Published online Apr 19, 2022. doi: 10.5498/wjp.v12.i4.623
Peer-review started: August 31, 2021
First decision: December 12, 2021
Revised: December 20, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 19, 2022
Processing time: 224 Days and 11 Hours
Psychiatric disorders are common but underdiagnosed in cancer survivors. Research suggests that tumor type has an effect on the prevalence of clinically relevant depression, anxiety, comorbid anxiety-depression and posttraumatic stress disorder (PTSD).
To identify studies that examined the prevalence of clinically relevant levels of depression, anxiety, comorbid anxiety-depression and PTSD for patients with one or more tumor sites and compare those prevalences between cancer subtypes.
Four databases (PubMed, PsycInfo, PubPsych and the Cochrane Database) were searched and resulted in a total of 2387 articles to be screened. To be included, a study must have investigated cancer-free and posttreatment survivors using tools to assess clinically relevant levels of the listed psychiatric comorbidities. All articles were screened by two authors with a third author reviewing debated articles.
Twenty-six studies on ten different tumor types fulfilled all inclusion criteria and were included in the review. The studies showed heterogeneity regarding the study characteristics, number of participants, time since diagnosis, and asse
Psychiatric comorbidities are more frequent in cancer survivors than in the general population, as reflected by the prevalence of depression, anxiety, comorbid anxiety-depression and PTSD across all tumor subtypes. Developing generalized screening tools that examine psychological distress in cancer survivors up to at least ten years after diagnosis could help to understand and address the psychological burden of cancer survivors.
Core Tip: Psychiatric disorders are a common comorbidity in cancer survivors, even years after diagnosis. Studies have found that tumor type has an effect on the prevalence of clinically relevant depression, anxiety, comorbid anxiety-depression and posttraumatic stress disorder. This systematic review compared the prevalence of these four psychiatric disorders in cancer survivors among tumor types. The results suggest that there are variations in the prevalence of all comorbidities across and within cancer types. A future direction should be the development of a screening tool to regularly assess cancer survivors’ psychological distress for at least 10 years after the initial disease.
- Citation: Bach A, Knauer K, Graf J, Schäffeler N, Stengel A. Psychiatric comorbidities in cancer survivors across tumor subtypes: A systematic review. World J Psychiatry 2022; 12(4): 623-635
- URL: https://www.wjgnet.com/2220-3206/full/v12/i4/623.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i4.623
Before, during and after treatment, patients with cancer are exposed to a variety of factors (physical constraints, fatigue, financial problems, etc.) that may impact their psychological state. This is in addition to the possible trauma caused by a cancer diagnosis and treatment. With the number of cancer survivors growing due to longevity and medical progress, the evaluation of long-term psychological aftereffects and their predispositions becomes more relevant[1]. Over the last decades, the examination of psychiatric comorbidities in cancer survivors has become a growing research field. According to several studies, tumor type can have an impact on the risk of developing a psychiatric comorbidity[2-4]. This paper aimed to review the literature about psychiatric comorbidities in cancer survivors across cancer types to identify their commonalities and differences.
Cancer survivors experience several challenges even after finishing acute treatment. Chemotherapy, radiation and other kinds of treatment often bear the risk of long-term side effects. This can lead to clinically relevant levels of psychological distress, and survivors have an increased risk for mood alterations compared to the general population[5]. The simultaneous presence of two or more clinical conditions is referred to as comorbidity, which requires special attention when strategizing treatment[6]. Some of the most frequent psychiatric comorbidities in long-term cancer survivors are depression, generalized anxiety disorder and posttraumatic stress disorder (PTSD), all of which can depend on the type of cancer.
The response to each cancer type calls for unique treatment plans and exposes survivors to a particular risk of recurrence. Therefore, cancer survivors of different tumor types are exposed to several burdens, not only during the acute treatment phase but also after the treatment is finished. Studies have found that patients with specific tumor types may experience more psychological distress than others. Muzzatti et al[4] found that survivors with a history of breast cancer showed more anxiety and depression than those with a history of lymphoma or genitourinary tumors. Similarly, Götze et al[3] described that breast and skin cancer survivors showed the highest levels of anxiety and depression, whereas prostate cancer survivors showed the lowest levels. Another study showed significant variation in psychological distress across cancer types[7]. In contrast, there are studies that did not find a significant difference between cancer sites and clinical levels of depression, anxiety or PTSD[8-10]. In these studies, other patient characteristics, such as sex and age at the time of diagnosis, were proposed to have an influence on the prevalence of psychiatric comorbidities. Another argument by Deimling et al[10] is that with increasing time since diagnosis, cancer type and treatment-specific stressors are removed, and psychological stressors become more homogeneous.
Identifying whether there are specific influences on distress depending on a survivor’s cancer site could help to identify necessary adjustments to survivorship programs and medical follow-up treatments. To do so, it is important to know which patient characteristics and tumor entities have an effect on psychological distress and further effects on the development of psychiatric disorders due to disease-related burdens. Additionally, this could provide more insight into cancer site-specific psychological guidelines for the psychological care of cancer survivors after treatment.
Therefore, this systematic review aimed to identify studies that examined clinically relevant levels of depression, anxiety, comorbid anxiety-depression and PTSD across tumor types.
The systematic review was conducted according to the PRISMA statement criteria[11]. The review protocol is registered in PROSPERO, the International Prospective Register of Systematic Reviews (CRD42021253430).
We searched four databases between February 8th and 19th, 2021: PubMed, PsycInfo, PubPsyc and the Cochrane Database. Articles published in any year were included. Our search terms were as follows: [(Psychiatric OR psych*) AND (comorbidity OR disorder)] AND (cancer OR tumor OR neoplasm OR oncolog*) AND (survivor OR survivorship OR long-term).
The eligibility criteria were based on the five PICOS dimensions. P: The participants were cancer survivors with the following characteristics: Adults at the time of cancer diagnosis and not in (primary) acute treatment. Survivors were defined according to the World Health Organization (WHO) as patients who have had cancer and are, following treatment, now cured of the disease[12]. This implies that all studies where all/a subpopulation(s) of survivors were still in active treatment were excluded. I: Studies with any kind of intervention were excluded. C: A control group was not necessary. O: The outcomes were the prevalence of psychiatric comorbidities, more specifically, the clinically relevant levels of depression, anxiety, comorbid anxiety-depression and PTSD. S: The study designs included in the review were observational, cross-sectional and longitudinal designs.
The exclusion criteria were: (1) Studies with no cancer patients; (2) Studies with no survivors; (3) Studies with no psychiatric/psychological assessment; (4) Studies with a patient group < 18 years old at the time of cancer diagnosis; (5) Studies not in accordance with the predefined study designs; (6) Articles with missing information or not written in English; (7) Studies including an intervention; and (8) Studies that did not separate the different tumor types.
After removing duplicates, the articles were screened for relevant titles by two authors. For papers where the first two authors did not agree, a third author decided. The remaining articles were screened for abstracts again by the two authors, with the third author reviewing debated articles. Then, all three authors came to a consensus. One author screened the remaining articles for the full texts. The studies that were considered eligible were included in the review, and the relevant data were extracted.
The quality of each study was assessed according to the study design, participant selection and method of patient evaluation[13].
The included papers showed high heterogeneity in the number of participants, time since diagnosis and assessment tools used (Table 1). Furthermore, there were a limited number of articles per tumor site (e.g., 4 articles related to breast cancer vs 1 article related to brain tumors). Therefore, this review aimed to perform a descriptive data analysis rather than a meta-analysis. The descriptive analysis focused on the prevalence of the mentioned psychiatric comorbidities in cancer survivors with a focus on similarities and differences among the tumor types.
| Assessment tool | Used in study (No. of occurrences) | |
| EDS | Edinburgh Depression Scale | 1 (1) |
| HADS | Hospital Anxiety and Depression Scale | 1, 3, 5, 6, 8, 9, 15, 19, 21, 22, 23 (11) |
| SCID | Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders | 1, 22, 23, 26 (4) |
| PTSD-(inventory) scale | Self-report scale based on DSM-III-R criteria with items corresponding to PTSD symptoms | 2, 16 (2) |
| SCL-90 | Symptom Checklist 90 | 2 (1) |
| PCL-C/PCL-S | Posttraumatic Stress Disorder Checklist-Civilian Version/Posttraumatic Stress Disorder Checklist-Specific | 3, 5, 13, 14 (4) |
| UW-QOL | The University of Washington Quality of Life instrument - brief, self-administered questionnaire to analyze rates of depression | 11 (1) |
| IES | Impact of Event Scale | 7, 20 (2) |
| BDI | Beck Depression Inventory | 4, 18, 20 (3) |
| PHQ-9 | Patient Health Questionaire-9 | 10, 17 (2) |
| GAD-7 | Generalized Anxiety Disorder 7 | 10, 17, 24 (3) |
| GDS-SF/GDS-15 | Geriatric Depression Scale-Short Form/Geriatric Depression Scale-15 | 12, 25 (2) |
| DASS | Depression-Anxiety-Stress-Scale | 24 (1) |
| MINI | Mini International Neuropsychiatric Interview | 4 (1) |
| SAI | Spielberger State Anxiety Inventory | 20 (1) |
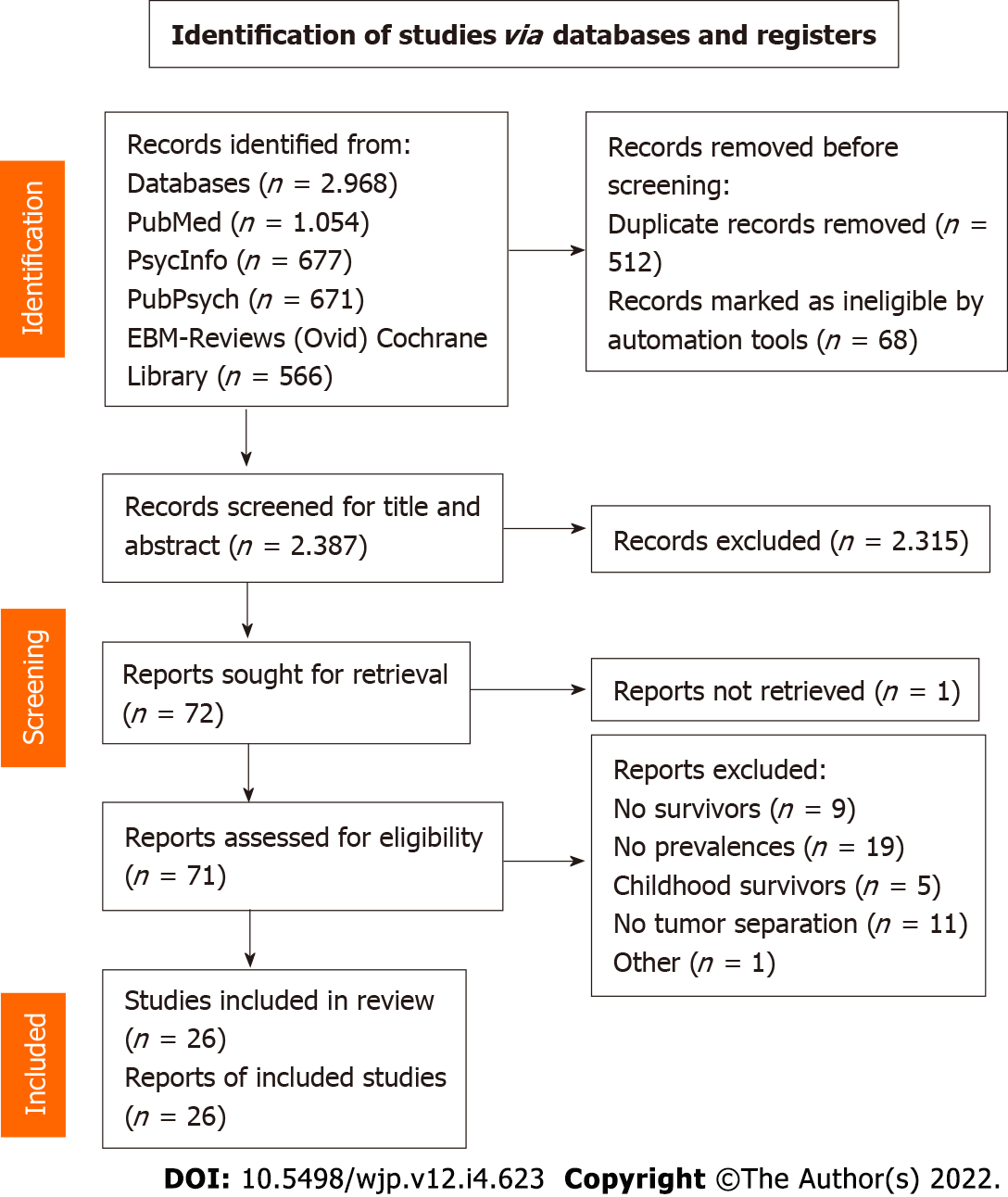
The literature search of the four scientific databases provided 2968 results. After removing duplicates, 2387 articles were left for screening. Title screening reduced the number to 102 articles, which was further filtered to 72 for full text screening. Finally, 26 studies were considered relevant to the topic and were included in the review (Figure 1). Table 2 shows the extracted data (reference, tumor type, study population, time since diagnosis, screening tools to assess psychiatric comorbidity, prevalence of comorbidity and potential bias) of the included articles. Several studies had to be excluded after full text screening because of missing reports of the prevalence in percentages and instead reporting the mean results of the questionnaires.
| No. | Ref./country
| Tumor site | Number of participants/gender/age in years | Time since diagnosis | Parameters and tests (related to psychiatric disorders) | Key results: Prevalence of clinical levels of: Anxiety/depression/PTSD/comorbid anxiety-depression | Possible bias |
| 1 | Alexander et al[26], 2010; United Kingdom | Breast | n = 200; 100% female; mean: 58.1, range: 29-89 | Mean time since last treatment: 10.1 mo | EDS; HADS; SCID | Depression: 9%; anxiety: 3.5%; comorbid: 1.5 | Selection bias; response bias |
| 2 | Amir et al[28], 2002; Israel | Breast | n = 39; 100% female; range: 37-60 | ≥ 5 yr | PTSD-scale; SCL-90 | Full PTSD: 18%; partial PTSD: 56% (additional) | Selection bias; response bias |
| 3 | Mehnert et al[25], 2008; Germany | Breast | n = 1083; 100% female; mean: 61.8, range: 31-81 | Average: 47 mo | HADS; PCL-C | Moderate to high anxiety: 38% (high: 20.1%); moderate to high depression: 22% (high: 11.3%); PTSD: 12% | Selection bias; response bias |
| 4 | Qiu et al[42], 2012; China | Breast | n = 505; 100% female; mean: 52.02 | Mean time after surgery: 17.6 mo | BDI; MINI | Depression: 20.59% | Response bias; performance bias |
| 5 | Vazquez et al[16], 2020; United States | Breast | n = 700; 100% female; median: 37, range: 17-40 | 144 d: HADS; 30 mo (PCL-S) | PCL-S; HADS | PTSS: 6.3%; depression: 8%; anxiety: 23% | Selection bias; response bias |
| 6 | Dahl et al[24], 2005; Norway | Testicular | n = 1408; 100% male; mean: 44.6 | Mean: 11.3 yr | HADS | Anxiety: 19.2%; depression: 9.7%; comorbid: 6.8% | Selection bias; response bias |
| 7 | Dahl et al[17], 2016; Norway | Testicular | n = 1418; 100% male; mean: 44.6 | Mean: 11 yr | IES | Full PTSD: 4.5%; partial PTSD: 6.4%; probable PTSD (combination of the 2): 10.9% | Selection bias; response bias |
| 8 | Fosså et al[43], 2003; Norway | Testicular | n = 791; 100% male; median: 44, range: 23-75 | Median: 12 yr | HADS | Anxiety: 19%; depression: 9% | Selection bias; response bias |
| 9 | Thorsen et al[15], 2005; Norway | Testicular | n = 1260; 100% male; median: 42 | Mean: 11 years | HADS | Anxiety: 20.2%; depression 9.7% | Selection bias; response bias |
| 10 | Vehling et al[19], 2016; Germany | Testicular | n = 164; 100% male; mean: 44.4 | Mean: 11.6 yr | GAD-7; PHQ-9 | Anxiety: 6.1%; depression: 7.9% | Selection bias; response bias |
| 11 | Chen et al[33], 2013; United States | Head and neck | n = 211; 58% male; median: 57, range: 21-93 | Disease free at least 1 yr | UW-QOL | Depression: 17% | Response bias |
| 12 | Lambert et al[21], 2005; United States | Head and neck | n = 694; 84.6% male; mean: 61.8 | At least 6 mo | GDS-SF | Depression: 44.1% | Selection bias; response bias |
| 13 | Moschopoulou et al[44], 2018; United Kingdom | Head and neck | n = 93; 58.1% male; mean: 66 | Mean: 6 yr | PCL-C | PTSD: 11.8% | Selection bias; response bias |
| 14 | Black et al[45], 2005; United Kingdom | Hodgkin’s lymphoma non- Hodgkin’s lymphoma; acute leukemia | n = 36; 50% female; adults | ? - complete remission | PCL-C | PTSD: 17% | Selection bias; response bias |
| 15 | Daniëls et al[46], 2014; The Netherlands | Hodgkin’s lymphoma | n = 180; 55% male; median: 46 | Mean: 4.6 yr | HADS | Anxiety: 23%; depression: 18% | Selection bias; response bias |
| 16 | Geffen et al[35], 2003; Israel | Hodgkin’s lymphoma; non-Hodgkin’s lymphoma | HD: n = 8; nHL: n = 36; 46% male; median: 51; range: 27-80 | At least 2 yr after treatment completion | PTSD-inventory scale | Full or partial PTSD: 32%; full PTSD: 18%; partial PTSD: 13% (additional) | Selection bias; response bias |
| 17 | Kuba et al[47], 2019; Germany | Hematological | n = 922; 57% male; range: 18-85 | 3 yr | PHQ-9; GAD-7 | Anxiety: 9%; depression: 15% | Selection bias; response bias |
| 18 | Han et al[22], 2013; Korea | Stomach | n = 391; 72.9% male; mean: 54.5 | Mean (time since operation): 27.4 mo | BDI | Depression: 43.9% | Selection bias; response bias |
| 19 | Hanprasertpong et al[48], 2017; Thailand | Cervical | n = 700; 100% female; mean: 53 | Completion of treatment 3 mo - 10 yr before study | HADS | Anxiety: 20.46%; depression: 9.44% | Selection bias; response bias |
| 20 | Urbaniec et al[18], 2011; Australia | Gynecological | n = 45; 100% female; mean: 56.7, range: 23-83 | Mean: 4 yr; range: 0.9-11.6 yr | BDI-II; SAI; IES-Revised | Anxiety: 28.9%; depression: 20%; probable PTSD: 15.6 | Selection bias; response bias |
| 21 | Krajewski et al[49], 2018; Germany | Melanoma | n = 561; 51.2% male; mean: 62.1 | 4 yr | HADS | Anxiety: 10.2%; depression: 10.3% | Selection bias; response bias |
| 22 | Rogiers et al[27], 2020; Belgium | Melanoma | n =25; 28% male; median: 58, range: 28-86 | Median: 30 mo | SCID-IV-CV; HADS | HADS: Anxiety: 32%; depression: 20%; comorbid: 12%. SCID: PTSD: 48%; depression: 0% | Selection bias; response bias; performance bias |
| 23 | Rogiers et al[14], 2020; Belgium | Melanoma | n = 17; 29% male; median: 57, range: 33-86 | Median: 5.6 yr | SCID-IV-CV; HADS | HADS: Anxiety: 35%; depression: 41%; comorbid: 30%. Interview: PTSD: 35%; depression: 11.76% | Selection bias; response bias; performance bias |
| 24 | Nicol et al[23], 2019; Australia | Brain | n = 65; 35.4% male; mean: 49.97; range: 22-75 | Mean: 5.29 yr | DASS-Depression; GAD-7 | Anxiety: 58.5%; depression: 43.1% | Selection bias; response bias |
| 25 | Recklitis et al[50], 2014; United States | Prostate | n = 693; 100% male; mean: 67.1 | Range: 3-8 yr | GDS-15 | Depression: 15% | Selection bias; response bias |
| 26 | Uchitomi et al[51], 2003; Japan | Lung | n = 212; 60.4% male; mean: 62.1, range: 22-83 | 1 mo after surgery | SCID, Revised; POMS scale | Depression: 8% | Selection bias; performance bias |
The studies included in the review were assessed for possible risks of bias. The natures of the study designs analyzed here are known to favor certain biases[13]. Table 2 shows the reviewed studies with the study design (self-report questionnaire, personal interview, etc.) and possible type of bias. All studies used a cross-sectional design, with only some having a matched comparison group, and therefore bear the risk of selection bias. Two qualities of the reviewed studies presented a risk of response bias: Cancer survivors with specific (psychological or physical) symptoms may be more likely to respond to a study invitation, and most studies were self-report questionnaires. Performance bias may have occurred in the studies that used personal interviews. Exclusion bias may be present in the studies where a specific group of participants was not included in the results (Table 2).
There was a wide range of study characteristics within the included articles. We extracted data for ten different broad tumor sites. For each site, the number of articles included were as follows: Breast (5), gynecological/cervical (2), hematological (4), testicular (5), prostate (1), head and neck (3), stomach (1), melanoma (3), brain (1) and lung (1). The number of participants ranged between 17[13] and 1260[14]. The studies were published between 2002 and 2020. The age of the participants ranged between 18 and 93 years. Seven studies included only women, and six studies included only men because of the specificity of the cancer site. The remaining thirteen studies included both men and women.
In the studies, psychiatric comorbidities were evaluated with a variety of assessment tools, including questionnaires and personal interviews. Table 1 shows all the assessment tools and their abbreviations with regard to the study they were used in. The most common questionnaire was the Hospital Anxiety and Depression Scale (HADS), which was used in eleven of the 26 studies. The assessment tool used in each article is shown in the summary of the findings (Table 2). Most articles included the screening of more than one psychiatric comorbidity (e.g., depression and anxiety), while others focused on only one.
The studies included in this review ranged from 144 d[16] to more than 11 years since diagnosis[17-19]. Some studies found an effect of time since cancer diagnosis and psychological distress. According to Mols et al[20], depressive symptoms declined over time, whereas anxiety scores stayed stable across a 4-year period.
Twenty-one of the 26 articles assessed the prevalence of depression in cancer survivors, including all ten tumor sites. Table 3 shows the studies organized by tumor site and the extracted percentages for clinical levels of depression. Comparing the prevalences among tumor types, a high variability, between 7.9% and 48%, can be seen. Whereas most tumor sites showed a range between 8% and 22% for clinical levels of depression, four cancer subtypes showed a much higher prevalence (above 40%): Head and neck[21], stomach[22], melanoma[14] and brain[23] cancer.
| No | Tumor site | Key result in % | Ref. | |||
| Depression | Anxiety | Comorbid anxiety-depression | PTSD | |||
| 1 | Breast | 9 | 3.5 | 1.5 | - | Alexander et al[26], 2010 |
| 2 | - | - | - | 18 | Amir et al[28], 2002 | |
| 3 | 22 | 38 | - | 12 | Mehnert et al[25], 2008 | |
| 4 | 20.6 | - | - | - | Qiu et al[42], 2012 | |
| 5 | 8 | 23 | - | 6.3 | Vazquez et al[16], 2020 | |
| 6 | Testicular | 9.7 | 19.2 | 6.8 | - | Dahl et al[24], 2005 |
| 7 | - | - | - | 4.5 | Dahl et al[17], 2016 | |
| 8 | 9 | 19 | - | - | Fosså et al[43], 2003 | |
| 9 | 9.7 | 20.2 | - | - | Thorsen et al[15], 2005 | |
| 10 | 7.9 | 6.2 | - | - | Vehling et al[19], 2016 | |
| 11 | Head and neck | 17 | - | - | - | Chen et al[33], 2013 |
| 12 | 44.1 | - | -- | - | Lambert et al[21], 2005 | |
| 13 | - | - | 11.8 | Moschopoulou et al[44], 2018 | ||
| 14 | Hematological | - | - | - | 17 | Black et al[45], 2005 |
| 15 | 18 | 23 | - | - | Daniels et al[21], 1976 | |
| 16 | - | - | - | 18 | Geffen et al[35], 2003 | |
| 17 | 15 | 9 | - | - | Kuba et al[47], 2019 | |
| 18 | Stomach | 43.9 | - | - | - | Han et al[22], 2013 |
| 19 | Cervical, gynecological | 9.4 | 20.5 | - | - | Hanprasertpong et al[48], 2017 |
| 20 | 20 | 28.9 | - | 15.6 | Urbaniec et al[18], 2011 | |
| 21 | Melanoma | 10.3 | 10.2 | - | - | Krajewski et al[49], 2018 |
| 22 | 20 | 32 | 12 | 48 | Rogiers et al[27], 2020 | |
| 23 | 41 | 35 | 30 | 35 | Rogiers et al[14], 2020 | |
| 24 | Brain | 43.1 | 58.5 | - | - | Nicol et al[23], 2019 |
| 25 | Prostate | 15 | - | - | - | Recklitis et al[50], 2014 |
| 26 | Lung | 8 | - | - | - | Uchitomi et al[51], 2003 |
Furthermore, within one cancer site, the prevalence varied. For testicular cancer survivors, the prevalence of depression was relatively stable across the four studies included in the review (between 7.9%[19] and 9.7%[15,24]). For patients with breast cancer, the prevalence varied between 8%[16] and 22%[25].
Fifteen of the eligible studies assessed the prevalence of clinical levels of anxiety in cancer survivors. Among these, six different tumor types were assessed: Breast, testicular, hematological, cervical/ gynecological, melanoma and brain tumors (Table 3). The percentage for anxiety ranged between 3.5% and 58.5%. A study on brain tumor survivors showed a high prevalence of clinical levels of anxiety of almost 60%[23], whereas across the other tumor sites, the prevalence ranged between 6.1%[19] and 20.2%[15].
Only four of the 26 articles assessed the prevalence of comorbid anxiety-depression in cancer survivors (Table 3). The included tumor types were breast, testicular and melanoma (2 studies). The two studies assessing the prevalence in melanoma patients showed a prevalence of comorbid anxiety-depression in up to 40%[14] of survivors. The smallest prevalence was found in breast cancer survivors, with a study indicating a prevalence of comorbid anxiety-depression of 1.5%[26].
Ten studies assessed PTSD in cancer survivors across 6 different tumor types. Whereas testicular cancer survivors showed a comparably low level of full PTSD with a prevalence of 4.5%[17], the two studies including melanoma patients showed numbers as high as 48%[14,27]. For breast cancer survivors, the prevalence ranged from 6.3%[16] to 18%[28].
This systematic review aimed to describe differences and commonalities between psychiatric comorbidities in cancer survivors across ten tumor types. Twenty-six studies that matched all the inclusion criteria and provided the prevalence of at least one of the four psychiatric comorbidities as a percentage were included.
Studies on psychological distress in cancer survivors found that there are risk factors for developing clinical levels of mood disorders. A systematic review on the prevalence of depression in breast cancer survivors reported several factors associated with depression: Fatigue, low income or poor financial status, low education level and younger age[29]. A review with testicular cancer survivors found that poorer psychological health was related to living alone, being unemployed or having a low socio-economic status and experiencing worse symptoms/side effects[30].
We observed differences across the studies in the prevalence of psychiatric comorbidities after a cancer diagnosis, even when patients were no longer in treatment and there was no sign of disease recurrence. It was not clear whether these differences were partly caused by the type of cancer. Other factors, such as the time since diagnosis, participant demographics, and the assessment tool, may have similarly influenced the prevalence of clinical levels of depression, anxiety, and PTSD. Andrykowski et al[31] found a wide range of reported anxiety and depression levels in cancer survivors, which was due to challenges in identifying the rate of psychological distress in cancer survivors. One of the difficulties is the variation in detecting a psychiatric disorder due to the range of screening tools and criteria. The studies in this review used a variety of assessment tools. Furthermore, the studies demonstrated a wide range of sample sizes and participant demographics, including the risk factors mentioned above. The country of origin has similarly been shown to have an effect on cancer survivors’ psychological distress. For example, a comparison between Hong Kong Chinese and German Caucasian women with breast cancer showed that greater unmet psychological needs were detected in Germany[32].
Our results show a higher prevalence of depression in cancer survivors than in the general population. Whereas some of the studies reported prevalences in the normal range, more than half of the prevalences were 15% or higher. Furthermore, one longitudinal study[33] found that the prevalence of depression did not differ significantly over the course of five years for head and neck cancer survivors. A comparison of cancer types regarding depression showed consistently lower levels of depression in testicular cancer survivors than in breast cancer survivors, where the prevalence varied from 8% to 22%. Patients with several tumor entities, namely, head and neck, stomach, melanoma and brain tumors, demonstrate higher levels of depression, between 41% and almost 50%, indicating the need for special support for these groups of cancer survivors.
Anxiety scores were reported by 15 studies and showed a very wide range of prevalences from 3.5% to almost 60%. Moreover, our review showed that anxiety prevalence was higher than the prevalence of clinical levels of depression. Similarly, among United States adults, data on anxiety disorders shows a higher prevalence than the prevalence of depression[34]. The highest prevalences of anxiety were seen in breast, melanoma and brain tumor survivors, although one study on breast cancer survivors reported a prevalence as low as 3.5%. The study by Nicol et al[23] with brain tumor survivors reported an especially high number of survivors showing clinically relevant levels of anxiety, with a prevalence of 58.5%.
Comorbid anxiety-depression was assessed in only four of the 26 included studies. A useful comparison among cancer types is therefore difficult. In contrast to the two studies on breast and testicular cancer survivors that reported a prevalence of 1.5% and 6.8%, respectively, the prevalences in two studies with melanoma survivors were higher (up to 40%)[14,27]. For all three of the previously mentioned psychiatric comorbidities, melanoma survivors seemed to show relatively high prevalences, which might indicate a distinctive demand for psychological support for this survivor group.
Ten studies examined posttraumatic stress syndrome in cancer survivors. Geffen et al[35] compared survivors who either had Hodgkin’s disease or non-Hodgkin’s lymphoma with a matched control group that had experienced at least one traumatic life event. They did not find significant differences between the survivors and control group in the occurrence of posttraumatic stress symptoms, suggesting that a cancer diagnosis might have the same impact as experiencing a traumatic event. Again, studies on melanoma cancer survivors showed a particularly high prevalence of PTSD (35% and 48%), which was assessed by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Clinical Version, at a median time of 30 mo and 5.6 years after the diagnosis, respectively[14,27]. Another study investigated the occurrence of PTSD in testicular cancer survivors 11 and 19 years after diagnosis and found that the prevalence of clinically relevant PTSD symptomatology was reduced by more than half at the latter time point[17].
This review contains some limitations, with the most obvious being the limited number of studies per cancer site. Since we employed stringent inclusion and exclusion criteria, many studies were not included in the review. It was important to include only cancer survivors based on the WHO definition, meaning that the survivors were not going through acute treatment. This exclusion criterion was chosen to ensure that the prognosis and side effects of the treatment were not likely to influence the results of a psychiatric assessment. Several studies included a noteworthy number of survivors who still received some kind of treatment, from radiotherapy to immunotherapy[3,36]. This limitation is likely influenced by the lack of a unique definition of cancer survivorship[37], which may have complicated the literature search.
Some studies have already investigated psychiatric comorbidities across different cancer types[4,9]. These studies did not include the separate prevalences per tumor type in their papers and therefore could not be reported in this review. Several studies reported the mean results on the questionnaires; however, the prevalence of clinical levels of depression, anxiety or PTSD could not be extracted. This review focused on four types of psychiatric comorbidities in cancer survivors, which represent the most common mental health disorders. Less common psychiatric comorbidities, such as acute psychosis, are likely present in cancer survivors (although at very low prevalence) but were beyond the scope of this review. Future work should address these.
We explored the extracted data with a focus on differences among cancer types. The studies that were reviewed displayed a high heterogeneity in key study characteristics (e.g., the number of participants, time since diagnosis, assessment tools), which may have had a significant influence on the results and was not considered in our review. The various screening tools possibly measure psychological distress and clinical relevance in a way that cannot be easily compared[31]. A systematic review on the HADS indicated that the assessment tool might underestimate true levels of anxiety and depressive symptoms because it does not include somatic symptoms[38]. This may have impacted the generalizability of the HADS-based results.
The increased prevalence of clinical levels of psychological distress for cancer survivors remains an issue to be adequately addressed. Whereas many survivorship programs are being developed, the specific needs of cancer survivors depending on their own personal experiences have not yet been widely explored. Beutel et al[39] suggest general screening even 10 years after diagnosis, which would show the objective and subjective needs of each cancer survivor. Götze et al[40] supported this recommendation following their examination of emotional distress in cancer survivors. They compared a group of survivors five years after diagnosis with a group 10 years post-diagnosis and found no significant difference in emotional distress between the groups. However, a significant difference between tumor entities was detected, with breast and skin cancer survivors showing the highest levels of anxiety and depression and prostate cancer survivors showing the lowest levels. Furthermore, Kypriotakis et al[41] compared long-term cancer survivors of different tumor sites at four different time points. They found that cancer stage at the time of diagnosis was a significant predictor of initial depressive symptoms. Therefore, a future direction could be the development of screening tools to repeatedly measure cancer survivors’ psychological distress up to 10 years after the last acute treatment phase. According to Beutel et al[39], such screening would include survivors who are below the threshold of a mental disorder but still have difficulties adjusting to being a cancer survivor.
The articles included in this review showed high heterogeneity in several study characteristics (the number of participants, time since diagnosis, assessment tools, etc.) and showed that psychological distress in survivors is dependent on multiple factors. We aimed to describe the differences among tumor types, which were limited by missing data and/or the lack of a clear definition for survivorship. More research is needed that evaluates the specific psychological needs of cancer survivors and how to address them in survivor programs. Future research should have a clear definition of cancer survivorship and take participant characteristics such as the tumor subtype, the time since diagnosis and demographics into account. Furthermore, our results strongly suggest future guidelines for psychiatric and distress screenings for at least ten years after a cancer diagnosis, even when there is no sign of recurrence.
Psychiatric disorders are common but underdiagnosed in cancer survivors. Research suggests that tumor type has an effect on the prevalence of clinically relevant depression, anxiety, comorbid anxiety-depression and posttraumatic stress disorder (PTSD) symptoms.
Detecting differences in the prevalence of four common mental disorders that can occur as a comorbidity in cancer survivors might lead to a better understanding of cancer survivors’ psychological distress. This might help to address the psychological concerns of cancer survivors more effectively.
The aim of this review was to identify studies in which clinically relevant levels of common mental disorders in cancer survivors were examined. The prevalence rates were compared among different cancer types.
Four databases were searched for studies that investigated cancer-free, posttreatment survivors with screening tools that assess clinically relevant levels of four common mental disorders. Two authors screened all articles, with a third author reviewing debated articles.
Twenty-six studies were included in the article and indicated the prevalence of one or more of the four mental disorders. Ten different tumor types were examined in the included papers. Generally, all four comorbidities show higher prevalences in cancer survivors than in the general population. The studies showed heterogeneity regarding the study characteristics, number of participants, time since diagnosis, and assessment tools. Each comorbid disorder had a variable prevalence across tumor subtypes. Within one cancer site, the prevalence also varied considerably among the studies.
Psychiatric comorbidities are high in cancer survivors relative to the general population, as reflected by the prevalences of depression, anxiety, comorbid anxiety-depression and PTSD across all tumor types. This enhanced distress is clinically relevant even years after a cancer diagnosis. The lack of a concise definition of cancer survivorship likely contributes to the high heterogeneity among studies focusing on cancer survivors’ psychological distress, which might hinder significant comparisons among studies.
Developing generalized screening tools that examine psychological distress in cancer survivors for at least ten years after diagnosis could help to understand and address the psychological burdens of the survivors.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cawthorpe DR, Canada; Wang D, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Mayer DK, Nasso SF, Earp JA. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017;18:e11-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 2. | Hartung TJ, Brähler E, Faller H, Härter M, Hinz A, Johansen C, Keller M, Koch U, Schulz H, Weis J, Mehnert A. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Götze H, Köhler N, Taubenheim S, Lordick F, Mehnert A. Polypharmacy, limited activity, fatigue and insomnia are the most frequent symptoms and impairments in older hematological cancer survivors (70+): Findings from a register-based study on physical and mental health. J Geriatr Oncol. 2019;10:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Muzzatti B, Giovannini L, Romito F, Cormio C, Barberio D, Abate V, De Falco F, Annunziata MA. Psychological health in long-term cancer survivorship: an Italian survey on depression and anxiety. Psychol Health Med. 2017;22:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Yi JC, Syrjala KL. Anxiety and Depression in Cancer Survivors. Med Clin North Am. 2017;101:1099-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 6. | Jakovljević M, Ostojić L. Comorbidity and multimorbidity in medicine today: challenges and opportunities for bringing separated branches of medicine closer to each other. Psychiatr Danub. 2013;25 Suppl 1:18-28. [PubMed] |
| 7. | Boyes AW, Girgis A, D'Este C, Zucca AC. Flourishing or floundering? J Affect Disord. 2011;135:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Wachen JS, Patidar SM, Mulligan EA, Naik AD, Moye J. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014;23:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Bamonti PM, Moye J, Naik AD. Pain is associated with continuing depression in cancer survivors. Psychol Health Med. 2018;23:1182-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Deimling GT, Kahana B, Bowman KF, Schaefer ML. Cancer survivorship and psychological distress in later life. Psychooncology. 2002;11:479-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47144] [Article Influence: 2946.5] [Reference Citation Analysis (0)] |
| 12. | World Health Organization. Diagnosis and treatment. [cited 15 July 2021]. Available from: https://www.who.int/cancer/treatment/en. |
| 13. | Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 2014;122:711-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 316] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 14. | Rogiers A, Leys C, Lauwyck J, Schembri A, Awada G, Schwarze JK, De Cremer J, Theuns P, Maruff P, De Ridder M, Bernheim JL, Neyns B. Neurocognitive Function, Psychosocial Outcome, and Health-Related Quality of Life of the First-Generation Metastatic Melanoma Survivors Treated with Ipilimumab. J Immunol Res. 2020;2020:2192480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Thorsen L, Nystad W, Stigum H, Dahl O, Klepp O, Bremnes RM, Wist E, Fosså SD. The association between self-reported physical activity and prevalence of depression and anxiety disorder in long-term survivors of testicular cancer and men in a general population sample. Support Care Cancer. 2005;13:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Vazquez D, Rosenberg S, Gelber S, Ruddy KJ, Morgan E, Recklitis C, Come S, Schapira L, Partridge AH. Posttraumatic stress in breast cancer survivors diagnosed at a young age. Psychooncology. 2020;29:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Dahl AA, Østby-Deglum M, Oldenburg J, Bremnes R, Dahl O, Klepp O, Wist E, Fosså SD. Aspects of posttraumatic stress disorder in long-term testicular cancer survivors: cross-sectional and longitudinal findings. J Cancer Surviv. 2016;10:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Urbaniec OA, Collins K, Denson LA, Whitford HS. Gynecological cancer survivors: assessment of psychological distress and unmet supportive care needs. J Psychosoc Oncol. 2011;29:534-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Vehling S, Mehnert A, Hartmann M, Oing C, Bokemeyer C, Oechsle K. Anxiety and depression in long-term testicular germ cell tumor survivors. Gen Hosp Psychiatry. 2016;38:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Mols F, Schoormans D, de Hingh I, Oerlemans S, Husson O. Symptoms of anxiety and depression among colorectal cancer survivors from the population-based, longitudinal PROFILES Registry: Prevalence, predictors, and impact on quality of life. Cancer. 2018;124:2621-2628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 21. | Lambert MT, Terrell JE, Copeland LA, Ronis DL, Duffy SA. Cigarettes, alcohol, and depression: characterizing head and neck cancer survivors in two systems of care. Nicotine Tob Res. 2005;7:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Han KH, Hwang IC, Kim S, Bae JM, Kim YW, Ryu KW, Lee JH, Noh JH, Sohn TS, Shin DW, Yun YH. Factors associated with depression in disease-free stomach cancer survivors. J Pain Symptom Manage. 2013;46:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Nicol C, Ownsworth T, Cubis L, Nguyen W, Foote M, Pinkham MB. Subjective cognitive functioning and associations with psychological distress in adult brain tumour survivors. J Cancer Surviv. 2019;13:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Dahl AA, Haaland CF, Mykletun A, Bremnes R, Dahl O, Klepp O, Wist E, Fosså SD. Study of anxiety disorder and depression in long-term survivors of testicular cancer. J Clin Oncol. 2005;23:2389-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Mehnert A, Koch U. Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. J Psychosom Res. 2008;64:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Alexander S, Palmer C, Stone PC. Evaluation of screening instruments for depression and anxiety in breast cancer survivors. Breast Cancer Res Treat. 2010;122:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Rogiers A, Leys C, De Cremer J, Awada G, Schembri A, Theuns P, De Ridder M, Neyns B. Health-related quality of life, emotional burden, and neurocognitive function in the first generation of metastatic melanoma survivors treated with pembrolizumab: a longitudinal pilot study. Support Care Cancer. 2020;28:3267-3278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Amir M, Ramati A. Post-traumatic symptoms, emotional distress and quality of life in long-term survivors of breast cancer: a preliminary research. J Anxiety Disord. 2002;16:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Zainal NZ, Nik-Jaafar NR, Baharudin A, Sabki ZA, Ng CG. Prevalence of depression in breast cancer survivors: a systematic review of observational studies. Asian Pac J Cancer Prev. 2013;14:2649-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 30. | Smith AB, Rutherford C, Butow P, Olver I, Luckett T, Grimison P, Toner G, Stockler M, King M. A systematic review of quantitative observational studies investigating psychological distress in testicular cancer survivors. Psychooncology. 2018;27:1129-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Andrykowski MA, Lykins E, Floyd A. Psychological health in cancer survivors. Semin Oncol Nurs. 2008;24:193-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Lam WW, Au AH, Wong JH, Lehmann C, Koch U, Fielding R, Mehnert A. Unmet supportive care needs: a cross-cultural comparison between Hong Kong Chinese and German Caucasian women with breast cancer. Breast Cancer Res Treat. 2011;130:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Chen AM, Daly ME, Vazquez E, Courquin J, Luu Q, Donald PJ, Farwell DG. Depression among long-term survivors of head and neck cancer treated with radiation therapy. JAMA Otolaryngol Head Neck Surg. 2013;139:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Substance Abuse and Mental Health Data Archive. National Survey on Drug Use and Health (NSDUH). 2019. [cited 15 July 2021]. Available from: https://www.datafiles.samhsa.gov/dataset/national-survey-drug-use-and-health-2019-nsduh-2019-ds0001. |
| 35. | Geffen DB, Blaustein A, Amir MC, Cohen Y. Post-traumatic stress disorder and quality of life in long-term survivors of Hodgkin's disease and non-Hodgkin's lymphoma in Israel. Leuk Lymphoma. 2003;44:1925-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Jung A, Crandell JL, Nielsen ME, Mayer DK, Smith SK. Post-traumatic stress disorder symptoms in non-muscle-invasive bladder cancer survivors: A population-based study. Urol Oncol. 2021;39:237.e7-237.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Marzorati C, Riva S, Pravettoni G. Who Is a Cancer Survivor? J Cancer Educ. 2017;32:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 38. | Cosco TD, Doyle F, Ward M, McGee H. Latent structure of the Hospital Anxiety And Depression Scale: a 10-year systematic review. J Psychosom Res. 2012;72:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 39. | Beutel ME, Fischbeck S, Binder H, Blettner M, Brähler E, Emrich K, Friedrich-Mai P, Imruck BH, Weyer V, Zeissig SR. Depression, anxiety and quality of life in long-term survivors of malignant melanoma: a register-based cohort study. PLoS One. 2015;10:e0116440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Götze H, Friedrich M, Taubenheim S, Dietz A, Lordick F, Mehnert A. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support Care Cancer. 2020;28:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 41. | Kypriotakis G, Deimling GT, Piccinin AM, Hofer SM. Correlated and Coupled Trajectories of Cancer-Related Worries and Depressive Symptoms among Long-Term Cancer Survivors. Behav Med. 2016;42:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Qiu J, Yang M, Chen W, Gao X, Liu S, Shi S, Xie B. Prevalence and correlates of major depressive disorder in breast cancer survivors in Shanghai, China. Psychooncology. 2012;21:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Fosså SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Moschopoulou E, Hutchison I, Bhui K, Korszun A. Post-traumatic stress in head and neck cancer survivors and their partners. Support Care Cancer. 2018;26:3003-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 45. | Black EK, White CA. Fear of recurrence, sense of coherence and posttraumatic stress disorder in haematological cancer survivors. Psychooncology. 2005;14:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Daniëls LA, Oerlemans S, Krol AD, Creutzberg CL, van de Poll-Franse LV. Chronic fatigue in Hodgkin lymphoma survivors and associations with anxiety, depression and comorbidity. Br J Cancer. 2014;110:868-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Kuba K, Esser P, Mehnert A, Hinz A, Johansen C, Lordick F, Götze H. Risk for depression and anxiety in long-term survivors of hematologic cancer. Health Psychol. 2019;38:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Hanprasertpong J, Geater A, Jiamset I, Padungkul L, Hirunkajonpan P, Songhong N. Fear of cancer recurrence and its predictors among cervical cancer survivors. J Gynecol Oncol. 2017;28:e72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Krajewski C, Benson S, Elsenbruch S, Schadendorf D, Livingstone E. Predictors of quality of life in melanoma patients 4 years after diagnosis: Results of a nationwide cohort study in Germany. J Psychosoc Oncol. 2018;36:734-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Recklitis CJ, Zhou ES, Zwemer EK, Hu JC, Kantoff PW. Suicidal ideation in prostate cancer survivors: understanding the role of physical and psychological health outcomes. Cancer. 2014;120:3393-3400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Uchitomi Y, Mikami I, Nagai K, Nishiwaki Y, Akechi T, Okamura H. Depression and psychological distress in patients during the year after curative resection of non-small-cell lung cancer. J Clin Oncol. 2003;21:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |