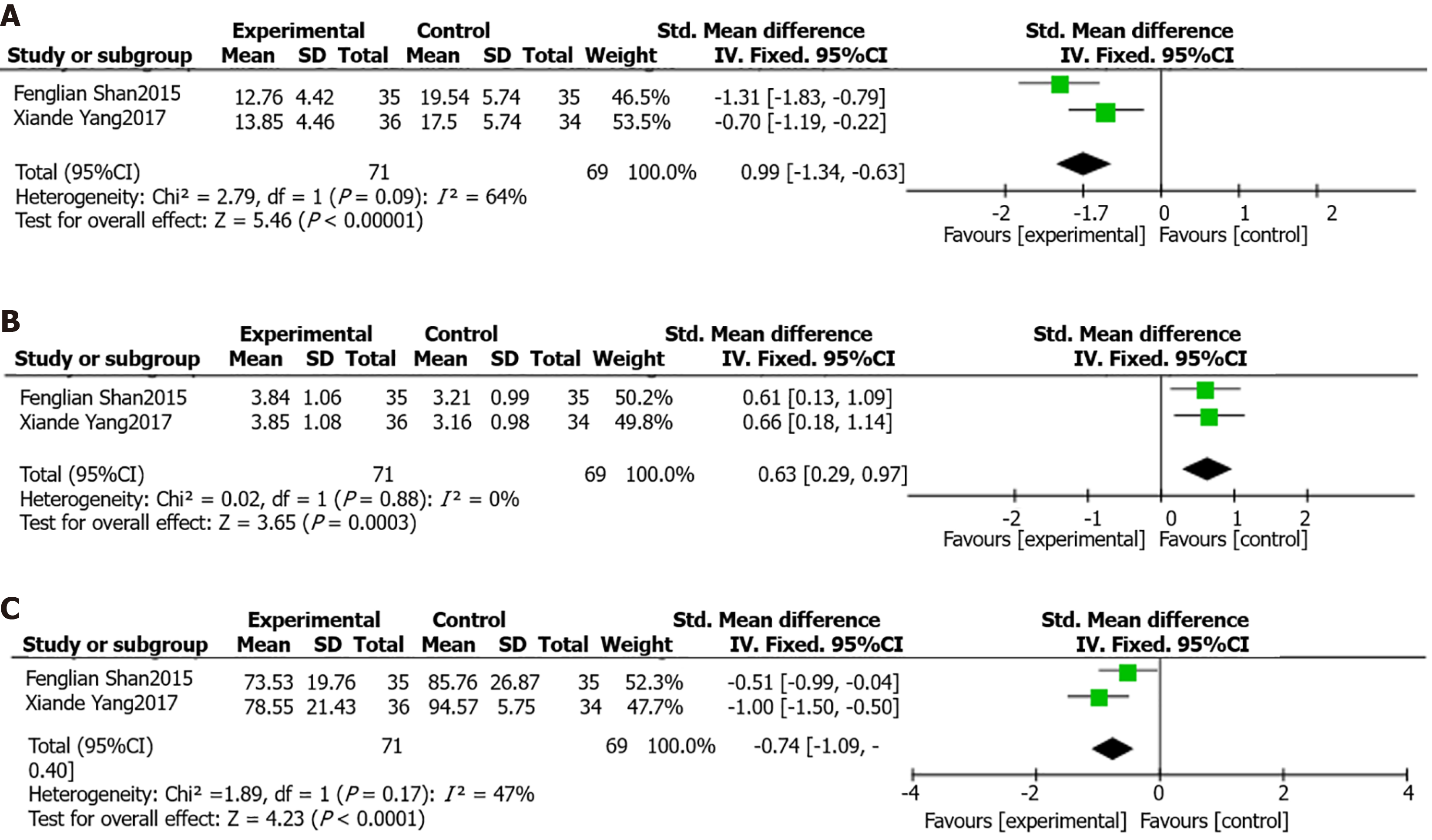
Cure time of illness: There are two literatures that analyzed the cure time of patients, and the heterogeneity between the two groups showed that P = 0.09, I2 = 64%, and there was heterogeneity. Meta-analysis using the random-effects model showed that the cure time of the experimental group was better than that of the control group, and the time was shorter (OR = -0.99, 95%CI: -1.34 to -0.63, P < 0.0001), as shown in Figure 4A.
Hospitalization expenses: Two studies analyzed the hospitalization expenses of patients, and the heterogeneity between the two groups was P = 0.88, I2 = 0% and there was no heterogeneity. Meta-analysis using the fixed effects model showed that the hospitalization expenses of the experimental group were higher than those of the control group (OR = 0.63, 95%CI: 0.29-0.97, P = 0.0003), as shown in Figure 4B.
Time of remission: Two studies analyzed the remission time of patients, and the heterogeneity test between the two groups showed that P = 0.17, I2 = 47%, and there was no heterogeneity; therefore, the fixed-effect model meta-analysis showed that the remission time of the experimental group was significantly shorter than that of the control group (OR = -0.74, 95%CI: -1.09 to 0.40, P < 0.0001), as shown in Figure 4C.
Funnel diagram hint: The scatter points are symmetrical and distributed in an inverted funnel shape; therefore, the funnel diagram suggests that there is no publication bias, as shown in Figure 5.
Imipenem, a carbapenem antibiotic, can effectively inhibit the synthesis of bacterial cell walls by closely combining with penicillin-binding proteins, further exerting its antibacterial effect[14]. Imipenem is stable against plasmid- or chromosome-mediated lactamases produced by most gram-positive and gram-negative bacteria and can show post-antibiotic effects on Pseudomonas aeruginosa. In addition, imipenem also has significant antibacterial activity against bacteria producing extended-spectrum β-lactamases[15], making it one of the best drugs to treat serious bacterial infectious diseases. However, the wide application of imipenem in the clinic has also caused controversy regarding mental disorders and adverse reactions. The purpose of this study was to explore the potential mechanisms of imipenem-induced mental disorders and evaluate their value in practical applications to provide a more scientific and comprehensive medical basis for clinical work.
Seven articles were included in this review, with 273 participants in the control group and 277 in the experimental group. The results showed that the probability of mental disorders in the experimental group was significantly higher than that in the control group after treatment. However, the experimental group was superior to the control group in terms of treatment efficiency, cure time, and remission time. In addition, the hospitalization expenses of patients in the experimental group were relatively high. All data were statistically significant (P < 0.05). However, there was no significant difference in adverse reactions between the two groups (P > 0.05).
The control group was treated with conventional drugs, and the study group was treated with imipenem or imipenem on the basis of the control group. The results showed that imipenem use might lead to mental disorders in patients; however, this was not observed in the control group. The analysis reason is that this may be related to the high affinity of C2 side chain groups in the molecular structure of imipenem for gamma-aminobutyric acid (GABA) receptors. This affinity hinders the binding of GABA and its receptor, thus reducing neuronal discharge inhibition[16]. Therefore, the balance between excitability and inhibition of synaptic transmission is broken to some extent, which further interferes with the nerve inhibition of GABA[17], increasing the excitability of the central nervous system and leading to symptoms, such as restlessness, involuntary movement of limbs, and even seizures[18]. When the organism is infected by gram-negative bacteria, endotoxin binds to the body’s Toll-like receptor to activate the Toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-κB signaling pathway to release a large number of inflammatory factors, triggering an inflammatory response of the organism, thereby increasing the permeability of the blood-brain barrier, imipenem molecules enter the brain tissue system with the circulation of the blood, and the structure of the carbapenems α-position of the carbon atoms on the substitute for the special side chain is closely related. This side chain structure combines with the inhibitory neurons in the central nervous system, causing the central inhibitory transmitter to be blocked, which enhances central excitation to a certain extent[19]; moreover, factors such as the alkaline strength of the amino group, the distance from the carboxyl group to the base, and the spatial structure around the amino group are all factors that affect the role of central inhibitory transmitter in the structure of imipenem molecule, which ultimately leads to hallucinations, hallucinations, and orientation altered orientation and other neurologic adverse effects. This suggests that the inflammatory response of the body may affect the efficacy of imipenem and the occurrence of adverse effects. This study also found that imipenem may cause adverse reactions in other systems, such as renal function. This is primarily because most drugs are excreted through the kidneys. If renal function declines, the excretion rate of drugs will decrease, which will lead to an increase in blood drug concentration, further increasing the permeability of the blood-cerebrospinal fluid barrier and drug concentration in the brain tissue. Therefore, renal dysfunction is considered to be an important cause of drug-induced neurotoxicity. Although antibacterial drugs exert strong antibacterial effects, they can cause serious adverse reactions. In this study, there was no significant difference in the occurrence of adverse reactions between the control and study groups, which further emphasizes that when using antibacterial drugs, such as imipenem, it is necessary to comprehensively consider their efficacy and potential risks to ensure patient safety.
The results of the present study significantly revealed the efficient efficacy of imipenem. The reason was analyzed as follows: Imipenem can effectively inhibit the synthesis of virulent bacteria by binding tightly to penicillin-binding proteins on the cell membrane, thus destroying the structural integrity of virulent bacteria. In addition, imipenem can combine with the cell walls of anaerobic bacteria to inhibit their growth and reproduction, thus showing superior efficacy in treating mixed infections of aerobic and anaerobic bacteria. In addition, imipenem was effective against Pseudomonas aeruginosa. Owing to its unique side chain group, imipenem can penetrate the outer membrane of cells and the body of bacteria, play an antibacterial role, and show good antibacterial effects against multidrug-resistant bacteria[20].
The mechanism of adverse reactions in the study is unclear and there is selective bias. There are some differences in the condition, course of disease, and age of the participants, which affect the results of meta-analysis to some extent. There were methodological defects, the quality was low, and there may be potential bias, which may have affected the research results to some extent. The specific methods, usage and course of treatment of the participants were inconsistent, and the results may be biased. The quality of literature was low, the sample size was small, and there were too few detection indicators, thus limiting the accuracy of argumentation. The severity of infection of the patients involved in the included papers was not analyzed in this study, which will affect the results of Meta-analysis to some extent. The routine medication of patients and the specific dose of imipenem were not analyzed in this study when patients were analyzed, and the specific efficacy of imipenem in the results of Meta-analysis may also be biased due to the differences in the types of routine medication and dose of patients in the included papers. This study only analyzed the therapeutic effect of imipenem, and did not analyze the incidence of adverse reactions, such as psychiatric disorders, during treatment with imipenem, so the safety of the use of imipenem needs to be further studied. The study only analyzed the clinical data of the seven papers involved, and did not explore the specific mechanism of mental disorders induced by imipenem from the animal and cellular levels, so further experiments are needed to explore the specific pathways of action. The literature included in this study was only in Chinese and English, which led to the fact that relevant studies from other countries were not included, and the selection of data for this study was limited.
According to existing literature, although imipenem is effective in the treatment of serious bacterial infectious diseases, it may also lead to neurotoxic side effects, such as mental disorders. Therefore, when using imipenem, it is necessary to strictly control the dosage and closely monitor the patient’s nervous system. For patients with preexisting nervous system diseases, drugs should be used with caution to prevent the disease from worsening. However, owing to the limitations of this study, to evaluate the efficacy and safety of imipenem more comprehensively, multicenter, randomized, double-blind, large-sample randomized controlled trials should be carried out in the future to further verify and demonstrate the existing results.
| 1. | Jin SP, Lan L, Li CJ. [Imine south/west division of his ding adverse reactions summary of recent literature]. Zhongguo Yaowu Lanyong Fangzhi Zazhi. 2014;2. |
| 2. | Li P. [Clinical pharmacists participated in the treatment of epilepsy induced by imipenem cilastatin: a case report]. Zhongguo Xiandai Yaowu Yingyong. 2015;9. |
| 3. | Wen AP, Li DD, Bao XL. [Incidence and risk factors of central nervous system adverse reactions induced by imipenem/Cilastatin in adult critically ill patients]. Zhongguo Yaowu Yingyong Yu Jiance. 2019;16. |
| 4. | Zhu ZD, Chen DQ, Wang LY, Wang WL. [Analysis of nervous system adverse reactions induced by imipenem/Cilastatin in patients with heart failure and severe infection]. Zhongguo Xiandai Yaowu Yingyong. 2008. |
| 5. | Xiao HL, Cao BW, He H, Yin CH. [Meta-analysis of efficacy and safety of meropenem and imipenem in the treatment of moderate and severe pulmonary infection]. Zhongguo Ganran Yu Hualiao Zazhi. 2010;10:264-269. [DOI] [Full Text] |
| 6. | Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: A systematic review. Neurology. 2015;85:1332-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Yang XD. [Effect of imipenem/Cilastatin and norvancomycin in the treatment of patients with lower respiratory tract infection]. Zhongguo Chufangyao. 2017;100-101. |
| 8. | Shan FL, Shao ZW, Shan F. [Effect of imipenem/Cilastatin combined with norvancomycin on patients with lower respiratory tract infection]. Zhongguo Yiyuan Ganranxue Zazhi. 2015;25:1047-1049. [DOI] [Full Text] |
| 9. | Chen MF. [Clinical analysis of imipenem-cilastatin in the treatment of 46 cases of severe infection]. Zhejiang Yixue. 2010;264-265. |
| 10. | Xu SH, Wang W, Huang H, Zhang Y. [Clinical study of meropenem in the treatment of ventilator-associated pneumonia]. Zhonghua Yiyuan Ganranxue Zazhi. 2005;15. [DOI] [Full Text] |
| 11. | Liu Y, Liu XM. [Comparison of clinical efficacy and safety of meropenem in the treatment of severe pneumonia caused by extended-spectrum β -lactamases producing bacteria]. Zhongguo Jijiu Yixue. 2004;. |
| 12. | Yu BX, Liu YN, Wang R. [Clinical study of meropenem and imipenem/cilastatin in the treatment of lower respiratory tract infection]. Zhonghua Yiyuan Ganranxue Zazhi. 2003;13:72-74. [DOI] [Full Text] |
| 13. | Chen YQ, Xiang SG, Leng B, Zhou H, Wang XT, Qiao YD. [Observation and study on the curative effect of meropenem in the treatment of elderly bacterial lower respiratory tract nosocomial infection]. Zhongguo Kangganran Hualiao Zazhi. 2005;5:373-375. [DOI] [Full Text] |
| 14. | Neo HY, Tan KT, Caroline C, Wei-Han Ng D, Pei-Ying Ho E, Lim JP, Hsien-Xiong Lee R, Choon-Kiat Tan N, Chien-Boon Lye D, Ding YY. Higher rates of carbapenem-related seizures in older hospitalised adults. Intern Med J. 2020;50:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Sagan O, Yakubsevitch R, Yanev K, Fomkin R, Stone E, Hines D, O'Donnell J, Miller A, Isaacs R, Srinivasan S. Pharmacokinetics and Tolerability of Intravenous Sulbactam-Durlobactam with Imipenem-Cilastatin in Hospitalized Adults with Complicated Urinary Tract Infections, Including Acute Pyelonephritis. Antimicrob Agents Chemother. 2020;64:e01506-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Salmon-Rousseau A, Martins C, Blot M, Buisson M, Mahy S, Chavanet P, Piroth L. Comparative review of imipenem/cilastatin versus meropenem. Med Mal Infect. 2020;50:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 17. | Martínez Delgado S, Mínguez Sabater A, Ladrón Abia P, Aguilera Sancho-Tello MV, García Eliz M, Conde I. Ertapenem neurotoxicity in liver transplantation. Rev Esp Enferm Dig. 2022;114:240-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Guo X, Guo M, Li J, Cui X. Central nervous system adverse events of ceftazidime/avibactam: A retrospective study using Food and Drug Administration Adverse Event Reporting System. J Clin Pharm Ther. 2022;47:2369-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Sang D, Wang JY. [Clinical analysis and treatment of epilepsy caused by Imipenem/cilastatin sodium]. Zhongguo Yaoshi. 2018;32:975-978. [DOI] [Full Text] |
| 20. | Titov I, Wunderink RG, Roquilly A, Rodríguez Gonzalez D, David-Wang A, Boucher HW, Kaye KS, Losada MC, Du J, Tipping R, Rizk ML, Patel M, Brown ML, Young K, Kartsonis NA, Butterton JR, Paschke A, Chen LF. A Randomized, Double-blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults With Hospital-acquired or Ventilator-associated Bacterial Pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis. 2021;73:e4539-e4548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |