Published online Oct 19, 2024. doi: 10.5498/wjp.v14.i10.1573
Revised: September 11, 2024
Accepted: September 23, 2024
Published online: October 19, 2024
Processing time: 173 Days and 1.8 Hours
Major depressive disorder (MDD) is a substantial global health concern, and its treatment is complicated by the variability in individual response to antidepressants.
To consolidate research and clarify the impact of genetic variation on MDD treatment outcomes.
Adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, a systematic search across PubMed, EMBASE, Web of Science, and the Cochrane Library was conducted without date restrictions, utilizing key terms related to MDD, serotonin 1A receptor polymorphism (5-HTR1A), C-1019G polymorphism, and antidepressant response. Studies meeting inclusion criteria were thoroughly screened, and quality assessed using the Newcastle-Ottawa Scale. Statistical analyses, including χ2 and I² values, were used to evaluate heterogeneity and fixed-effect or random-effect models were applied accordingly.
The initial search yielded 1216 articles, with 11 studies meeting criteria for inclusion. Analysis of various genetic models showed no significant association between the 5-HTR1A C-1019G polymorphism and antidepressant efficacy. The heterogeneity was low to moderate, and no publication bias was detected through funnel plot symmetry and Egger's and Begg's tests.
This meta-analysis does not support a significant association between the 5-HTR1A C-1019G polymorphism and the efficacy of antidepressant treatment in MDD. The findings call for further research with larger cohorts to substantiate these results and enhance the understanding of antidepressant pharmacogenetics.
Core Tip: This study investigates whether the serotonin 1A receptor polymorphism C-1019G polymorphism influences antidepressant efficacy in major depressive disorder (MDD). Despite extensive research, our meta-analysis involving 11 selected studies shows no significant correlation between this genetic variation and treatment outcomes. These findings underscore the complexity of antidepressant pharmacogenetics and highlight the need for further large-scale studies to clarify the role of genetic factors in MDD treatment response. This work contributes to the ongoing discussion and development of personalized medicine strategies in psychiatry.
- Citation: Wu HN, Zhu SY, Zhang LN, Shen BH, Xu LL. Association between 5-HTR1A gene C-1019G polymorphism and antidepressant response in patients with major depressive disorder: A meta-analysis. World J Psychiatry 2024; 14(10): 1573-1582
- URL: https://www.wjgnet.com/2220-3206/full/v14/i10/1573.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i10.1573
Major depressive disorder (MDD) is a prevalent and incapacitating mental health illness that impacts millions globally. MDD is marked by enduring sorrow, diminished interest, and various mental and physical issues, severely affecting daily functioning and overall quality of life[1,2]. Despite its great prevalence and severity, the pathophysiology of MDD is only poorly comprehended, highlighting the necessity for ongoing study into its molecular foundations[3,4]. The management of MDD predominantly entails the use of antidepressant pharmacotherapy, designed to mitigate symptoms and enhance patient outcomes. The clinical efficacy, safety, and tolerance of these drugs differ significantly among patients. This heterogeneity can be ascribed to individual genetic differences, contextual influences, and the intricate structure of the condition itself. As a result, a significant percentage of patients fail to attain a satisfactory response to initial therapy, necessitating attempts with various drugs and combinations, frequently yielding minimal success.
The serotonin 1A receptor polymorphism (5-HTR1A) gene has garnered significant interest among the several genetic variables associated with the diversity in antidepressant response. The 5-HT1A receptor, encoded by this gene, is pivotal in the serotonergic system, a primary target for most antidepressant medications[5]. Polymorphisms in the 5-HTR1A gene, namely the C-1019G variation, are posited to affect the effectiveness of antidepressant therapy[6]. This po
Considering the potential ramifications of these findings for personalized medicine in the treatment of MDD, a thorough comprehension of the association between the 5-HTR1A C-1019G polymorphism and antidepressant efficacy is essential. This systematic review and meta-analysis endeavor to consolidate existing research on this subject, addressing the discrepancies in the literature and offering a more conclusive determination regarding the impact of this genetic variation on treatment outcomes in MDD. By clarifying this relationship, we aspire to aid in the formulation of more effective, personalized treatment strategies for individuals afflicted by this debilitating condition.
This systematic review demonstrated strict compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards[8]. A systematic search was performed across four electronic databases: PubMed, EMBASE, Web of Science, and the Cochrane Library, on September 19, 2023, without constraints on publication date. The formulated search strategy included essential phrases such as MDD, 5-HTR1A, C-1019G polymorphism, and antidepressant response. These terms were carefully selected to comprehensively address the elements of the Patient, Intervention, Comparison, Outcome framework and guarantee a complete compilation of relevant papers for the meta-analysis. Publications in every language were taken into account. A comprehensive manual examination of the bibliographies of pertinent papers was conducted to uncover any further studies.
Inclusion criteria: Studies that specifically investigated the association between the 5-HTR1A gene C-1019G poly
Studies that provided clear data on the genotype of the 5-HTR1A C-1019G polymorphism.
Exclusion criteria: Research involving animal models or in vitro experiments.
Non-peer-reviewed articles, conference abstracts, editorials, and literature reviews.
Studies with a primary focus on other psychiatric disorders, even if they included a subset of patients with MDD.
Publications not available in full text or those that required translation from languages other than English, due to resource constraints.
In the meta-analysis, literature screening and data extraction were performed independently by two reviewers, who subsequently cross-verified their results. In cases of disagreement, the reviewers engaged in discussions to achieve consensus, and a third-party reviewer was consulted when necessary. The extracted data included various parameters: (1) Authorship; (2) Publication year; (3) Sample size; (4) Participant age; (5) Ethnicity; (6) Country; (7) Clinical diagnosis; (8) Diagnostic criteria; (9) Treatment efficacy criteria; (10) Medication duration; (11) Types of antidepressants utilized; and (12) The distribution of the 5-HTR1A gene C1019G polymorphism. When the required data were not available in published reports, the original study investigators were contacted via email to request the pertinent unpublished information.
The meta-analysis involved a thorough evaluation of study quality by two independent assessors using the Newcastle-Ottawa Scale (NOS)[9]. This measure, noted for its robustness, assesses research across three essential domains: (1) Selection; (2) Comparability; and (3) Outcome, each contributing to a thorough awareness of potential biases within the investigations. Following this comprehensive evaluation, studies were assigned ratings from 0 to 9 according to their compliance with these criteria. The score interpretation was organized as follows: (1) A score ranging from 0 to 3 denoted low-quality studies; (2) Scores from 4 to 6 indicated moderate quality; and (3) Studies with scores from 7 to 9 were classified as high quality.
The meta-analysis conducted statistical evaluations to assess the heterogeneity among studies. This was accomplished using χ2 statistics, with the degree of heterogeneity measured by the I2 value. A situation in which the I2 value was below 50% and the P value was 0.10 or greater signified an absence of substantial heterogeneity, resulting in the utilization of the fixed-effect model for determining the aggregated effect size. An I2 value of 50% or above, or a P value below 0.10, indicates considerable heterogeneity. In these situations, the random-effects model was employed to ascertain the aggregated effect size. An examination of the funnel plot's symmetry was performed to assess potential publishing bias. A symmetrical arrangement of data points around the apex in the funnel plot would indicate a negligible impact of publication bias on the results of the meta-analysis. Additionally, Egger's linear regression test functioned as a quantitative instrument for identifying publication bias. All statistical tests were bilateral, with a P value threshold established at less than 0.05 for statistical significance. The data analysis utilized Stata version 17 (StataCorp, College Station, TX, United States).
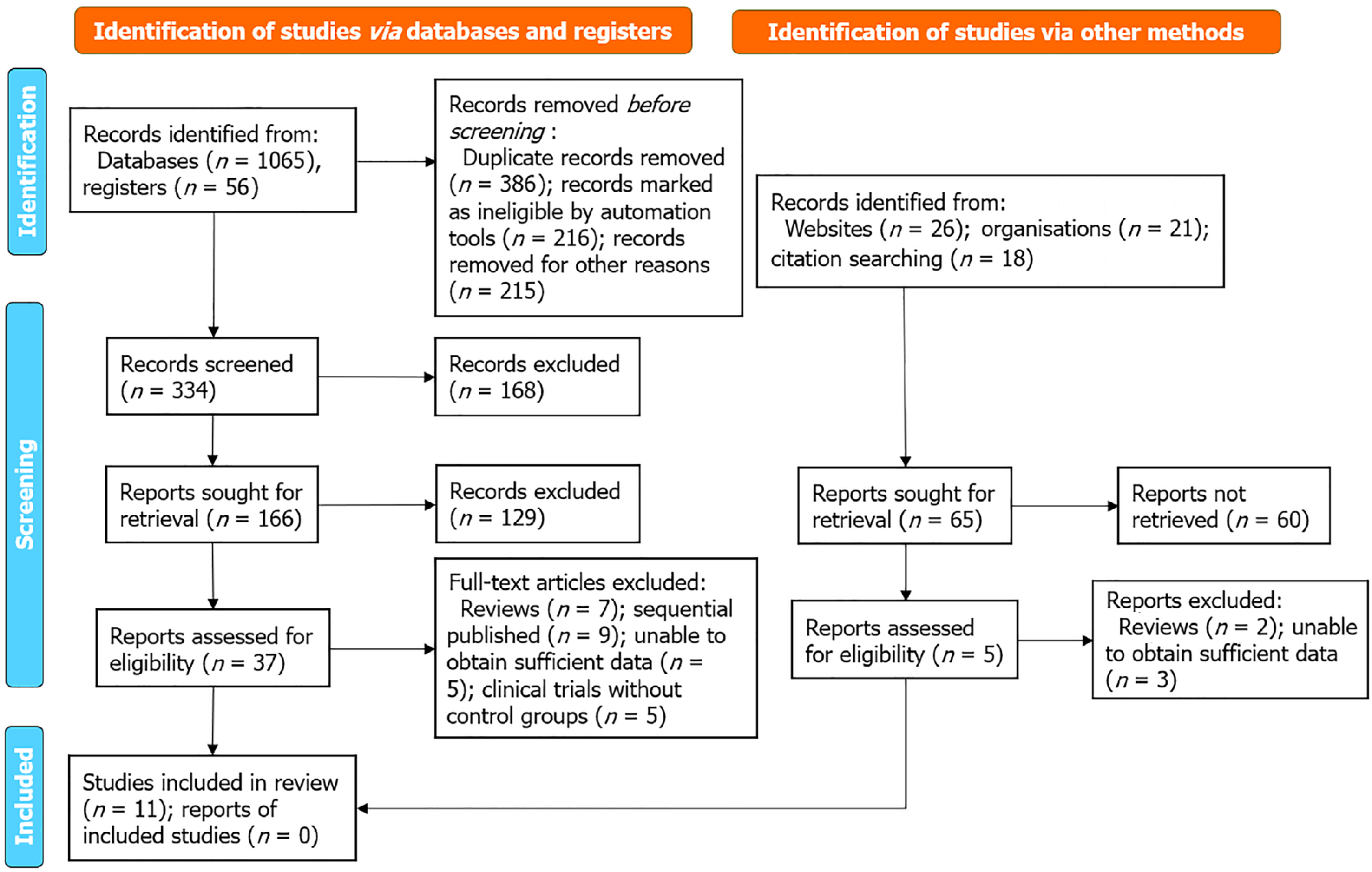
During the initial phase of this systematic review and meta-analysis, a thorough search across various electronic databases yielded 1216 articles potentially relevant to the study. A deduplication algorithm was then applied to eliminate duplicate entries, ensuring the uniqueness of each study included. This was followed by a detailed screening of titles and abstracts, guided by well-defined inclusion and exclusion criteria. These criteria encompassed various aspects such as study methodology, demographic characteristics of the participants, clinical outcomes measured, and the research methods' overall quality. After this preliminary screening, 42 articles were shortlisted for a more detailed evaluation. This next stage involved an independent, full-text review by multiple investigators to ensure an impartial and comprehensive assessment of each article. During this in-depth examination, 31 articles were excluded for specific reasons: (1) Review articles (n = 9); (2) Sequentially published works (n = 9); (3) Studies with insufficient data for analysis (n = 8); and (4) Clinical trials without control groups (n = 5). Consequently, 11 articles were identified that satisfactorily met the stringent criteria outlined in the research protocol[10-20]. These selected studies were then included in the final meta-analysis, as illustrated in Figure 1.
The meta-analysis encompasses a broad spectrum of international studies that investigated the efficacy of antidepressant medication in MDD, as diagnosed by the diagnostic and statistical manual of mental disorders, fourth edition criteria. The included studies, published between 2004 and 2022, examined populations in Asia (India, South Korea, Japan, and China) and Europe (United Kingdom, Italy, and Finland), as well as multi-country studies including Caucasian populations from Belgium, Austria, and Israel. Participant ages across studies varied, with an average age ranging from 35.0 years ± 10.3 years to 88.16 years ± 3.80 years. The gender distribution within the sample sizes, where reported, included both male and female subjects, with total sample sizes ranging from 19 individuals to 283 individuals. The studies utilized a variety of scales to measure antidepressant efficacy, including the Hamilton Depression Scale, with both the 17-item and 21-item versions, the montgomery and asberg depression rating scale, the hamilton depression rating scale, and the geriatric depression scale. Treatment durations varied, with some studies conducting assessments over a period as brief as 2 weeks, while others extended up to 12 weeks. A diverse array of antidepressant medications was analyzed across the studies, including Citalopram, Escitalopram, Mirtazapine, Fluoxetine, Paroxetine, Sertraline, and combinations thereof, along with selective serotonin reuptake inhibitors, Selective Serotonin and Norepinephrine Reuptake Inhibitors, Venlafaxine, and Fluvoxamine. This collection of studies provides a comprehensive dataset for evaluating the impact of various antidepressant treatments on MDD, taking into account a range of demographic variables and clinical outcomes. The data will be instrumental in assessing the overall effectiveness of antidepressants in a diverse, international cohort of patients with MDD (Table 1).
| First author | Year | Country | Population | Diagnostic criteria | Age (mean ± SD) (years) | Sample size (man/female) | Efficacy criteria | Treatment duration (weeks) | Antidepressant medication |
| Ramesh | 2022 | India | Asian | DSM-IV | 39.26 ± 11.52 | 125 (73/52) | HAMD | 8 | SER, ESC, FLU, PAR |
| Scutt | 2018 | United Kingdom | Caucasian | DSM-IV | 88.16 ± 3.80 | 19 (5/14) | Geriatric depression scale | 4 | CIT |
| Basu | 2015 | India | Asian | DSM-IV | 35.0 ± 10.3 | 55 (32/23) | MADRS | 6-8 | ESC |
| Chang | 2014 | South Korea | Asian | DSM-IV | NA | 283 (254/290) | HAMD-17 | 12 | Mirtazapine |
| Serretti | 2013 | Italy | Caucasian | DSM-IV | 43.93 ± 15.93 | 117 (80/37) | Hamilton depression rating scale | 8 | AD |
| Zhao | 2012 | China | Asian | DSM-IV | 45.75 ± 7.28 | 85 (28/57) | HAMD-21 | 4 | SER |
| Noro | 2010 | Belgium, Austria, Israel | Caucasian | DSM-IV | NA | 206 | HAMD-17 | 4 | AD |
| Illi | 2009 | Finland | Caucasian | DSM-IV | 44.4 ± 13.9 | 86 | MADRS | 6 | FLU, PAR, CIT |
| Kato | 2009 | Japan | Asian | DSM-IV | 45.8 ± 14.4 | 137 (75/62) | HAMD | 6 | Selective serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors |
| Lin | 2009 | China | Asian | DSM-IV | 38.4 ± 12.6 | 101 (26/75) | HAMD | ≥ 2 | Venlafaxine, SER, PAR, FLU |
| Serretti | 2004 | Italy | Caucasian | DSM-IV | NA | 262 (173/89) | HAMD-21 | 6 | Fluvoxamine |
The methodological quality of the papers included in the meta-analysis was meticulously assessed using the NOS, a thorough instrument intended to evaluate the quality of non-randomized research, especially within systematic reviews and meta-analyses. The evaluation criteria encompassed three primary categories: (1) Selection of research groups; (2) Comparability of groups; and (3) Determination of either the exposure or result of interest for case-control or cohort studies, respectively. The study revealed a dispersion of NOS ratings that indicates predominantly excellent methodological quality. Two studies received a score of 7, indicating good quality, three studies were rated 8 points, and the bulk, consisting of six research, achieved the highest score of 9 points, denoting very high quality. Significantly, none of the studies employed blinding, and there was no indication of allocation concealment, features that could possibly introduce bias. Nonetheless, it was noted that none of the research exhibited indications of financing biases, implying that financial issues did not affect the study results. Moreover, all trials presented comprehensive outcome data, and there were no signs of early termination bias or baseline imbalances among participants. This thorough assessment resulted in a summary risk of bias and associated ratios, as outlined in Table 2 of the meta-analysis[10-20].
| Ref. | Selection | Comparability | Outcome | Total score | |||||
| Representativ-eness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome | Comparability of cohorts | Assessment of outcome | Was follow-up long enough | Adequacy of follow up of cohorts | ||
| Ramesh et al[16] | 1 | 1 | 1 | 1 | 1,1 | 1 | 1 | 1 | 9 |
| Scutt et al[17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Basu et al[10] | 1 | 1 | 1 | 1 | 1,1 | 1 | 1 | 1 | 9 |
| Chang et al[11] | 1 | 1 | 1 | 1 | 1,1 | 1 | 1 | 1 | 9 |
| Serretti et al[19] | 1 | 1 | 1 | 1,1 | 1 | 1 | 1 | 8 | |
| Zhao et al[20] | 1 | 1 | 1 | 1 | 1,1 | 1 | 1 | 1 | 9 |
| Noro et al[15] | 1 | 1 | 1 | 1 | 1,1 | 1 | 1 | 8 | |
| Illi et al[12] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Kato et al[13] | 1 | 1 | 1 | 1 | 1,1 | 1 | 1 | 1 | 9 |
| Lin et al[14] | 1 | 1 | 1 | 1 | 1,1 | 1 | 1 | 1 | 9 |
| Serretti et al[18] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
For the allelic model comparing the G allele to the C allele, the odds ratio (OR) was 0.86 with a 95%CI: 0.69-1.12, suggesting no significant association (P = 0.23). The model utilized for heterogeneity was random due to the moderate level of heterogeneity among the studies (I² = 46.46%), with a significant heterogeneity P value (P = 0.02). Both Egger's and Begg's tests showed P values of 0.64 and 0.83, respectively, indicating no significant publication bias. In the dominant model (CG vs CC), a fixed-effects approach yielded an OR of 0.81 (95%CI: 0.71–1.09), with the association remaining non-significant (P = 0.13). The heterogeneity was modest (I² = 15.81%), and not statistically significant (P = 0.26). Egger's and Begg's tests suggested a low probability of publication bias with P values of 0.10 and 0.09, respectively. The recessive model (GG vs CC) was analyzed using a random-effects model due to significant heterogeneity (I² = 40.64%, P = 0.04), and the OR was 0.80 (95%CI: 0.60–1.31). The association was not statistically significant (P = 0.37), and publication bias tests were non-indicative of bias (Egger's P = 0.25, Begg's P = 0.21).
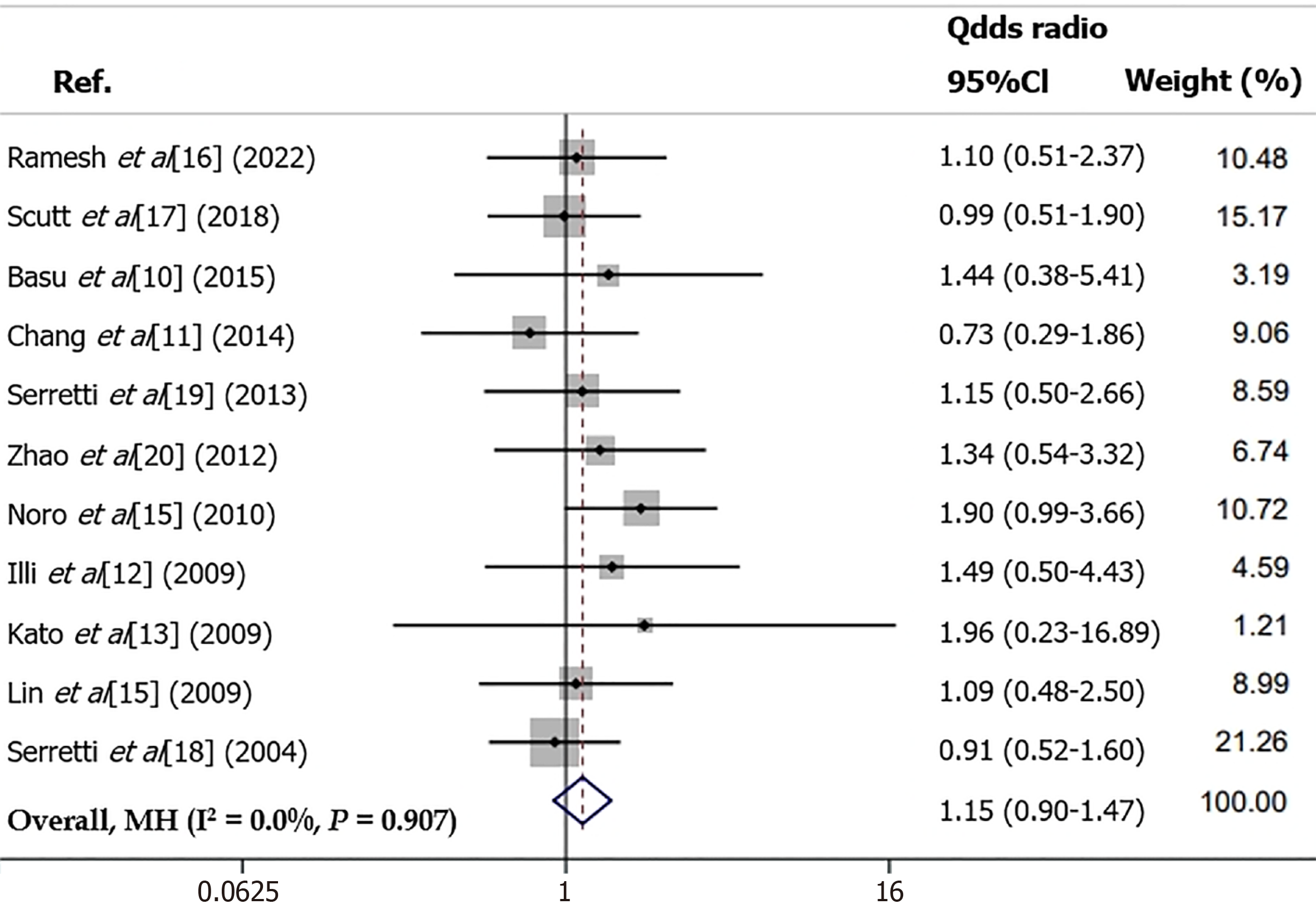
The combined dominant model (CG+GG vs CC) also applied a random-effects model because of substantial heterogeneity (I² = 33.66%, P = 0.04) and reported an OR of 0.80 (95%CI: 0.59–1.09), with a non-significant association (P = 0.23). Tests for publication bias returned P values of 0.10 for Egger's and 0.08 for Begg's, indicating a low likelihood of bias. The locus model (GG vs CG+CC) using a fixed-effects model (due to moderate heterogeneity, I² = 0.00%, P = 0.907) indicated an OR of 1.15 (95%CI: 0.90–1.47) with a non-significant association (P = 0.20, Figure 2). Publication bias was not suggested by the Egger's (P = 0.29) and Begg's tests (P = 0.16). Lastly, the unfitted model (CC+GG vs GC), analyzed with a fixed-effects model due to non-significant heterogeneity (I² = 0.00%, P = 0.52), showed an OR of 0.97 (95%CI: 0.82–1.20) with a non-significant association (P = 0.95). The publication bias tests were inconclusive (Egger's P = 0.64, Begg's P = 0.21) (Table 3).
| Genetic model | Odds ratio | 95%CI | P value (association) | Model type (heterogeneity) | P value (heterogeneity) | I² (heterogeneity) | Egger's test P value | Begg's test P value |
| Allelic Model (G vs C) | 0.86 | 0.69–1.12 | 0.23 | Random | 0.02 | 46.46 | 0.64 | 0.83 |
| Dominant model (CG vs CC) | 0.81 | 0.71–1.09 | 0.13 | Fixed | 0.26 | 15.81 | 0.10 | 0.09 |
| Recessive model (GG vs CC) | 0.80 | 0.60–1.31 | 0.37 | Random | 0.04 | 40.64 | 0.25 | 0.21 |
| Dominant model (CG+GG vs CC) | 0.80 | 0.59–1.09 | 0.23 | Random | 0.04 | 33.66 | 0.10 | 0.08 |
| Locus model (GG vs CG+CC) | 1.15 | 0.90–1.47 | 0.20 | Fixed | 0.907 | 0.00 | 0.29 | 0.16 |
| Unfitted model (CC+GG vs GC) | 0.97 | 0.82–1.20 | 0.95 | Fixed | 0.52 | 0.00 | 0.64 | 0.21 |
The meta-analysis did not reveal a significant impact of the 5-HTR1A C-1019G polymorphism on the efficacy of antidepressant treatment across various genetic models. The analyses demonstrate a consistent lack of statistically significant association, with low to moderate heterogeneity and minimal indications of publication bias across studies. This suggests that the C-1019G polymorphism in the 5-HTR1A gene may not be a major determinant of the clinical response to antidepressant medication in the assessed effective treatment group.
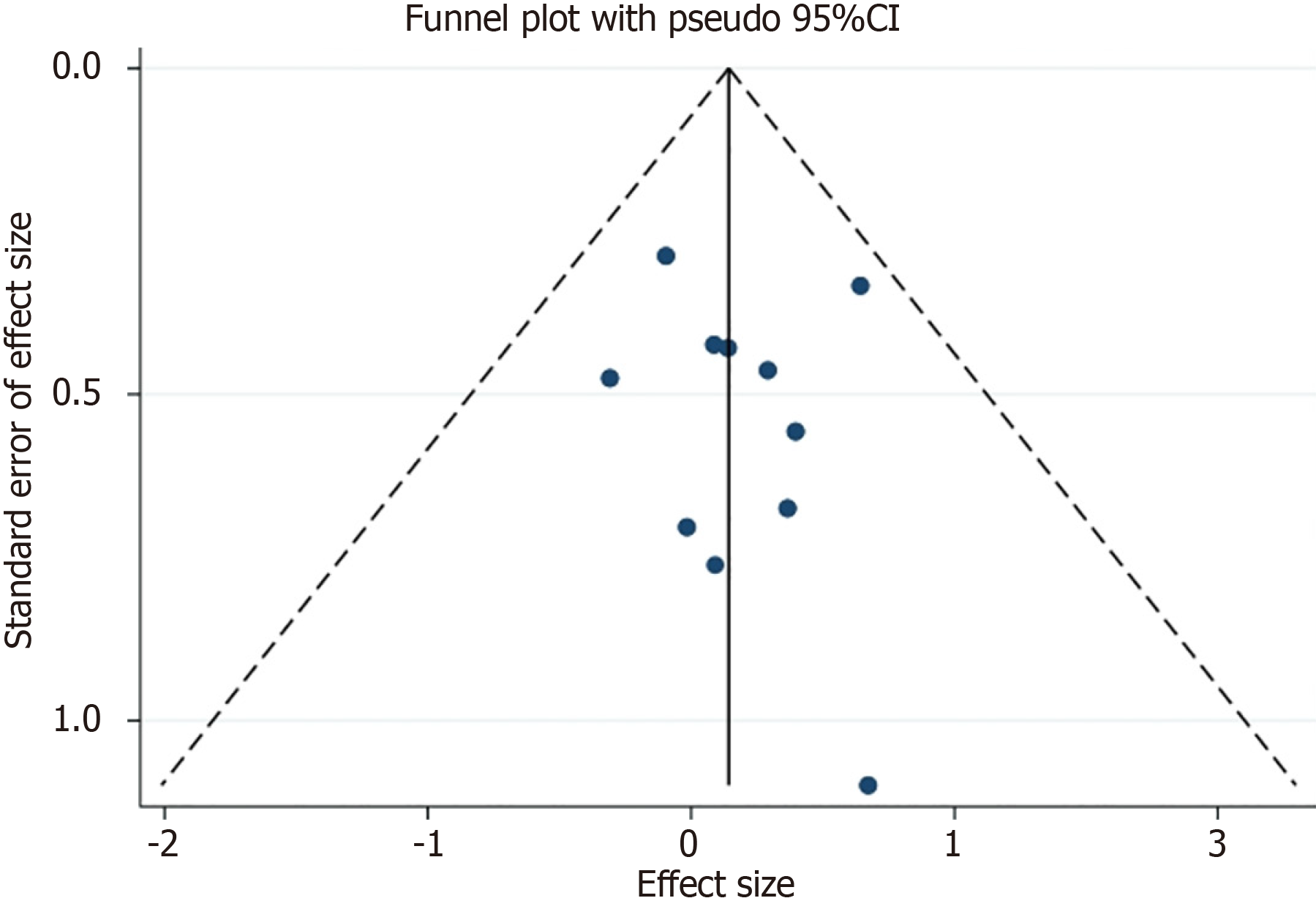
Publication bias within the meta-analysis was systematically evaluated to ascertain the robustness and reliability of the pooled results. Funnel plots, which visually depict the correlation between study size and effect estimates, were created for each analysis. The symmetrical arrangement of papers in these plots did not indicate publication bias, as asymmetry in such plots typically signifies this bias (Figure 3). Additionally, Egger's linear regression test was utilized as a statistical method to quantitatively evaluate the likelihood of publication bias among the papers. The test findings were non-significant for all evaluated variables (P > 0.05), hence reinforcing the lack of publication bias in this meta-analysis. These findings bolster the validity of the meta-analytic results, indicating that the estimated effects are not significantly affected by unpublished research or selective reporting.
The novelty of this study lies in its comprehensive synthesis of international data, spanning diverse ethnic populations and clinical contexts, making it one of the few meta-analyses to thoroughly assess the role of the 5-HTR1A C-1019G polymorphism in antidepressant efficacy. This provides valuable insight into personalized treatment strategies in psychiatric care. Although the polymorphism itself may not be a major determinant of therapeutic outcomes, the study’s findings emphasize the importance of exploring other genetic and environmental factors in MDD treatment response, further supporting the need for precision medicine in this field. The potential clinical application of these findings lies in guiding future genetic research, where a broader array of biomarkers could enhance antidepressant treatment opti
MDD continues to be a critical global health issue, marked by its high prevalence and considerable impact on disability and mortality rates. The intricacy of its pathophysiology, encompassing genetic, environmental, and psychosocial components, hampers its management[21,22]. The 5-HT1A receptor, encoded by the 5-HTR1A gene, is implicated in the pathophysiology of depression and the pharmacodynamics of antidepressants among hereditary variables. The C-1019G polymorphism in the promoter region of the 5-HTR1A gene has garnered research interest because of its possible influence on receptor production and function[23]. This polymorphism is posited to affect the risk of developing MDD and to impact the efficacy of antidepressant treatment. The G allele is specifically correlated with modified 5-HT1A receptor density and may be connected with differences in antidepressant effectiveness. This study's originality is in its thorough and quantitative evaluation of the 5-HTR1A C-1019G polymorphism's effect on antidepressant effectiveness, enhancing the precision medicine strategy in psychiatric care[24]. This research emphasizes the necessity of combining genetic, clinical, and environmental aspects to enhance treatment options for MDD. Our findings support expanded genetic screening and comprehensive patient evaluation to improve therapeutic outcomes, highlighting the complexity and variability of MDD treatment responses.
The present meta-analysis examined the pharmacogenetic foundations of antidepressant efficacy, specifically emphasizing the 5-HTR1A gene C-1019G polymorphism, which is a significant focus in the investigation of MDD. Although the biological plausibility of the polymorphism influencing serotonergic neurotransmission exists, our findings indicate that its presence does not substantially impact the efficacy of antidepressants in people with a positive clinical response. The consistent absence of a meaningful connection across diverse genetic models, ranging from allelic to locus, highlights the complexity of treatment responses in MDD. Despite the odds ratios being near unity across models, none achieved statistical significance, suggesting that the C-1019G polymorphism may not be a dependable biomarker for forecasting treatment results. This observation remained valid despite the modest heterogeneity across the included studies, indicated by the I² values, which demonstrated variability but did not undermine the overall result.
Despite the limited number of studies included in this meta-analysis (n = 11), the results provide robust evidence regarding the association between the 5-HTR1A gene C-1019G polymorphism and antidepressant response in MDD. The statistical analyses reveal that none of the genetic models demonstrated a significant association between the polymorphism and antidepressant efficacy across various populations, with the allelic model (OR: 0.86, 95%CI: 0.69–1.12, P = 0.23), dominant model (OR: 0.81, 95%CI: 0.71–1.09, P = 0.13), and recessive model (OR: 0.80, 95%CI: 0.60–1.31, P = 0.37) all yielding non-significant results. The credibility of the meta-analysis is bolstered by the absence of publication bias, as confirmed by Egger's and Begg's tests, and supported by symmetrical funnel plot distributions. This is critical as publication bias can often distort the apparent effects in genetic association studies. Furthermore, despite moderate heterogeneity in some models, these findings suggest that the studies provide a representative and unbiased sample of the available evidence. The clinical implications of these results are significant, indicating a multifactorial model of antidepressant response where a single nucleotide polymorphism is not a decisive factor. This insight may encourage clinicians to adopt a more holistic approach, integrating genetic, environmental, and psychosocial factors when devising treatment strategies for MDD.
This meta-analysis possesses multiple limitations. The research focused exclusively on the 5-HTR1A C-1019G polymorphism, perhaps neglecting the effects of additional genetic variations or gene-environment interactions. The analysis relied on published data, which may be influenced by publication bias, despite statistical tests indicating otherwise. The variability in participants' demographics and the antidepressants utilized among the included trials may have influenced the results. Ultimately, the very limited sample sizes in certain genetic models may have diminished the statistical power to identify minor effects.
The current meta-analysis concluded that there is no statistically significant correlation between the 5HTR1A gene C-1019G polymorphism and the effectiveness of antidepressant treatment. Future research with larger sample sizes is necessary to confirm and possibly refine these results, thereby improving our comprehension of the genetic determinants affecting antidepressant response.
| 1. | Rybak YE, Lai KSP, Ramasubbu R, Vila-Rodriguez F, Blumberger DM, Chan P, Delva N, Giacobbe P, Gosselin C, Kennedy SH, Iskandar H, McInerney S, Ravitz P, Sharma V, Zaretsky A, Burhan AM. Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety. 2021;38:456-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Trivedi MH. Major Depressive Disorder in Primary Care: Strategies for Identification. J Clin Psychiatry. 2020;81:UT17042BR1C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | McIntyre RS, Lee Y, Carmona NE, Subramaniapillai M, Cha DS, Lee J, Lee JH, Alageel A, Rodrigues NB, Park C, Ragguett RM, Rosenblat JE, Almatham F, Pan Z, Rong C, Mansur RB. Characterizing, Assessing, and Treating Cognitive Dysfunction in Major Depressive Disorder. Harv Rev Psychiatry. 2018;26:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 645] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 5. | Basu A, Chadda RK, Sood M, Kaur H, Kukreti R. A preliminary association study between serotonin transporter (5-HTTLPR), receptor polymorphisms (5-HTR1A, 5-HTR2A) and depression symptom-clusters in a north Indian population suffering from Major Depressive Disorder (MDD). Asian J Psychiatr. 2019;43:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Hong CJ, Chen TJ, Yu YW, Tsai SJ. Response to fluoxetine and serotonin 1A receptor (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2006;6:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Dong ZQ, Li XR, He L, He G, Yu T, Sun XL. 5-HTR1A and 5-HTR2A genetic polymorphisms and SSRI antidepressant response in depressive Chinese patients. Neuropsychiatr Dis Treat. 2016;12:1623-1629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40322] [Article Influence: 10080.5] [Reference Citation Analysis (2)] |
| 9. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 10. | Basu A, Chadda RK, Sood M, Kaur H, Kukreti R. Association of serotonin transporter (SLC6A4) and receptor (5HTR1A, 5HTR2A) polymorphisms with response to treatment with escitalopram in patients with major depressive disorder: A preliminary study. Indian J Med Res. 2015;142:40-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Chang HS, Lee HY, Cha JH, Won ES, Ham BJ, Kim B, Lee MS. Interaction of 5-HTT and HTR1A gene polymorphisms in treatment responses to mirtazapine in patients with major depressive disorder. J Clin Psychopharmacol. 2014;34:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Illi A, Setälä-Soikkeli E, Viikki M, Poutanen O, Huhtala H, Mononen N, Lehtimäki T, Leinonen E, Kampman O. 5-HTR1A, 5-HTR2A, 5-HTR6, TPH1 and TPH2 polymorphisms and major depression. Neuroreport. 2009;20:1125-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Kato M, Fukuda T, Wakeno M, Okugawa G, Takekita Y, Watanabe S, Yamashita M, Hosoi Y, Azuma J, Kinoshita T, Serretti A. Effect of 5-HT1A gene polymorphisms on antidepressant response in major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Lin E, Chen PS, Chang HH, Gean PW, Tsai HC, Yang YK, Lu RB. Interaction of serotonin-related genes affects short-term antidepressant response in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Noro M, Antonijevic I, Forray C, Kasper S, Kocabas NA, Lecrubier Y, Linotte S, Mendlewicz J, Montgomery S, Snyder L, Souery D, Verbanck P, Zohar J, Massat I. 5HT1A and 5HT2A receptor genes in treatment response phenotypes in major depressive disorder. Int Clin Psychopharmacol. 2010;25:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Ramesh V, Venkatesan V, Ramasamy B. Role of serotonin transporter and receptor gene polymorphisms in treatment response to selective serotonin reuptake inhibitors in major depressive disorder. Hum Psychopharmacol. 2022;37:e2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Scutt G, Overall A, Scott R, Patel B, Hachoumi L, Yeoman M, Wright J. Does the 5-HT(1A) rs6295 polymorphism influence the safety and efficacy of citalopram therapy in the oldest old? Ther Adv Drug Saf. 2018;9:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Serretti A, Artioli P, Lorenzi C, Pirovano A, Tubazio V, Zanardi R. The C(-1019)G polymorphism of the 5-HT1A gene promoter and antidepressant response in mood disorders: preliminary findings. Int J Neuropsychopharmacol. 2004;7:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Serretti A, Fabbri C, Pellegrini S, Porcelli S, Politi P, Bellino S, Menchetti M, Mariotti V, Demi C, Martinelli V, Cappucciati M, Bozzatello P, Brignolo E, Brambilla P, Pae CU, Balestrieri M, De Ronchi D. No effect of serotoninergic gene variants on response to interpersonal counseling and antidepressants in major depression. Psychiatry Investig. 2013;10:180-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Zhao X, Jin Q, Wu L, Huang Y, Li J, Zhu G. Sertraline (Zoloft) response in major depressive disorder is not associated with three 5-HT1A receptor gene polymorphisms (rs6295, rs10042486, or rs1364043) in Chinese-Han patients. Psychiatr Genet. 2012;22:261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Jackson WC, Papakostas GI, Rafeyan R, Trivedi MH. Recognizing Inadequate Response in Patients With Major Depressive Disorder. J Clin Psychiatry. 2020;81:OT19037BR2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Schramm E, Klein DN, Elsaesser M, Furukawa TA, Domschke K. Review of dysthymia and persistent depressive disorder: history, correlates, and clinical implications. Lancet Psychiatry. 2020;7:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 23. | Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y, Sun N, Wang S, Shen Y. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2009;114:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G, Brodsky B, Arango V, Brent DA, Mann JJ. Human 5-HT1A receptor C(-1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol. 2004;7:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |